Abstract
IL-18 has a well-established role in pro-inflammatory responses in the islets in type 1 diabetes. Here, we identify a distinctive role for IL-18 in expanding pathogenic T cells in the periphery of NOD mice. Well in advance of disease onset, the periphery of IL-18-deficient mice exhibits reduced T cell turnover, an increased prevalence of naïve and quiescent T cells, emergence of fewer effector T cells, and disease protection. Islet-reactive T cells fail to become activated in the lymphoid organs of mice lacking IL-18 and their rapid expansion is inhibited. IL-18 secretion by antigen presenting cells increases with advancing disease and is required for expression of its receptor on T cells. Our results demonstrate that induction of the IL-18 receptor reflects a critical stage of autoreactive T cell activation and expansion on the pathway toward effector T cell differentiation. This study therefore assigns a novel role to IL-18 for expanding the pool of islet-destructive T cells during pre-diabetes. This report highlights a new basic mechanism in type 1 diabetes pathogenesis and suggests that targeting the IL-18 pathway should be explored as a potential treatment strategy.
Keywords: Type 1 diabetes, T cells, IL-18
1. Introduction
The NOD mouse model has been instrumental in studies of the pathogenesis of type 1 diabetes, an autoimmune disease resulting from T cell-mediated destruction of the beta cells in the pancreatic islets. Type 1 diabetes is characterized by the over-production of Th1 cytokines, such as IFN-γ and IL-12. IFN-γ is produced by pancreas-infiltrating T cells in NOD mice [1] where it promotes homing of inflammatory cells to this site [2, 3] and contributes directly to β cell apoptosis [4, 5]. IL-12 participates in the development of type 1 diabetes by stimulating the infiltration of APC and T cells into the pancreas [6]. Notably however, neither IFN-γ signaling nor IL-12 expression is required for autoimmunity in NOD mice [1, 7, 8] and IFN-γ injection can paradoxically reduce the severity of insulitis [9, 10]. More recently, the Th17 subset of CD4+ T cells, which produce the pro-inflammatory cytokines IL-17A and IL-17F, has been implicated in type 1 diabetes [11], although their roles in disease pathogenesis are still controversial [12, 13]. Thus, the hierarchy of cytokine involvement in the pathogenesis of type 1 diabetes requires further clarification, particularly to provide insight into possible therapeutic strategies.
The objective of the current study was to investigate the role of IL-18, also known as the IFN-γ inducing factor [14], utilizing the NOD mouse model of type 1 diabetes. Production of bioactive IL-18 involves caspase-1 cleavage of the inactive precursor molecule (pro-IL-18) within inflammasomes in response to innate stimuli [15]. The receptor for IL-18 (IL-18R) is expressed constitutively at low or negligible levels on peripheral T cells but is upregulated upon stimulation by IL-12 [16–18]. APC producing IL-12 and IL-18 therefore stimulate antigen independent proliferation and IFN-γ secretion by T cells [19–22]. Interestingly, IL-18 is also produced by mouse beta cells, and its upregulation correlates with islet damage in an allograft model and with advancing insulitis in NOD mice, preceding a rise in IFN-γ expression in inflamed islets [23–25]. Indeed, IL-18 is considered a biomarker of tissue injury in numerous autoimmune and inflammatory disorders [26].
In this report, we present a novel perspective on the role of IL-18 in autoimmunity as a factor that exerts a primary role in self-reactive T cell activation. IL-18 is indispensable for the spontaneous development of autoimmunity in NOD mice, and in its absence, a sizeable pool of islet-reactive T cells never accrues. IL-18 production by APC correlates with advancing disease and drives vigorous expansion of self-reactive T cells in the lymphoid organs during pre-diabetes, leading to the accumulation of islet-reactive T cells. We also demonstrate that IL-18R expression identifies autoreactive T cells that divide rapidly in response to IL-18. Hence, this report provides new insight into the T cell population that precipitates type 1 diabetes and presents a novel perspective on the role of IL-18 in autoimmunity.
2. Materials and Methods
2.1. Mice
All mice were maintained under specific pathogen-free conditions in the rodent breeding colonies at The Scripps Research Institute (La Jolla, CA) and the University of Nebraska Medical Center (Omaha, NE) where these studies were conducted. B6.IL18KO mice [27] were backcrossed to the NOD genetic background for 8 generations utilizing a speed congenic screening strategy to select progeny at each cross. Fluorescent genotyping was carried out to verify that the N8 mice were NOD-derived at all regions containing Idd loci linked to diabetes susceptibility. The exception was the 20cM interval encompassing the Idd2 locus on mouse chromosome 9, since IL-18 is itself a primary candidate for the Idd2 susceptibility gene [28]. B6 and IL-18KO B6 mice were originally obtained from The Jackson Laboratories (Bar Harbor, ME). 8.3NODScid mice [29] were kindly provided by Dr. Pere Santamaria (University of Calgary, Alberta, Canada). BDC2.5 NOD mice [30] were a kind gift from Drs. Diane Mathis and Christophe Benoist (Harvard University, Cambridge, MA). Female mice were used for all experiments. All animal experiments were performed in compliance with the regulations of IACUC.
2.2. Diabetes Incidence
Diabetes was assessed by weekly measurement of venous blood glucose levels using Glucometer Elite strips (Bayer, Elkhart, IN). Mice with two consecutive blood glucose measurements >300 mg/dl were considered diabetic.
2.3. Histological analysis of islets
Freshly isolated pancreas tissue was removed from NOD, IL-18KO NOD or NODScid mice, fixed in 10% neutral buffered formalin, and embedded in paraffin. Tissues were cut into 4 µm thick sections from two levels through the tissue, sampled at intervals of at least 120 µm apart to permit analysis of independent islet infiltrates. The sections were stained with H&E or with Ab against insulin or glucagon (10 ng/mL; DAKO, Carpinteria, CA) followed by counterstaining with hematoxylin. The islets were scored by a blinded observer. The extent of islet infiltration was categorized as follows: “no insulitis”, “peri-insulitis”, indicated by peri-vascular and peri-islet infiltrates, “insulitis”, defined by the presence of visible infiltrates, or “destroyed”, marked by islet remnants. To identify islet remnants, insulin-stained sections were matched to serial sections stained for glucagon. For experiments using frozen tissue sections, pancreata were snap-frozen in liquid nitrogen, embedded in OCT compound, and stored at −70° C. Preparation of serial frozen sections was performed with a Leica Cryostat (Leica, Canada). The sections were cut at 10 µm and stained with anti-CD4 and anti-CD8 Ab (eBioscience, San Diego, CA). Adjacent sections were stained with the appropriate secondary Ab.
2.4. Isolation and culture of islets
Islets were isolated from NOD pancreas tissue as described previously [31]. For detection of IL-18 secretion, the islets were cultured in RPMI 1640 medium supplemented with 10% FCS, 50 units/mL penicillin, and 50 µg/mL streptomycin for 48 h.
2.5. Adoptive transfers of NOD and IL-18KO NOD cells to monitor diabetes incidence
Single cell suspensions were prepared from spleens or pancreatic lymph nodes from 6–8 wk old NOD and IL-18KO NOD mice or 4–6 wk-old BDC2.5 NOD mice followed by lysis of red blood cells using ammonium chloride lysis buffer. Splenocytes or pancreatic lymph node-derived cells were counted, suspended in PBS, and injected i.v. into 5–7 wk old NODScid recipient mice. In some experiments, CD4+ cells were enriched from pooled pancreatic lymph nodes by negative selection using magnetic separation columns (Miltenyi Biotec, Auburn, CA) for injection into NODScid recipients. Some recipients were also treated daily with recombinant murine IL-18 (2.5 µg/mouse/d i.p., MBL International, Woburn, MA) or an equivalent volume of PBS. Some recipients were also treated with IL-18BP:Fc (30 µg/mouse i.p., R&D Systems, Minneapolis, MN) or IgG1:Fc (30 µg/mouse i.p., R&D Systems) every other day beginning on day 0 at the time of adoptive transfer. The recipients were then euthanized at the indicated time points following adoptive transfer and their lymphoid organs or pancreata were harvested for analysis.
2.6. Adoptive transfers of 8.3 NODScid and BDC2.5 NOD splenocytes to measure T cell activation
Splenocytes from 4–6 wk old 8.3 NODScid or BDC2.5 NOD mice were pooled for red blood cell lysis. CFSE labeling was performed by incubating 5 × 107 cells/mL in PBS with 5mM CFSE (Molecular Probes, Eugene, OR) for 10 min at 37°C. The CFSE reaction was quenched by adding 5 mL of cold FCS and the cells were washed twice in PBS prior to i.v. injection of 2.0 × 107 cells into 6–9 wk old NOD, IL-18KO NOD or NODScid recipients. The recipients were age-matched for each experiment. Experiments were also conducted in which the adoptive transfer recipients were treated with IL-18 (2.5 µg/mouse/d i.p.), IL-18BP:Fc (30 µg/mouse/d i.p., R&D Systems), IgG1:Fc control protein (30 µg/mouse/d i.p., R&D Systems) or an equivalent volume of PBS. These reagents were administered daily, beginning at the time of adoptive transfer. The efficacy of IL-18BP at neutralizing IL-18 bioactivity was verified by stimulating splenocytes from IL-18BP-treated mice in vitro with anti-CD3/anti-CD28 and measuring the intracellular IFN-γ expression by flow cytometry. This dose of IL-18BP led to a 60–70% reduction in the percentages of IFN-γ+ T cells when compared to PBS injection (data not shown). A sample of the injected donor cells was taken for flow cytometry to verify that 100% of the injected cells were CSFE+ (data not shown). 8.3 NODScid and BDC2.5 NOD T cells were also stained with Ab against Vβ8.1/8.2 and Vβ4, respectively, and co-stained with anti-CD44 Ab (all from BD Pharmingen, San Diego, CA) for flow cytometry in order to demonstrate that the majority (70–75%) of the injected cells were CD44low (data not shown).
Pancreatic lymph nodes, inguinal lymph nodes, and spleens from the recipient mice were harvested at three or five days following adoptive transfer, single cell suspensions were prepared in sterile Hanks medium containing 5% FCS, and the cells were stained for flow cytometry. In some experiments, the data were used to estimate the number of CFSE-labeled cells that homed to the lymphoid organs before expansion by dividing the number of cells in each CSFE division peak by 1/(2×) (where × represents the cell division number within the peak) and adding the sum for all the peaks [32]. The absolute numbers of transgenic T cells present in each lymphoid organ were calculated by multiplying the total lymphocyte counts for each organ, as determined by trypan blue exclusion, by the percentages of gated cells from flow cytometry.
2.7. Ab and flow cytometry
Red blood cell-lysed single cell suspensions were prepared from the pancreatic lymph nodes, inguinal lymph nodes, and spleens of individual mice and the cells were counted by trypan blue exclusion. Fc receptors were blocked by incubation with anti-CD16/CD32 Ab (BD Pharmingen) and cells were incubated on ice for 30 min with Ab directed against cell surface molecules or with the appropriate isotype control Ab. The anti-mouse IL-18Rα Ab was purchased from R&D Systems; all other Ab were purchased from BD Pharmingen or eBioscience (San Diego, CA). To assess apoptosis, freshly isolated single cell suspensions of spleens and pancreatic lymph nodes were stained with Ab against cell surface markers and with annexin V and 7-AAD according to the manufacturer’s instructions (BD Biosciences). Flow cytometry was performed using a FACSCaliber cytometer and CellQuest software or digital LSR II with DiVa and FlowJo software.
2.8. BrdU labeling experiments
Mice were given 0.8 mg/mL BrdU (Sigma, St. Louis, MO) dissolved in sterile drinking water for 4 or 6 consecutive days, changed daily. Cell surface staining was done as described followed by intracellular staining for detection of BrdU incorporation according to the manufacturer’s instructions (BD Pharmingen).
2.9. Analysis of intracellular and secreted cytokines
Splenocytes (1–2 × 106 cells/well) were activated in vitro with plate (48-well) bound anti-CD3 Ab (1 µg/ml, 145-2C11 clone) and soluble anti-CD28 Ab (1 µg/ml, 7.51 clone) in complete RPMI 1640 (containing 10% FCS, 50 units/ml penicillin and 50 µg/ml streptomycin, 10 mM HEPES buffer, 2 mM l-glutamine) in the presence of GolgiPlug™ (BD Pharmingen, 1 µL/ml). Five h post-stimulation, the cells were harvested, washed and processed for intracellular IFN-γ and IL-17F staining according to the manufacturer's protocol (BD Pharmingen) and flow cytometry was performed. Experiments were also carried out using unstimulated lymphocytes as negative controls (data not shown).
For measurement of IFN-γ production by in vivo-stimulated cells, NOD and IL-18KO NOD mice were injected i.v. with LPS from Salmonella Minnesota (50 µg/mouse; Alexis Biochemicals, San Diego, CA). Control mice were injected with an equivalent volume of PBS. At 3 h post-injection, the spleens were harvested, single cell suspensions were prepared and the cells were cultured for an additional 3 h in complete RPMI 1640 medium supplemented with 10% FCS and Golgi-Plug™ (1 µL/mL; BD Pharmingen).
For analysis of IL-18 secretion, cells were cultured in complete RPMI medium and supernatant samples were collected after 40–48 h and tested using an IL-18 ELISA kit (R&D Systems).
2.10. Statistics
Statistical analyses were performed using the two-sample, unpaired Student’s t test (two-tailed) to determine the level of significance. The Kaplan-Meier survival test was used for comparisons of the cumulative diabetes incidence. In all cases, p<0.05 was considered significant. All data are expressed as the average ± SD in the text.
3. Results
3.1. IL-18 expression is necessary for diabetes pathogenesis in NOD mice
Insulin-producing beta cells secrete IL-18, which in turn induces IFN-γ production by T cells [24, 25]. However, whether IL-18 influences T cells in the periphery has not been explored. Interestingly, APC from healthy mice constitutively produce IL-18 [23, 33, 34]; therefore, we explored the possibility that this cytokine mediates early effects on autoreactivity prior to the onset of beta cell damage. To define the relevant compartments of IL-18 production in NOD mice, freshly isolated cells from NOD lymphoid organs were cultured without cell-activating stimuli and their ability to secrete IL-18 was measured, an approach which has been shown to reflect the magnitude of the IL-18 responses that have been elicited in vivo [23, 34, 35]. When splenic APC (MHC class II+ cells) were purified from 4 and 10 wk old NOD mice and cultured for 40 h, we observed that bioactive IL-18 was secreted constitutively (i.e. in the absence of addition of a maturation stimulus) and the quantities of IL-18 detected increased with the age of mice examined (Fig. 1A). There was also an age-related increase in the quantities of IL-18 produced by NOD pancreatic lymph node (panLN)-derived cells in comparisons of 4 and 10 wk-old mice (Fig. 1B). Interestingly, panLN-derived cells from NOD mice secreted larger quantities of IL-18 in comparison to splenic APC despite the fact that the latter population was enriched for APC (Fig. 1A and 1B). In addition, NOD islets were cultured from 13–15 wk old NOD mice and IL-18 secretion was barely detectable; however, by 18 wk of age, IL-18 secretion by cultured islets had increased approximately 9-fold (Fig. 1C). These experiments demonstrate that cells in both the periphery and the islets of NOD mice produce IL-18.
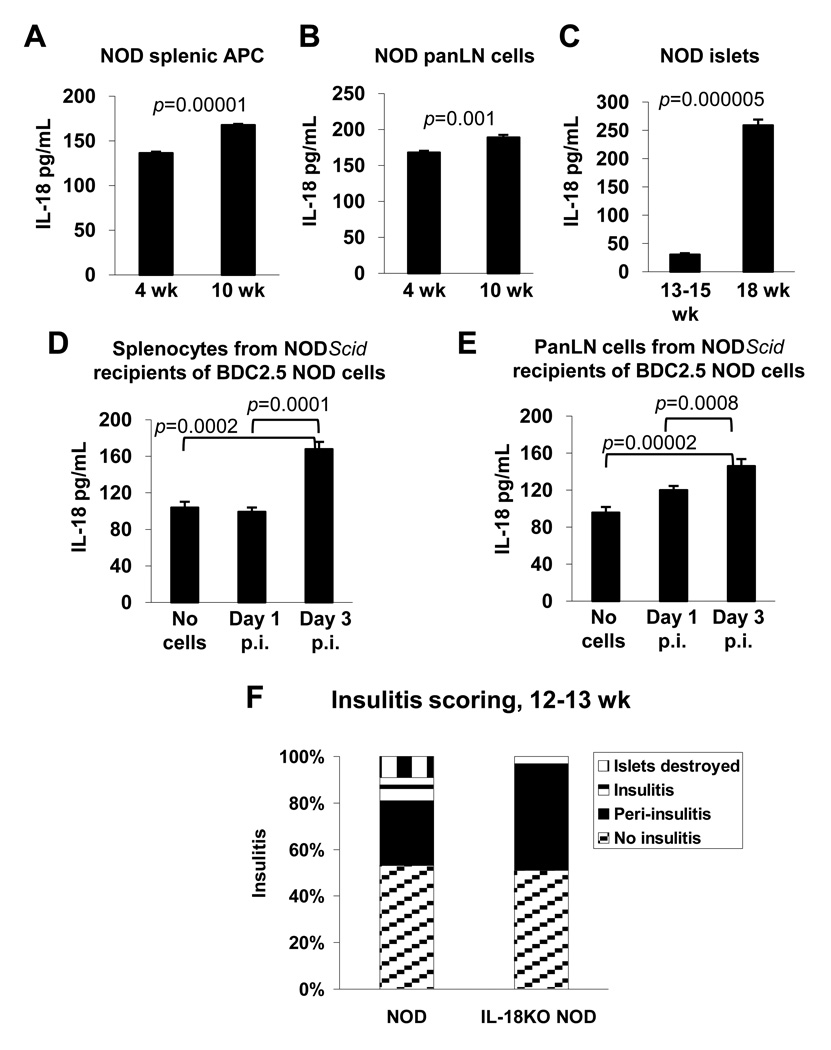
Fig. 1. IL-18 is required for type 1 diabetes pathogenesis.
(A–E) Freshly isolated cells from various tissues were tested for their ability to secrete IL-18 in cultures consisting of complete RPMI-1640 medium without addition of cell-activating agents. The following cell populations were tested for IL-18 production after 48 h of culture: (A) splenic APC from 4 or 10 wk old NOD mice, (B) panLN-derived cells from 4 or 10 wk old NOD mice, and (C) islets from 13–15 or 18 wk old NOD mice. In (D–E), splenocytes or panLN cells from NODScid mice that were injected with BDC2.5 NOD donor splenocytes (2 × 107 cells injected/recipient) were tested for IL-18 secretion following 40 h of culture. The cells were harvested from the NODScid recipients on days 1 or 3 post-injection (p.i.) of BDC2.5 NOD cells, or from mice that were not injected with donor cells (“no cells”). Each of the experiments in (A–E) were repeated twice with similar results, utilizing splenocytes and pooled PLN-derived cells from 3 age-matched mice in each experiment, and islet preparations from 6 mice. Cells from lymphoid organs were cultured at 106 cells/mL in duplicate wells for analysis of cytokine secretion, except in (E) where 7.0 × 105 cells/mL were cultured. (F) Islets from 12–13 wk-old NOD (n=150) and IL-18KO NOD mice (n=168) were graded for insulitis according to the following criteria: no insulitis, peri-insulitis, insulitis, or destroyed islets.
We also explored whether IL-18 secretion by APC is associated with T cell activation. Immune deficient NODScid mice that lack endogenous T and B cells were injected with diabetogenic CD4+ T cells from BDC2.5 NOD mice which carry rearranged α and β chain genes from a diabetogenic CD4+ T cell clone isolated from a diabetic NOD mouse [30]. Splenocytes and panLN-derived cells were harvested from the NODScid recipient mice on day 3 following adoptive transfer, which represents the peak of donor T cell expansion (data not shown), and the cells were cultured to measure IL-18 secretion. Comparisons were drawn to cells taken from NODScid mice on day 1 following adoptive transfer, and to cells from control mice that were not injected with BDC2.5 NOD cells. Interestingly, we found that the splenocytes (Fig. 1D) and panLN-derived cells (Fig. 1E) harvested on day 3 following adoptive transfer secreted significantly higher quantities of IL-18 compared to the cells taken on day 1 or from non-injected NODScid mice. These results suggest that the quantities of IL-18 that are secreted by APC are augmented during self-reactive T cell activation.
To explore whether there was a causal link between IL-18 expression and the development of type 1 diabetes, we bred IL-18-deficient C57BL/6 (B6) mice onto the NOD genetic background for 8 generations to generate the IL-18KO NOD strain. IL-18 is a candidate gene within the Idd2 diabetes susceptibility locus on mouse chromosome 9 [28]. The Idd2 locus was identified in NOD mice expressing resistance alleles from the NON strain (non-obese normal) but this genetic region was not protective in crosses with the B6 strain [36]. Since we used the B6 genetic background to generate our IL-18KO NOD colony, our approach has enabled us to knockout a candidate Idd2-associated gene while avoiding the influences of IL-18-linked resistance alleles on the disease process. We observed resistance to autoimmunity in 100% of female IL-18KO NOD mice (n=19) whereas 85% of NOD females (n=16) succumbed to disease during the 34-wk blood glucose-monitoring period (p=0.000002). Histological analysis of pancreata at 12–13 wk of age revealed that IL-18KO NOD mice developed significant peri-insulitis but few islets exhibited insulitis, whereas islet destruction had commenced by this age in NOD mice [Fig. 1F; Supplementary Fig. 1A histology showing disrupted insulin staining in NOD islets and uniform staining in IL-18KO NOD islets]. Preservation of islet integrity was also verified in 20 wk-old IL-18KO NOD mice by which time significant destruction was evident in NOD mice [Supplementary Fig. 1B]. Immunohistochemistry prepared from pancreata from 10 wk-old mice demonstrated the presence of CD4+ and CD8+ infiltrates in NOD islets [Supplementary Fig. 1C], whereas T cell infiltration of IL-18KO NOD islets was limited [Supplementary Fig. 1C]. IL-18 expression is therefore required for the emergence of islet-destructive CD4+ and CD8+ T cell populations in NOD mice.
3.2. T cells from IL-18KO NOD mice induce diabetes when injected into NODScid mice
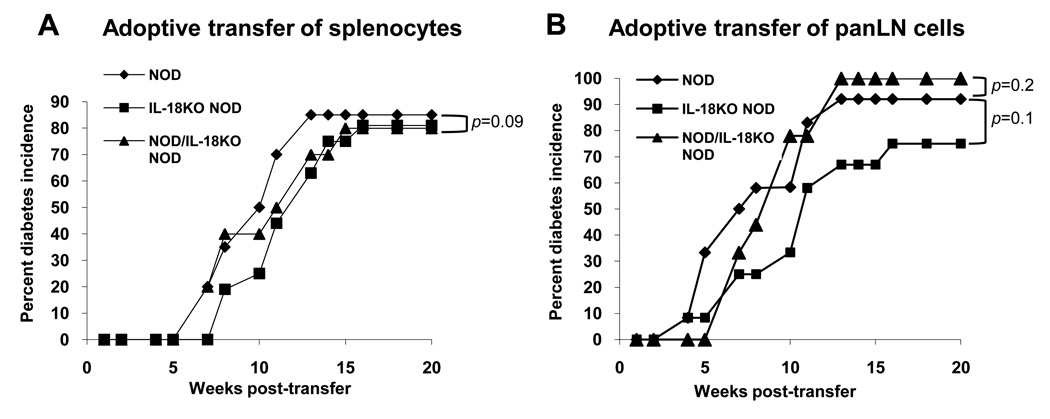
To determine whether T cells from IL-18KO NOD mice can induce diabetes in an IL-18-sufficient environment, we performed adoptive transfers of splenocytes or panLN cells from six-wk old NOD and IL-18KO NOD mice into NODScid mice, and monitored the incidence of diabetes. The results showed that NOD and IL-18KO NOD splenocytes displayed a similar ability to induce diabetes in NODScid recipient mice (Fig. 2A). Similarly, there was no significant difference in the ability of panLN-derived cells from NOD and IL-18KO NOD mice to induce diabetes in NODScid mice (Fig. 2B). Additionally, when the donor cell inoculum consisted of mixtures of NOD and IL-18KO NOD cells, there was a similar incidence and kinetics of disease progression as that observed in recipients of NOD cells alone (Fig. 2A and 2B), indicating that the IL-18KO NOD cells are not acting as immune regulators. These results demonstrate that T cells from NOD and IL-18KO NOD mice have equivalent diabetogenic potential in NODScid recipient mice, and that NOD T cells therefore require IL-18 to become activated.
Fig. 2. IL-18KO NOD lymphoid organs contain pathogenic T cells that mediate disease in NODScid recipient mice.
(A) To compare the pathogenicity of T cells from the spleen, NOD and IL-18KO NOD splenocytes (3 ×107 cells/recipient; n=20 and 16 recipients, respectively) or a mixture of splenocytes from NOD and IL-18KO NOD mice (1.5 ×107 cells from each strain/recipient; n=10 recipients) were injected into NODScid recipient mice and diabetes onset was monitored. p=0.09 for comparisons of the diabetes incidence in recipients of NOD vs. IL-18KO NOD cells. (B) To compare the pathogenicity of panLN-derived cells, NOD and IL-18KO NOD panLN cells (2 × 107 cells/recipient; n=12 recipients/group) or a mixture of NOD panLN cells (107 cells/recipient) plus purified CD4+ cells from IL-18KO NOD panLN (8 × 106 cells/recipient; n=9) were injected into NODScid recipients for analysis of the percent diabetes incidence.
3.3. The periphery of IL-18KO NOD mice contains more naïve T cells and fewer activated T cells
To determine how IL-18 expression affects peripheral T cells, we assessed the spleens of 4–6 wk-old NOD and IL-18KO NOD mice, prior to the onset of inflammation in the islet milieu that could impact T cells systemically. Strikingly, IL-18KO NOD spleens contained significantly more cells than NOD spleens (Table I). Analysis of the cellular composition of the spleens revealed that the numbers of CD4+ and CD8+ cells were substantially increased in IL-18KO NOD mice (Table I) whereas the numbers of B cells (B220+), NK cells (CD3− DX5+), NKT cells (CD3+ DX5+), and APC (MHC class II+), were comparable to those in NOD mice (data not shown). Interestingly, the splenic cellularity and the numbers of CD4+ cells were higher in 9–10 wk-old NOD mice compared to 4–6 wk-old NOD mice. In contrast, in IL-18KO NOD spleens, the cell numbers remained stable between 4–6 and 9–10 wk of age (Table I).
Table I.
Numbers of CD4+ and CD8+ T cells in NOD and IL-18KO NOD lymphoid organs.
| NOD | IL-18 KO NOD | NOD | IL-18KO NOD | ||
|---|---|---|---|---|---|
| 4–6 wk | 4–6 wk | 9–10 wk | 9–10 wk | ||
| Total cells (× 106) | Spleen | 49.9 ± 19.1 | 68.4 ± 24.5 * | 62.8 ± 6.7 | 75.7 ± 6.9 * |
| n=20 | n=20 | n=9 | n=9 | ||
| panLN | 2.2 ± 1.0 | 1.8 ± 0.9 | 2.7 ± 0.6 | 1.8 ± 0.8 * | |
| n=21 | n=21 | n=8 | n=8 | ||
| No. CD4+ (× 106) | Spleen | 14.0 ± 3.6 | 25.4 ± 10.3 * | 19.8 ± 3.1 | 25.9 ± 4.6 * |
| n=20 | n=20 | n=9 | n=9 | ||
| panLN | 1.0 ± 0.5 | 1.0 ± 0.5 | 1.3 ± 0.2 | 0.9 ± 0.1 * | |
| n=21 | n=21 | n=8 | n=8 | ||
| No. CD8+ (× 106) | Spleen | 6.2 ± 1.6 | 12.6 ± 6.1 * | 7.6 ± 2.1 | 13.1 ± 3.2 * |
| n=20 | n=20 | n=9 | n=9 | ||
| panLN | 0.51 ± 1.0 | 0.36 ± 0.2 * | 0.66 ± 0.1 | 0.39 ± 0.1 * | |
| n=21 | n=21 | n=8 | n=8 |
Absolute numbers of each cell type were calculated by multiplying the cellular percentages obtained by flow cytometry by the total cell counts for each organ from individual mice. Data are presented as the mean ± SD where n equals the number of mice examined. For each individual mouse, a minimum of 200, 000 gated lymphocytes, 30, 000 CD4+ cells and 10, 000 CD8+ cells were enumerated by flow cytometry.
p<0.05 for comparisons of NOD and IL-18KO NOD cells within a particular age category.
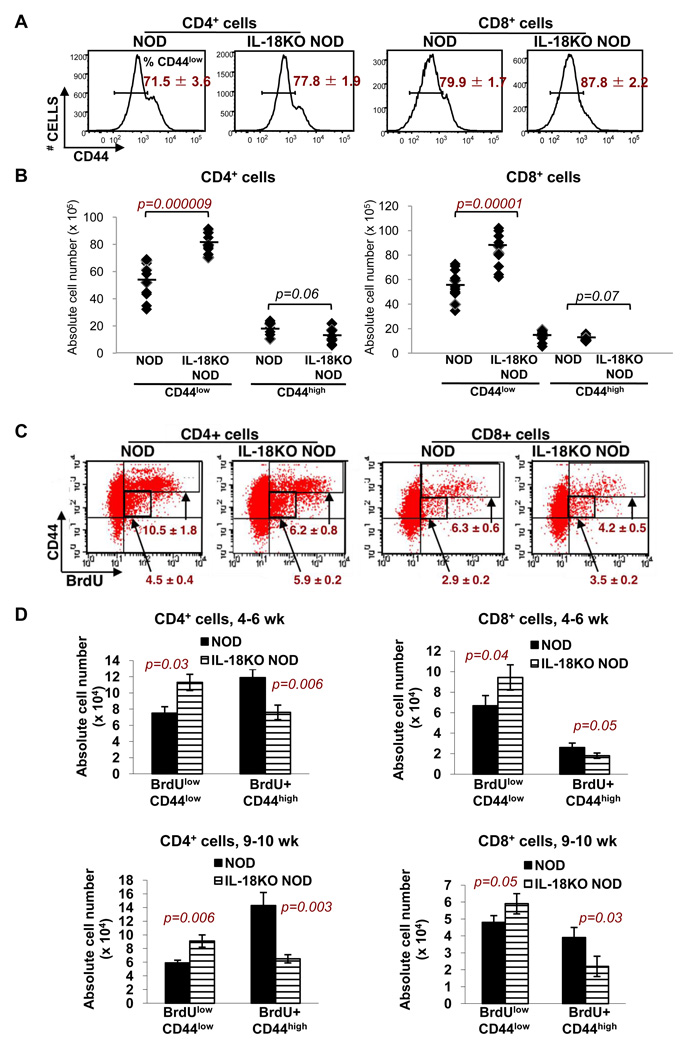
Evaluation of T cell subsets in NOD and IL-18KO NOD mice revealed that the percentages of CD44low (naïve) cells (Fig. 3A) and numbers of CD4+ CD44low and CD8+ CD44low cells (Fig. 3B) were increased in IL-18KO NOD spleens. In mice that had been given BrdU in their drinking water to measure T cell proliferation, the percentages (Fig. 3C) and numbers (Fig. 3D) of BrdUlow CD44low cells were also increased in IL-18KO NOD spleens. The BrdUlow CD44low population is believed to include the recent thymic emigrants (RTE), which have incorporated only low quantities of BrdU in the thymus due to competition between BrdU and nucleotides released from dying thymocytes [37]. Between 4–6 and 9–10 wk of age, the numbers of BrdUlow CD44low cells declined in NOD and IL-18KO NOD spleens, a finding that is consistent with reduced thymic output as a function of aging (Fig. 3D).
Fig. 3. IL-18 expression promotes T cell activation and expansion.
(A) Flow cytometry was used to compare the percentages of CD44low T cells in the spleens of 4–6 wk-old NOD and IL-18KO NOD mice. (B) The percentages of T cell subsets determined in (A) were used to calculate the absolute numbers (× 105) of CD4+ CD44low, CD4+ CD44high, CD8+ CD44low, and CD8+ CD44high cells in NOD and IL-18KO NOD spleens. Each data point represents an individual mouse and the lines denote the averages. (C) To assess T cell proliferation, 4–6 wk-old NOD and IL-18KO NOD mice were given BrdU in their drinking water for four consecutive days. The dot plots depict the percentages of BrdUlow CD44low and BrdU+ CD44high cells (gated regions) in the CD4+ and CD8+ splenic T cell populations. (D) The percentages of cell subsets determined by flow cytometry were used to calculate the absolute numbers (× 104) of BrdUlow CD44low and BrdU+ CD44high cells in 4–6 and 9–10 wk-old mice. All of these results are representative of 8–12 mice/strain. Statistically significant differences between NOD and IL-18KO NOD mice are indicated in red font.
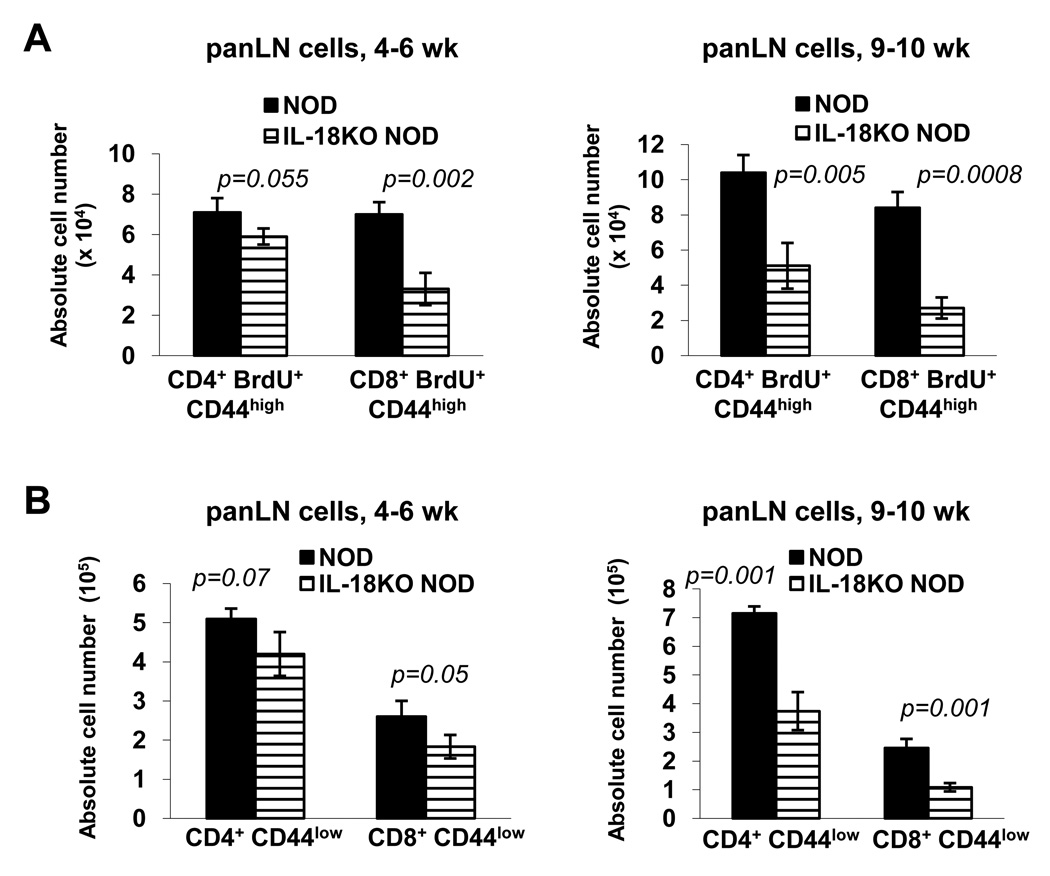
Comparison of the total antigen experienced T cell compartment in 4–6 wk-old NOD and IL-18KO NOD mice revealed that there was a marked deficit in the percentages of CD44high CD45RBlow and CD44high CD11ahigh cells in the CD4+ and CD8+ populations, respectively, in IL-18KO NOD spleens (Supplementary Fig. 2). There was a trend toward reduced total numbers of CD44high cells in IL-18KO NOD spleens, although the data did not reach statistical significance (p=0.06 and p=0.07 for CD4+ and CD8+ cells, respectively) (Fig. 3B). However, there were significant reductions in the percentages of BrdU+ CD44high cells (Fig. 3C) and in the numbers of CD4+ BrdU+ CD44high and CD8+ BrdU+ CD44high in IL-18KO NOD vs. NOD spleens (Fig. 3D). The numbers of CD4+ BrdU+ CD44high and CD8+ BrdU+ CD44high cells increased with age based on comparisons of 4–6 wk old and 9–10 wk-old NOD mice, whereas there was no age-related change in their numbers in IL-18KO NOD mice (Fig. 3D). Similarly, in the panLN where islet-reactive T cells selectively accumulate, the numbers of CD4+ BrdU+ CD44high and CD8+ BrdU+ CD44high cells were reduced in the IL-18KO NOD strain at 4–6 wk and 9–10 wk of age as compared to NOD mice (Fig. 4A). Additionally, the panLN in IL-18KO NOD mice contained fewer total CD8+ cells at 4–6 wk of age, and fewer CD4+ and CD8+ cells at 9–10 wk of age compared to NOD mice (Table I). The total numbers of CD8+ CD44low (naïve) T cells were reduced in IL-18KO NOD vs. NOD panLN at 4–6 weeks of age; however, the numbers of both CD4+ CD44low and CD8+ CD44low cells were significantly reduced at 9–10 wk of age (Fig. 4B). Hence, IL-18KO NOD mice harbor more circulating CD4+ and CD8+ T cells due to increased numbers of naïve T cells, yet contain fewer activated T cells.
Fig. 4. IL-18 expression increases the numbers of activated T cells in the panLN.
(A) Flow cytometry to determine the absolute numbers (× 104) of CD4+ BrdU+ CD44high and CD8+ BrdU+ CD44high cells in the panLN of 4–6 wk-old and 9–10 wk-old NOD and IL-18KO NOD mice that were treated with BrdU in their drinking water for four days. (B) Flow cytometric evaluation of the numbers (×105) of CD4+ CD44low and CD8+ CD44low cells in the panLN of 4–6 wk and 9–10 wk old mice. These data were derived using 8 mice/strain.
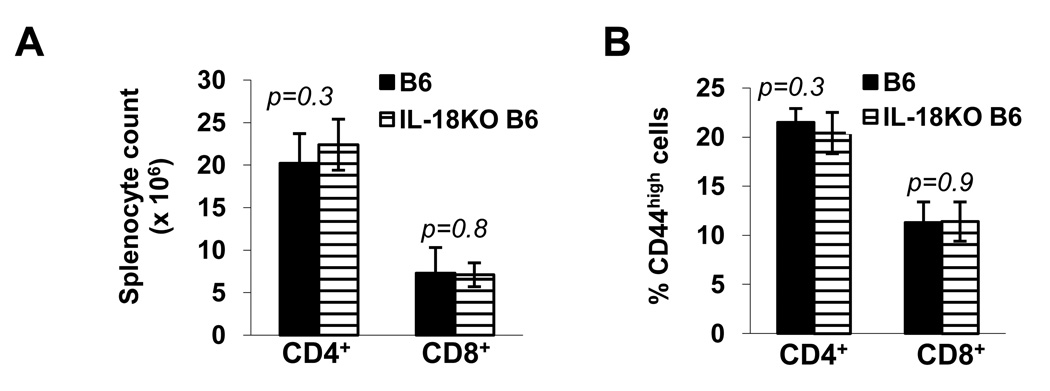
To gauge whether the effects of IL-18 on peripheral T cells were specific to the NOD strain, we also compared T cell compartments in 5–6 wk-old, autoimmune-resistant B6 and IL-18KO B6 mice. Our results showed that B6 and IL-18KO B6 spleens contained comparable total numbers of CD4+ and CD8+ T cells (Fig. 5A), and equivalent percentages of CD44high cells within these subsets (Fig. 5B). Therefore, IL-18 expression has a distinctive influence in regulating the peripheral T cell numbers and subset composition in the NOD strain.
Fig. 5. IL-18 expression does not affect the splenic T cell population in B6 mice.
The absolute numbers of CD4+ and CD8+ cells in spleens from 5–6 wk-old B6 and IL-18KO B6 mice (A) and the percentages of CD44high cells (B) in these populations were compared. These data were derived using 8 mice/strain.
3.4. Turnover of antigen experienced T cells is increased by IL-18 expression
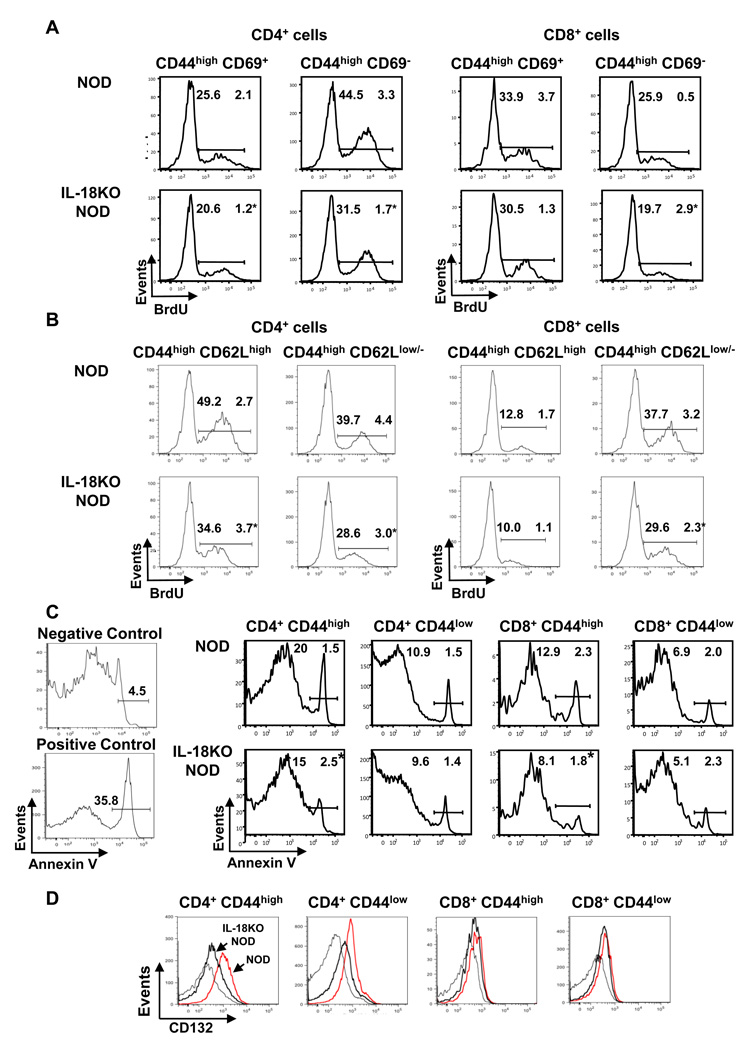
Despite comparable numbers of CD44high cells in NOD and IL-18KO NOD spleens, the finding that proliferation of this population was reduced in IL-18KO NOD mice suggested that the dynamics of T cell turnover differed between the strains. Flow cytometry was conducted to assess proliferation within the CD44high CD69+ and CD44high CD69− populations; these cells represent recently activated T cells and vigorously expanding T cells, respectively [38]. In the CD4+ population, IL-18KO NOD mice displayed reduced percentages of BrdU+ cells in the CD44high CD69+ and CD44high CD69− subsets as compared to NOD mice (Fig. 6A). Analysis of CD8+ cells revealed that the CD44high CD69− population contained reduced percentages of BrdU+ cells in IL-18KO NOD mice, whereas proliferation was equivalent in the CD44high CD69+ subsets in NOD and IL-18KO NOD mice (Fig. 6A). We also examined subsets of T cells expressing CD62L (L-selectin), which exhibits activation-linked downregulation [39]; CD44high CD62Lhigh cells represent the central memory cells, whereas effector T cells are CD44high CD62low/− [40]. We observed that the frequencies of BrdU+ cells were reduced in the CD44high CD62Lhigh (central memory) and CD44high CD62Llow/− (effector) CD4+ cells and in the CD44high CD62Llow/− population of CD8+ T cells in IL-18KO NOD spleens (Fig. 6B). Notably however, these differences in T cell populations in NOD and IL-18KO NOD spleens were not observed in the panLN (data not shown). Taken together, these results show that in the absence of IL-18, vigorous expansion of activated CD4+ and CD8+ effector T cells in spleens is reduced, as is the early activation of CD4+ T cells.
Fig. 6. T cells in the IL-18KO NOD periphery exhibit reduced turnover compared to NOD mice.
(A,B) To identify the proliferating T cell populations by flow cytometry, first the splenic CD4+ and CD8+ populations and then the CD44high CD69+ and CD44high CD69− populations (A) and the CD44high CD62Lhigh and CD44high CD62Llow/− cells were gated (B). The histograms show the percentages of BrdU+ cells within these populations in NOD and IL-18KO NOD mice. These data are representative of 4 mice/strain and were repeated twice with similar results. (C) Comparison of apoptosis in NOD and IL-18KO NOD splenic T cell populations was performed by gating on the 7-AAD− (live) cells and analyzing the percentages of annexin V+ cells. NOD thymocytes and NOD panLN-derived cells served as positive and negative control samples, respectively, for annexin V staining. These results are representative of 4 mice/group and are representative of three independent experiments. (D) Analysis of CD132 expression on the indicated splenocyte populations from NOD (red lines) and IL-18KO NOD (thick black lines) mice relative to isotype control staining (thin black lines). These results are representative of 8 mice/group. (*) denotes a statistically significant difference between NOD and IL-18KO NOD mice for the indicated population.
To investigate the stability of the T cell pool, we also examined apoptosis of T cell populations by flow cytometry. The data showed that significantly reduced proportions of CD4+ CD44high and CD8+ CD44high cells from IL-18KO NOD spleens were apoptotic in comparison to NOD controls (Fig. 6C). However, there were no differences in the percentages of apoptotic CD44low (naïve) cells in NOD and IL-18KO NOD spleens (Fig. 6C). Interestingly also, CD4+ CD44low and CD4+ CD44high T cells from IL-18KO NOD spleens expressed lower levels of CD132 (common gamma chain; γc), the common receptor subunit that governs responsiveness to cytokines important for T cell survival and proliferation [41] (Fig. 6D). Taken together, these data reveal that IL-18 expression promotes short-lived effector T cells.
3.5. Islet reactive T cells undergo IL-18-driven T cell expansion
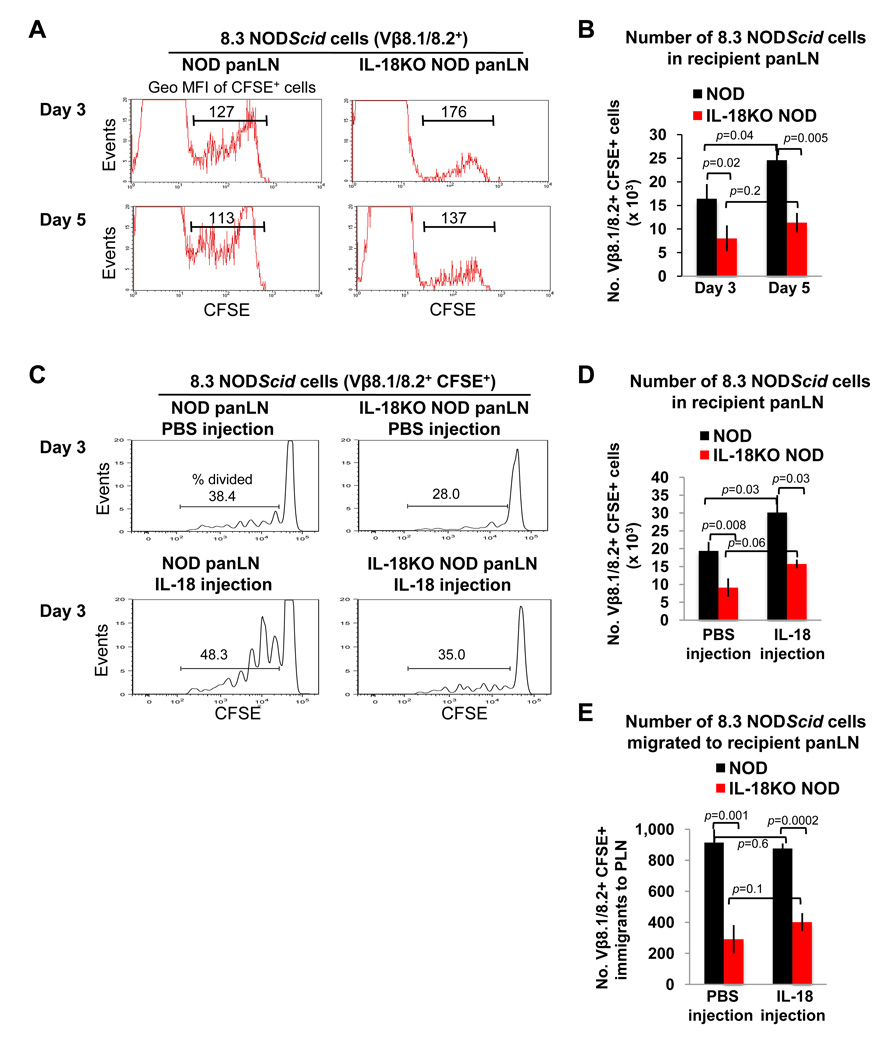
Next we sought to identify how the absence of IL-18 would impact the activity of diabetogenic T cells by adoptively transferring CFSE-labeled splenocytes from TCR transgenic donor mice into NOD and IL-18KO NOD recipients. We first obtained donor T cells from 8.3 NODScid mice, which harbor diabetogenic CD8+ islet-reactive T cells [29]. When CFSE-labeled splenocytes from 8.3 NODScid mice were injected into NOD and IL-18KO NOD recipients, the donor CD8+ T cells proliferated less in IL-18KO NOD panLN, as demonstrated by the limited dilution of the CFSE label on days 3 and 5 after the adoptive transfer (Fig. 7A). Accordingly, the total numbers of the donor T cells were also reduced in IL-18KO NOD vs. NOD panLN on days 3 and 5 following transfer (Fig. 7B).
Fig. 7. Injected 8.3 NODScid T cells undergo IL-18-driven proliferation in the pancreatic lymph nodes of NOD recipient mice.
CFSE-labeled 8.3 NODScid splenocytes were injected intravenously into NOD (n=4) and IL-18KO NOD (n=3) mice (107 cells/recipient), and the panLN from recipient mice were harvested 3 or 5 days later for flow cytometry. (A) The histograms delineate the CFSE+ cells and the geo MFI of the CFSE label on gated Vβ8.1/8.2+ cells in representative mice. (B) Summary of the total numbers of Vβ8.1/8.2+ CFSE+ cells (× 103) in the panLN of these recipients. (C) NOD and IL-18KO NOD mice were injected with 8.3 NODScid splenocytes (107 cells/recipient) and also received daily injections of PBS or IL-18. The histograms show the percentages of CFSElow (proliferating) cells within the Vβ8.1/8.2+ CFSE+ gate on day 3 following transfer. (D) Summary of the total numbers of Vβ8.1/8.2+ CFSE+ cells (× 103) in the panLN of the adoptive transfer recipients that received PBS or IL-18 injections. (E) Estimation of the numbers of Vβ8.1/8.2+ CFSE+ cells that migrated into the panLN of NOD and IL-18KO NOD recipient mice by day 3 following transfer (refer to Materials and Methods for the method of calculating the numbers of migrated donor T cells). All experiments were repeated at least twice (3–4 mice per group) and yielded similar results.
To test whether the concentrations of IL-18 affect islet-reactive T cell expansion, NOD and IL-18KO NOD mice were injected with 8.3 NODScid splenocytes and were co-treated with recombinant IL-18 or PBS. These elevated systemic IL-18 levels led to increased percentages of donor T cells that divided in NOD panLN (Fig. 7C) and increased absolute numbers of mitotically active donor T cells (Fig. 7D). IL-18 injection also bolstered the fractions of donor T cells that divided in IL-18KO NOD panLN; however, the percentages of dividing T cells (Fig. 7C) and the total donor T cell numbers (Fig. 7D) were still reduced in comparison to IL-18-injected NOD recipients. Lastly, we used these CFSE dilution peaks (Fig. 7C) to estimate the numbers of CFSE-labeled donor T cells that migrated into the panLN prior to cellular division (using the formula in Materials and Methods). This analysis revealed that physiologic IL-18 expression yielded an increase in the numbers of donor T cells that migrated into NOD panLN; however, the effects of IL-18 injection and endogenous IL18 were not additive in enhancing donor T cell migration to this tissue in NOD mice (Fig. 7E).
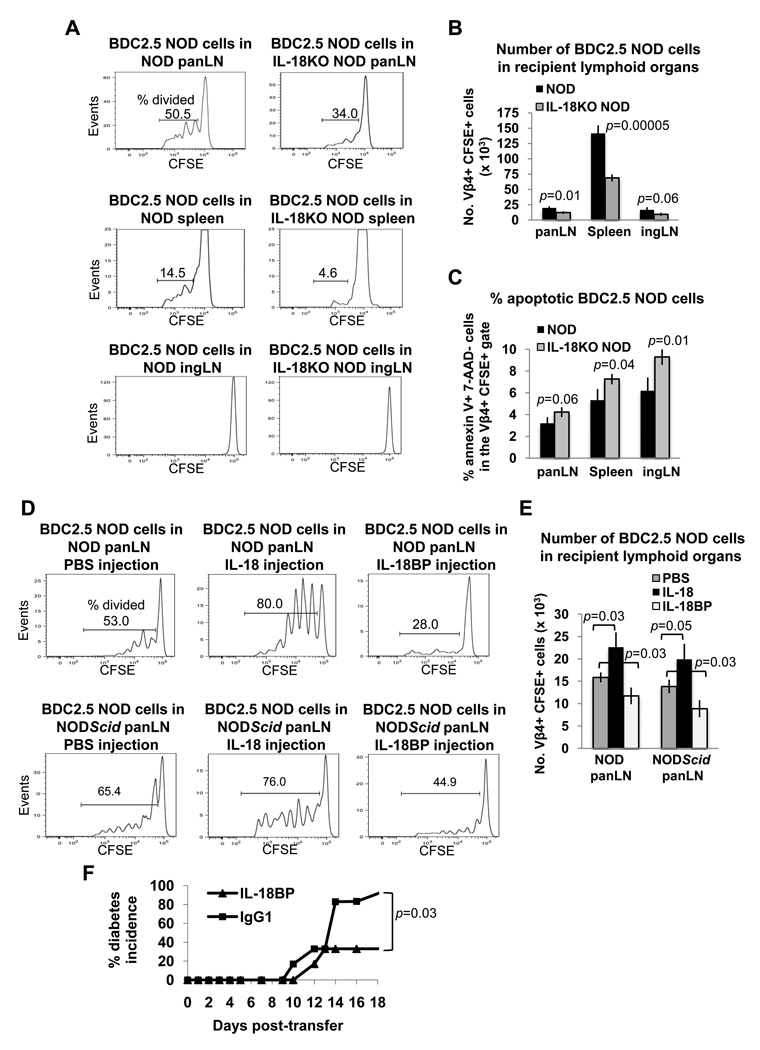
Experiments were also conducted to study the impact of IL-18 on islet-reactive CD4+ T cells by injecting CFSE-labeled splenocytes from BDC2.5 NOD mice into NOD and IL-18KO NOD recipient mice. Analysis of the panLN and spleens on day 3 following adoptive transfer revealed that donor T cell proliferation was restricted in IL-18KO NOD lymphoid organs, as judged by the limited dilution of the CFSE label (Fig. 8A) and the reduced donor T cell numbers in comparison to NOD recipient mice (Fig. 8B). In the inguinal lymph nodes (ingLN), where islet antigen is absent, BDC2.5 NOD donor T cells did not proliferate in either strain (Fig. 8A), and their total recovery in NOD and IL-18KO NOD ingLN was not significantly different (Fig. 8B). Interestingly however, when apoptosis was assessed by flow cytometry, the donor CD4+ T cells exhibited markedly impaired survival in IL-18KO NOD spleens and ingLN, although their survival was not significantly affected by IL-18 expression in the panLN (p=0.06) (Fig. 8C). Therefore, the IL-18KO NOD periphery may be inhibitory to the survival of the adoptively transferred islet-reactive T cells.
Fig. 8. IL-18 expression promotes extensive division of BDC2.5 NOD T cells in NOD and NODScid mice.
(A) CFSE-labeled splenocytes from BDC2.5 NOD mice were injected into NOD and IL-18KO NOD mice (2.0 × 107 cells/recipient) and the recipient lymphoid organs were harvested 3 days later for flow cytometric analysis of the percentages of CFSElow (proliferating) cells in the Vβ4+ CFSE+ gates. (B) Summary of the total numbers of Vβ4+ CFSE+ cells (× 103) in the recipient lymphoid organs. (C) Flow cytometric assessment of the percentages of apoptotic (annexin V+ 7-AAD−) cells in the Vβ4+ CFSE+ populations in recipient lymphoid organs. (D) NOD and NODScid mice were injected with BDC2.5 NOD splenocytes and were also given daily injections of PBS, IL-18 or IL-18BP. On day 3 following transfer, the percentages of CFSElow (proliferating) donor T cells were assessed in the Vβ4+ CFSE+ populations. (E) The total numbers of Vβ4+ CFSE+ cells (× 103) in recipient lymphoid organs were calculated. All experiments utilized three mice per treatment group and were repeated at least twice. (F) To test the effect of IL-18 neutralization on the diabetic potential of BDC2.5 NOD cells, NODScid recipient mice were each injected with 2.5 × 107 splenocytes from BDC2.5 NOD donors and either IL-18BP (6 recipients) or IgG1 control protein (6 recipients) beginning on the day of adoptive transfer and each day thereafter. The percentages of diabetic mice are shown.
Our studies were also extended to explore the importance of IL-18 for autoreactive T cell activation in immune deficient NODScid mice that were injected with diabetogenic BDC2.5 NOD cells. IL-18 injection led to accelerated islet infiltration by BDC2.5 NOD cells on day 6 following adoptive transfer compared to that observed in PBS-treated NODScid recipients (Supplementary Fig. 3). IL-18 injection into NOD and NODScid recipients of BDC2.5 NOD cells increased the percentages of proliferating donor T cells in the panLN (Fig. 8D), reflected by increased donor T cell numbers in this tissue (Fig. 8E), and these cells underwent more divisions than in PBS-treated control mice as evident by the extent of CFSE dilution (Fig. 8D). We also utilized IL-18 binding protein (IL-18BP), which neutralizes endogenous IL-18 activity, to investigate the role of IL-18 expression on autoreactive T cell expansion. When IL-18BP was administered to the adoptive transfer recipients, the percentages of mitotically active donor T cells were significantly reduced in NOD and NODScid panLN (Fig. 8D), as were the total donor T cell numbers in comparison to PBS-treated recipients (Fig. 8E). We also tested the effect of IL-18BP on autoimmunity mediated by adoptively transferred BDC2.5 NOD cells in NODScid recipient mice. This analysis showed that there was a significantly reduced incidence of disease in IL-18BP-treated recipients compared to the recipients that were treated with IgG1 control protein (Fig. 8F). Hence, IL-18 also promotes aggressive T cell division and autoimmunity in immune deficient NODScid recipients of diabetogenic T cells.
3.6. IL-18Rα receptor expression is a hallmark of activated T cells
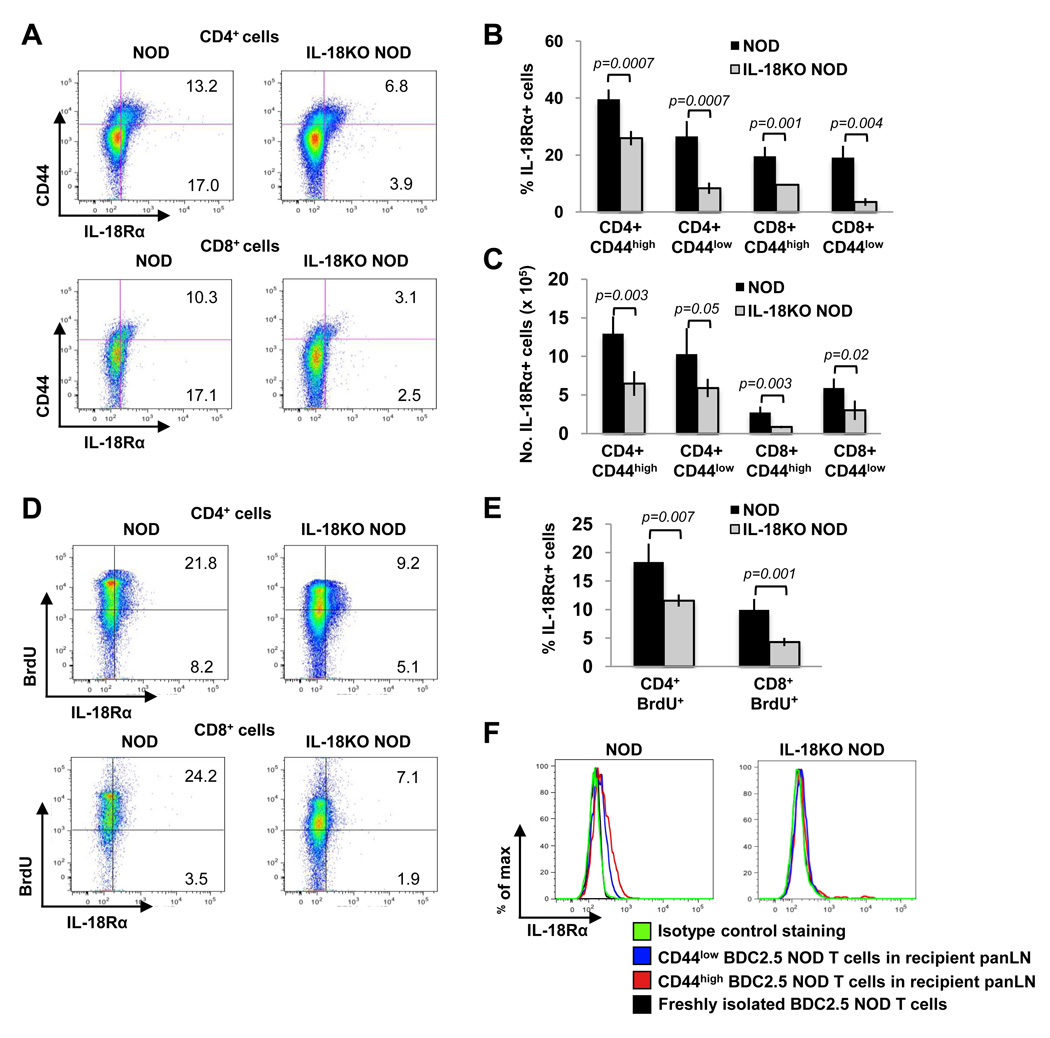
To identify the IL-18-responsive cells present in NOD mice, we also examined the expression patterns of the ligand binding (α) subunit of its receptor, IL-18Rα. Flow cytometry revealed that IL-18Rα was expressed at low levels on a minority of CD4+ CD44low and CD8+ CD44low cells in NOD mice (Fig. 9A and 9B). CD44high cells in NOD mice expressed higher levels of IL-18Rα than CD44low cells based on a shift toward higher fluorescence intensities of IL-18Rα staining on the former cells (Fig. 9A). A comparison of NOD and IL-18KO NOD T cells revealed that IL-18 deficiency conferred a striking reduction in the percentages of IL-18Rα+ cells in the CD44high populations (Fig. 9A and 9B) and in the absolute numbers of CD4+ CD44high IL-18Rα+ and CD8+ CD44high IL-18Rα+ cells (Fig. 9C). Moreover, the CD44low IL-18Rα+ population was also drastically diminished in IL-18KO NOD mice in terms of both percentages (Fig. 9A and 9B) and absolute numbers (Fig. 9C). BrdU incorporation assays showed that a proportion of the mitotically active CD4+ and CD8+ T cells in NOD mice were IL-18Rα+ (Fig. 9D and 9E). In contrast, the dividing T cells in IL-18KO NOD mice contained significantly fewer IL-18Rα+ cells (Fig. 9E). Therefore, IL-18Rα upregulation is a characteristic of activated and proliferating T cells, and this population is diminished in the IL-18KO NOD strain.
Fig. 9. IL-18Rα is upregulated on activated and proliferating T cells in IL-18-expressing NOD mice.
(A) Flow cytometry delineating the percentages of CD44high IL-18Rα+ cells (upper right quadrants) and the CD44low IL-18Rα+ cells (lower right quadrants) in NOD and IL-18KO NOD mice. (B, C) Summary of the percentages (B) and absolute numbers (× 105) (C) of IL-18Rα+ cells in the indicated T cell populations. (D) Flow cytometry to compare the percentages of BrdU+ IL-18Rα+ cells (upper right quadrants) and BrdU− IL-18Rα+ cells (lower right quadrants) in T cells from NOD and IL-18KO NOD mice that were given BrdU in their drinking water for six days. (E) Summary of the percentages of IL-18Rα+ cells in the BrdU+ populations. All of the results presented in (A–E) are representative of 4 mice/group from one of two independent experiments. (F) NOD (n=4) and IL-18KO NOD mice (n=2) were injected with splenocytes from BDC2.5 NOD mice. IL-18Rα expression was analyzed on the CD44low and CD44high donor T cells (Vβ4+ CFSE+ gated) that were present in the panLN three days later. The IL-18Rα levels on donor T cells were compared to those on freshly isolated Vβ4+ CFSE+ cells prior to adoptive transfer. Staining with isotype control Ab on gated CD44high donor T cells is shown.
To evaluate IL-18Rα expression on a known autoreactive T cell population, we examined its expression on BDC2.5 NOD cells on day 3 following adoptive transfer into NOD or IL-18KO NOD recipient mice. Significantly, CD44low T cells in the panLN of NOD recipients exhibited low levels of IL-18Rα expression, whereas this receptor was undetectable on freshly isolated BDC2.5 NOD cells prior to adoptive transfer (Fig. 9F). Moreover, CD44high cells expressed higher levels of IL-18Rα compared to the CD44low cells in NOD panLN (Fig. 9F). In contrast, neither the CD44low cells nor the CD44high T cells from BDC2.5 NOD mice expressed IL-18Rα in IL-18KO NOD recipients (Fig. 9F). These observations demonstrate that IL-18 regulates the expression of its own receptor on pathogenic T cells during their activation.
3.7. IL-18 skews the effector cytokine profile from IL-17F toward IFN-γ production
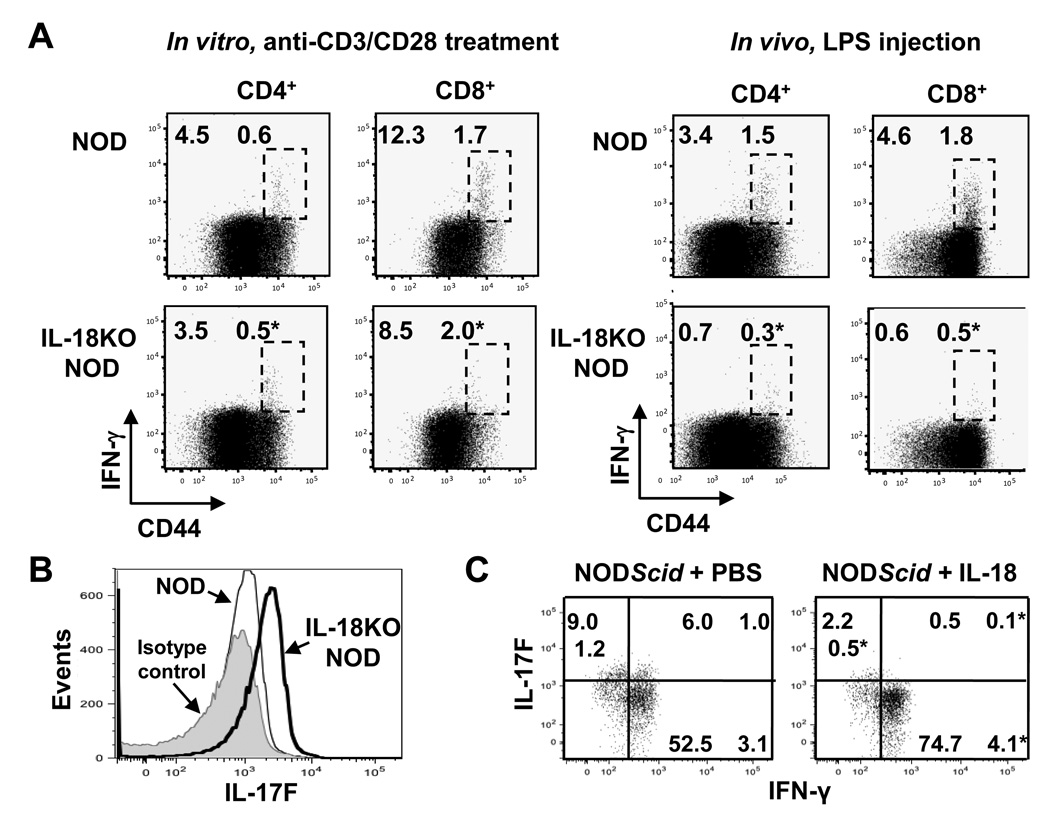
Lastly, we asked whether IL-18-mediated T cell expansion is accompanied by a distinctive cytokine profile of responding T cells. Concurring with the role for IL-18 in augmenting IL-12-induced IFN-γ production, IL-18KO NOD cells demonstrated reduced IFN-γ expression upon stimulation with anti-CD3/anti-CD28 in vitro or LPS treatment in vivo (Fig. 10A). Intriguingly however, we found that in vitro-stimulated T cells from IL-18KO NOD mice instead expressed significantly higher levels of IL-17F in comparison to NOD cells (Fig. 10B).
Fig. 10. The T cell cytokine profile shifts from IL-17F toward IFN-γ production under the influence of IL-18.
(A) NOD and IL-18KO NOD splenocytes were cultured with anti-CD3/CD28 and brefeldin A for 5 hours (n=8 spleens/strain). Alternatively, LPS (50 µg /mouse, i.v.) was administered to NOD (n=4) and IL-18KO NOD (n=3) mice and splenocytes were harvested 3 hours later and cultured with brefeldin A for an additional 3 h. Flow cytometry was used to measure the percentages of IFN-γ+ CD44high cells (delineated by the boxed regions). (B) Comparison of IL-17F expression by CD4+ cells that were activated in vitro with anti-CD3/CD28. The histogram shows isotype control staining (shaded) and IL-17F staining in NOD (thin line) and IL-18KO NOD (bold line) cells. These results are representative of 8 mice/strain. (C) BDC2.5 NOD splenocytes were injected into NODScid mice along with daily injections of PBS or IL-18 (n=3 mice/group). Four days later, the spleens were harvested and the lymphocytes were cultured with anti-CD3/CD28 for analysis of IL-17F and IFN-γ expression in Vβ4+ CD62Llow/− cells. This experiment was repeated twice with similar results. (*) denotes a statistically significant difference between NOD and IL-18KO NOD mice or PBS and IL-18 treatment groups for the indicated T cell population.
In NODScid adoptive transfer recipients of BDC2.5 NOD cells, islet damage is reportedly facilitated by the conversion from IL-17-producing cells, which contribute to pancreatic inflammation, to IFN-γ+ T cells [12, 13]. Following adoptive transfer of BDC2.5 NOD cells into NODScid mice, we administered IL-18 or PBS, harvested the spleens on day 4, and restimulated the cells in vitro for analysis of cytokine expression in donor T cells. IL-18 treatment led to the disappearance of donor T cells that expressed IL-17F and co-expressed IL-17F and IFN-γ in favor of higher percentages of cells that exclusively expressed IFN-γ (Fig. 10C). The observations that IL-18 stimulates the proliferation of islet-reactive T cells as well as stimulating effector T cell cytokine production are indicative of a necessary role for this cytokine in directing key elements of autoimmune pathogenesis.
4. Discussion
This report assigns novel functions for IL-18 in T cell-mediated autoimmunity and dispels the long-standing notion that IL-18 merely serves a supplementary role alongside IL-12 and IFN-γ in promoting Th1 responses. Here we show that IL-18 promotes naïve T cell activation and vigorous T cell expansion resulting from self-antigen encounter. In parallel, IL-18 promotes the terminal differentiation of effector T cells, inducing a switch from IL-17-producers into IFN-γ expressing cells. These functions render IL-18 uniquely necessary for diabetes pathogenesis in the NOD mouse, unlike IL-12 and IFN-γ, which are redundant in genetically deficient mice. IL-18 upregulation has long been associated with intra-islet inflammation [23, 24, 28]; however, our discovery that IL-18 is fundamental for the expansion of the earliest autoreactive T cells during pre-diabetes provides a novel perspective of its role in type 1 diabetes. Significantly also, the levels of IL-18 and the frequencies of IL-18R-expressing T cells parallel the course of disease and T cell activation in NOD mice. Hence, IL-18 should now be considered a key initiator of T cell-mediated autoimmunity and prospectively also, a target for intervention strategies.
IL-18KO NOD mice harbor increased proportions and numbers of naive T cells in the periphery, including increased numbers of CD44low BrdUlow cells as compared to NOD mice. The larger T cell pool size in IL-18KO NOD mice appears to be enacted through increased production of RTE or enhanced stimulation by APC rather than enhanced survival, since the frequencies of apoptotic naïve T cells are similar in NOD and IL-18KO NOD mice. A pertinent question provoked by this work is the mechanism by which IL-18 limits the size of the naïve T cell pool in NOD spleens. IL-18 in conjunction with IL-12 promotes thymocyte apoptosis [42], suggesting that there may be increased thymocyte differentiation and thymic output in IL-18KO NOD mice. It is also plausible that the IL-18-mediated effects on T cell numbers and subsets might intersect with the homeostatic pathways that govern T cell growth and differentiation through the common gamma chain binding cytokines. This possibility is suggested by the fact that IL-18KO NOD mice exhibit reduced T cell turnover, and peripheral CD4+ T cells in IL-18KO NOD mice also have reduced CD132 expression levels. Support for this idea also comes from our finding that IL-18 bolsters T cell division and promotes autoimmunity in lymphopenic NODScid mice in which homeostasis-driven proliferation of injected diabetogenic T cells generates effector T cells [43].
Our laboratory has previously shown that the NOD mouse demonstrates mild T cell lymphopenia, chronic immune activation, and instability of peripheral T cell compartments [44]. The current study identifies IL-18 as a factor that destabilizes the peripheral T cell pool, leading to the generation of mitotically active and short-lived effector T cells in NOD mice. These effects of IL-18 on peripheral T cell subsets have never been documented previously, as they are apparent on the NOD genetic background but not in the B6 strain. The large naïve T cell pool in IL-18KO NOD mice could conceivably limit the numbers of activated T cells that form, thereby prohibiting disease progression. However, the inhibited T cell proliferation in IL-18KO NOD mice may be only partially space-dependent, since expansion of adoptively transferred T cells in IL-18KO NOD mice can be bolstered by injection of IL-18, albeit still to a lesser degree compared to its effects in NOD mice. Interestingly however, we observed that the newly introduced islet-reactive CD4+ T cells survived poorly in the peripheral lymphoid organs of IL-18KO NOD mice. In contrast, endogenous CD44high T cells exhibited enhanced survival in IL-18KO NOD vs. NOD mice. Hence, competition with endogenous T cells for lymphoid resources in the overly full periphery of IL-18KO NOD mice might contribute to the poor survival of adoptively transferred T cells relative to that in NOD mice.
It is already established that IL-18 perpetuates the inflammation that occurs within the islets [23, 24, 28]. This is probably due to the earliest role demonstrated for IL-18 as an inducer of IFN-γ expression. Of note, our data are not suggestive of a role for IL-18 in modifying the activity of regulatory T cells in NOD mice. Our results instead support a role for IL-18 in the transition of Th17 cells into IFN-γ producing T cells, or alternatively, in the stimulation of a distinct T cell population that predominantly produces IFN-γ, the cytokine which is required to evoke disease [12, 13]. Our data are also consistent with a previous report where increased numbers of Th17 cells were observed in IL-18-deficient mice that were also deficient in apolipoprotein E [47]. In another study IL-18 was found to promote the secretion of pro-inflammatory cytokines from Th17 cells [48]. Furthermore, our data reveal that IL-18 expression promotes increased proliferation of effector T cells, defined by a CD44high CD62Llow/− phenotype, in both the CD4+ and CD8+ T cell compartments, and also promotes increased division of CD4+ CD44high CD62Lhigh cells in NOD mice. Intriguingly, higher percentages of the effector CD8+ T cells proliferate as compared to central memory CD8+ T cells in NOD spleens, and the extent of effector T cell division is reduced in IL-18KO NOD mice, suggesting that this population is associated with emerging autoreactivity.
One intriguing finding from this work is that IL-18 regulates the expression of its receptor on T cells. To date, the known inducers of IL-18R expression are TCR ligation combined with IL-12-mediated stimulation [18]. We observed that IL-18KO NOD mice harbor reduced frequencies of IL-18Rα-expressing cells within the naïve and antigen-experienced T cell compartments in comparison to NOD mice. IL-18 likely exerts a direct role in promoting the expansion of the IL-18Rα-expressing T cell population; however, the residual IL-18Rα+ cells in IL-18KO NOD mice may comprise a population that responds to innate stimuli and proliferates TCR-independently [19]. The effect of IL-18 on IL-18Rα expression may also be indirect, particularly for naïve T cells that rely upon APC stimulation. For example, IL-18 may be required for activating IL-18R-expressing APC. In the absence of complete immune activation, there may be limited release of IL-12, which in turn leads to failed induction of IL-18Rα on T cells. The idea that IL-18 promotes signals for APC activation is suggested by a previous report in which injection of NOD mice with an IL-18 expression plasmid led to increased IL-12 and CD86 mRNA expression levels in the periphery and elevated IFN-inducible protein-10 mRNA expression in the pancreas [49]. In this sense, the reduced frequencies of IL-18-responsive T cells may reflect the absence of strongly stimulated APC in IL-18KO NOD mice. This concept is also supported by our observation that when BDC2.5 NOD cells were transferred into NOD recipient mice, the levels of IL-18Rα quickly increased, indicating that receptor expression accompanies T cell activation, whereas IL-18Rα was not expressed on T cells in IL-18KO NOD hosts.
An overview of previous studies has unveiled a complex role for IL-18 in autoimmunity. Administration of IL-18BP has protective effects in experimentally induced T1D [50, 51]. Daily recombinant IL-18 administration suppressed disease in one report [52], whereas IL-18 plasmid DNA injection exacerbated disease in another [49]. Given the differing method, dose and timing of IL-18 administration, variations in cytokine bioavailability in lymphoid compartments probably underlie these discrepancies. Caspase-1-deficient NOD mice, which do not process IL-18 and IL-1β and exhibit impaired IL-1α production, exhibit a normal incidence of diabetes compared to wild-type mice [53], possibly due to compensatory upregulation of alternate pro-diabetogenic pathways in this particular strain, as is suggested by their preserved IFN-γ production. There has also been an interesting report that IL-18-deficient mice on a B6 genetic background succumb to type 2 diabetes at six months of age, a defect that is corrected by intra-cranial administration of recombinant IL-18 [54]. Since our IL-18KO NOD colony remained normoglycemic for at least one year (unpublished observations), there may be genetic variability in the mechanisms that govern central glucose homeostasis.
Collectively, these data illuminate a central role for IL-18 in T cell activation and expansion in the initiation of type 1 diabetes pathogenesis, thereby highlighting a novel mechanism of disease. Perhaps most importantly, our experiments in the NOD mouse might provide mechanistic insight into clinical disease, where increased serum levels of IL-18 have been identified in patients with type 1 diabetes [55]. Additionally, our identification of IL-18Rα expression as a defining characteristic of autoreactive T cells may be applicable to human type 1 diabetes as a marker of disease susceptibility or as a therapeutic target for recent onset disease.
Supplementary Material
Acknowledgments
We thank Dr. Marcie Kritzik for critical reading of this manuscript.
Sources of support: AM was supported by a postdoctoral fellowship from The Larry L. Hillblom Foundation. This work was supported by NIH grant RO1AI064325 to NS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing interests.
References
- 1.Trembleau S, Penna G, Gregori S, Chapman HD, Serreze DV, Magram J, et al. Pancreas-infiltrating Th1 cells and diabetes develop in IL-12-deficient nonobese diabetic mice. J Immunol. 1999;163:2960–2968. [PubMed] [Google Scholar]
- 2.Savinov AY, Wong FS, Chervonsky AV. IFN-gamma affects homing of diabetogenic T cells. J Immunol. 2001;167:6637–6643. doi: 10.4049/jimmunol.167.11.6637. [DOI] [PubMed] [Google Scholar]
- 3.Hill NJ, Van Gunst K, Sarvetnick N. Th1 and Th2 pancreatic inflammation differentially affects homing of islet-reactive CD4 cells in nonobese diabetic mice. J Immunol. 2003;170:1649–1658. doi: 10.4049/jimmunol.170.4.1649. [DOI] [PubMed] [Google Scholar]
- 4.Campbell IL, Iscaro A, Harrison LC. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988;141:2325–2329. [PubMed] [Google Scholar]
- 5.Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, et al. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 6.Trembleau S, Penna G, Gregori S, Giarratana N, Adorini L. IL-12 administration accelerates autoimmune diabetes in both wild-type and IFN-gamma-deficient nonobese diabetic mice, revealing pathogenic and protective effects of IL-12-induced IFN-gamma. J Immunol. 2003;170:5491–5501. doi: 10.4049/jimmunol.170.11.5491. [DOI] [PubMed] [Google Scholar]
- 7.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes. 2000;49:2007–2011. doi: 10.2337/diabetes.49.12.2007. [DOI] [PubMed] [Google Scholar]
- 8.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 9.Campbell IL, Oxbrow L, Harrison LC. Reduction in insulitis following administration of IFN-gamma and TNF-alpha in the NOD mouse. J Autoimmun. 1991;4:249–262. doi: 10.1016/0896-8411(91)90022-5. [DOI] [PubMed] [Google Scholar]
- 10.Sobel DO, Han J, Williams J, Yoon JW, Jun HS, Ahvazi B. Gamma interferon paradoxically inhibits the development of diabetes in the NOD mouse. J Autoimmun. 2002;19:129–137. doi: 10.1006/jaut.2002.0604. [DOI] [PubMed] [Google Scholar]
- 11.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 15.Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog. 2009;5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn HJ, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, et al. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 17.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, et al. IL-12 upregulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 18.Smeltz RB, Chen J, Hu-Li J, Shevach EM. Regulation of interleukin (IL)-18 receptor alpha chain expression on CD4(+) T cells during T helper (Th)1/Th2 differentiation. Critical downregulatory role of IL-4. J Exp Med. 2001;194:143–153. doi: 10.1084/jem.194.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tough DF, Zhang X, Sprent J. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J Immunol. 2001;166:6007–6011. doi: 10.4049/jimmunol.166.10.6007. [DOI] [PubMed] [Google Scholar]
- 20.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 21.Chakir H, Lam DK, Lemay AM, Webb JR. "Bystander polarization" of CD4+ T cells: activation with high-dose IL-2 renders naive T cells responsive to IL-12 and/or IL-18 in the absence of TCR ligation. Eur J Immunol. 2003;33:1788–1798. doi: 10.1002/eji.200323398. [DOI] [PubMed] [Google Scholar]
- 22.Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- 23.Lewis EC, Dinarello CA. Responses of IL-18- and IL-18 receptor-deficient pancreatic islets with convergence of positive and negative signals for the IL-18 receptor. Proc Natl Acad Sci U S A. 2006;103:16852–16857. doi: 10.1073/pnas.0607917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frigerio S, Hollander GA, Zumsteg U. Functional IL-18 Is produced by primary pancreatic mouse islets and NIT-1 beta cells and participates in the progression towards destructive insulitis. Horm Res. 2002;57:94–104. doi: 10.1159/000057959. [DOI] [PubMed] [Google Scholar]
- 25.Rothe H, Hibino T, Itoh Y, Kolb H, Martin S. Systemic production of interferon-gamma inducing factor (IGIF) versus local IFN-gamma expression involved in the development of Th1 insulitis in NOD mice. J Autoimmun. 1997;10:251–256. doi: 10.1006/jaut.1997.0135. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 28.Rothe H, Jenkins NA, Copeland NG, Kolb H. Active stage of autoimmune diabetes is associated with the expression of a novel cytokine, IGIF, which is located near Idd2. J Clin Invest. 1997;99:469–474. doi: 10.1172/JCI119181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 31.Hill NJ, Stotland A, Solomon M, Secrest P, Getzoff E, Sarvetnick N. Resistance of the target islet tissue to autoimmune destruction contributes to genetic susceptibility in Type 1 diabetes. Biol Direct. 2007;2:5. doi: 10.1186/1745-6150-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci U S A. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito H, Esashi E, Akiyama T, Inoue J, Miyajima A. IL-18 produced by thymic epithelial cells induces development of dendritic cells with CD11b in the fetal thymus. Int Immunol. 2006;18:1253–1263. doi: 10.1093/intimm/dxl058. [DOI] [PubMed] [Google Scholar]
- 36.McAleer MA, Reifsnyder P, Palmer SM, Prochazka M, Love JM, Copeman JB, et al. Crosses of NOD mice with the related NON strain. A polygenic model for IDDM. Diabetes. 1995;44:1186–1195. doi: 10.2337/diab.44.10.1186. [DOI] [PubMed] [Google Scholar]
- 37.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlub TE, Venturi V, Kedzierska K, Wellard C, Doherty PC, Turner SJ, et al. Division-linked differentiation can account for CD8+ T-cell phenotype in vivo. Eur J Immunol. 2009;39:67–77. doi: 10.1002/eji.200838554. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 41.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Galan MC, Bream JH, Farr A, Young HA. Synergistic effect of IL-2, IL-12, and IL-18 on thymocyte apoptosis and Th1/Th2 cytokine expression. J Immunol. 2005;174:2796–2804. doi: 10.4049/jimmunol.174.5.2796. [DOI] [PubMed] [Google Scholar]
- 43.Le Campion A, Gagnerault MC, Auffray C, Becourt C, Poitrasson-Riviere M, Lallemand E, et al. Lymphopenia-induced spontaneous T-cell proliferation as a cofactor for autoimmune disease development. Blood. 2009;114:1784–1793. doi: 10.1182/blood-2008-12-192120. [DOI] [PubMed] [Google Scholar]
- 44.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 45.Tomura M, Itoh K, Kanagawa O. Naive CD4+ T lymphocytes circulate through lymphoid organs to interact with endogenous antigens and upregulate their function. J Immunol. 2010;184:4646–4653. doi: 10.4049/jimmunol.0903946. [DOI] [PubMed] [Google Scholar]
- 46.Iwai Y, Hemmi H, Mizenina O, Kuroda S, Suda K, Steinman RM. An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation. PLoS One. 2008;3:e2404. doi: 10.1371/journal.pone.0002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pejnovic N, Vratimos A, Lee SH, Popadic D, Takeda K, Akira S, et al. Increased atherosclerotic lesions and Th17 in interleukin-18 deficient apolipoprotein E-knockout mice fed high-fat diet. Mol Immunol. 2009;47:37–45. doi: 10.1016/j.molimm.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898–2906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 49.Oikawa Y, Shimada A, Kasuga A, Morimoto J, Osaki T, Tahara H, et al. Systemic administration of IL-18 promotes diabetes development in young nonobese diabetic mice. J Immunol. 2003;171:5865–5875. doi: 10.4049/jimmunol.171.11.5865. [DOI] [PubMed] [Google Scholar]
- 50.Nicoletti F, Di Marco R, Papaccio G, Conget I, Gomis R, Bernardini R, et al. Essential pathogenic role of endogenous IL-18 in murine diabetes induced by multiple low doses of streptozotocin. Prevention of hyperglycemia and insulitis by a recombinant IL-18-binding protein: Fc construct. Eur J Immunol. 2003;33:2278–2286. doi: 10.1002/eji.200323864. [DOI] [PubMed] [Google Scholar]
- 51.Zaccone P, Phillips J, Conget I, Cooke A, Nicoletti F. IL-18 binding protein fusion construct delays the development of diabetes in adoptive transfer and cyclophosphamide-induced diabetes in NOD mouse. Clin Immunol. 2005;115:74–79. doi: 10.1016/j.clim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Rothe H, Hausmann A, Casteels K, Okamura H, Kurimoto M, Burkart V, et al. IL-18 inhibits diabetes development in nonobese diabetic mice by counterregulation of Th1-dependent destructive insulitis. J Immunol. 1999;163:1230–1236. [PubMed] [Google Scholar]
- 53.Schott WH, Haskell BD, Tse HM, Milton MJ, Piganelli JD, Choisy-Rossi CM, et al. Caspase-1 is not required for type 1 diabetes in the NOD mouse. Diabetes. 2004;53:99–104. doi: 10.2337/diabetes.53.1.99. [DOI] [PubMed] [Google Scholar]
- 54.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 55.Katakami N, Kaneto H, Matsuhisa M, Yoshiuchi K, Kato K, Yamamoto K, et al. Serum interleukin-18 levels are increased and closely associated with various soluble adhesion molecule levels in type 1 diabetic patients. Diabetes Care. 2007;30:159–161. doi: 10.2337/dc06-1768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.