Abstract
Several laboratories have independently isolated mitotically active germ cells, termed female germline stem cells or oogonial stem cells (OSCs), from adult mouse ovaries. However, a recent study using Ddx4-Cre;Rosa26 reporter mice concluded that such germ cells do not exist. Given the disparity in conclusions drawn in this study compared with others, we felt it was important to re-assess the utility of Ddx4-Cre;Rosa26 reporter mice for identification of OSCs in adult mouse ovaries. Transgenic Ddx4-Cre mice were crossed with Rosa26tdTm/tdTm mice to drive restricted tomato red (tdTm) gene expression in cells in which the Ddx4 gene promoter has been activated. Crude dispersion of ovaries from recombined offspring generated cell fractions containing tdTm-positive immature oocytes, which are incapable of proliferation and thus probably represent the uncharacterized reporter-positive ovarian cells identified in the paper Zhang et al. (2012) as being mitotically inactive. Dispersed ovaries further subjected to fluorescence-activated cell sorting yielded a large population of non-germline tdTm-positive cells, indicative of promoter ‘leakiness’ in the Ddx4-Cre mouse line. Nonetheless, a small percentage of these tdTm-positive cells exhibited externalized (extracellular, ec) expression of Ddx4 protein (ecDdx4-positive), expressed markers of primitive germ cells but not of oocytes, and actively proliferated in culture, all of which are characteristic features of OSCs. Thus, crude dispersion of ovaries collected from Ddx4 gene promoter-driven reporter mice is not, by itself, a reliable approach to identify OSCs, whereas the same ovarian dispersates further subjected to cell sorting strategies yield purified OSCs that can be expanded in culture.
Keywords: Ddx4, germline stem cell, oogonial stem cell, oogenesis, oocyte
Introduction
A decade ago, several initial lines of evidence using mice as a model were published, which challenged the longstanding paradigm in reproductive biology that female mammals lose the capacity for germ cell renewal around the time of birth (Johnson et al., 2004). Subsequently, many independent studies have corroborated and extended these early findings (reviewed in Woods and Tilly, 2013a, b). Some of these studies reported the successful isolation of mitotically active germ cells from adult mouse, rat, cow, monkey or human ovaries using magnetic-assisted cell sorting (MACS) or fluorescence-activated cell sorting (FACS) approaches (Zou et al., 2009, 2011; Pacchiarotti et al., 2010; Zhang et al., 2011; White et al., 2012; Anderson et al., 2013; Woods and Tilly, 2013a; Dunlop et al., 2014; Wolff et al., 2014; Zhou et al., 2014). Additionally, intragonadal transplantation studies, essentially identical to those used for 20 years to establish the identity of spermatogonial stem cells in mammalian testes (Brinster and Zimmermann, 1994; Brinster and Avarbock, 1994; Hermann et al., 2012), have shown that these adult ovary-derived germ cells, termed female germline stem cells or oogonial stem cells (OSCs), differentiate into fully functional eggs that ovulate and fertilize to produce viable embryos and offspring (Zou et al., 2009; Zhang et al., 2011; White et al., 2012; Woods and Tilly, 2013a; Zhou et al., 2014). Parallel in vitro studies of mammalian OSCs have established several characteristic features of these cells, including stable long-term proliferative potential (Zou et al., 2009, 2011; Pacchiarotti et al., 2010; Zhang et al., 2011; White et al., 2012; Anderson et al., 2013; Woods and Tilly, 2013a; Wolff et al., 2014; Zhou et al., 2014), inherent gene expression profile differences between embryonic primordial germ cells (PGCs) and OSCs (Imudia et al., 2013), and responsiveness of OSCs to endogenous cues that activate meiotic gene expression and the subsequent formation of immature oocytes (Park et al., 2013a; Woods and Tilly, 2013a).
However, the emerging field of mammalian OSC biology is not without controversy (Tilly et al., 2009; Woods et al., 2013), as would be expected of any major paradigm shift, and this has been refueled in part by a recent study from Liu and colleagues (Zhang et al., 2012). By evaluating the ovaries of a genetic mouse model in which expression of a fluorescent reporter gene is driven by the promoter of the germ cell-specific gene, Ddx4 (DEAD-box polypeptide 4; also referred to as mouse vasa homolog or Mvh), Zhang et al. (2012) claimed their findings refute a large body of work regarding the existence of mitotically active germ cells in post-natal mammalian ovaries (Johnson et al., 2004; Zou et al., 2009, 2011; Pacchiarotti et al., 2010; Zhang et al., 2011; White et al., 2012; Anderson et al., 2013; Imudia et al., 2013; Park et al., 2013a; Woods and Tilly, 2013a, b; Dunlop et al., 2014; Wolff et al., 2014; Zhou et al., 2014). That study, which received extensive attention (Hamzelou, 2012; Underwood, 2012; Yong, 2012), used a genetic approach reliant on the generation of recombined offspring derived from crossing two transgenic mouse lines: Ddx4-Cre and Rosa26rbw/rbw. The resultant Cre-mediated recombination event at the Rosa26rbw/+ locus triggers an irreversible switch from ubiquitous green fluorescent protein expression in all cells of the parental reporter mouse line to restricted expression of red fluorescent protein (RFP), orange fluorescent protein (OFP) or cyan fluorescent protein (CFP) in any cells that have at any point in development activated the Ddx4 gene promoter. Assuming the fragment of the Ddx4 gene promoter used to drive Cre expression in this transgenic mouse line is, like the endogenous Ddx4 gene, germ cell-specific (Fujiwara et al., 1994), recombined offspring should exhibit restricted RFP, OFP or CFP expression in all cells of the germ lineage.
Liu and colleagues presented four sets of experimental outcomes in their study using these mice (Zhang et al., 2012); however, three of the four data sets were concerned with either transplantation of transgenic fetal ovary-derived cells into wild-type adult ovaries or ex vivo study of post-natal ovary-derived cells that were identified as Ddx4-negative in recombined offspring. These experiments do not address the question of whether OSCs, which clearly express Ddx4 (Zou et al., 2009, 2011; Pacchiarotti et al., 2010; White et al., 2012), exist in adult ovaries. The main case tendered by Zhang et al. (2012) against the existence of OSCs in mice centers on their data which show only fluorescent images of RFP-expressing (Ddx4-Cre recombined) cells in 24-, 48- and 72-h cultures of cell fractions obtained by crude dispersion of post-natal day 8 ovaries from recombined offspring. Assessment of the RFP-expressing cells did not reveal evidence of active mitosis over the first 3 days of culture. It was therefore concluded that the germ cells identified in post-natal ovaries as having activated the Ddx4 gene promoter do not proliferate, and consequently conclusions drawn from prior studies that have purified mitotically active germ cells from post-natal mouse ovaries are incorrect (Zhang et al., 2012).
Unfortunately, the identity of the recombined cells present in the crudely dispersed ovarian cell fractions that were studied in short-term cultures were not discerned. Based on the relatively large size of the RFP-expressing cells shown in Fig. 2 of their paper (Zhang et al., 2012), it is possible that these cells were immature oocytes, which express the Ddx4 gene and thus would be RFP-positive, but are no longer capable of mitosis. Furthermore, the authors did not reproduce the experiments they claim to invalidate, by simply enriching for or purifying OSCs from ovaries of their recombined mice as others have done (Zou et al., 2009, 2011; Pacchiarotti et al., 2010; White et al., 2012). Detailed methods for mouse OSC purification are available and fully validated, and would have removed the possibility of interpretational ambiguity resulting from oocyte contamination in any end-point analysis directed at detection or analysis of premeiotic germ cells (reviewed in Woods and Tilly, 2013a).
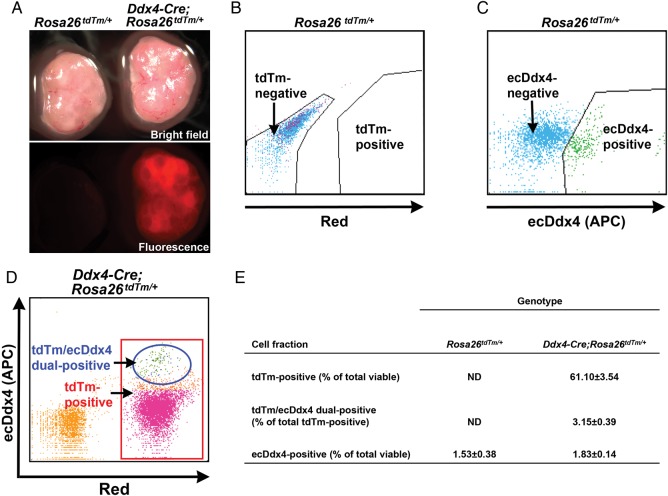
Figure 2.
FACS-based detection of ecDdx4 identifies OSCs in ovarian cell fractions from adult Ddx4-Cre;Rosa26tdTm/+ mice. (A) Representative bright-field and direct fluorescence images of ovaries collected from Rosa26tdTm/+ (left) and Ddx4-Cre;Rosa26tdTm/+ (right) mice at 60 days of age. (B and C) Representative FACS plots from analysis of cell fractions prepared from adult Rosa26tdTm/+ ovaries, sorted based on tdTm fluorescence (B) or APC-coupled antibody-based detection of ecDdx4 expression (C). (D) Representative FACS plot from analysis of cell fractions prepared from adult Ddx4-Cre;Rosa26tdTm/+ ovaries, sorted based on both ecDdx4 (APC, y-axis) and tdTm (x-axis) expression. (E) Quantitative yield of viable tdTm-positive cells or ecDdx4-positive cells (expressed as a percentage of total viable cells sorted), or of ecDdx4/tdTm dual-positive cells (expressed as a percentage of the total tdTm-positive cell population), following FACS of adult Rosa26tdTm/+ and adult Ddx4-Cre;Rosa26tdTm/+ mouse ovaries (mean ± SEM of combined data from three independent replicates, using different mice in each experiment).
Given these questions, and the discordance in the conclusions drawn by Zhang et al. (2012) compared with those of other laboratories (Johnson et al., 2004; Zou et al., 2009, 2011; Pacchiarotti et al., 2010; Zhang et al., 2011; White et al., 2012; Anderson et al., 2013; Imudia et al., 2013; Park et al., 2013a; Woods and Tilly, 2013a, b; Dunlop et al., 2014; Wolff et al., 2014; Zhou et al., 2014), we felt it was imperative to more comprehensively examine the utility of the Ddx4-Cre mouse line employed by these authors for the detection of OSCs in ovaries of fluorescent reporter mice. Here, we show that the methods used by Liu and colleagues lacked certain key control experiments, leading to an erroneous conclusion that mitotically active germ cells do not exist in post-natal mouse ovaries. We further show that by employing available sorting techniques to purify OSCs (Zou et al., 2009; White et al., 2012; Woods and Tilly, 2013a), ovaries of genetically recombined offspring resulting from crosses of Ddx4-Cre mice with Rosa26 reporter mice do contain mitotically active germ cells that are RFP-positive.
Materials and Methods
Animals
Rosa26tdTm/tdTm female mice (JAX stock 007914) and Ddx4-Cre male mice (JAX stock 006954) were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). The Rosa26tdTm/tdTm mice harbor a loxP-flanked STOP-cassette preventing transcription of the downstream CAG promoter-driven RFP variant, tdTomato (tdTm). In recombined (Ddx4-Cre;Rosa26tdTm/+) mice, Ddx4 promoter-driven expression of Cre recombinase excises the STOP-cassette to permanently activate expression of the tdTm gene, which should be restricted to Ddx4-expressing germ lineage cells. All procedures reported herein were reviewed and approved by the appropriate institutional animal care and use committee.
Preparation of crude ovarian cell fractions
Ovaries from post-natal Day 8 Ddx4-Cre;Rosa26tdTm/+ female mice were collected, minced, dissociated with trypsin, and then filtered through a 40-μm-pore cell strainer to remove large oocytes, debris and cell aggregates, exactly as described previously (Zhang et al., 2012). The resultant cell fractions were analyzed for the presence of immature oocytes by evaluating expression of oocyte-specific genes (see ‘Gene Expression Analysis’).
Isolation and culture of OSCs
Ovaries from 2-month-old Rosa26tdTm/+ and Ddx4-Cre;Rosa26tdTm/+ female mice were processed for FACS-based isolation of viable ecDdx4-positive cells (viz. OSCs), as described (White et al., 2012; Woods and Tilly, 2013a). Sorted OSCs were then established as pure germ cell cultures without somatic feeder cells (White et al., 2012; Woods and Tilly, 2013a). Fluorescence images of whole ovaries before dispersion, and of purified OSC colonies formed after 1 or 2 weeks of culture in vitro, were taken and analyzed with Spot Advanced Software (Diagnostic Instruments, Sterling Heights, MI, USA).
Cell proliferation analysis
Purified tdTm-expressing cells were seeded onto glass coverslips coated with poly-l-lysine, and then cultured with 10 μM BrdU (Sigma-Aldrich, St. Louis, MO, USA) for 48 h. The cells were then gently rinsed with 1×-concentrated phosphate-buffered saline prior to fixation in 1% paraformaldehyde for dual immunofluorescence-based detection of bromodeoxyuridine (BrdU) incorporation (mitotically active cells) and RFP expression, as described (White et al., 2012).
Gene expression analysis
Total RNA was isolated from ovaries (intact or crude dispersates) and testes using TRIzol reagent (Invitrogen—Life Technologies, Grand Island, New York, USA), and from freshly isolated ecDx4-positive cells (OSCs) using the RNeasy Plus Micro kit (Qiagen, Valencia, CA, USA). Each pool of RNA was then quantify and assessed by reverse transcription–polymerase chain reaction (RT–PCR) using Platinum Taq polymerase (Invitrogen—Life Technologies) for the presence of germ cell-specific and oocyte-specific mRNAs. Sequences of the forward and reverse primers used, along with GenBank accession numbers of the corresponding genes, are as reported previously (White et al., 2012).
Data presentation and analysis
All experiments were independently replicated at least three times using different mice or cell preparations in each experimental replicate. Where appropriate, qualitative images representative of outcomes obtained in the replicate experiments are provided.
Results
Crudely dispersed Ddx4-Cre;Rosa26tdTm/+ ovarian cell fractions contain oocytes
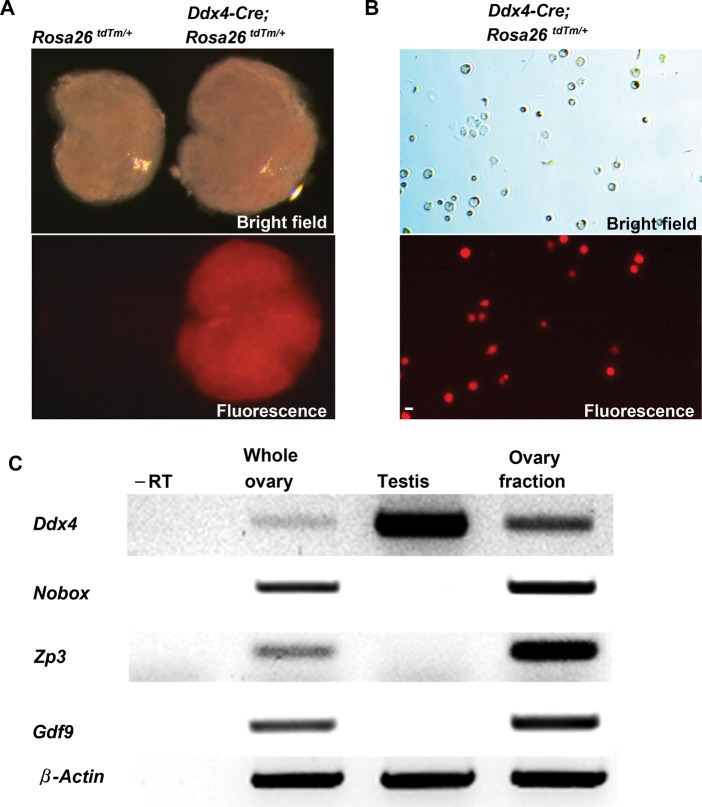
We first replicated the experiment presented by Zhang et al. (2012) in Fig. 2 of their paper using the same Ddx4-Cre transgenic mouse line crossed with transgenic mice carrying the Rosa26tdTm/tdTm reporter locus as a target for Cre-mediated recombination to drive restricted tdTm fluorescence gene expression in only those cells that have activated the Ddx4 promoter. Ovaries from recombined offspring were collected on post-natal Day 8 and subsequently processed through the trypsin-based dispersion procedure detailed in their paper (Zhang et al., 2012). Prior to dispersion, we observed a generalized and widespread pattern of tdTm fluorescence in recombined ovaries (Fig. 1A), which would not be expected of a transgenic reporter designed to mimic endogenous Ddx4 expression and thus be specific for germ cells. This finding led us to speculate that the Ddx4-Cre mouse line is prone to ‘leaky expression’ in non-germline cells, which was confirmed in subsequent FACS experiments (see next section below). Dispersion of ovaries by trypsin, mechanical disruption and filtration through a 40-μm cell-strainer, exactly as reported by Zhang et al. (2012), produced crude cell fractions containing uncharacterized debris and cell aggregates (Fig. 1B). Not surprisingly, we found that these fractions also contained immature oocytes, as determined by PCR analysis of a panel of oocyte-specific markers (Fig. 1C).
Figure 1.
Assessment of Ddx4-Cre;Rosa26tdTm/+ mouse ovaries reveals both reporter ‘leakiness’ and oocyte contamination in crudely dispersed cell fractions. (A) Representative bright-field and direct fluorescence images of ovaries collected from Rosa26tdTm/+ (non-recombined offspring, reporter not activated; left panels) and Ddx4-Cre;Rosa26tdTm/+ (recombined offspring, reporter activated; right panels) mice at post-natal Day 8. (B) Representative bright-field and direct fluorescence images of crudely dissociated cell fractions prepared from Ddx4-Cre;Rosa26tdTm/+ ovaries, exactly as described (Zhang et al., 2012). Scale bar, 50-μm. (C) Expression analysis of Ddx4 (germ cell marker), oocyte-specific genes (Nobox, Gdf9, Zp3) and β-actin (housekeeping gene) in post-natal Day 8 whole ovary, adult testis and the crudely dissociated post-natal Day 8 ovary cell fractions shown in (B) (–RT, PCR analysis of ovary cell fraction RNA sample processed without reverse transcription to rule out the possibility of target gene amplification from genomic DNA contamination).
Identification of OSCs in Ddx4-Cre; Rosa26tdTm/+ ovaries by FACS
Based on the issues encountered thus far, we elected to refine the strategy for identification of premeiotic germ cells in recombined ovaries by incorporating a recently detailed FACS-based method for the purification of OSCs (White et al., 2012; Woods and Tilly, 2013a). This approach takes advantage of the fact that the C-terminus of the Ddx4 protein is exposed on the outer plasma membrane of OSCs, but becomes localized entirely in the cytoplasm upon differentiation of the cells into oocytes (Zou et al., 2009; White et al., 2012; Imudia et al., 2013; Woods and Tilly, 2013a). Accordingly, antibodies that target the C-terminus of Ddx4 can be used with FACS to obtain from dispersed ovaries a purified fraction of viable OSCs, which are extracellular Ddx4 protein (ecDdx4)-positive; these cells are completely free of contaminating oocytes, which are ecDdx4-negative but still positive for Ddx4 gene expression (White et al., 2012; Woods and Tilly, 2013a). The other significant modification we made was to move away from trypsin-based digestion of ovarian tissue used by Liu and colleagues (Zhang et al., 2012), since this approach would have a very high likelihood of stripping away the Ddx4 antigen exposed on the plasma membrane of OSCs and consequently prevent antibody (FACS or MACS)-based collection of the cells (Woods and Tilly, 2013a).
Similar to ovaries harvested from recombined mice on post-natal Day 8 (Fig. 1A), ovaries collected from Ddx4-Cre;Rosa26tdTm/+ mice during adulthood (post-natal Day 60) also exhibited a generalized and widespread pattern of tdTm fluorescence (Fig. 2A). After dissociation using a collagenase-based protocol (White et al., 2012; Woods and Tilly, 2013a), we analyzed the resultant ovarian cell fraction by FACS for tdTm expression, APC-coupled ecDdx4 protein expression, or the two end-points together. As anticipated, ovaries of non-recombined (Rosa26tdTm/+) female offspring derived from crosses of Ddx4-Cre and Rosa26tdTm/tdTm mice did not contain any cells positive for tdTm expression (Fig. 2B and F); however, a small population of ecDdx4-postive cells (1.53% of the total viable cells sorted) was readily detectable in dispersed Rosa26tdTm/+ ovaries (Fig. 2C and F). Also as expected based on the widespread tdTm fluorescence in recombined (Ddx4-Cre;Rosa26tdTm/+) ovaries at collection (Figs 1A and 2A), more than 60% of the viable cells sorted from these ovaries were tdTm-positive (Fig. 2D and F). In addition, of the viable cells comprising the tdTm-positive fraction, only 3.15% were dual-positive for both tdTm and ecDdx4 expression (Fig. 2E and F). This ecDdx4-positive population represented 1.83% of the total viable cells sorted from dispersed Ddx4-Cre;Rosa26tdTm/+ ovaries (Fig. 2F). The yield of ecDdx4-positive cells from ovaries of 2-month-old Rosa26tdTm/+ mice (non-recombined) and Ddx4-Cre;Rosa26tdTm/+ mice (recombined) is fully consistent with data from past studies of OSC yield from adult mouse ovaries (White et al., 2012; Imudia et al., 2013; Woods and Tilly, 2013a).
Cells positive for both tdTm and ecDdx4 are mitotically active germ cells
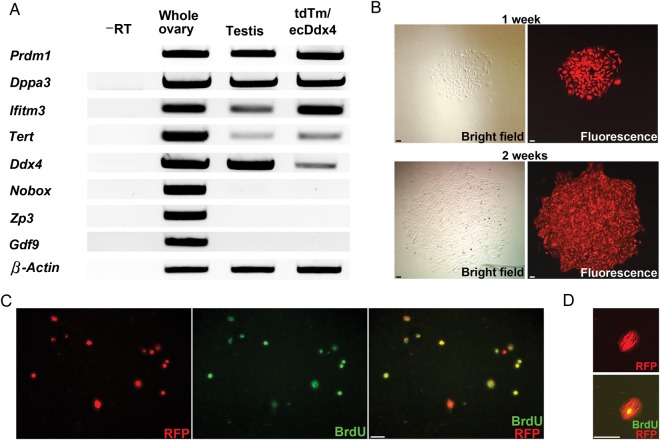
The purified tdTm/ecDdx4 dual-positive cell fraction obtained by FACS from dissociated Ddx4-Cre;Rosa26tdTm/+ ovaries expressed the primitive germ cell markers, Prdm1 (PR domain containing 1 with ZNF domain; also referred to as Blimp1), Dppa3 (developmental pluripotency-associated 3; also referred to as Stella), Ifitm3 (interferon-induced transmembrane protein 3; also referred to as Fragilis), Tert (telomerase reverse transcriptase) and Dazl (deleted in azoospermia-like), but did not express any of a panel of widely accepted oocyte-specific markers [Nobox (newborn ovary homeobox), Zp3 (zona pellucida glycoprotein 3), Gdf9 (growth differentiation factor 9)] (Fig. 3A). Levels of Ddx4 mRNA in freshly isolated tdTm/ecDdx4 dual-positive cells were low, but could be detected with increased PCR cycle number. In addition, plating of tdTm/ecDdx4 dual-positive cells in culture resulted in the formation of visibly fluorescent germ cell colonies within 1 week, which rapidly expanded in numbers with continued culture (Fig. 3B). Dual immunostaining analysis of RFP expression and BrdU incorporation confirmed that the vast majority of tdTm-positive germ cells plated in culture were actively dividing (Fig. 3C and D).
Figure 3.
Ovaries of adult Ddx4-Cre;Rosa26tdTm/+ reporter mice contain mitotically active germ cells. (A) Representative expression analysis of primitive germline markers (Prdm1, Dppa3, Ifitm3, Tert, Ddx4) and oocyte markers (Nobox, Zp3, Gdf9) in adult whole ovary, adult testis and ecDdx4/tdTm dual-positive cells isolated by FACS from ovaries of adult Ddx4-Cre;Rosa26tdTm/+ mice (–RT, PCR analysis of ecDdx4/tdTm dual-positive cell RNA sample processed without reverse transcription to rule out the possibility of target gene amplification from genomic DNA contamination; β-actin, internal sample loading control). (B) Time sequence analysis of a typical germ cell colony formed after plating ecDdx4/tdTm dual-positive cells isolated by FACS from adult Ddx4-Cre;Rosa26tdTm/+ ovaries, which doubles in size between Week 1 (upper panels) and Week 2 (lower panels) of culture. Scale bars, 50-μm. (C and D) Assessment of proliferation by dual detection of RFP expression (red) and BrdU incorporation (green) in cultures of ecDdx4/tdTm dual-positive cells isolated by FACS from adult Ddx4-Cre;Rosa26tdTm/+ ovaries and cultured without a somatic feeder cell layer. Scale bars: C, 50-μm; D, 20-μm.
Discussion
Over the last decade, an increasing number of academic (Johnson et al., 2004; Zou et al., 2009, 2011; Zhang et al., 2011; White et al., 2012; Anderson et al., 2013; Imudia et al., 2013; Park et al., 2013a, b; Woods and Tilly, 2013a, b; Dunlop et al., 2014; Zhou et al., 2014), industry (Pacchiarotti et al., 2010) and government (e.g. National Institutes of Health; Wolff et al., 2014) laboratories have produced experimental evidence questioning the decades-old belief that female mammals are incapable of post-natal oocyte renewal (Zuckerman, 1951). However, this line of study remains a source of contention in the field of reproductive biology (Tilly et al., 2009; Hamzelou, 2012; Underwood, 2012; Yong, 2012; Gura, 2012; Woods et al., 2013), despite the fact that the female equivalent of spermatogonial stem cells in males have not only been purified from ovaries of adult mice, rats, cows, monkeys and humans (Zou et al., 2009, 2011; Pacchiarotti et al., 2010; Zhang et al., 2011; White et al., 2012, Anderson et al., 2013; Dunlop et al., 2014; Wolff et al., 2014; Zhou et al., 2014), but also shown by intraovarian transplantation studies in mice and rats to possess the capacity to differentiate into fully functional eggs (Zou et al., 2009; Zhang et al., 2011; White et al., 2012; Park et al., 2013b; Zhou et al., 2014). Countering these reports, Liu and colleagues recently claimed that their experiments using Ddx4-Cre;Rosa26rbw/+ reporter mice provide strong genetic proof that post-natal mouse ovaries do not contain mitotically active germ cells (Zhang et al., 2012).
The first data set offered by these authors, shown in Fig. 1 of their paper (Zhang et al., 2012), assessed the fate of crudely dispersed Rosa26rbw/+ (viz. non-recombined) fetal ovary-derived cell fractions transplanted into ovaries of post-natal wild-type mice. The rationale for this experiment is not clear; the oocyte-forming capacity of embryonic PGCs in adult ovaries have little relevance to the question of whether adult ovaries contain mitotically active germ cells. Recent studies have shown PGCs have a gene expression profile more closely matched to embryonic stem cells rather than adult ovary-derived OSCs (Imudia et al., 2013), indicating that the two germ cell populations are quite distinct. Furthermore, it is already well established that PGCs require developmentally matched ovarian somatic cells to generate follicles (Lei et al., 2006). With respect to this latter point, Liu and colleagues injected only dispersed fetal ovary fractions into adult ovaries rather than separate injections of PGCs or embryonic ovarian somatic cells, which would be feasible with their genetic recombination model. With both fetal cell lineages present in post-natal ovaries after injection, it is expected that all new follicles formed would contain donor-derived oocytes surrounded by donor-derived somatic cells, as these embryonic-derived cells are natural and prerequisite partners for folliculogenesis (Lei et al., 2006; Hayashi et al., 2012).
The second experimental data set from Zhang et al. (2012), involves a direct analysis of post-natal ovaries for evidence of mitotically active germ cells. However, three issues uncovered in our study weaken clear interpretation of the experimental data presented by these authors. First, in our hands Cre-mediated recombination in Ddx4-Cre;Rosa26 fluorescent reporter mice is ‘leaky’ and not specific for germ cells. Although the commercial source of Ddx4-Cre mice used in our study and that of Liu and colleagues (Zhang et al., 2012) is the same, it is possible that variation exists in the specificity of Cre expression driven by the Ddx4 promoter amongst different mice in this particular transgenic line (Gallardo et al., 2007). Even if this were the case, a second issue in their study design is that the crudely dispersed ovarian cell fractions were contaminated with immature oocytes, which would be genetically recombined but incapable of proliferation. Hence, the mitotically inactive RFP-expressing cells studied by these authors are in all likelihood immature oocytes. The third issue is that Zhang et al. (2012) did not separate OSCs away from oocytes (and other cell types if there is promoter leakiness), using well-validated protocols that have been successfully employed with ovaries from a variety of mammalian species (Zou et al., 2009, 2011; White et al., 2012; Anderson et al., 2013; Woods and Tilly, 2013a; Dunlop et al., 2014; Wolff et al., 2014; Zhou et al., 2014). After publication of the Zhang et al. (2012) study, the senior author of this report subsequently clarified that his laboratory was unable to repeat the technique of Ddx4 antibody-based sorting, thus necessitating use of an alternative strategy to track potential germ cells in post-natal mouse ovaries (Gura, 2012). Since Liu and colleagues used trypsin to disperse ovaries for analysis (Zhang et al., 2012), it is not surprising that subsequent isolation of cells based on detection of a cell surface protein epitope (viz. extracellular Ddx4) would prove problematic (Woods and Tilly, 2013a). We found that replacing trypsin with collagenase and further processing the ovarian dispersates by FACS using a C-terminal Ddx4 antibody (White et al., 2012; Woods and Tilly, 2013a), RFP-expressing OSCs were readily detectable in adult ovaries of Ddx4-Cre;Rosa26 fluorescent reporter mice. Further, these cells, as reported previously by others and us (Zou et al., 2009, 2011; Zhang et al., 2011; White et al., 2012; Anderson et al., 2013; Woods and Tilly, 2013a; Wolff et al., 2014; Zhou et al., 2014), can be easily established in culture for propagation in vitro.
The third and fourth data sets tendered by Liu and colleagues, shown in Fig. 3 and Figure 4 of their paper (Zhang et al., 2012), are devoted to in vitro analyses of unidentified post-natal ovarian cells that did not undergo recombination in their Ddx4-Cre;Rosa26rbw/+ offspring. The absence of recombination at the Rosa26rbw/+ locus indicates that the cells under analysis for these final sets of experiments had never activated the Ddx4 gene promoter, leaky or otherwise. Accordingly, the gene expression and growth characteristics of these non-germline cells have no bearing on questions about OSCs or the oocytes that OSCs generate, all which are positive for Ddx4 expression at the mRNA and protein levels (Zou et al., 2009, 2011; Pacchiarotti et al., 2010; White et al., 2012; Woods and Tilly, 2013a).
It is worthwhile noting that a second genetic mouse study was published in 2013 in the same journal as the Zhang et al. (2012) paper, which also concluded that post-natal mouse ovaries lack mitotically active germ cells capable of de novo oogenesis (Lei and Spradling, 2013). With our re-assessment of the Zhang et al. (2012) study completed, we have now turned our attention to a similar re-assessment of the experimental designs and results reported by Lei and Spradling (2013). While these new experiments will take some time to complete, several key issues are already apparent with the elliptical arguments and data sets tendered by these authors in disputing the existence of OSCs in adult mouse ovaries (Woods and Tilly, 2013a, b). Most notably, the genetic approach of labeling all cell types at low frequency does not provide a ‘sensitive lineage-labeling system’ to ‘directly identify germline stem cells’ (Lei and Spradling, 2013) since this non-targeted approach makes it impossible to fate-map if a given oocyte arises from the subsequent differentiation of a labeled OSC. This report also did not enrich for or purify OSCs from ovaries of their mice prior to concluding from indirect experiments that such germ cells do not exist (Lei and Spradling, 2013). As we demonstrate here, if genetic reporter mouse lines are coupled with OSC sorting strategies (Woods and Tilly, 2013a), direct evidence for the existence of mitotically active germ cells, which are fully capable of differentiating into functional eggs following intraovarian transplantation in vivo (Zou et al., 2009; Zhang et al., 2011; White et al., 2012; Park et al., 2013b; Zhou et al., 2014), continues to emerge.
Authors' roles
E.S.P. and J.L.T. designed the experiments. E.S.P. conducted the experiments. E.S.P. and J.L.T analyzed the results, wrote the manuscript and approved the final manuscript for submission.
Funding
This study was supported by the U.S. National Institutes of Health (National Institute on Aging Method to Extend Research in Time Award R37-AG012279); and, the Glenn Foundation for Medical Research.
Conflict of interest
E.S.P. has nothing to disclose; J.L.T. discloses interest in intellectual property described in United States Patent 7195775, United States Patent 7850984, United States Patent 7955846 and United States Patent 8642329, and is a scientific co-founder of OvaScience, Inc. (Cambridge, MA, USA).
Acknowledgements
This study was initiated while the authors were at Massachusetts General Hospital and Harvard Medical School, and completed at Northeastern University.
References
- Anderson RA, McLaughlin M, Woods DC, Tilly JL, Telfer EE. Evaluation of oogonial stem cells and neo-oogenesis in girls and women with Turner Syndrome: a pilot study. Hum Reprod. 2013;28(Supplement):52. [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop CE, Bayne RA, McLaughlin M, Telfer EE, Anderson RA. Isolation, purification, and culture of oogonial stem cells from adult human and bovine ovarian cortex. Lancet. 2014;383:S45. [Google Scholar]
- Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci USA. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gura T. Reproductive biology: fertile mind. Nature. 2012;491:318–320. doi: 10.1038/491318a. [DOI] [PubMed] [Google Scholar]
- Hamzelou J. 2012. Debate over existence of ovarian stem cells heats up. NewScientist http://www.newscientist.com/article/dn22062-debate-over-existence-of-ovarian-stem-cells-heats-up.html#.UtrZnfYo5T4. (13 July 2014, date last accessed)
- Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imudia AN, Wang N, Tanaka Y, White YAR, Woods DC, Tilly JL. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil Steril. 2013;100:1451–1458. doi: 10.1016/j.fertnstert.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci USA. 2013;110:8585–8590. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Zhang H, Jin S, Wang F, Fu M, Wang H, Xia G. Stage-specific germ-somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J Cell Physiol. 2006;208:640–647. doi: 10.1002/jcp.20702. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 (BMP4) promotes mammalian oogonial stem cell differentiation via SMAD1/5/8 signaling. Fertil Steril. 2013a;100:1468–1475. doi: 10.1016/j.fertnstert.2013.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E-S, Woods DC, White YAR, Tilly JL. Oogonial stem cells isolated from ovaries of adult transgenic female mice generate functional eggs and offspring following intraovarian transplantation. Reprod Sci. 2013b;20(Supplement):75A–76A. [Google Scholar]
- Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood E. 2012. Stem cell study scrambles egg debate, again. Science Now http://news.sciencemag.org/2012/07/stem-cell-study-scramblesegg-de.html. (10 July 2014, date last accessed)
- White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EF, Libfraind LL, Weitzel P, Woods DC, Feng Y, Tilly JL, DeCherney AH, Tisdale JF. Oogonial stem cells generate mature oocytes in an autologous Rhesus macaque transplantation model. Reprod Sci. 2014;21(Supplement):119A. [Google Scholar]
- Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013a;8:966–988. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. Germline stem cells in adult mammalian ovaries. In: Sanders S, editor. Ten Critical Topics in Reproductive Medicine. Washington, DC: Science/AAAS; 2013b. pp. 10–12. [Google Scholar]
- Woods DC, White YAR, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view towards the future. Reprod Sci. 2013;20:7–15. doi: 10.1177/1933719112462632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. 2012. Ovarian stem cell debate. The Scientist http://www.the-scientist.com/?articles.view/articleNo/32332/title/Ovarian-Stem-Cell-Debate/ (9 July 2014, date last accessed)
- Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–141. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA. 2012;109:12580–12585. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang L, Kang JX, Xie W, Li X, Wu C, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20:271–281. doi: 10.1093/molehr/gat081. [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20:2197–2204. doi: 10.1089/scd.2011.0091. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. The number of oocytes in the mature ovary. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]