Abstract
Staphylococcus aureus is a significant cause of hospital and community acquired pneumonia and causes secondary infection after influenza A. Recently, patients with hyper-IgE syndrome, who often present with S. aureus infections of the lung and skin, were found to have mutations in STAT3, required for Th17 immunity, suggesting a potential critical role for Th17 cells in S. aureus pneumonia. Indeed, IL-17R−/− and IL-22−/− mice displayed impaired bacterial clearance of S. aureus compared with that of wild-type mice. Mice challenged with influenza A PR/8/34 H1N1 and subsequently with S. aureus had increased inflammation and decreased clearance of both virus and bacteria. Coinfection resulted in greater type I and II IFN production in the lung compared with that with virus infection alone. Importantly, influenza A coinfection resulted in substantially decreased IL-17, IL-22, and IL-23 production after S. aureus infection. The decrease in S. aureus-induced IL-17, IL-22, and IL-23 was independent of type II IFN but required type I IFN production in influenza A-infected mice. Furthermore, overexpression of IL-23 in influenza A, S. aureus-coinfected mice rescued the induction of IL-17 and IL-22 and markedly improved bacterial clearance. These data indicate a novel mechanism by which influenza A-induced type I IFNs inhibit Th17 immunity and increase susceptibility to secondary bacterial pneumonia.
Community-acquired and healthcare-associated pneumonia represent a significant cause of morbidity and mortality in the United States and the world. Pneumonia is the leading cause of death in children worldwide, resulting in nearly two million deaths per year (1). A number of causative organisms have been described in patients, including both Gram-positive and Gram-negative bacteria. Among these, Staphylococcus aureus infections have been found to be prevalent at rates as high as 45% (2). The recent increase in the occurrence of methicillin-resistant S. aureus (MRSA) has increased further the importance of understanding disease pathogenesis induced by this bacterium. The host response to bacteria is largely triggered by TLR ligands stimulating the production of inflammatory mediators and the recruitment of phagocytic cells to the lung. Several components of the innate immune system have been identified as key mediators of bacterial clearance (3–5). The role of T cells in bacterial pneumonia is more unclear. HIV patients with depleted CD4+ T cells are more susceptible to bacterial infection in the lung (6, 7). In mice, CD4+ and γδ T cells have been shown to play a role in immunity versus Klebsiella pneumoniae (8, 9). In addition, vaccination strategies were effective in bacterial pneumonia (10), further suggesting a role for memory T cells. These data indicate that the host response in bacterial pneumonia requires multiple cell types and likely both arms of innate and adaptive immunity.
Recently, the Th17 subset of T cells has been described as producing high levels of the proinflammatory cytokines IL-17 and IL-22 (11-13). Th17 cells are characterized by high expression of the transcription factors retinoid orphan receptor (ROR)α and RORγT driven by IL-6 and TGF-β signaling through STAT3 and SMAD pathways, respectively (12). The cytokine IL-23 has been implicated in Th17 cell regulation, proliferation, and cytokine production. STAT3 activation, driven by IL-6 and IL-23, is required for terminal Th17 differentiation and IL-22 production (14, 15). A role for STAT3 in T cells in the context of bacterial infection has emerged recently. Patients with hyper-IgE syndrome (Job’s syndrome) were shown to have STAT3 mutations (16). Consequently, these patients fail to develop Th17 cells or produce IL-17A, resulting in S. aureus infection of the skin and lung (17). These patients appear to have enhanced susceptibility to S. aureus due to a specific requirement for IL-17 and IL-22 signaling in the epithelium (18), suggesting a specific role for Th17 immunity in host defense against this pathogen.
A primary consequence of Th17 polarization and recruitment to the lung is production of IL-17 and IL-22. IL-17A, also termed IL-17, is known to signal through the heteromeric IL-17R complex to drive production of neutrophil growth factors and chemokines, including IL-6, G-CSF, keratinocyte chemoattractant, and MIP-2/IL-8. Thus, a primary consequence of IL-17A production in vivo is neutrophil accumulation, and IL-17R−/− mice are deficient in neutrophil recruitment to the airways in lung infection models (19). Overexpression of IL-17A by adenovirus augments the host response to K. pneumoniae (19). In addition to Th17 cells, lung-resident γδT cells produce high levels of IL-17 after Escherichia coli challenge, and IL-17 is critical for neutrophil recruitment and bacterial clearance (20, 21). These data confirm a role for IL-17 in recruiting neutrophils to the airways and in the clearance of infection in the lung. Another Th17-derived cytokine, IL-22 signals though the activation of the STAT3 pathway by binding a heteromeric receptor consisting of IL-22R1 and IL-10R2 (22). IL-22 is known to induce the production of several classes of antimicrobial peptides, such as β-defensin 2 and 3, S100A7–9, RegIIIαβγ, and lipocalin 2 (13, 23-26). IL-22 also has been shown to be required for immunity against K. pneumoniae (26). In that study, IL-22, along with IL-17, was shown to induce G-CSF, IL-6, and antimicrobial peptide production in the lung. These data suggest that IL-22 plays a critical role in regulating inflammation and promoting bacterial clearance in lung immunity. Although the role of IL-17A and IL-17F in promoting the clearance of S. aureus in mucocutaneous infection has recently been demonstrated recently (27), the impact of IL-17– and IL-22–mediated host defense against S. aureus pneumonia is unknown.
Influenza represents a highly contagious family of respiratory viruses that infect 5–20% of the U.S. population yearly and account for as many as 30,000 deaths annually. The majority of influenza A infection is not fatal; rather most patients appear to fully recover within 2 wk of infection. Due to this, there tends to be an assumption that the changes and damage that occur in response to infection are transient. However, it is well documented that influenza infection enhances susceptibility to secondary bacterial infections by altering bacterial adhesions (28), TLR expression (29, 30), and the pathogen-associated molecular pattern receptors (31) on epithelial cells. Recently, induction of type I or type II IFNs by influenza A has been shown to inhibit clearance of pneumococcal pneumonia in mice (32, 33). In addition, exacerbation of S. aureus pneumonia by preceding influenza A infection has been demonstrated (34, 35). These data indicate that influenza A infection enhances the lung’s susceptibility to secondary bacterial pneumonia. Bacterial sepsis represents a significant cause of death in the United States, 200,000 deaths annually, and the primary cause and location of sepsis are Gram-positive bacteria and the lung (36, 37). Several studies have linked temporally the onset of influenza A infection and the incidence of S. aureus pneumonia (38). Furthermore, the presence of preceding influenza-like symptoms has been shown to correlate with increased mortality in MRSA-infected patients (39). These data suggest that a primary severe consequence of influenza A infection is secondary S. aureus pneumonia. However, the role of Th17 cells in promoting immunity against S. aureus in the context of influenza A infection has not been examined previously.
Materials and Methods
Mice
Six- to 8-wk-old wild-type (WT) C57BL/6 mice were purchased from Taconic. IFN-γ−/− and C57BL/6 control mice were purchased from The Jackson Laboratory. IL-17Ra−/− (40), IL-17A−/− (41), IL-17F−/− (Genentech), IL-22−/− (24), and IFN-αR−/− (42) have been reported previously. Mice were maintained under pathogen-free conditions. All of the studies were performed on age- and sex-matched mice. All of the animal studies were conducted with approval from the University of Pittsburgh Institutional Animal Care and Use Committee.
S. aureus infection
S. aureus (American Type Culture Collection 49775) producing γ-hemolysin and Panton-Valentine leukocidin was purchased from the American Type Culture Collection. S. aureus was cultured as detailed by American Type Culture Collection instructions in casein hydrolysate yeast extract containing-modified medium, overnight for 18 h to stationary growth phase. Mice were inoculated with 108 CFU of S. aureus (in 50 μl sterile PBS) by oropharyngeal aspiration, and lungs were harvested 24 or 48 h later.
Influenza A PR/8/34 H1N1 infection
Influenza A PR/8/34 H1N1 was propagated in chicken eggs as described previously (43). Mice were infected with 100 PFU of influenza A PR/8/34 H1N1 (in 40 μl sterile PBS) from a frozen stock or control PBS by oropharyngeal aspiration. Infected mice were incubated for 6 d, at which time mice received S. aureus inoculum or control PBS. After an additional 24 or 48 h, lungs were harvested. Viral burden was determined by quantitative real-time RT-PCR on lung RNA for viral matrix protein as described previously (44).
Adenoviral IL-23 infection
Generation of adenovirus expressing IL-23 has been described previously (45). Mice were infected with adenovirus (5 × 108 PFU in 50 μl sterile PBS) expressing IL-23 or enhanced GFP (EGFP) (control) by oropharyngeal aspiration 4 d after influenza A infection. After three additional days, lungs were harvested.
Analysis of lung inflammation
At the indicated time points, mouse lungs were lavaged with 1 ml sterile PBS for inflammatory cell differential counts. The cranial lobe of the right lung was homogenized in sterile PBS by mechanical grinding. The resulting lung homogenate was used for bacterial colony counting and cytokine analysis by Lincoplex (Millipore) or by ELISA for IL-22 and IL-23 (R&D Systems). The middle and caudal lobes of the right lung were snap-frozen and homogenized under liquid nitrogen for RNA isolation by standard TRIzol extraction. RNA analysis was performed by standard RT-PCR using Assay on Demand Taqman probes and primers (Applied Biosystems, Foster City, CA). Mouse spleens were homogenized in sterile PBS and were used for bacterial colony counts.
IL-17 flow cytometry
For intracellular IL-17 staining, whole mouse lungs were digested with collagenase as described (44). Lung cells were stimulated with PMA (50 ng/ml) plus ionomycin (750 ng/ml) for 5 h at 37°C. Cells then were stained with fluorescent conjugated Abs for CD4, CD8, CD49b (DX5), and γδTCR (BD Biosciences). After surface marker staining, the cells were fixed and permeabilized (BD Biosciences), and intracellular staining was performed using anti–IL-17A Ab (BD Biosciences).
Bone marrow dendritic cell culture
Bone marrow dendritic cells were isolated from C57BL/6 mice as described previously (46). After 7 d in culture with MEM containing GM-CSF, nonadherent cells were used for assays. Dendritic cells were stimulated with peptidoglycan from S. aureus (Sigma-Aldrich).
CD11c+ dendritic cell isolation
Lungs from infected mice were cut into small pieces (~2 mm) and digested with collagenase as noted above. Digested lungs then were forced through a 70-μm filter, and RBCs were lysed. CD11c+ and CD11c− cells then were isolated using Miltenyi positive selection microbeads per the manufacturer’s instructions (Mitlenyi Biotec, Auburn, CA). After isolation, RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA).
Statistical analysis
All of the data are presented as the mean ± SEM. Significance was tested by unpaired t test (for two means) or one-way ANOVA (for multiple data groups) followed by Tukey posthoc test. Data were analyzed using the Microsoft Excel software package. Mouse survival data were analyzed by log-rank test using the Graph Pad Prism software package.
Results
Efficient clearance of S. aureus from the lung requires the Th17 pathway
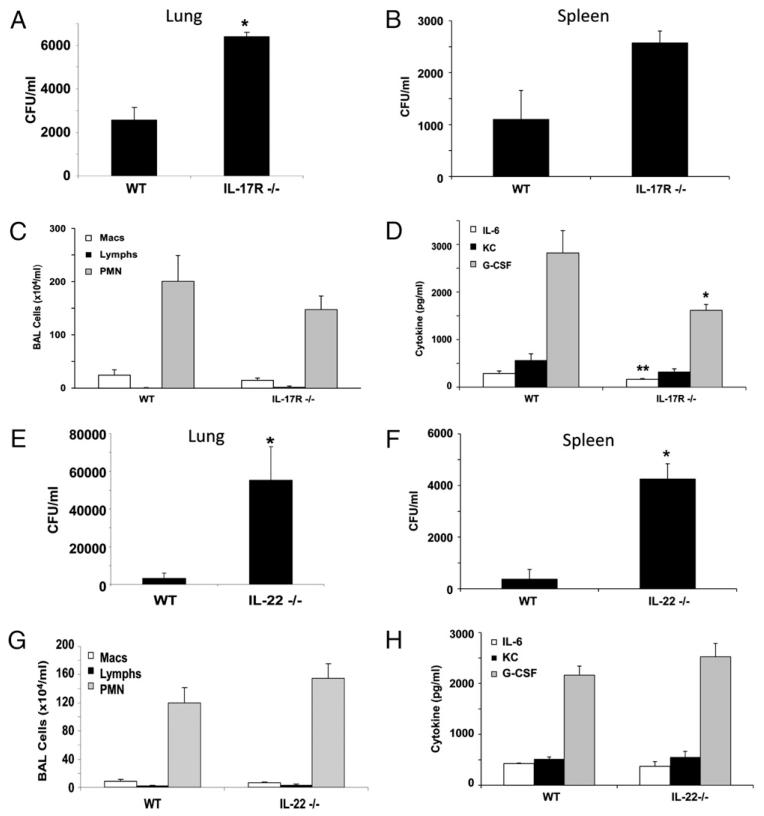
Because hyper-IgE syndrome patients due to STAT3 mutations that impair Th17 immunity develop skin and lung S. aureus infections, we investigated the role of Th17 effector cytokines or their receptors in S. aureus pneumonia. To test this, we infected WT C57BL/6, IL-17A−/−, IL-17F−/−, IL-17R−/−, and IL-22−/− mice with 108 CFU of S. aureus (Fig. 1, Supplemental Figs. 1, 2). S. aureus induced robust airway inflammation in WT mice characterized by neutrophil chemokine production and neutrophil recruitment to the airways (Fig. 1C, 1D). Abrogation of IL-17R signaling resulted in significantly increased bacterial burden in the lung (48 h postinfection) and attenuated neutrophil chemokine production (at both 24 and 48 h postinfection) (Fig. 1A, 1D, Supplemental Fig. 1C). In addition, IL-17R deletion resulted in a trend toward increased bacterial dissemination to the spleen versus that in WT mice (Fig. 1B). A similar phenotype of impaired bacterial clearance was evident in IL-17A−/− and IL-17F−/− mice (Supplemental Fig. 2), suggesting that both of these IL-17 ligands contribute to this response. Next, we examined the role of IL-22 in immunity versus S. aureus pneumonia. IL-22−/− mice also had impaired clearance of S. aureus from the lung (48 h postinfection) and increased bacterial dissemination to the spleen (Fig. 1E, 1F). However, deletion of IL-22 did not impair neutrophil recruitment or cytokine production (Fig. 1G, 1H, Supplemental Fig. 1E, 1F). In addition, attenuation of IL-17 or IL-22 did not alter production of the proinflammatory cytokine TNF-α at the 24 h time point (Supplemental Fig. 3). These data suggest that the impaired clearance of S. aureus in Th17 pathway knockout mice is not solely due to a lack of neutrophil recruitment to the lung, because attenuation of IL-17 or IL-22 both result in decreased bacterial clearance despite differing effects on inflammation.
FIGURE 1.
IL-17 and IL-22 are required for efficient clearance of S. aureus from the lung. C57BL/6, IL-17R−/−, or IL-22−/− mice were challenged with 108 CFU of S. aureus for 24 (C, D, G, H) or 48 h (A, B, E, F). All of the experiments were repeated once; representative data are presented. A and E, Bacterial colony counts in the cranial lobe of the right lung (n = 4). B and F, Bacterial colony counts in the spleen (n = 4). C and G, Lavage cell differential counts (n = 6 IL-17R−/−, n = 4 IL-22−/−). D and H, Th17-induced cytokines in lung homogenate (n = 6 IL-17R−/−, n = 4 IL-22−/−). *p< 0.05; **p< 0.10 versus WT.
Preceding influenza A infection worsens S. aureus pneumonia
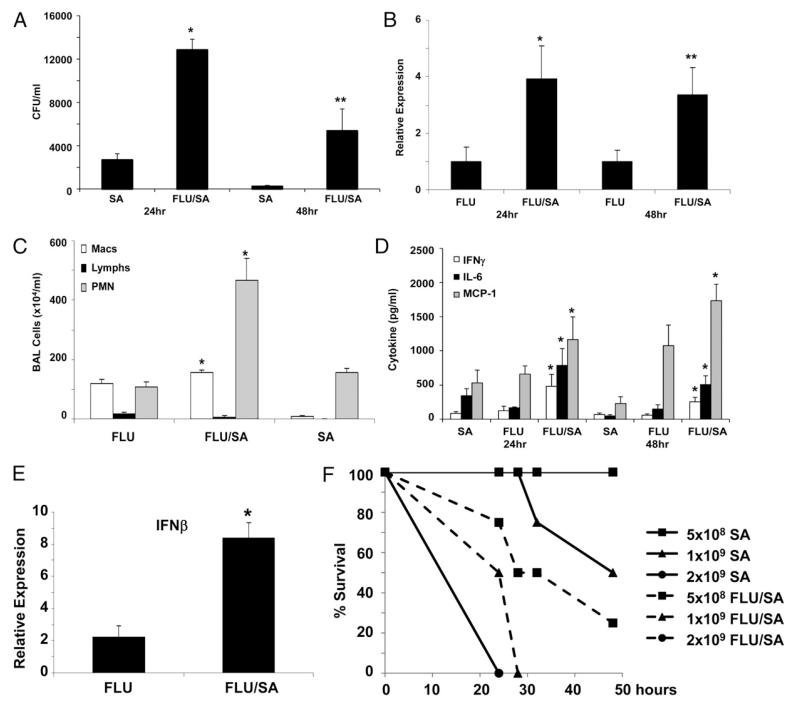
In a recent review, the presence of preceding influenza-like symptoms in patients with S. aureus pneumonia correlated with increased mortality (39). These data support the concept that superinfection with S. aureus may be a severe consequence of influenza. To test this hypothesis in an animal model, C57BL/6 mice were challenged with 100 PFU of influenza A PR/8/34 H1N1 for 6 d followed by 108 CFU of S. aureus; after 24 or 48 h, bacterial and viral clearance as well as lung inflammation were assessed. Preceding influenza A infection resulted in attenuated clearance of S. aureus in the lung (Fig. 2A). Interestingly, viral clearance also was inhibited in double-infected mice (Fig. 2B). Coinfection with influenza and S. aureus increased both macrophage and neutrophil recruitment to the lung as well as IL-6 and MCP-1 production (Fig. 2C, 2D). These data suggest that coinfection suppresses both viral and bacterial immunity in the lung, despite increasing inflammation. Production of both type I and II IFNs were enhanced by coinfection (Fig. 2D, 2E). Previous data have shown an important role for IFNs in the suppression of Streptococcus pneumoniae immunity (32, 33); however, the impact of the IFN response in S. aureus coinfection is unknown. To further demonstrate the impact of preceding influenza A infection on S. aureus pneumonia, we challenged mice with influenza A or vehicle as described above followed by 5 × 108 to 2 × 109 CFU of S. aureus (Fig. 2F). The highest quantity of S. aureus induced mortality in both influenza-preinfected and control mice; however, influenza A increased mortality to S. aureus throughout the time course. These data indicate that Influenza A infection worsens the outcome of secondary S. aureus pneumonia.
FIGURE 2.
Influenza A infection increases the susceptibility to S. aureus pneumonia. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 d, mice then were challenged with 108 CFU of S. aureus for 24 or 48 h (n = 4), all of the experiments were repeated twice, and representative data are presented. A, Bacterial colony counts in the upper right lobe of the lung. B, Influenza matrix protein expression in lung RNA. C, Lavage cell differential counts (24 h). D, Cytokine concentrations in lung homogenate. E, IFN-β gene expression in lung RNA (24 h). *p< 0.05; **p< 0.10 versus influenza A or S. aureus alone. F, Mortality curve for S. aureus challenged (solid lines) versus influenza A, S. aureus-coinfected (dashed lines) mice (n = 4). p< 0.05 versus S. aureus alone.
Influenza A inhibits Th17 pathway activation by secondary bacterial challenge
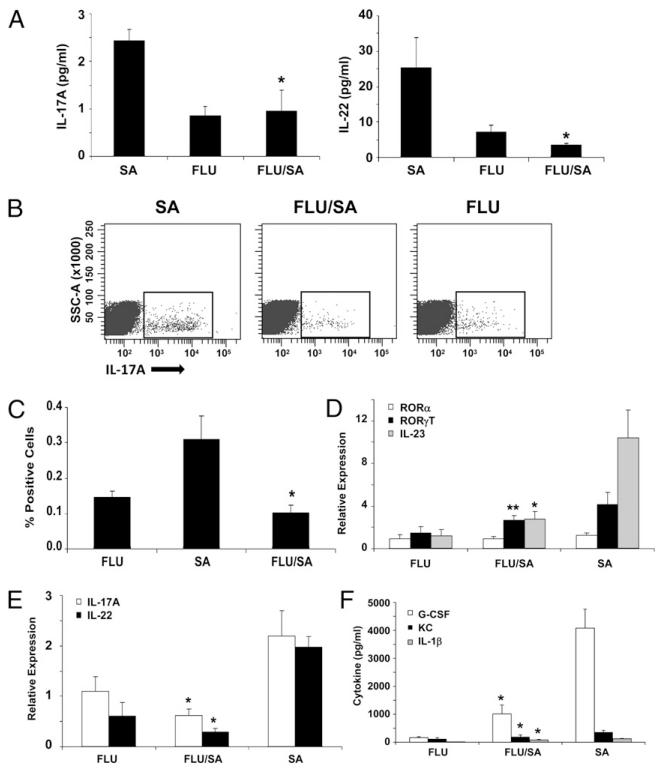
Because the Th17 pathway was shown to be critical for host defense against S. aureus and influenza A worsened S. aureus pneumonia, we proposed that influenza A infection may inhibit Th17-mediated immunity in the lung. To test this, we challenged mice with influenza A followed by S. aureus as outlined in the above section. S. aureus infection alone induced a Th17 effector cytokine response (Fig. 3, Supplemental Fig. 4). S. aureus enhanced IL-17A and IL-22 protein and gene expression in the lung as well as production of Th17 cytokine-induced chemokines (Fig. 3). In addition, S. aureus increased the number of IL-17A+ cells in the lung, and cell surface staining revealed that the majority of IL-17A–producing cells were CD4+ or γδ T cells (Fig. 3B, 3C, Supplemental Fig. 4). S. aureus also induced expression of the Th17 transcription factor RORγT and the Th17-promoting cytokine IL-23 (Fig. 3D). IL-23 has been shown to be required for IL-17A production by both Th17 and γδ T cells (47, 48). These data confirm that S. aureus induces a Th17 pathway immune response in the lung, and data in Fig. 1 demonstrate that in the absence of the Th17 pathway bacterial clearance is delayed. Importantly, preceding influenza infection significantly inhibited the S. aureus-driven Th17 pathway activation. Influenza A infection resulted in substantially decreased IL-17A and IL-22 protein and gene expression, decreased IL-17A+CD4+ and γδ T cells, and decreased RORγT and IL-23 expression. These data illustrate a potential mechanism by which influenza A inhibits bacterial immunity by suppressing the Th17 response in the lung.
FIGURE 3.
Influenza A inhibits the Th17 pathway induction by S. aureus. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 d, mice then were challenged with 108 CFU of S. aureus for 24 h (n = 4), all of the experiments were repeated twice, and representative data are depicted. A, IL-17 and IL-22 protein concentrations in lung homogenate. B and C, Total IL-17A–producing cells in lung by flow cytometry. D and E, Th17 pathway gene expression in lung RNA. F, Th17 pathway cytokine concentrations in lung homogenate. *p< 0.05; **p< 0.10 versus S. aureus alone.
Type I IFN induced by influenza A inhibits Th17 activation
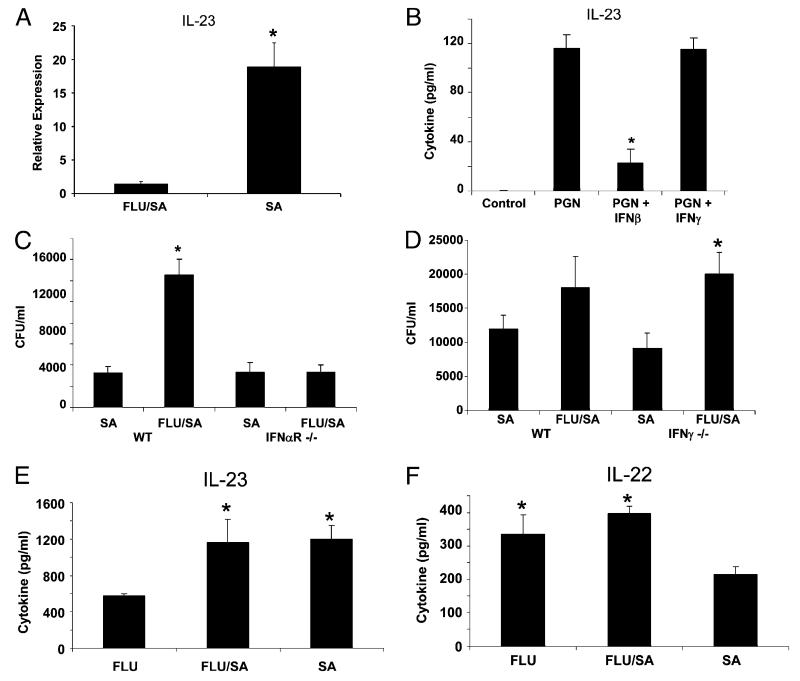
To determine the mechanism by which influenza A inhibits Th17 immunity, we focused on the elevated production of type I and II IFNs during coinfection. This mechanism has been implicated in increased susceptibility to S. pneumoniae infection and may be related to the observations in this study. We observed that preceding influenza infection suppressed the production of IL-23 induced by S. aureus infection, which is required for the production of Th17 cytokines and the maintenance of Th17 cells in the lung. A likely source of IL-23 in the lung is dendritic cells that drive Th17 polarization. To further demonstrate influenza-induced suppression of dendritic cell IL-23 production, we compared IL-23 expression in CD11c+ lung dendritic cells from S. aureus or coinfected mice. S. aureus induced IL-23 expression in CD11c+ dendritic cells, and this was inhibited by preceding influenza A infection (Fig. 4A). Next, we proposed that S. aureus TLR2 ligands, such as peptidoglycan or lipoteichoic acid, may stimulate IL-23 production in dendritic cells. Furthermore, we proposed that type I or II IFNs may inhibit IL-23 production by dendritic cells, thus attenuating the Th17 pathway. To test this mechanism in vitro, we derived bone marrow dendritic cells and stimulated them with peptidoglycan in the presence or absence of type I or II IFN (Fig. 4B). Peptidoglycan from S. aureus increased IL-23 production by dendritic cells; this induction was inhibited by the type I IFN IFN-β but not by the type II IFN IFN-γ. These data suggest that influenza A-induced type I IFN may inhibit the Th17 pathway in the lung. To then determine whether type I or II IFN was essential for the exacerbation of S. aureus pneumonia in the lung, we challenged IFN-αR−/− or IFN-γ−/− mice with influenza A followed by S. aureus. Deletion of either type I or II IFN resulted in suppressed lung inflammation during coinfection compared with that in WT mice (Supplemental Fig. 5). Type II IFN knockout mice still displayed exacerbation of S. aureus pneumonia in double-challenged mice (Fig. 4D). These data are consistent with a lack of suppression of IL-23 production in dendritic cells treated with IFN-γ. However, knockout of type I IFN signaling resulted in a loss of effects on S. aureus pneumonia, suggesting that type I IFNs are required for influenza A-induced worsening of bacterial immunity in the lung (Fig. 4C). Furthermore, preceding influenza A infection failed to inhibit IL-23 or IL-22 production in IFN-αR−/− mice (Fig. 4E, 4F). These data support a mechanism by which influenza A induces type I IFN, which through inhibition of IL-23 production attenuates Th17 immunity in the lung. The results of this molecular cascade lead to impaired antibacterial host defense and enhanced susceptibility to secondary infection.
FIGURE 4.
Inhibition of the Th17 response by influenza A requires type I IFN. Mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 d followed by 108 CFU of S. aureus for 24 h. CD11c+ dendritic cells were isolated from the lung of S. aureus or coinfected mice, and IL-23 expression was determined by RT-PCR. A, CD11c+ dendritic cell IL-23 expression (n = 7, 6, respectively). Bone marrow-derived dendritic cells were stimulated for 48 h with Staphylococcus peptidoglycan (20 μg/ml) in the presence or absence of IFN-β (10 U/ml) or IFN-γ (5 ng/ml) (n = 3), all of the experiments were repeated twice, and representative data are presented. B, IL-23 production in the media as measured by ELISA. C57BL/6, IFN-αR−/−, or IFN-γ−/− mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 d, mice then were challenged with 108 CFU of S. aureus for 24 h (n = 4), all of the experiments were repeated once, and representative data are presented. C and D, Bacterial colony counts in the upper right lobe of the lung. E and F, IL-22 and IL-23 production in lung homogenate from IFN-αR−/− mice as measured by ELISA. *p< 0.05 versus S. aureus alone (A versus influenza A/S. aureus; B versus peptidoglycan; D versus influenza A).
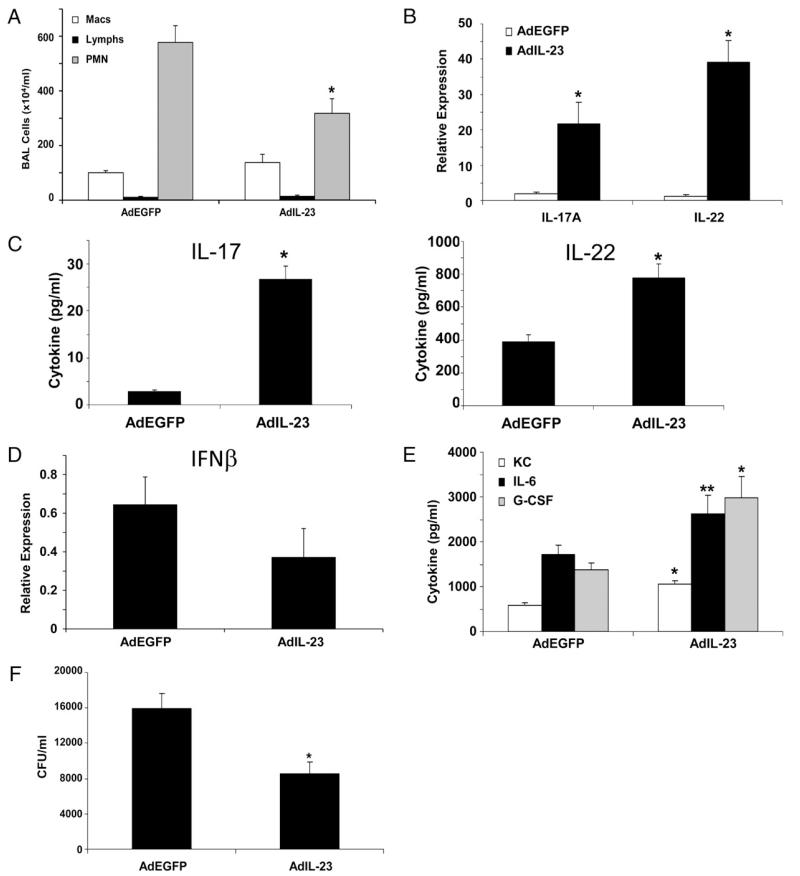
Exogenous IL-23 rescues Th17 activation and improves S. aureus clearance
As our mechanism proposes, decreased IL-23 production in influenza A, S. aureus-coinfected mice leads to exacerbated bacterial pneumonia by inhibiting Th17 immunity. To test this pathway, we overexpressed IL-23 in influenza A, S. aureus-coinfected mice, which we would predict would rescue Th17 immunity and improve bacterial clearance. IL-23 overexpression resulted in decreased lung inflammation, increased production of IL-17A and IL-22 protein and mRNA, and increased Th17 cytokine-induced chemokines compared with those in control adenovirus-infected mice (Fig. 5). This elevation of Th17 pathway immunity increased the clearance of S. aureus in mice infected with both influenza A and bacteria. In addition, exogenous IL-23 did not significantly alter type I IFN production induced by influenza A (Fig. 5D). These data further provide evidence for the mechanism outlined in this study. Influenza A inhibition of the Th17 pathway was rescued by overexpression of IL-23, which restored bacterial immunity in the lung and prevented increased susceptibility to secondary infection. These data suggest a potential therapeutic benefit of boosting Th17 immunity in patients with primary influenza A infection to reduce the risk of complicating secondary infections.
FIGURE 5.
Overexpression of IL-23 rescues the influenza-induced defect in Th17 induction and improves clearance of S. aureus. C57BL/6 mice were infected with 100 PFU of influenza A PR/8/34 or vehicle for 6 d, on Day 4 mice were inoculated with 5 × 108 PFU of adenovirus expressing IL-23 or EGFP, mice then were challenged with 108 CFU of S. aureus for 24 h (n = 6), all of the experiments were repeated twice, and representative data are presented. A, Lavage cell differential counts. B, IL-17A and IL-22 gene expression in lung RNA. C, IL-17A and IL-22 protein production in lung homogenate. D, IFN-β expression in lung tissue by RT-PCR. E, Th17 pathway cytokine levels in lung homogenate. F, Bacterial colony counts in the upper right lobe of the lung. *p< 0.05; **p< 0.10 versus adenoviral EGFP (AdEGFP).
Discussion
The findings of this study demonstrate a molecular and cellular mechanism by which influenza A infection impairs host defense against secondary S. aureus challenge. In addition, the data confirm a critical role for the Th17 pathway in promoting immunity versus S. aureus pneumonia. The vast majority of severe and fatal influenza infections are related to secondary bacterial pneumonia (49), indicating the importance of understanding compromised immune defense in this context. Furthermore, recent findings during the current H1N1 pandemic have shown the presence of secondary S. aureus infections in fatal influenza A cases in young adults. In our study, type I IFN induced by influenza A inhibits IL-23 production, which is required for Th17 pathway activation. Exogenous IL-23 during influenza A infection increased Th17 pathway activation and improved the immune response to secondary S. aureus challenge. These observations may have implications for the attenuation of host defense against a number of pathogens, because the role of the Th17 pathway has been demonstrated in numerous bacterial challenge models and in fungal immunity.
The observation that influenza A worsens susceptibility to S. aureus was reported many years ago (50); however, the molecular mechanisms for this phenotype have remained unclear. Further, pathologic synergism between influenza A and S. aureus products has been identified (51-53). Recently, influenza A was shown to exacerbate secondary S. aureus infection in mice as measured by decreased bacterial clearance and increased mortality (34). In that study, influenza was shown to inhibit NK cell production of TNF-α, which resulted in impaired antimicrobial function of macrophages. In our S. aureus model, IL-17R−/− or IL-22−/− mice had no change in TNF-α production in the lung induced by S. aureus at 24 h postchallenge compared with that in control mice despite worsened clearance of S. aureus. It is possible that TNF-α levels differed at earlier time points after S. aureus challenge. Further investigation is required to determine the role of TNF-α in our model. In addition, we observed a greater neutrophil response to S. aureus compared with macrophage recruitment, suggesting a potential role for neutrophils in the host response. In the context of secondary S. pneumoniae infection, preceding influenza A infection inhibited neutrophil recruitment to the lung, potentially explaining the increased susceptibility to bacterial infection (32). In addition, it has been reported that influenza A results in lasting desensitization to TLR ligands, resulting in decreased proinflammatory cytokine production (30). However, in our study, we observed increased neutrophilia and macrophage recruitment in coinfected mice, indicating that inflammatory cell recruitment alone does not explain the differences in S. aureus clearance. These data suggest that alternate mechanisms for S. aureus clearance are being suppressed by influenza A infection, which will require further investigation.
Previous work in our laboratory confirmed that IL-17 is required for acute lung injury during influenza A infection (44). IL-17R−/− mice displayed decreased lung inflammation, decreased weight loss, and decreased mortality after a lethal influenza A challenge. Despite the suppression of lung injury, IL-17R−/− mice did not fail to clear the virus from the lung, suggesting a therapeutic benefit of IL-17 neutralization in viral pneumonia. However, in light of our current data, inhibition of IL-17 during viral infection may lead to enhanced susceptibility to secondary bacterial challenge. These data indicate that modulation of the IL-17 pathway in the context of influenza A infection may have opposing effects on lung injury and susceptibility to further infection. In our study, exogenous IL-23 given 4–6 d after influenza A resulted in enhanced Th17 activation and suppressed airway neutrophilia. Increased Th17 activation may have been expected to increase inflammation, and the mechanism for the observed inhibition is unclear. These data suggest that the timing of Th17 pathway modulation may be critical to achieving the desired effect of reducing the risk of infection and minimizing lung injury.
Two recent studies have shown a role for type I or II IFNs in inhibiting the clearance of secondary S. pneumoniae infection after influenza, suggesting a critical mechanism for the repression of bacterial immunity (32, 33). In our study, the type II IFN pathway had no effect on IL-23 production or bacterial clearance. However, type I IFN robustly inhibited IL-23 levels produced by dendritic cells in response to S. aureus ligands. In addition, attenuation of type I IFN signaling resulted in a loss of influenza A exacerbation of S. aureus pneumonia and increased Th17 pathway activation. In support of this finding, the inhibition of Th17 polarization by type I IFN has been reported in human T cells (54). These data also suggest a broader mechanism by which other mucosal pathogens that induce type I IFN responses may inhibit Th17 immunity worsening secondary infections due to a variety of bacterial and fungal pathogens. Furthermore, a recent study has shown that highly virulent MRSA strains directly induce type I IFN production (55). This may represent a novel pathway by which S. aureus can directly inhibit host defense via Th17 pathway inhibition.
Due to the often severe consequences of secondary bacterial infections after influenza A on both lung function and the development of sepsis, a better understanding of the molecular pathways involved in host defense is critical. The impact of the Th17 pathway in promoting immunity against S. aureus appears clear; however, the molecular mechanisms involved remain elusive. Future studies will define the specific pathways and cell types critical for bacterial clearance. Inhibition of Th17 immunity in the lung by influenza A could have an impact on susceptibility to a variety of pathogens, expanding the impact of these studies presented in this work. In addition, the role of Th17 immunity in additional mucosal tissues is well described, suggesting a broader application of these data beyond the lung. The therapeutic potential of manipulating Th17 immunity in this context is yet to be determined and will require future investigation. This approach may be exploited to improve patient outcomes in severe cases of viral and bacterial coinfection.
Supplementary Material
Acknowledgments
We thank Allison Logar and Megan Blanchard of the Children’s Hospital of Pittsburgh Flow Cytometry Core for expertise in the analysis of IL-17–producing cells.
This work was supported by a Parker B. Francis Foundation Fellowship (to J.F.A.), a Children’s Hospital of Pittsburgh Research Advisory Committee Start-up grant (to J.F.A.), and National Heart, Lung and Blood Institute Grant R01HL079142 (to J.K.K.).
Abbreviations used in this article
- EGFP
enhanced GFP
- MRSA
methicillin-resistant Staphylococcus aureus
- ROR
retinoid orphan receptor
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 2.Carratalà J, Garcia-Vidal C. What is healthcare-associated pneumonia and how is it managed? Curr. Opin. Infect. Dis. 2008;21:168–173. doi: 10.1097/QCO.0b013e3282f4f248. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JA, Fischer AJ, McCray PB., Jr. Innate immune functions of the airway epithelium. Contrib. Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 4.Cazzola M, Matera MG, Pezzuto G. Inflammation—a new therapeutic target in pneumonia. Respiration. 2005;72:117–126. doi: 10.1159/000084039. [DOI] [PubMed] [Google Scholar]
- 5.Marriott HM, Dockrell DH. The role of the macrophage in lung disease mediated by bacteria. Exp. Lung Res. 2007;33:493–505. doi: 10.1080/01902140701756562. [DOI] [PubMed] [Google Scholar]
- 6.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen- host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AJ, O’Donnell AE. HIV-related pulmonary infections: a review of the recent literature. Curr. Opin. Pulm. Med. 2003;9:210–214. doi: 10.1097/00063198-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lanzke N, Kleinwächter R, Kerschischnik S, Sargsyan L, Groneberg DA, Kamradt T, Liesenfeld O, Krenn V, Sander M, Spies C. Differential effects of ethanol on IFN-γamma- and TNF-alpha-producing splenic T lymphocytes in a murine model of gram-negative pneumonia. Addict. Biol. 2007;12:59–68. doi: 10.1111/j.1369-1600.2006.00042.x. [DOI] [PubMed] [Google Scholar]
- 9.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J. Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 10.Chen WH, Kang TJ, Bhattacharjee AK, Cross AS. Intranasal administration of a detoxified endotoxin vaccine protects mice against heterologous Gram-negative bacterial pneumonia. Innate Immun. 2008;14:269–278. doi: 10.1177/1753425908095959. [DOI] [PubMed] [Google Scholar]
- 11.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin. Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 13.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 15.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 17.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J. Immunol. 2008;181:2071–2075. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- 21.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 22.Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 25.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 26.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 28.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 2003;187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 30.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulding J, R Snelgrove,, J Saldana,, A Didierlaurent,, Cavanagh M, Gwyer E, Wales J, Wissinger EL, Hussell T. Respiratory infections: do we ever recover? Proc. Am. Thorac. Soc. 2007;4:618–625. doi: 10.1513/pats.200706-066TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 34.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, Xing Z. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J. Immunol. 2010;184:2048–2056. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- 35.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J. Infect. Dis. 2010;201:508–515. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 38.Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr. Opin. Infect. Dis. 2004;17:185–191. doi: 10.1097/00001432-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Vardakas KZ, Matthaiou DK, Falagas ME. Comparison of community-acquired pneumonia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus producing the Panton-Valentine leukocidin. Int. J. Tuberc. Lung Dis. 2009;13:1476–1485. [PubMed] [Google Scholar]
- 40.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 42.García-Sastre A, Durbin RK, Zheng H, Palese P, Gertner R, Levy DE, Durbin JE. The role of interferon in influenza virus tissue tropism. J. Virol. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braciale TJ. Immunologic recognition of influenza virus-infected cells. I. Generation of a virus-strain specific and a cross-reactive subpopulation of cytotoxic T cells in the response to type A influenza viruses of different subtypes. Cell. Immunol. 1977;33:423–436. doi: 10.1016/0008-8749(77)90170-8. [DOI] [PubMed] [Google Scholar]
- 44.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Happel KI, Lockhart EA, Mason CM, Porretta E, Keoshkerian E, Odden AR, Nelson S, Ramsay AJ. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect. Immun. 2005;73:5782–5788. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 49.DeLeo FR, Musser JM. Axis of coinfection evil. J. Infect. Dis. 2010;201:488–490. doi: 10.1086/650304. [DOI] [PubMed] [Google Scholar]
- 50.Jakab GJ, Warr GA, Knight ME. Pulmonary and systemic defenses against challenge with Staphylococcus aureus in mice with pneumonia due to influenza A virus. J. Infect. Dis. 1979;140:105–108. doi: 10.1093/infdis/140.1.105. [DOI] [PubMed] [Google Scholar]
- 51.Tashiro M, Ciborowski P, Klenk HD, Pulverer G, Rott R. Role of Staphylococcus protease in the development of influenza pneumonia. Nature. 1987;325:536–537. doi: 10.1038/325536a0. [DOI] [PubMed] [Google Scholar]
- 52.Tashiro M, Ciborowski P, Reinacher M, Pulverer G, Klenk HD, Rott R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology. 1987;157:421–430. doi: 10.1016/0042-6822(87)90284-4. [DOI] [PubMed] [Google Scholar]
- 53.Zhang WJ, Sarawar S, Nguyen P, Daly K, Rehg JE, Doherty PC, Woodland DL, Blackman MA. Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice. J. Immunol. 1996;157:5049–5060. [PubMed] [Google Scholar]
- 54.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J. Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 55.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O’Seaghdha M, Soong G, Schindler C, Prince A. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J. Clin. Invest. 2009;119:1931–1939. doi: 10.1172/JCI35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.