Abstract
Mutations of FIG4 are responsible for Yunis-Varón syndrome, familial epilepsy with polymicrogyria, and Charcot-Marie-Tooth type 4J neuropathy (CMT4J). Although loss of the FIG4 phospholipid phosphatase consistently causes decreased PtdIns(3,5)P2 levels, cell-specific sensitivity to partial loss of FIG4 function may differentiate FIG4-associated disorders. CMT4J is an autosomal recessive neuropathy characterized by severe demyelination and axonal loss in human, with both motor and sensory involvement. However, it is unclear whether FIG4 has cell autonomous roles in both motor neurons and Schwann cells, and how loss of FIG4/PtdIns(3,5)P2-mediated functions contribute to the pathogenesis of CMT4J. Here, we report that mice with conditional inactivation of Fig4 in motor neurons display neuronal and axonal degeneration. In contrast, conditional inactivation of Fig4 in Schwann cells causes demyelination and defects in autophagy-mediated degradation. Moreover, Fig4-regulated endolysosomal trafficking in Schwann cells is essential for myelin biogenesis during development and for proper regeneration/remyelination after injury. Our data suggest that impaired endolysosomal trafficking in both motor neurons and Schwann cells contributes to CMT4J neuropathy.
INTRODUCTION
In yeast and mammalian cells, the Fig4/FIG4 phospholipid phosphatase controls the generation and turnover of the PtdIns(3,5)P2 phosphoinositide, a regulator of membrane and protein trafficking at the level of the endosome–lysosome axis. Loss of Fig4/FIG4 causes a decrease of PtdIns(3,5)P2 levels and defects in multiple pathways in the endomembrane system. Typical cellular features associated with Fig4/FIG4 loss are the enlargement of late endosomes–lysosomes (LE/LY) and cytosolic vacuolization (1–3).
In human, recessive mutations in FIG4 are responsible for the neurodegenerative Yunis-Varón syndrome, familial epilepsy with polymicrogyria, and Charcot-Marie-Tooth type 4J (CMT4J) neuropathy (3–10). Haploinsufficiency of FIG4 may also be a risk factor for amyotrophic lateral sclerosis (ALS) (4).
Yunis-Varón syndrome is a severe disorder with autosomal recessive inheritance characterised by skeletal and structural brain abnormalities and facial dysmorphism (5). FIG4 mutations identified in Yunis-Varón patients are nonsense or missense mutations that abolish FIG4 enzymatic activity, thus resulting in complete loss of FIG4 function (5,9). Recently, a homozygous missense mutation causing partial loss of FIG4 function was demonstrated to co-segregate with polymicrogyria, psychiatric manifestations and epilepsy in a consanguineous Moroccan family, thus suggesting a role for FIG4 in the regulation of cortical brain development (10). ALS is a severe neurological disorder characterized by selective neurodegeneration of lower and upper motor neurons. ALS patients carrying mutations in FIG4 are heterozygous for a null allele (deletions or splice site mutations leading to frameshift) or for missense mutations which alter FIG4 enzymatic activity (4). Patients with CMT4J neuropathy display a variable degree of severity. Early onset CMT4J shows asymmetrical motor and sensory neuropathy, which is usually rapid in progression. Late onset CMT4J displays a prevalent motor and asymmetric neuropathy, which is a typical feature of lower motor neuron disease rather than of CMT neuropathy (6). However, in both early and late onset CMT4J, the reduction of nerve conduction velocity (NCV) and the presence of onion bulbs in nerve biopsy suggest a demyelinating type of CMT, thus being classified in the CMT4 subclass (6–8). CMT4J patients are compound heterozygous for one missense mutation and one loss-of-function mutation. The I41T allele is the most frequent CMT4J missense mutation, and partially affects FIG4 enzymatic activity by destabilizing the protein (3,11).
Overall, these disorders indicate that, despite the ubiquitous expression, loss of FIG4 affects specific cell types with distinct pathogenetic mechanisms. This cell-specific effect might be due to the impact of the different mutations on the FIG4 enzymatic activity/stability and/or to the impairment of cell-specific functions within the endolysosome axis. These aspects have been only partially elucidated using the Fig4-null mouse models generated so far. For example, while available mouse models clearly indicate a predominant role for Fig4 in neurons, the onion bulbs and active demyelination/remyelination observed in CMT4J patient biopsies would be consistent with a cell autonomous role for FIG4 in Schwann cells as well (6–8).
Here, we report the generation and characterization of mouse mutants with conditional inactivation of Fig4 in either motor neurons or Schwann cells, two cell types affected in the CMT4J neuropathy. We found that Fig4 loss in motor neurons causes neuronal and axonal degeneration, whereas the Fig4-Schwann cell conditional mutant displays a general trafficking impairment, leading to a defect in autophagy-mediated degradation and demyelination. We also exploited the Fig4-Schwann cell conditional mutant to investigate whether trafficking through the endolysosome axis contributes to myelin biogenesis during development and to regeneration/remyelination. Our in vitro and in vivo data suggest that altered LE/LY homeostasis in Schwann cells impairs both active myelination and nerve regeneration.
RESULTS
Loss of Fig4 in motor neurons in vivo leads to neuronal and axonal degeneration
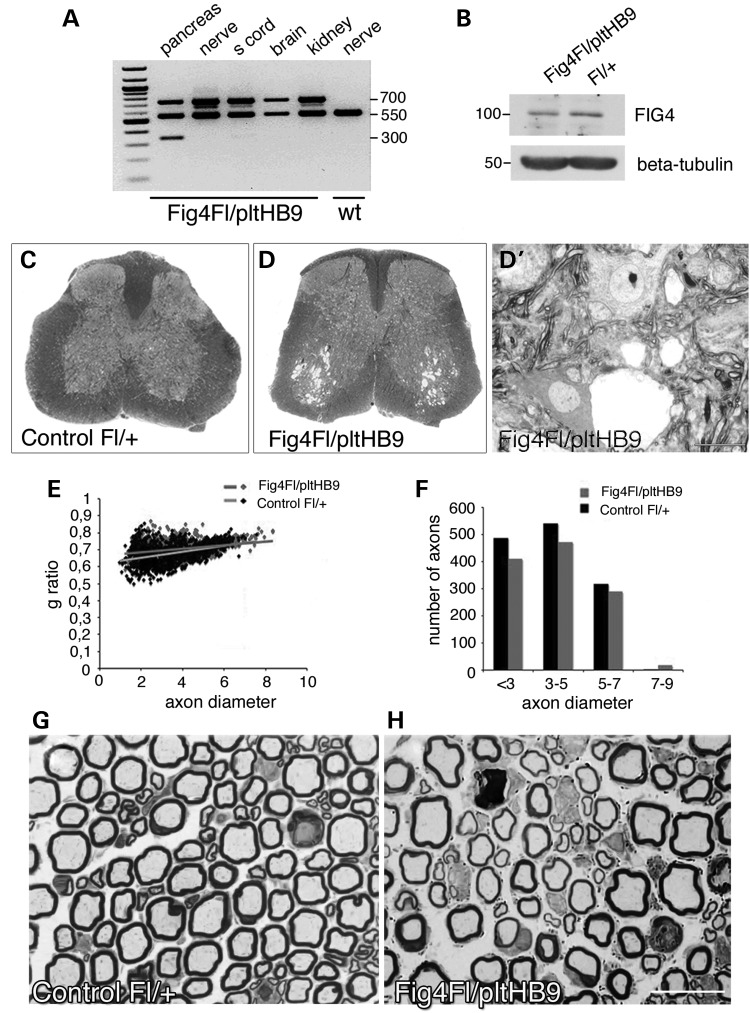
CMT4J patients initially display a prevalent motor and asymmetric neuropathy, which is a typical feature of a lower motor neuron disease rather than of demyelinating CMT neuropathies (6,7). This observation suggests that lower motor neurons are vulnerable to loss of Fig4. Mutants investigated thus far include the Fig4plt/plt mouse (a spontaneous mutant with global Fig4 loss), the Fig4Floxed/Floxed, Syn-Cre conditional mutant lacking Fig4 specifically in neurons and the Fig4plt/plt, NSE-Fig4(tg) mouse overexpressing Fig4 specifically in neurons under the control of the neuron-specific promoter NSE. Analysis of all of these mutants demonstrates that Fig4 plays an important role in neurons (1,3,12). However, in the Fig4plt/plt mouse, spinal motor neurons were among the last neurons to exhibit vacuolization, being largely preserved at P21 but filled with vacuoles at 6 weeks of age (3,13). The lethality of the Fig4plt/plt mice ∼6 weeks of age did not permit further evaluation of the Fig4 loss-of-function phenotype in motor neurons. Thus, for a more specific assessment of Fig4 in motor neurons and their peripheral projections, we generated Fig4Floxed/plt, HB9-Cre mice, in which the HB9-Cre transgene produces somatic recombination at embryonic day 9.5 (E9.5) in motor neurons and in the pancreas (14–17). To achieve maximal efficiency of HB9-Cre-mediated recombination, we generated compound heterozygous mice carrying one null allele (Fig4plt, global Fig4 deficiency) and one Floxed allele at the Fig4 locus. Heterozygous Fig4plt/+ mice and homozygous Fig4Flox/Flox mice are normal in survival and morphology, as previously reported (3,12,18). PCR analysis of genomic DNA demonstrated HB9-Cre-mediated excision of Fig4 in the pancreas and partial excision in the spinal cord, which also contains non-neuronal cells (Fig. 1A). Western blot analysis of lysates from ventral horns and motor roots of spinal cords also showed decreased Fig4 expression in Fig4Floxed/plt, HB9-Cre mice (Fig. 1B). Fig4Floxed/plt, HB9-Cre spinal cords at P30 and P90 display extensive cell vacuolization in the ventral horn where motor neurons are located (Fig. 1C and D′ and data not shown). Moreover, quadriceps nerves from Fig4Floxed/plt, HB9-Cre mice displayed mild hypomyelination with increased g-ratio (the ratio between axon diameter and fibre diameter) at P30 (Fig. 1E and F; g-ratio: Fig4Floxed/plt, HB9-Cre 0.71 ± 0.0004, 1189 fibres; controls Fig4Fl/+ 0.68 ± 0.003, 1350 fibres; n = 4, P = 0.0057). This was also observed at P90, when signs of axonal degeneration and fibre loss were evident (Fig. 1G and H; number of fibres at P90: Fig4Floxed/plt, HB9-Cre 477 ± 11.5 and controls Fig4Fl/+ 536 ± 7.9, n = 3, P = 0.01). At 6 and 12 months of age, these Fig4Floxed/plt, HB9-Cre mice were viable and clinically indistinguishable from control mice, and did not display tremor or gross behavioural impairment.
Figure 1.
Conditional ablation of Fig4 specifically in motor neurons. (A) PCR analysis of genomic DNA from Fig4Floxed/plt, HB9-Cre mice and controls. A 300-bp recombination band was detected in the pancreas where HB9 is highly expressed. A faint band is also present in spinal cord, which contains other cells in addition to motor neurons where recombination occurs. (B) Western blot analysis demonstrated decreased Fig4 expression in lysates from motor roots and ventral horn of mutant mice at P30. (C and D′) Toluidine blue staining of spinal cords from Fig4Floxed/plt, HB9-Cre mice at P30 shows vacuolization in the ventral horns where motor neurons are located (L4–L5). (E and F) G-ratio analysis of quadriceps nerves at P30 indicates reduction of myelin thickness in Fig4Floxed/plt, HB9-Cre mouse nerves. The total number of fibres and axon diameter distribution are normal in Fig4Floxed/plt, HB9-Cre P30 quadriceps nerves. (G and H) Semithin section analysis of quadriceps nerves at P90 shows hypomyelination and reduced density of fibres in mutant Fig4Floxed/plt, HB9-Cre nerves. Bar in (D′) is 10 µm and in (H) is 10 µm.
Consistent with the observations with HB9-Cre, we also observed motor neuron vacuolization in spinal cords of Fig4Floxed/plt, Olig2-Cre mice (Supplementary Material, Fig. S1), where the Olig2 promoter drives Cre expression starting at E10.5 in Shh (Sonic Hedgehog) responsive domains of the neural tube that give rise to motor neurons as well as oligodendrocyte precursor cells (OPCs) (19,20).
These results demonstrate that Fig4 has a cell autonomous role in motor neurons since Fig4Floxed/plt, HB9-Cre mutants show neuronal and axonal degeneration, leading to mild hypomyelination likely as a secondary consequence of altered axo-glial communication. Moreover, the fact that demyelination is not observed in the nerves of the motor neuron conditional Fig4Floxed/plt, HB9-Cre mouse supports the hypothesis that loss of Fig4 in Schwann cells may contribute to demyelinating CMT4J.
Loss of Fig4 in Schwann cells in vivo causes a progressive demyelinating neuropathy
To directly assess a Schwann cell autonomous role of Fig4, we generated both Fig4Floxed/Floxed, P0-Cre and Fig4Floxed/plt, P0-Cre mice, in both of which Fig4 is specifically ablated in Schwann cells starting from E13.5 (17,21–23).
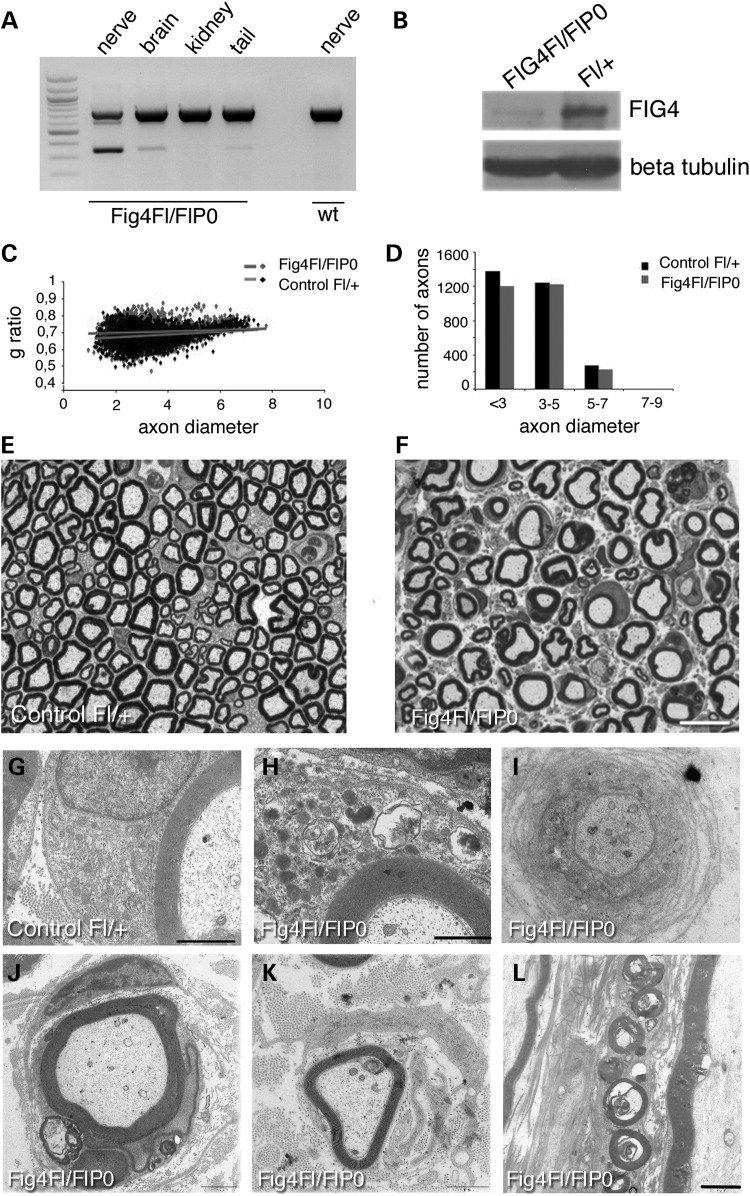
To demonstrate P0-Cre-mediated ablation of Fig4 in Schwann cells of Fig4Floxed/Floxed, P0-Cre mouse nerves, we performed PCR analysis of genomic DNA from different tissues. Recombination of the Floxed allele in sciatic nerve and brain was detected by generation of a 300 bp PCR product (Fig. 2A). Western blot analysis confirmed reduced Fig4 expression in Fig4Floxed/Floxed, P0-Cre mouse sciatic nerve (Fig. 2B). Morphological analysis of sciatic nerve revealed an accumulation of organelles and of lipidic material/vesicles in the cytoplasm of myelinating Schwann cells in Fig4Floxed/Floxed, P0-Cre nerve fibres at P30, P60 and 4 months of age, suggesting a general trafficking impairment (Fig. 2F and H and Fig. 3B). The assembly of myelin membrane during myelination depends on polarized trafficking of lipids and proteins through endocytic routes (24,25). Consistent with this, Fig4Floxed/Floxed, P0-Cre mutant sciatic nerves also displayed reduced myelin thickness and increased g-ratio values at P60 (Fig. 2C and D), suggesting that the regulation of endocytic trafficking through the endolysosomal axis is essential for myelination (g-ratio: Fig4Floxed/Floxed, P0-Cre 0.71 ± 0.005, 2655 fibres; controls Fig4Floxed/+ 0.68 ± 0.005, 2832 fibres, n = 4, P = 0.009). In older Fig4Floxed/Floxed, P0-Cre nerves at 4 months we observed progression of the phenotype with demyelinating features including onion bulbs (1% of the total number of fibres in sciatic nerves—onion bulbs have never been observed in control nerves), myelin degeneration (Fig. 2I–L), and a further reduction in the myelin thickness when compared with developmental stages (g-ratio: Fig4Floxed/Floxed, P0-Cre 0.72 ± 0.001, 880 fibres and controls Fig4Floxed/+ 0.68 ± 0.001, 1416 fibres, n = 3; P = 6 28178E−06). Finally, at 4 months demyelination was also associated with fibre loss (total number of fibres: Fig4Floxed/Floxed, P0-Cre 2368 ± 56.45 fibres and controls Fig4Floxed/+ 2978 ± 152.9 fibres, n = 3; P = 0.0191; Figs. 2F and 3B).
Figure 2.
Schwann cell conditional ablation of Fig4 causes developmental hypomyelination and demyelination in the Fig4Floxed/Floxed, P0-Cre mouse. (A) PCR analysis on genomic DNA from Fig4Floxed/Floxed, P0-Cre mice and controls. A 300-bp recombination band was detected in the nerve of mutants but not in wild type. The faint 300-bp band in the brain probably indicates recombination in cranial nerves (21,22) and in Schwann cells in the tail nerve. (B) Western blot analysis shows reduction of Fig4 in mutant sciatic nerves at P30. (C and D) G-ratio analysis of sciatic nerves at P60 indicates decreased myelin thickness and hypomyelination in Fig4Floxed/Floxed, P0-Cre mouse nerves. (E and F) Semithin section analysis of sciatic nerves at 4 months showed the presence of vesicles/myelin debris in the Schwann cell cytoplasm of mutant myelinated fibres, observed in ultrastructural analysis at P30 in (H), when compared with a control at the same age (G). (I and L) Ultrastructural analysis of sciatic nerves at 4 months showed the presence of demyelination such as onion bulbs (I–K) and myelin debris (L). Bar in (E and F) is 10 µm, in (G) is 0.7 µm and in (H) is 0.88 µm. Bar in (L) is 0.5 μm for (I) and 2 μm for (J–L).
Figure 3.
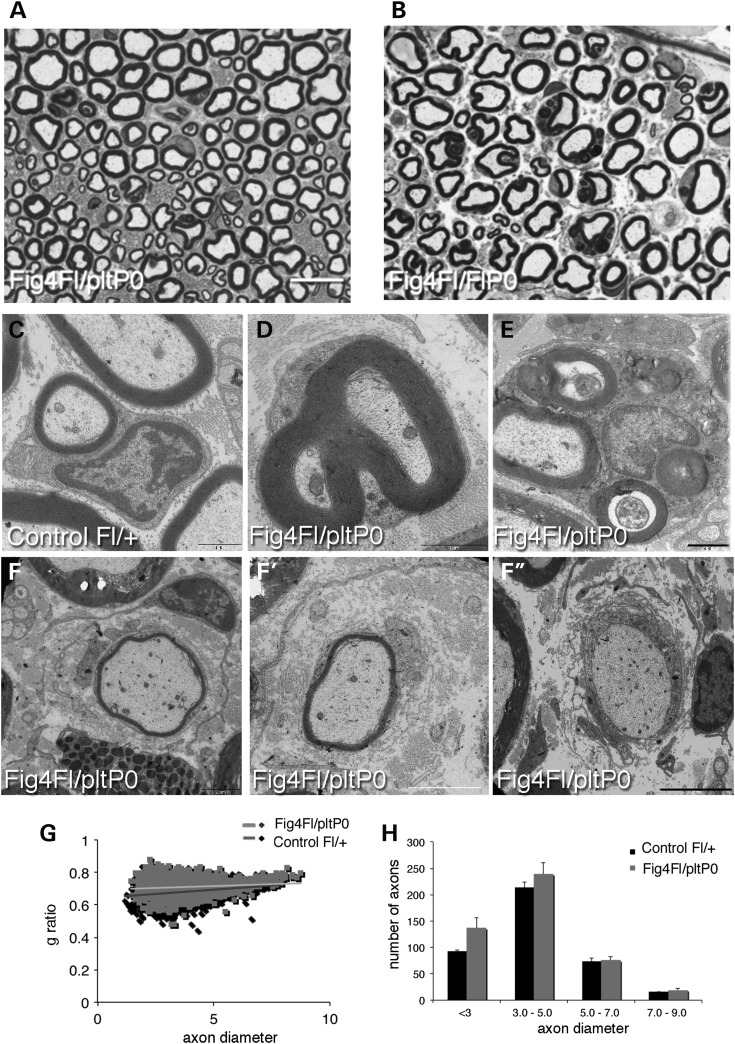
Schwann cell conditional ablation of Fig4 in the Fig4Floxed/plt, P0-Cre mouse resulted in a milder phenotype. (A and B) Semithin section analysis of sciatic nerves of Fig4Floxed/plt, P0-Cre when compared with Fig4Floxed/Floxed, P0-Cre at 4 months. The percentage of fibres carrying vesicles/myelin debris in the Schwann cell cytoplasm is higher in Fig4Floxed/Floxed, P0-Cre mice. Myelinated fibres from Fig4Floxed/plt, P0-Cre sciatic nerves at 8 months showing demyelinating features such as redundant myelin (D), myelin degeneration (E), and onion bulbs (F–F′′). Fig4Floxed/plt, P0-Cre sciatic nerves analysed at P30 (G and H) have increased g-ratio values indicating developmental hypomyelination. Bar in (A) is 10 µm for (A and B); bar in (F′′) is 1.7 µm for (C); 1 µm for (D); 1.7 µm for (E); 3 µm for (F); 2.4 µm in (F′) and 5 µm for (F′′).
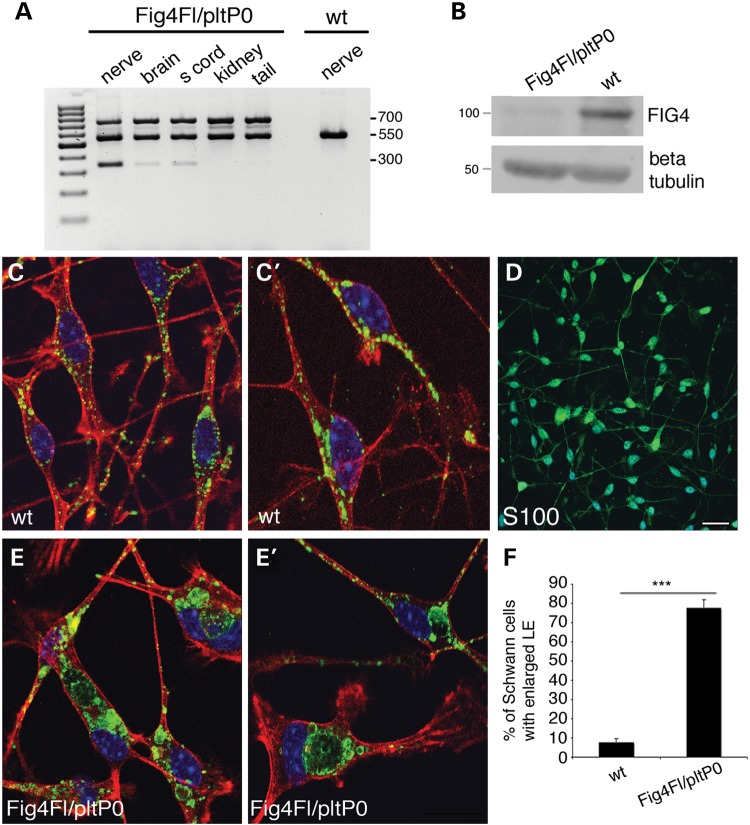
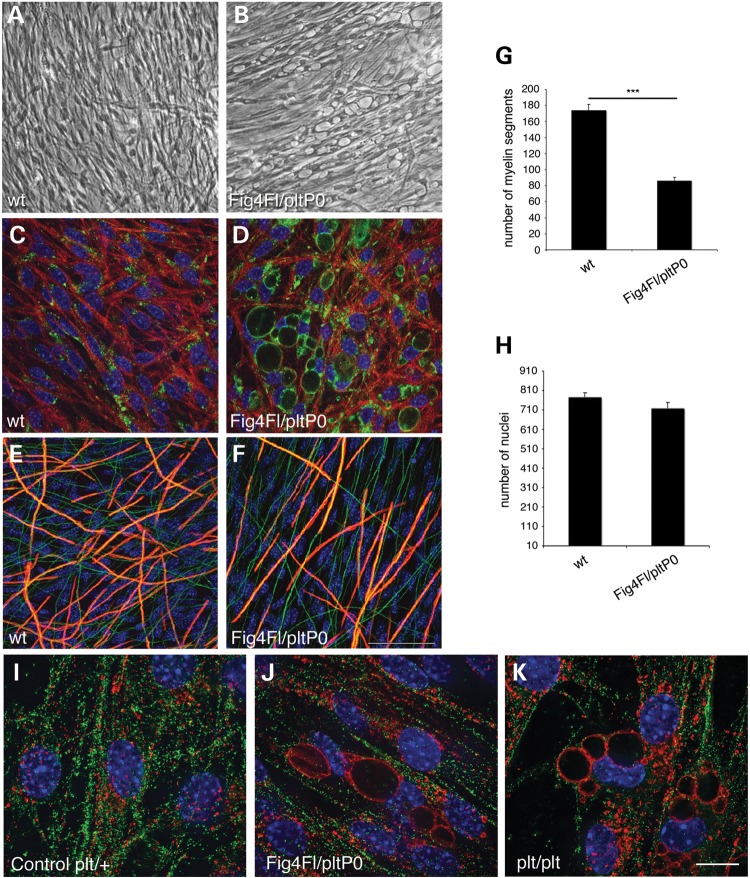
Morphological analysis of sciatic nerve from Fig4Floxed/plt, P0-Cre mice also revealed abnormal cytosolic enlarged vacuoles, accumulation of vesicles, organelles and myelin debris (Fig. 3A and B). Fig4Floxed/plt, P0-Cre mouse nerves also exhibit mild hypomyelination with increased g-ratio values observed at P30 (Fig. 3G and H) that is slightly worsened at 8 months of age, together with occasional onion bulbs (Fig. 3F–F′′; g-ratio at P30: Fig4Floxed/plt, P0-Cre 0.70 ± 0.003, 2161 fibres and controls Fig4Floxed/+ 0.68 ± 0.003, 2488 fibres, n = 4, P = 0.009 and controls Fig4Floxed/plt 0.68 ± 0.002, n = 2300 fibres, P = 0.001; g-ratio at 8 months: Fig4Floxed/plt, P0-Cre 0.71 ± 0.001, 1640 fibres and controls Fig4Floxed/+ 0.68 ± 0.001, 2023 fibres; n = 5, P = 1.12833E−06). Note that Fig4plt/+ nerves have normal g-ratio values at both P30 and 4.5 months (data not shown) and as previously reported (18). Fibre loss was not observed in Fig4Floxed/plt, P0-Cre sciatic nerves at 8 months (total number of fibres: Fig4Floxed/plt, P0-Cre 2279 ± 72.5 and controls Fig4Floxed/+ 2229 ± 12.5; n = 4, P = 0.52). Neurophysiological analysis was consistent with a demyelinating neuropathy with reduced NCV (Fig4Floxed/plt, P0-Cre 29.07 ± 0.579 and controls Fig4Floxed/+ 40.84 ± 0.773, P = 1.91553E−06) and increased F-wave latency (Fig4Floxed/plt, P0-Cre 6.25 ± 0.2903 and controls Fig4Floxed/+ 4.957 ± 0.105, n = 8, P = 0.0031). Cre-mediated recombination was demonstrated in Fig4Floxed/plt, P0-Cre by PCR analysis of genomic DNA and by western blot analysis of nerve lysates, which clearly demonstrated a strong reduction of Fig4 expression (Fig. 4A and B). To further assess the efficiency of P0-Cre-mediated recombination, we cultured primary mouse Schwann cells established from Fig4Floxed/plt, P0-Cre and control nerves at P3. Immunohistochemistry for LAMP1, which is a marker of LE/LY, demonstrated that 70% of mutant Schwann cells carried enlarged LE/LY (Fig. 4C–F), thus confirming loss of Fig4/PtdIns(3,5)P2-mediated control of LE/LY homeostasis in these cells.
Figure 4.
P0-Cre-mediated recombination efficiency in the Fig4Floxed/plt, P0-Cre model. (A) PCR analysis on genomic DNA from Fig4Floxed/plt, P0-Cre mice and controls. A 350-bp recombination band was detected in the nerve of mutants but not in wild type. The 350-bp faint band in the brain and spinal cord indicates recombination in cranial and spinal nerves of brain and spinal cord, respectively. (B) Western blot analysis of nerve homogenates at P30 indicates decreased Fig4 expression in sciatic nerves of Fig4Floxed/plt, P0-Cre mice. (C–F) Purified Schwann cells from Fig4Floxed/plt, P0-Cre mouse nerves at P3 indicate the presence of enlarged LE/LY-LAMP1 positive (green in C, C′, E, E′; red is phalloidin; blue is DAPI) in mutant cells, a feature of Fig4 loss and PtdIns(3,5)P2 decrease. (D) S100 staining marks Schwann cells in the culture. Bar in (D) is 20 µm for (C, C′, E, E′) and 50 µm for (D).
We attribute the somewhat milder phenotype of Fig4Floxed/plt, P0-Cre mice, compared with Fig4Floxed/Floxed, P0-Cre mice, to the greater contribution of genetic background from strain C57BL/6J, which is known to exacerbate Fig4-null associated features (1,3,26). The Fig4plt allele was maintained in a mixed strain background whereas the Fig4Floxed/Floxed, P0-Cre genotype was enriched in the C57BL/6J strain (3,12).
Overall, these findings demonstrate that Schwann cells are vulnerable to loss of Fig4 and that loss of Fig4 in Schwann cells reproduces demyelinating features of human CMT4J neuropathy.
Loss of Fig4 in Schwann cells in vivo impairs nerve regeneration
Loss of Fig4 in Schwann cells was shown above to be associated with demyelination and progressive axonal loss. Since impaired regeneration may also contribute to axonal loss in CMT neuropathies, we investigated whether impaired trafficking through the endolysosome axis in Fig4-null Schwann cells could affect remyelination and nerve regeneration. To this aim, we exploited the milder Fig4Floxed/plt P0-Cre mouse model to prevent developmental degeneration that might interfere with nerve regeneration after injury. Sciatic nerves from Fig4Floxed/plt, P0-Cre mice and controls were crushed at 2 months of age, a time point at which myelination and axonal integrity are largely normal, and morphological analysis was performed 60 days after injury (Fig. 5A). As expected, regeneration of axons and myelin was nearly complete in control nerves. In contrast, in Fig4Floxed/plt, P0-Cre crushed nerves, we observed a reduced number of medium and large calibre fibres and total fibres, and the presence of myelin debris (Fig. 5A and B; Fig4Floxed/plt, P0-Cre 2537 fibres when compared with control Fig4Floxed/+ 1782 fibres, n = 4 and P = 0.002). Heterozygous Fig4plt/+ crushed nerves were indistinguishable from controls (18) (and data not shown).
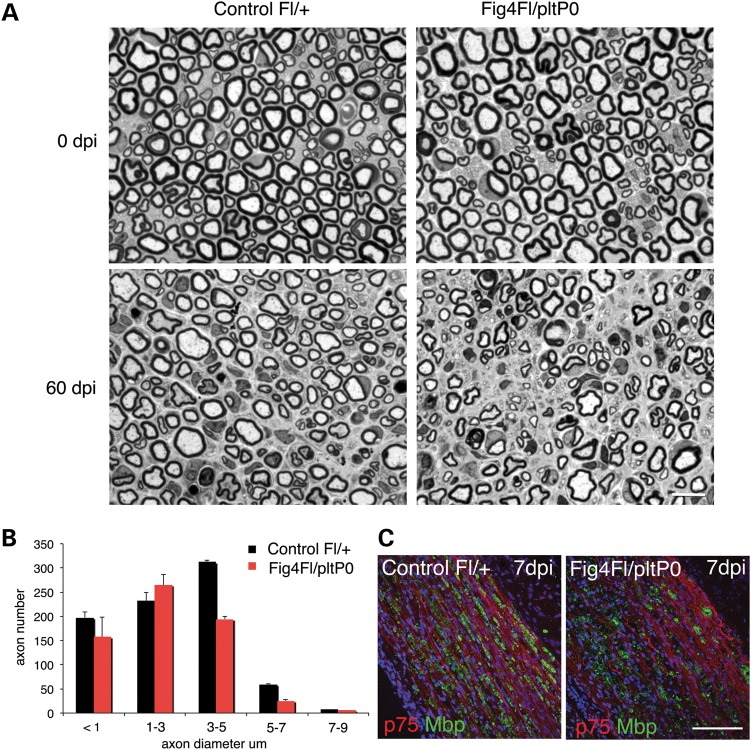
Figure 5.
Nerve regeneration is impaired in the Fig4Floxed/plt, P0-Cre conditional knock out. (A) Semithin section analysis of sciatic nerves from controls (left) and Fig4Floxed/plt, P0-Cre mutant nerves (right panels) analysed at 60 dpi (days post injury-crush performed at 2.5 months) indicates that regeneration is impaired in this mutant. Fibre loss is also observed in mutant nerves after injury. (B) The distribution of the number of axons per diameter indicates that fibres in the range of 3–5 µm (P = 0.006) and 5–7 µm of diameter (P = 0.001) were significantly decreased in the Fig4Floxed/plt, P0-Cre nerves after crush. (C) Staining of Fig4Floxed/plt, P0-Cre nerves at 7 dpi using anti-MBP antibody (green) which marks myelin and anti-p75 (red), which labels trans-differentiating Schwann cells. Bar in (A) is 20 µm; in (C) is 100 µm.
Since specific loss of Fig4 in Schwann cells appears to cause an endolysosomal trafficking impairment, we asked whether the altered regeneration of myelinated fibres could be caused by delayed clearance of myelin debris by Schwann cells. Immunohistochemistry of nerves at 7 days after injury demonstrated that MBP protein level was similar between non-regenerating Fig4Floxed/plt, P0-Cre nerves and controls, indicating normal myelin clearance (Fig. 5C). This finding was also supported by morphological analysis of semithin sections (data not shown).
In conclusion, loss of Fig4 in Schwann cells impairs regeneration of myelinated fibres suggesting that Fig4 and PtdIns(3,5)P2 homeostasis are necessary for efficient nerve regeneration.
Loss of Fig4 in Schwann cells impairs endolysosomal homeostasis resulting in hypomyelination in vitro
We showed above that loss of Fig4 specifically in Schwann cells causes a general trafficking defect, an impairment of active myelination during development, and demyelination. To determine how loss of Fig4 in Schwann cells and the consequent impairment of PtdIns(3,5)P2-mediated trafficking affect myelination during development, we established myelin-forming Schwann cell/DRG neuron co-culture explants from Fig4Floxed/plt, P0-Cre mice, in which P0-Cre-mediated recombination should be more efficient in vitro due to the presence of a single Floxed allele. Under myelinating conditions, following ascorbic acid treatment, most Schwann cells displayed enlarged LAMP1-positive LE/LY (Fig. 6A–D). Although Fig4Floxed/plt, P0-Cre explants were able to myelinate, the number of MBP-positive segments was significantly reduced when compared with control cultures (Fig. 6E–G). The Schwann cell number was similar between mutant and control (Fig. 6H). This finding is consistent with the observed hypomyelination in mutant mice and confirms that reduced PtdIns(3,5)P2 in Schwann cells results in impaired myelination. Note that myelin production was equivalent in Fig4+/+, Fig4plt/+ and Fig4plt/Floxed cultures, consistent with the normal in vivo phenotype of Fig4plt/+ mice (data not shown).
Figure 6.
Schwann cell/DRG neuron co-cultures from Fig4Floxed/plt, P0-Cre mice are hypomyelinated. (A and B) Bright field images showing vacuolization of Schwann cells in explants established from Fig4Floxed/plt, P0-Cre mouse embryos, which corresponds to LE/LY compartment LAMP1 positive (LAMP1 green, red phalloidin and blue DAPI) in (C and D). (E–H) Vacuolated Schwann cells produced less myelin segments after 7 days of ascorbic acid treatment when compared with control cultures (red is MBP, green is neurofilament and blue is DAPI), n = 6–8 explants per genotype/three different experiments. (I and J) Myelin protein zero, P0, does not co-localize with LAMP1 in control and in Fig4Floxed/plt, P0-Cre Schwann cells (green is P0, red is LAMP1 and blue is DAPI), n = 4 explants per time point of ascorbic acid treatment, per genotype. Two different anti-P0 antibodies were used. Bar in (F) is 150 µm for (A and B) and 50 µm for (C–F); bar in (K) is 13 µm for (I–K).
To explore the molecular basis of hypomyelination, we determined whether loss of Fig4 in Schwann cells and the enlargement of LE/LY results in impaired exocytosis of myelin proteins, impaired myelin protein degradation or more general defects in the endomembrane system during active myelination. It has been recently suggested that P0 co-localizes with LAMP1-positive compartments at early stages of postnatal nerve development (27). Myelin protein zero (P0) may be stored in LE/LY compartments prior to exocytosis during myelin assembly, as proposed for PLP in oligodendrocytes in the CNS (28), or during remyelination after damage. To explore this possibility, we evaluated retention of P0 in the enlarged LE/LY compartment of Fig4-null Schwann cells. Explants were stained with anti-P0 and anti-LAMP1 antibodies after ascorbic acid treatment, at 1–6 days of ascorbic acid treatment. We did not observe co-localization of P0 and LAMP1 in mutant or control cultures (Fig. 6I–K).
We then investigated whether altered protein degradation or turnover contributes hypomyelination in mutant cells. To this aim, we determined levels of autophagic markers and ubiquitinated substrates by western blot analysis. We did not observe an elevation of LC3II/I, p62 or ubiquitinated substrates in Fig4Floxed/plt, P0-Cre explants when compared with controls after 7 days of ascorbic acid treatment (Supplementary Material, Fig. S2A–C), suggesting that hypomyelination is not a consequence of impaired protein turnover.
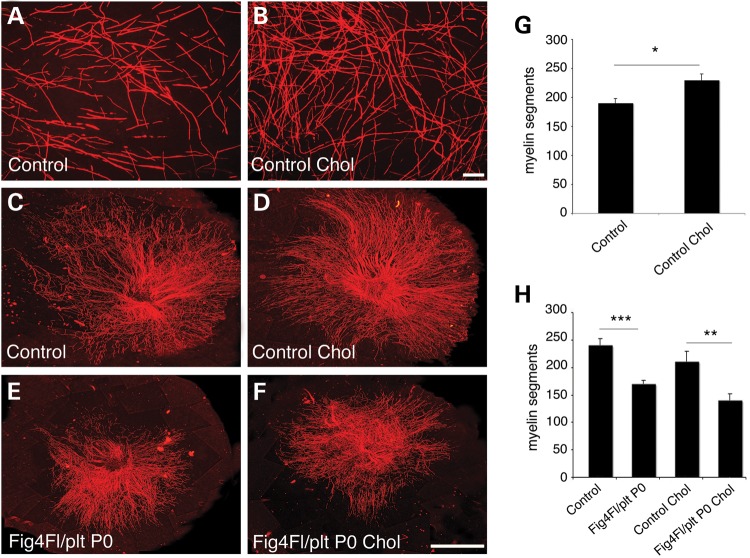
Cholesterol is known to facilitate the exit of P0 from the Golgi compartment and delivery to the plasma membrane, and addition of cholesterol to myelin-forming cultures accelerates myelination (29). We reasoned that if hypomyelination in Fig4Floxed/plt, P0-Cre mutant cultures was the consequence of impaired membrane trafficking in the endomembrane system, cholesterol delivery to the plasma membrane would itself be impaired and therefore addition of cholesterol to the culture medium would not rescue hypomyelination. Control cultures treated with cholesterol displayed enhanced myelination after 7 days of treatment, but not at 13 days, in agreement with previous reports (29) (Fig. 7A–D, and quantification in G). Consistent with our hypothesis, treatment of mutant explants with ascorbic acid in the presence of 20 µg/μl cholesterol did not rescue hypomyelination after 7 or 14 days of treatment (Fig. 7E and F, and quantification in H). This finding suggests that in mutant cells cholesterol trafficking and/or P0 delivery and assembly to the plasma membrane is not as efficient as in controls due to defects in the endolysosomal trafficking.
Figure 7.
Cholesterol supplementation does not rescue Fig4Floxed/plt, P0-Cre hypomyelination in vitro. (A and B) Cholesterol supplementation accelerates myelination of control explants after 7 days of ascorbic acid treatment, quantified in (G), P = 0.03305, n = 6. (C–F) Cholesterol supplementation does not rescue hypomyelination in Fig4Floxed/plt, P0-Cre cultures following 7 days of ascorbic acid treatment. (H) Cholesterol supplementation does not rescue hypomyelination also after 15 days of ascorbic acid treatment, as myelin segments in mutant cultures were decreased when compared with controls without cholesterol supplementation (P = 0.00071) and with cholesterol supplementation (P = 0.00868), n = 6. (H) Note that, after 15 days of cholesterol supplementation, control cholesterol-treated and untreated cultures produced the same amount of myelin, as previously reported (29). Red is MBP, myelin basic protein. Bar in (B) and (F) is 50 µm. Control genotypes are Fig4Floxed/plt and Fig4Floxed/Floxed.
Altered endolysosomal trafficking results in a block of autophagic progression and demyelination
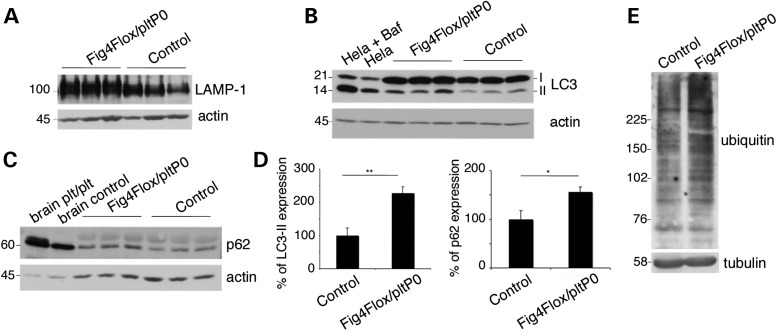
We then asked how loss of Fig4 in Schwann cells and the consequent impairment of PtdIns(3,5)P2-mediated trafficking can lead to the observed demyelination in the nerve. Fig4 loss and/or reduced activity of the PIKfyve kinase complex that generates PtdIns(3,5)P2 from PtdIns3P, leads to decreased PtdIns(3,5)P2 levels and enlargement of the LE/LY compartment (2,30–32). A block in autophagic progression has been reported in astrocytes and, to a lesser extent, in neurons of Fig4plt/plt mice (1,3,33). This defect may result from reduced fusion between enlarged LE/LY and autophagosomes to form autophagolysosomes (33). We thus asked whether there is a block of autophagy in Fig4-null Schwann cells. Western blot analysis of sciatic nerve lysates from Fig4Floxed/plt, P0-Cre mice at P30 revealed that the levels of LAMP1, LC3II and p62 proteins were significantly increased, consistent with a block in autophagic flux (Fig. 8A–D). Accumulation of ubiquitinated proteins is characteristic of impaired autophagy (34). We analysed levels of ubiquitinated proteins in P90 Fig4Floxed/plt, P0-Cre nerve lysates and found an increase of polyubiquitinated proteins in mutant nerves when compared with control (Fig. 8E).
Figure 8.
Block of autophagic progression in Fig4Floxed/plt, P0-Cre nerves. (A–D) LAMP1, LC3II/I and p62 levels are increased in P30 sciatic nerves of Fig4Floxed/plt, P0-Cre mice. An elevation of p62 indicates a block in autophagic progression in Schwann cells. In (B), lysates from HeLa cells starved and starved-treated using Bafalomycin, which blocks lysosomal-mediated degradation, were used as controls of LC3 II/I elevation. In (C), brain lysates from Fig4plt/plt and controls were used as a positive control of p62 increase, as already reported (1,33). (E) Lysates of sciatic nerves from Fig4Floxed/plt, P0-Cre mice and controls at P90 were stained using an anti-polyubiquitin antibody. Control genotypes are Fig4Floxed/plt and Fig4Floxed/Floxed.
The data demonstrate that Fig4 loss in Schwann cells causes both enlarged LE/LY and a block of autophagic flux, which may lead to demyelination.
DISCUSSION
The CMT4J neuropathy: cell autonomy of Fig4 in motor neurons and Schwann cells
Loss of human FIG4 causes a spectrum of inherited conditions suggesting that more than one cell type is vulnerable to impairment of FIG4/PtdIns(3,5)P2-mediated functions (3–7,9,10). This cell-specific sensitivity could be related to the impact of the different mutations on FIG4 enzymatic activity and protein stability, as well as to the impairment of different cellular functions in different cell types.
The CMT4J neuropathy is classified as CMT type 4 because of the autosomal recessive inheritance and of demyelinating features such as the reduction of NCV and the presence of onion bulbs in the nerve biopsies (6–8). However, late onset CMT4J patients initially display a predominantly motor and asymmetric neuropathy, suggesting a lower motor neuron involvement in the pathogenesis of this neuropathy (6).
In the mouse, the phenotypes of the global null Fig4plt/plt mutant and the Fig4Floxed/Floxed, Syn-Cre conditional null in neurons demonstrate the severe effect of PtdIns(3,5)P2 deficiency in neurons (1,3,12). Further, survival of Fig4plt/plt mice is rescued by the NSE-Fig4 transgenic mice, which express Fig4 specifically in neurons (12). Interestingly, among the different sub-population of neurons, DRG sensory neurons and autonomic ganglia are among the earliest affected neurons in the Fig4plt/plt mouse beginning by P1–P7, whereas motor neurons do not display typical pathological features related to PtdIns(3,5)P2 deficiency until 6 weeks of age (3,13). Whether FIG4 has a cell autonomous role in motor neurons and Schwann cells and how loss of FIG4/PtdIns(3,5)P2-mediated functions in these cells contribute to CMT4J pathogenesis have not been clearly defined. To address this gap in our knowledge we generated conditional mutants that selectively lack Fig4 in motor neurons or Schwann cells. Spinal cords of Fig4Floxed/plt, HB9-Cre and Fig4Floxed/plt, Olig2-Cre mice display motor neuron vacuolization, which is associated with axonal degeneration, hypomyelination and a decrease in the number of myelinated fibres in the quadriceps nerve. These findings suggest that Fig4 has a cell autonomous role in motor neurons and that loss of Fig4 in motor neurons contributes to the CMT4J neuropathy. In the motor neuron-specific knockout demyelinating features were not observed, supporting the hypothesis that demyelination is a consequence of specific loss of Fig4 in Schwann cells. Consistent with this, we also report that Fig4 conditional mutants in Schwann cells (Fig4Floxed/Floxed, P0-Cre and Fig4Floxed/plt, P0-Cre mice) exhibit accumulation of vesicles and organelles in the cytosol of myelinating cells indicative of a general trafficking impairment of the endolysosome axis, as well as enlargement of LE/LY and a block in autophagy. These cellular features are associated with impaired myelination during development, demyelination and defective regeneration, thus clearly demonstrating a primary involvement of Schwann cells in the pathogenesis of CMT4J neuropathy.
Demyelination in peripheral neuropathies can arise as a consequence of impaired endosomal trafficking and/or of a gain of toxic function. In addition to FIG4, several other proteins involved in the regulation of membrane trafficking are mutated in demyelinating CMT neuropathies and dys-regulation of endolysosomal trafficking may be a common mechanism at the basis of these disorders. These proteins regulate or are connected with PI metabolism, and include FRABIN/FGD4, SIMPLE, SH3TC2, MTMR2, MTMR5 and MTMR13 (1,35). For instance, mutated SIMPLE re-localizes from endosomal membranes to the cytosol where it exerts a dominant negative effect on the ESCRT protein complex (36,37). This in turn is thought to lead to defective ESCRT-mediated endosomal sorting and degradation of ErbB2/B3 receptors, loss of ERK signalling attenuation and demyelination.
Demyelination in peripheral neuropathies can also arise from activation of an integrated stress response (ISR) in Schwann cells. Several mutations in the myelin protein P0 responsible for demyelinating CMT1B cause misfolding of the protein, retention in the ER and UPR-mediated activation of ISR, which is maladaptive and leads to demyelination (38,39). Moreover, in the Tfam-KO Schwann cell mutant, mitochondria dysfunction causes ISR and demyelination (40). The Fig4Floxed/Floxed, P0-Cre and Fig4Floxed/plt, P0-Cre mutants that we generated with conditional ablation of Fig4 in Schwann cells displayed demyelinating features such as myelin debris and degeneration, onion bulbs and decrease of NCV, thus recapitulating demyelination of the CMT4J neuropathy. Demyelination in these models can be the consequence of a general endolysosomal trafficking impairment and loss of LE/LY homeostasis. We indeed demonstrated that Fig4-deficient Schwann cells display a block in autophagy, which progressively leads to an accumulation of ubiquitinated substrates. As in lysosomal storage disorders, accumulation of macromolecules, cholesterol and defective calcium release from the LE/LY compartment may affect the functioning of several organelles such as Golgi, ER and mitochondria thus ultimately leading to demyelination (41).
Endolysosomal trafficking and myelination
Myelin biogenesis relies on polarized trafficking and assembly of bulk of lipids and proteins (25). We exploited the mouse model with conditional ablation of Fig4 in Schwann cells to investigate whether the resulting impairment of endolysosomal trafficking would influence myelin biogenesis during development. We indeed observed decreased myelin thickness and hypomyelination both in vivo and in vitro as a result of the Fig4 deficiency in Schwann cells. During postnatal nerve development, the P0 myelin protein has been found to co-localize with the lysosomal cathepsin D in Schwann cells in vivo. Calcium-dependent lysosomal exocytosis has been suggested as a mechanism of P0 delivery to the plasma membrane during myelin biogenesis (27). We thought that hypomyelination in the conditionally deleted Schwann cells could result from the reduced delivery of P0 to the plasma membrane from the LE/LY compartment. However, we did not observe co-localization of P0 with LAMP1-positive LE/LY in either control or mutant Schwann cells at various stages of differentiation. We then hypothesized that the hypomyelination could result from a general impairment of trafficking occurring in Fig4-null Schwann cells. The observation that cholesterol supplementation does not rescue the hypomyelination in vitro supports this hypothesis. In normal conditions, cholesterol promotes the insertion of myelin P0 and other proteins into vesicles specifically destined for the developing myelin sheath. In Fig4-null Schwann cells, myelin proteins and/or cholesterol may be mis-trafficked and not properly delivered to the nascent myelin sheath causing hypomyelination.
Thus, we conclude that FIG4-mediated regulation of endolysosome trafficking in Schwann cells is important for generation of myelin during development, although the molecular mechanism remains to be clarified.
Therapeutical perspectives for CMT4J and pathologies associated to FIG4 loss
FIG4 loss leads to decreased PtdIns(3,5)P2 levels and to the enlargement of LE/LY. Loss of LE/LY homeostasis and vacuolization in models of PtdIns(3,5)P2 deficiency may arise as a consequence of Ca2+ retention in the lysosome, which is the other Ca2+ buffering organelle in addition to the ER (41,42). Indeed, PtdIns(3,5)P2 activates the cation channels TPC1 (two pore channel), TPC2 and MCONL1/TRPML1 (Mucolipin 1, transient receptor potential cation channel subfamily M, member 1) localized at LE/LY compartments (43). An exciting and intriguing therapeutic strategy for CMT4J and other human disorders associated with FIG4 deficiency would be to increase the activity of these channels to restore LE/LY homeostasis and dynamics. Indeed, synthetic compounds have been recently identified as activators of the MCONL1/TRPML1 channels, which promote the efflux of Ca2+ from the lysosomal lumen into the cytosol and trigger lysosomal exocytosis (43). However, different cell types may respond differently to these treatments depending on the level of expression of these channels and the elicited electrophysiological response. Mouse models of cell-specific Fig4 deficiency will therefore be useful for preclinical validation of these strategies.
MATERIALS AND METHODS
Ethics statement
All experiments involving animals were performed in accordance with Italian national regulations and covered by experimental protocols reviewed by local Institutional Animal Care and Use Committees (IACUC #525). Animals were generated and maintained in a SPF (specific pathogen free) Institutional facility.
Mice
Fig4plt/plt (global Fig4 deficiency) mice were maintained on CAST/Ei, C57BL/6J and 129 SV strain backgrounds. The generation of the Fig4Floxed allele has been already reported (12). The Fig4Floxed allele was detected by PCR with primers flanking exon 4 and the loxP sites. The forward primer, 5′-GAGGCAAGTACTCTACCAACTTAGC-3′ and reverse primer, 5′-CATGTGAACCTTGTTTCCCACACC-3′ detected fragments 558, 679 and 282 bp from wild type, Fig4Floxed and exon4-deleted alleles, respectively. Fig4Floxed/plt Olig2-Cre mice were obtained by crossing Fig4plt/+ with wild-type mice carrying the Olig2-Cre transgene. The resulting Fig4plt/+, Olig2-Cre genotype was then crossed with Fig4Floxed/Floxed mice to obtain the Fig4Floxed/plt, Olig2-Cre conditional knock out. The same strategy was followed to generate Fig4Floxed/plt, HB9-Cre and Fig4Floxed/plt, P0-Cre. Fig4Floxed/Floxed, P0-Cre mice were generated by crossing Fig4Floxed/Floxed or Fig4Floxed/+ with Fig4Floxed/+, P0-Cre mice. Genotyping was performed as already described (3,12,17). For all the experiments involving animals at least n = 4 animals per genotype of either sex were analysed. Fig4Floxed/Floxed mice have normal physiology and are indistinguishable from controls since they display normal nerve morphology including myelin thickness, and the absence of cell vacuolization in all cells analysed in both CNS and PNS also in aged mice (data not shown and as previously reported) (12). Fig4plt/+ heterozygous mice are normal as no vacuolization has been observed in any tissue analysed and myelin thickness is normal in peripheral nerves, as previously reported (3,18).
Morphological analysis
Semithin section and ultrastructural analysis of sciatic nerves was performed as previously reported (44). To perform morphometric analysis, digitalized images of fibre cross sections were obtained from corresponding levels of the sciatic nerves with a ×100objective and digital camera Leica DFC300F. Five images per animal were acquired and analysed with the Leica QWin software (Leica Microsystem). The g-ratio was determined by dividing the mean diameter of an axon (without myelin) by the mean diameter of the same axon including myelin. Statistical analysis was performed on the mean g-ratio values of the different nerves (animals) analysed per genotype. The quadriceps nerve, the motor branch of the femoral nerve, was dissected at the level of the inguinal ligament (45).
Sciatic nerve crush-lesion was performed as reported (46).
Antibodies
For western blot analysis and immunohistochemistry the following antibodies were used: goat anti-ChAT (Millipore); rat anti-LAMP1 (Iowa Hybridoma bank); Guinea pig anti-P62 (Progen); rabbit anti-LC3 (Sigma); rat anti-MBP (Chemicon); rabbit anti-NF-H (Millipore); mouse anti-TUJ1 (Promega); mouse anti-tubulin (Sigma); rabbit anti-actin (Sigma); mouse anti-FIG4 (Neuromab); rabbit anti-S100 (Sigma), rabbit anti-NGF receptor p75 (Millipore); chicken anti-P0 (Millipore) and mouse anti-P0 antibodies (kindly provided by J.J. Archelos); rabbit anti-ubiquitin (Santa Cruz); rhodamine phalloidin (Molecular Probes).
Protein lysates were prepared using a lysis buffer containing 1% Triton X-100 (or NP40 1% for autophagy marker evaluation), 50 mm Tris buffer, pH 8.0, 150 mm NaCl, 10 mm NaF, 1 mm Na vanadate, Complete (Roche) protease inhibitors.
Immunohistochemistry on sections
Spinal cord
Juvenile (P21) and adult (P120–210) mice were overdosed with ketamine/xylosine and transcardially perfused with ice-cold PBS and 4% PFA. Spinal cords were post-fixed in 4% PFA overnight, cryoprotected in 30% sucrose and frozen in OCT compound on dry ice. Lumbar spinal cords were sectioned at 30–40 µm at levels L3–L6 and stored in PBS. Free-floating immunohistochemistry was performed on 5–10 spinal cord sections per mouse. Sections were incubated overnight in primary antibody solution (Goat anti-ChAT, Millipore 1:100, 1.5% NDS in PBST-x) at 4°C on a rocker. After three washes in PBST-x sections were incubated in biotinylated donkey anti-goat secondary antibody in PBST-x (1:500, Jackson Immunoresearch) for 1 h. Followed by three washes in PBST-x, sections were incubated in ABC Vectashield kit solution (Vector, as per manufacturer's instructions) for 1 h. Antibody binding was visualized using diaminobenzidine kit (Vector Labs, as per manufacturer's instructions). Sections were mounted on glass slides, dehydrated in series of ethanol solutions and imaged using a Leica inverted bright field microscope.
Sciatic nerve
Immunofluorescence on cryosections was performed as described (47) and examined with confocal TCS SP5 laser-scanning confocal (Leica) or Olympus BX (Olympus Optical) fluorescent microscope, and Zeiss Axiovert S100 TV2 with Hamamatsu OrcaII-ER. For immunohistochemistry, sciatic nerves were removed and rapidly snap-frozen in liquid nitrogen, either unfixed or previously fixed in buffered 4% PFA.
Cell culture
Isolated mouse Schwann cells were prepared from P2–P3 pups. Sciatic nerves were dissected, washed in L15 medium and then incubated with trypsin 0.25% plus collagenase 130 U/ml (type I, Worthington) in DMEM for 50 min at 37°C. After incubation, DMEM plus 10% serum was added to block dissociation and cells were plated on poly-lysine/laminin coated coverslips in defined medium, DF [DF contains 1 : 1 Hams F12/DMEM supplemented with 100 µg/ml glutamine, 0.03% bovine serum albumin (BSA), 100 µg/ml transferrin, 16 µg/ml putrescine, 38 ng/ml dexamethasone, 60 ng/ml progesterone, 400 ng/ml thyroxine (T4), 5 ng/ml insulin (low insulin) or 5 µg/ml (high insulin), 10 ng/ml triiodothyronine (T3), 160 ng/ml selenium and 100 U/ml each of penicillin/streptomycin], 3% serum and Ara C. Schwann cells were further purified using DF without serum.
Myelin-forming Schwann cell/DRG neuron co-cultures were established from embryonic Day 13.5 mouse embryos as previously described (48,49). Following NB (Neurobasal, B27 supplement, glucose, NGF) medium treatment for 7 days to allow neuritogenesis and Schwann cell migration, myelination was induced by treatment for 7–15 days with ascorbic acid (final concentration, 50 μg/ml) (Sigma). Cholesterol (dissolved in ethanol) was added every other day to C-media (MEM, 10% serum, NGF, glucose) plus ascorbic acid at 20 μg/ml final concentration. Analysis of autophagy on co-cultures was performed by starving Schwann cell/DRG explants in HBSS for 6 h and then by adding 100 nm bafilomycin A1 (LC laboratories) for 2 h to block LC3/p62 lysosomal degradation.
Analysis of proteasome-mediated degradation was performed by treating co-cultures using MG132 proteasome inhibitor at 25 µm final concentration for 6 h.
For analysis of myelination, immunohistochemistry on Schwann cell/DRG neuron co-cultures was performed as follows: cells were fixed for 15 min in 4% paraformaldehyde, permeabilized for 15 min in ice-cold methanol at −20°C, blocked for 20 min with 10% normal goat serum (Dako), 1% BSA (Sigma) and then incubated with primary antibody for 1 h. After extensive washing, the coverslips where incubated with the secondary antibody for 30 min, washed and mounted. For double immunostaining with anti-NF-H and anti- MBP antibody, the coverslips were blocked with 1% BSA, 10% NGS for 20 min on ice, and primary antibodies were incubated overnight at 4°C. For LAMP1 staining, cells were permeabilized using 0.1% saponin after fixation.
Analysis of myelination
To quantify the amount of myelin, the number of MBP-positive segments in each explant/coverslip was assessed. As myelination is also a function of the amount of neurites/axons and of the Schwann cell number in the culture, the network of NF-H-positive filaments and the number of Schwann cells (DAPI) were also evaluated in each explant. Using a fluorescence microscope, at least 5–10 fields per cover were randomly acquired and MBP-positive myelinated fibres were counted per field. Average (among 5–10 fields) of MBP fibres was calculated per cover and statistical analysis was performed on different covers per condition/per experiment (SEM) in at least three different experiments. In the case of cholesterol treatment, the entire DRG was also reconstructed to evaluate the difference in the area occupied by myelinated segments in treated versus untreated explants. MBP-positive fibres and vacuolated organelles were acquired using TCS SP5 laser-scanning confocal (Leica) or Olympus BX (Olympus Optical) fluorescent microscope, and Zeiss Axiovert S100 TV2 with Hamamatsu OrcaII-ER. P0-LAMP1 co-localization analysis was performed using DeltaVision-Olympus IX70 with DeltaVision RT Deconvolution System.
Imaging and statistical analysis
Micrographs were acquired using a digital camera (Leica F300), and figures were prepared using Adobe Photoshop, version 7.0 and 8.0 (Adobe Systems). Statistical analysis was performed using the Student's t-test; two tails, unequal variance and α = 0.005 were used. Error bars in the graphs represent SEM.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
A.B. was supported by Telethon-Italy grant numbers GPP10007D, GGP12017; Association Française contre les Myopathies (AFM)-France grant number 16040/16922, and the ERA-Net for research programs on rare diseases (E-Rare JTC2011). S.C.P. was supported by Telethon-Italy grant numbers GGP10007B, GGP12024. Y.A.M. was supported by the Cellular and Molecular Biology Graduate Program Training Grant T32GM007315, 2011–2013 and the Training Program in Organogenesis T32HD007505, 2014–2015. M.H.M. and R.J.G. received support from National Institute of Health R01 GM24872 and R01 NS081281 and the Dr Miriam and Sheldon Adelson Foundation on Neurorepair and Rehabilitation to R.J.G. Funding to pay the Open Access publication charges for this article was provided by Telethon-Italy.
Supplementary Material
REFERENCES
- 1.Vaccari I., Dina G., Tronchere H., Kaufman E., Chicanne G., Cerri F., Wrabetz L., Payrastre B., Quattrini A., Weisman L.S., et al. Genetic interaction between MTMR2 and FIG4 phospholipid phosphatases involved in Charcot-Marie-Tooth neuropathies. PLoS Genet. 2011;7:e1002319. doi: 10.1371/journal.pgen.1002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCartney A.J., Zhang Y., Weisman L.S. Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. BioEssays. 2014;36:52–64. doi: 10.1002/bies.201300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow C.Y., Zhang Y., Dowling J.J., Jin N., Adamska M., Shiga K., Szigeti K., Shy M.E., Li J., Zhang X., et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow C.Y., Landers J.E., Bergren S.K., Sapp P.C., Grant A.E., Jones J.M., Everett L., Lenk G.M., McKenna-Yasek D.M., Weisman L.S., et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campeau P.M., Lenk G.M., Lu J.T., Bae Y., Burrage L., Turnpenny P., Roman Corona-Rivera J., Morandi L., Mora M., Reutter H., et al. Yunis-Varon syndrome is caused by mutations in FIG4, encoding a phosphoinositide phosphatase. Am. J. Hum. Genet. 2013;92:781–791. doi: 10.1016/j.ajhg.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson G., Lenk G.M., Reddel S.W., Grant A.E., Towne C.F., Ferguson C.J., Simpson E., Scheuerle A., Yasick M., Hoffman S., et al. Distinctive genetic and clinical features of CMT4J: a severe neuropathy caused by mutations in the PI(3,5)P phosphatase FIG4. Brain. 2011;134:1959–1971. doi: 10.1093/brain/awr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Chow C.Y., Sahenk Z., Shy M.E., Meisler M.H., Li J. Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain. 2008;131:1990–2001. doi: 10.1093/brain/awn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottenie E., Menezes M.P., Rossor A.M., Morrow J.M., Yousry T.A., Dick D.J., Anderson J.R., Jaunmuktane Z., Brandner S., Blake J.C., et al. Rapidly progressive asymmetrical weakness in Charcot-Marie-Tooth disease type 4J resembles chronic inflammatory demyelinating polyneuropathy. Neuromuscul. Disord. 2013;23:399–403. doi: 10.1016/j.nmd.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima J., Okamoto N., Shiraishi J., Nishimura G., Nakashima M., Tsurusaki Y., Saitsu H., Kawashima H., Matsumoto N., Miyake N. Novel FIG4 mutations in Yunis-Varon syndrome. J. Hum. Genet. 2013;58:822–824. doi: 10.1038/jhg.2013.104. [DOI] [PubMed] [Google Scholar]
- 10.Baulac S., Lenk G.M., Dufresnois B., Ouled Amar Bencheikh B., Couarch P., Renard J., Larson P.A., Ferguson C.J., Noe E., Poirier K., et al. Role of the phosphoinositide phosphatase FIG4 gene in familial epilepsy with polymicrogyria. Neurology. 2014;82:1068–1075. doi: 10.1212/WNL.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenk G.M., Ferguson C.J., Chow C.Y., Jin N., Jones J.M., Grant A.E., Zolov S.N., Winters J.J., Giger R.J., Dowling J.J., et al. Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet. 2011;7:e1002104. doi: 10.1371/journal.pgen.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson C.J., Lenk G.M., Jones J.M., Grant A.E., Winters J.J., Dowling J.J., Giger R.J., Meisler M.H. Neuronal expression of Fig4 is both necessary and sufficient to prevent spongiform neurodegeneration. Hum. Mol. Genet. 2012;21:3525–3534. doi: 10.1093/hmg/dds179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katona I., Zhang X., Bai Y., Shy M.E., Guo J., Yan Q., Hatfield J., Kupsky W.J., Li J. Distinct pathogenic processes between Fig4-deficient motor and sensory neurons. Eur. J. Neurosci. 2011;33:1401–1410. doi: 10.1111/j.1460-9568.2011.07651.x. [DOI] [PubMed] [Google Scholar]
- 14.Arber S., Han B., Mendelsohn M., Smith M., Jessell T.M., Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- 15.Li H., Arber S., Jessell T.M., Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat. Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- 16.Yang X., Arber S., William C., Li L., Tanabe Y., Jessell T.M., Birchmeier C., Burden S.J. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 17.Bolis A., Coviello S., Bussini S., Dina G., Pardini C., Previtali S.C., Malaguti M., Morana P., Del Carro U., Feltri M.L., et al. Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J. Neurosci. 2005;25:8567–8577. doi: 10.1523/JNEUROSCI.2493-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Q., Guo J., Zhang X., Bai Y., Wang L., Li J. Trauma does not accelerate neuronal degeneration in Fig4 insufficient mice. J. Neurol. Sci. 2012;312:102–107. doi: 10.1016/j.jns.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Lu Q.R., Yuk D., Alberta J.A., Zhu Z., Pawlitzky I., Chan J., McMahon A.P., Stiles C.D., Rowitch D.H. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 20.Dimou L., Simon C., Kirchhoff F., Takebayashi H., Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feltri M.L., D'Antonio M., Previtali S., Fasolini M., Messing A., Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann. N. Y. Acad. Sci. 1999;883:116–123. [PubMed] [Google Scholar]
- 22.Feltri M.L., D'Antonio M., Quattrini A., Numerato R., Arona M., Previtali S., Chiu S.Y., Messing A., Wrabetz L. A novel P0 glycoprotein transgene activates expression of lacZ in myelin-forming Schwann cells. Eur. J. Neurosci. 1999;11:1577–1586. doi: 10.1046/j.1460-9568.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- 23.Noseda R., Belin S., Piguet F., Vaccari I., Scarlino S., Brambilla P., Martinelli Boneschi F., Feltri M.L., Wrabetz L., Quattrini A., et al. DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J. Neurosci. 2013;33:15295–15305. doi: 10.1523/JNEUROSCI.2408-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anitei M., Pfeiffer S.E. Myelin biogenesis: sorting out protein trafficking. Curr. Biol. 2006;16:R418–R421. doi: 10.1016/j.cub.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Simons M., Trotter J. Wrapping it up: the cell biology of myelination. Curr. Opin. Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Lenk G.M., Meisler M.H. Mouse models of PI(3,5)P2 deficiency with impaired lysosome function. Methods Enzymol. 2014;534:245–260. doi: 10.1016/B978-0-12-397926-1.00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G., Zhang Z., Wei Z., Cheng Q., Li X., Li W., Duan S., Gu X. Lysosomal exocytosis in Schwann cells contributes to axon remyelination. Glia. 2012;60:295–305. doi: 10.1002/glia.21263. [DOI] [PubMed] [Google Scholar]
- 28.Trajkovic K., Dhaunchak A.S., Goncalves J.T., Wenzel D., Schneider A., Bunt G., Nave K.A., Simons M. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J. Cell Biol. 2006;172:937–948. doi: 10.1083/jcb.200509022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saher G., Quintes S., Mobius W., Wehr M.C., Kramer-Albers E.M., Brugger B., Nave K.A. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J. Neurosci. 2009;29:6094–6104. doi: 10.1523/JNEUROSCI.0686-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michell R.H., Heath V.L., Lemmon M.A., Dove S.K. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem. Sci. 2006;31:52–63. doi: 10.1016/j.tibs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Michell R.H. Inositol lipids: from an archaeal origin to phosphatidylinositol 3,5-bisphosphate faults in human disease. FEBS J. 2013;280:6281–6294. doi: 10.1111/febs.12452. [DOI] [PubMed] [Google Scholar]
- 32.Dove S.K., Dong K., Kobayashi T., Williams F.K., Michell R.H. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem. J. 2009;419:1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson C.J., Lenk G.M., Meisler M.H. Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum. Mol. Genet. 2009;18:4868–4878. doi: 10.1093/hmg/ddp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benbrook D.M., Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp. Oncol. 2012;34:286–297. [PubMed] [Google Scholar]
- 35.Bucci C., Bakke O., Progida C. Charcot-Marie-Tooth disease and intracellular traffic. Prog. Neurobiol. 2012;99:191–225. doi: 10.1016/j.pneurobio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S.M., Chin L.S., Li L. Charcot-Marie-Tooth disease-linked protein SIMPLE functions with the ESCRT machinery in endosomal trafficking. J. Cell Biol. 2012;199:799–816. doi: 10.1083/jcb.201204137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.M., Olzmann J.A., Chin L.S., Li L. Mutations associated with Charcot-Marie-Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome-autophagy pathways. J. Cell Sci. 2011;124:3319–3331. doi: 10.1242/jcs.087114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Antonio M., Musner N., Scapin C., Ungaro D., Del Carro U., Ron D., Feltri M.L., Wrabetz L. Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice. J. Exp. Med. 2013;210:821–838. doi: 10.1084/jem.20122005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pennuto M., Tinelli E., Malaguti M., Del Carro U., D'Antonio M., Ron D., Quattrini A., Feltri M.L., Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viader A., Sasaki Y., Kim S., Strickland A., Workman C.S., Yang K., Gross R.W., Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886–898. doi: 10.1016/j.neuron.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platt F.M., Boland B., van der Spoel A.C. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Evans E., Platt F.M. Lysosomal Ca(2+) homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium. 2011;50:200–205. doi: 10.1016/j.ceca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Samie M., Wang X., Zhang X., Goschka A., Li X., Cheng X., Gregg E., Azar M., Zhuo Y., Garrity A.G., et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell. 2013;26:511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrabetz L., Feltri M.L., Quattrini A., Imperiale D., Previtali S., D'Antonio M., Martini R., Yin X., Trapp B.D., Zhou L., et al. P(0) glycoprotein overexpression causes congenital hypomyelination of peripheral nerves. J. Cell Biol. 2000;148:1021–1034. doi: 10.1083/jcb.148.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frei R., Motzing S., Kinkelin I., Schachner M., Koltzenburg M., Martini R. Loss of distal axons and sensory Merkel cells and features indicative of muscle denervation in hindlimbs of P0-deficient mice. J. Neurosci. 1999;19:6058–6067. doi: 10.1523/JNEUROSCI.19-14-06058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Triolo D., Dina G., Lorenzetti I., Malaguti M., Morana P., Del Carro U., Comi G., Messing A., Quattrini A., Previtali S.C. Loss of glial fibrillary acidic protein (GFAP) impairs Schwann cell proliferation and delays nerve regeneration after damage. J. Cell Sci. 2006;119:3981–3993. doi: 10.1242/jcs.03168. [DOI] [PubMed] [Google Scholar]
- 47.Previtali S.C., Nodari A., Taveggia C., Pardini C., Dina G., Villa A., Wrabetz L., Quattrini A., Feltri M.L. Expression of laminin receptors in schwann cell differentiation: evidence for distinct roles. J. Neurosci. 2003;23:5520–5530. doi: 10.1523/JNEUROSCI.23-13-05520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taveggia C., Zanazzi G., Petrylak A., Yano H., Rosenbluth J., Einheber S., Xu X., Esper R.M., Loeb J.A., Shrager P., et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolis A., Coviello S., Visigalli I., Taveggia C., Bachi A., Chishti A.H., Hanada T., Quattrini A., Previtali S.C., Biffi A., et al. Dlg1, Sec8, and Mtmr2 regulate membrane homeostasis in Schwann cell myelination. J. Neurosci. 2009;29:8858–8870. doi: 10.1523/JNEUROSCI.1423-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.