Abstract
Parental behavior modifies neural, physiologic, and behavioral characteristics of both maternal and paternal mammals. These parenting-induced modifications extend to brain regions not typically associated with parental responses themselves but that enhance ancillary responses, such as foraging efficiency and predator avoidance. Here we hypothesized that male and female owl monkeys (Aotus spp.) with reproductive experience (RE) would demonstrate more adaptive ancillary behavioral and neuroendocrine responses than those of their nonRE counterparts. To assess cognitive skills and coping flexibility, we introduced a foraging strategy task, including a set of novel objects (coin holders) marked with different symbols representing different food rewards, to the animals. To assess endocrine responses, urine samples were assayed for cortisol and dehydroepiandrosterone (DHEA) levels and their ratios to determine physiologic measures of emotional regulation in RE and nonRE owl monkeys. Compared with nonRE monkeys, experienced parents had higher DHEA:cortisol ratios after exposure to habituation training and on the first day of testing in the foraging task. Both hormones play critical roles in the stress response and coping mechanisms, and a high DHEA:cortisol ratio usually indicates increased coping skills. In addition, RE monkeys exhibited more efficient foraging responses (by 4-fold) than did the nonRE mating pairs. We conclude that RE modifies relevant behavioral and hormonal responses of both maternal and paternal owl monkeys exposed to a challenging cognitive paradigm. Corroborating previous research demonstrating adaptive modifications in foraging efficiency and emotional responses in reproductively experienced rodents, the current results extend these findings to a monogamous primate species.
Abbreviations: DHEA, dehydroepiandrosterone; MDS, multidimensional scaling; RE, reproductive experience
Past research has elucidated the fundamental components of pregnancy and motherhood,39,57,58,78 not only emphasizing the crucial role of the quality of maternal care on infant development9,27,34 but also revealing how pregnancy and motherhood can reshape the neural, physiologic, and behavioral characteristics of animals.2,11,36,53 These parent-induced modifications also extend to brain regions that are not directly associated with maternal responses46,60,63 but instead are involved in enhancing the mother's efficiency in ancillary parental responses, such as foraging efficiency and predator avoidance. Interestingly, evidence of these maternally related neurobiologic modifications has been confirmed in several species, including humans.12,13,39,47,50 For example, compared with animals that lacked reproductive experience (RE), maternal rats exhibited enhanced foraging abilities in a spatial task37,40 as well as less behavioral and neural evidence of fear reactivity in an open-field task, as expressed by reduced c-fos immunoreactivity in the basolateral amygdala.72
Prior research suggests that stress hormones play an integrative role in parent-induced neurobiologic adaptations. Glucocorticoids, for example, have been shown to be suppressed in lactating females, due to blunted activity of the hypothalamic–pituitary–adrenal axis.6,24,64 Accordingly, the current study assessed the integrative role of both cortisol and dehydroepiandrosterone (DHEA) on adaptive parental responses. Both hormones play critical roles in the stress response and coping mechanisms.15 DHEA has been shown to be released parallel to cortisol during physical stress28 and has been associated with providing protection against the negative effects of prolonged exposure to glucocorticoids.54 Potentially related to the cognitive modifications observed in maternal animals, DHEA can act centrally to decrease glucocorticoid-induced neuronal death in the hippocampus and to promote neurogenesis in the dentate gyrus of the hippocampus and in sensory dorsal root ganglion neurons.49 Furthermore, the ratio between DHEA and cortisol has been found to be a reliable index of neuroprotection, because DHEA can increase the proliferation of progenitor cells in the adult hippocampus and also have antidepressant activity.49,61,70 Accordingly, in the current study, we hypothesized that an increased DHEA:cortisol ratio would be advantageous in parental owl monkeys (Aotus spp.) presented with the metabolically expensive challenges associated with caring for offspring.
Although females demonstrate more pronounced parent-induced effects, males experience hormonal alterations associated with copulation, pair-bonding, and paternal care, such as increased vasopressin levels.16,41,42,51,55 Research has identified specific neurobiologic modifications that accompany various degrees of paternal responsiveness, including distinct patterns of vasopressin receptor-binding sites and enhanced arginine-, vasopressin-, and oxytocin-immunoreactive cell bodies and fibers, as well as increased neuronal restructuring in the hippocampus.30,41,79,80 Increased levels of oxytocin and prolactin have been reported in paternal males as well.29,67,79,81,82
Owl monkeys (Aotus) are New World monkeys that are characterized by the extensive involvement of fathers in the care of the infant. These primates are small (weight, approximately 1 kg), nocturnal, generalist omnivores that consume fruits, leaves, flowers, insects, and small vertebrate prey.18,75 Owl monkeys possess prolonged, and probably exclusively, monogamous relationships between the mating pair, enforced through a high level of intrasexual competition.18,19,53 In biomedical research, owl monkeys have been studied primarily because of their high resistance to parasites, and they are comparative model of herpes virus infection in humans. Consequently, very little is known about the physiologic correlates of their paternal behavior. Due to these characteristics, owl monkeys represent an ideal species in which to investigate the role of RE in the cognitive and emotional responses of animals in captivity.
In light of past research emphasizing adaptive effects in reproductively experienced (RE) rodents, we hypothesized that adaptive ancillary behavioral and neuroendocrine responses would be greater in RE male and female owl monkeys than in their nonRE counterparts. To assess the monkeys’ cognitive skills and coping flexibility, we introduced a set of novel objects (coin holders) marked with different symbols representing different food rewards. Arguably, cognitive skills related to foraging are among the most critical adaptations in primates.17 Furthermore, cortisol and DHEA were evaluated to determine emotional responsivity and resilience in parental owl monkeys. Extending these parental investigations to a nonhuman primate species is necessary to determine the robustness of previously reported findings from laboratory rodents as well as to provide a reliable animal model of the effects of parity on the behavior of captive primates involved in biomedical research.
Materials and Methods
Subjects and study site.
Mated pairs of owl monkeys (Aotus spp.; 11 pairs; age, 5 to 21 y) were studied at the DuMond Conservancy for Primates and Tropical Forests (Miami, FL) during the summer of 2013. Pairs were classified as RE animals (n = 10, 5 male and 5 female) and nonRE (n = 12, 6 male and 6 female), according to the number of infants they had previously raised. Specifically, RE monkeys had given birth to an average of 2.2 infants (range, 1 to 5); all pregnancies were successful and the infants raised. We have no information on the specific parental behavior of these animals. NonRE monkeys had never given birth or raised infants. There was no significant age difference between the 2 groups (RE: 11.7 ± 4.9 y; range, 5 to 21 y; nonRE: 10.25 ± 4.2 y, range, 4 to 19 y; t20 = 0.74; P = 0.47). The nonRE pairs were given hormonal contraceptive to control the size of the colony. A combination of estrogens, progestins, and antiprogestins were administered to the nonRE pairs through their reproductive cycles (varying from several months to several years, depending on the age of the pair). These contraceptives are designed to act specifically on ovarian functions and thus have minimal or no effects on the HPA axis.71 Although most animals were born in captivity, a few were wild animals that have lived in captivity for more than a decade.
Each pair was housed outdoors in a wire-framed enclosure (approximate diameter, 3.0 m; height, 3.0 m) located in a natural hardwood hammock area exposed to natural fluctuations in external conditions (for example, light, temperature, and rainfall). The enclosures contained wooden nest-boxes, perches, and poles to allow natural locomotion. Animals were fed a fresh fruit and vegetable mix (Lab Diet Monkey Diet and Lab Diet New World Primate Diet, LabDiet, St Louis, MO) twice daily, once in the morning and once in the late afternoon. In addition, monkeys had access to naturally occurring flying and crawling arthropods and small vertebrates, as well as natural vegetation growing inside the enclosure. Water was supplied ad libitum. Animals were maintained in accordance with the Randolph-Macon College and DuMond Conservancy IACUC. This research was approved by the Randolph-Macon College and DuMond Conservancy IACUC.
Materials.
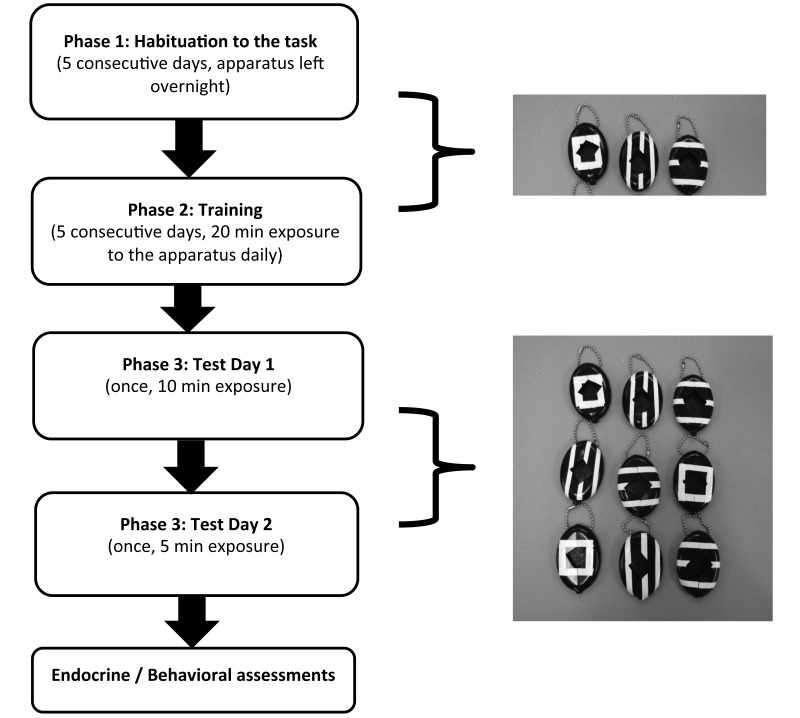
Black rubber squeeze coin holders (68 g each, Figure 1; sold through Amazon.com) were used to hide the marshmallow reward during the 3 experimental phases. Each coin holder was modified by enlarging the opening in the front to facilitate the monkeys’ retrieval of the marshmallow. Coin holders were sterilized before and after each use to avoid contamination. During the training trials, 3 coin holders were presented above the feeding platform of each cage. Three patterns were presented: (1) no value (NV): the coin holder did not contain a marshmallow and was marked with a white tape rectangle; (2) low value (LV): the coin holder contained a quarter of a small marshmallow hidden in the bottom portion and was marked with 3 horizontal white tape strips; (3) high value (HV): the coin holder contained 2 pieces of marshmallow hidden in the bottom part and was marked with 3 vertical white tape strips (Figure 1). Coin holders were attached to the cage wire with a 10.16 cm nickel-plated steel ball chain.
Figure 1.
Diagram of the timeline and procedures of the experiment, showing the 9 black-rubber squeeze coin holders used for the cognitive–foraging task. Each coin holder was modified by enlarging the hole in the front to facilitate the animals’ retrieval of the marshmallow. During the training trials, 3 coin holders were presented above the feeding platform of each cage. Three different patterns were presented: (1) no value: the coin holder did not have a marshmallow, and it was marked with a white tape rectangle; (2) low value: the coin holder had a quarter of a small marshmallow hidden in the bottom portion and was marked with 3 horizontal white tape strips; and (3) high value: the coin holder had 2 quarter pieces of marshmallow in the bottom and was marked with 3 vertical white strips. Coin holders were attached to cages by using 10.16-cm nickel-plated steel ball chains.
Plastic bowls (diameter, 19 cm; depth, 7 cm) and disposable pipettes were used to collect and transfer urine to 1.5-mL centrifuge tubes. Plastic bowls were used only once and then disposed. To facilitate urine collection, food rewards were given when the monkey urinated. Urine was collected in the morning between 0800 and 1000. A researcher entered the cage and waited underneath an animal until it urinated. Because the monkeys have been habituated to urinate at that time for routine medical checks, the waiting time was between 5 and 30 min.
Procedure.
The experiment was organized into 3 phases (Figure 1). All phases of the experiment were completed before the animals had their evening meal, to maximize the motivation to retrieve food. During phase 1 (habituation), monkeys were exposed to the 3 coin holders with different values (NV, LV, HV) for 5 consecutive days. To reinforce the association between the stimulus (coin holder with different symbols) and the reward (marshmallow), a piece of marshmallow was left visible in the central opening in both LV and HV holders. During this initial phase, the experiment began between 1930 and 2000, and all the coin holders were left in the cage overnight. The number of marshmallows retrieved was recorded the following day, when all the coin holders were retrieved, cleaned (soaked in soap and water), and marked again for the next exposure. During phase 1, the animals’ existing enrichment and feeding schedule was unmodified.
During phase 2 (training), monkeys again were exposed to the foraging stimuli (coin holders), but no marshmallow was visible, and the apparatus was presented in the cage for approximately 20 min, starting at 2000. The apparatus again consisted of 3 coin holders with different values (NV, LV, HV); the number of marshmallows retrieved was recorded; and all coin holders were cleaned and marked again for the following day. During phase 2 (and the subsequent phase 3), monkeys were not given enrichment toys during the afternoon prior to training with the coin holders. During this time, afternoon feedings were delayed until after the training exposure, to increase the animals’ motivation to retrieve the food rewards.
During the third and last phase of the experiment (foraging strategy assessment), the monkeys’ foraging strategies on exposure to 9 coin holders of various values were assessed. Specifically, in phase 3, testing was divided into 2 test stages: test day 1 (10 min exposure) and the test day 2 (final test, 5 min exposure). Between the 2 test days, animals were exposed to a 20-min trial to prime the animals for the final test. During all stages of the foraging strategy assessment, monkeys were presented with 9 coin holders (3 sets of 3 coin holders each for NV, LV, and HV) as depicted in Figure 1. After each test day, all coin holders were cleaned and marked again for the following day. So that animals’ responses during phase 3 without distraction from an external light source, the following behaviors were scored by a trained observer using a digital voice recorder and night-vision goggles (Night Owl Optics 5-Power NOXM50 Night Vision Monocular; iGEN, El Paso, TX): frequency of approaching the feeding areas with the coin holders (defined as animals being within arm's reach but not in contact with the apparatus); duration of proximity (defined as the animal's body length) with the apparatus; latency (defined as the time [in seconds] required to approach a coin holder), frequency and duration of contact with each of the 3 values of coin holders (NV, LV, HV); and number of pieces of marshmallow consumed.
Endocrine assessment.
To assess the physiologic responses to the experimental procedure and behavioral task, cortisol and DHEA metabolized in excreta were assessed by using fresh urine samples collected twice per animal between 0830 and 1000. The first sample was collected prior of the start of phase 1, and the second sample was obtained during phase 3, the morning after test day 1. Urine samples (0.5 to 3 mL) were collected from each animal and frozen unmixed in sealed containers at −80 °C until use. A total of 44 samples were collected and saved for cortisol and DHEA extraction and assay procedures.
Prior to assay, previously collected urine samples were thawed at room temperature and placed in a clean centrifuge tube. The contents of each tube then were mixed (Vortex Genie 2, Scientific Industries; Global, Port Washington, NY) for 30 s and subsequently centrifuged for 15 min at 1000 × g. By using a transfer pipette, 10-μL aliquots of urine were transferred to test tubes and diluted with 490 μL distilled water. Samples, controls, and standards were prepared in duplicate and added in 100-μL aliquots to the appropriate wells of the assay kits. Assay procedures were completed by using materials and protocols provided in an enzyme immunoassay kit (Assay Designs, Anne Arbor, MI). Samples were read by using an automated microplate reader (model ELX800, BioTek, Winooski, VT,) and KCjunior software (version 1.3, catalog no. 5270501, BioTek). Readings were assessed at a wavelength of 405 nm with correction at 490 nm.
The crossreactivity of the cortisol kit, as reported by the manufacturer, was 100% with cortisol and prednisolone (122%), 27.7% with corticosterone, 4% with 11-deoxycortisol, and negligible for other steroids (less than 1%). The crossreactivity of the DHEA kit was 100% with DHEA, 30% with DHEA sulfate, and negligible for other steroids (less than 1% for androstenedione, androsterone, and so forth). The sensitivity of the kit was 56.80 pg/mL for cortisol and 2.90 pg/mL for DHEA.
Creatinine concentration was measured by using a modified JafE endpoint assay to correct for intersubject variation in concentration.22,69 Cortisol and DHEA values are expressed as picograms per microgram of creatinine. Creatinine content was determined by using an enzyme immunoassay kit (Assay Designs). Urine was diluted 1:60 in distilled water, and duplicate 200-μL samples were added to 96-well microtiter plates. A 100-µL aliquot of picric acid–NaOH solution (equal volumes of 0.04 M picric acid and 0.75 M NaOH) was added to each sample, and the plates were shaken briefly. After 30 min, the absorbance at 490 nm was measured in the microplate reader. Intra- and interassay coefficients of variations were assayed in triplicate on each plate, and the results were between 1.4% and 3.9%, respectively.
Assay validation.
To demonstrate parallelism and accuracy of the cortisol and DHEA assays, each of 5 randomly selected urine samples was serially diluted (1:2 to 1:16). Percentage-binding data from the standard curve were plotted against logarithmic transformations of their dosages, and the resulting regression equation was compared with those of the dilution sequences. To assess dose response, 5 more urine samples were selected randomly, and unlabeled cortisol or DHEA (0, 2.5, 10, 40, or 160 pg) was added to a 10-μL sample aliquot.
Given that the primary adrenal androgen DHEA is excreted in the urine in its conjugated form (DHEA sulfate), 4 randomly selected samples (2 male and 2 female) were hydrolyzed and serially diluted (1:2 to 1:16). A parallelism test was performed to demonstrate the correlation between DHEA and DHEA sulfate. The correlation between the 2 slopes was r = 0.87, demonstrating that DHEA was an accurate indicator of excreted levels of DHEA sulfate.
The dose–response study generated a curve with a slope of 1.05 (r2 = 0.98, P < 0.001) for cortisol and a curve with a slope of 1.1 (r2 = 0.97, P < 0.001) for DHEA. Mean recoveries were 98.3% for cortisol and 99.9% for DHEA over a range of 2.5 to 160 pg. The slopes generated from the serially diluted samples in the parallelism study did not differ (cortisol: P = 0.88, DHEA: P = 0.53) from those of the standard curve. Intra- and interassay coefficients of variations were 5.9% and 9.5% for cortisol and 6.4% and 13.9% for DHEA.
Statistical analysis.
For descriptive purposes, 2-way ANOVA was used to determine the effects of RE and sex on each dependent measure (cortisol and DHEA levels, latency, frequency, and duration of contact with coin holders). Pearson correlation was used to investigate the relationship among the dependent variables. To test the physiologic and behavioral variation between test days 1 and 2, repeated-measures analysis was done. Because the 2 tests differed in duration, measures were normalized by time (behavioral response / unit of time).
Multidimensional scaling (MDS) analysis was conducted to provide a model of independent associations among the variables and yielded a visual representation (map) of the distance between variables. By using correlations, the relationships (that is, proximities) among variables can be displayed graphically. The variables are represented by a set of points in 2D or 3D (a map). Therefore, the closer 2 or more variables are, the more highly correlated they are, whereas the farther apart they are, the less correlated they are. To map all of the variables into a desired space, some lack of fit (that is, s stress) has to be accepted; the values of s-stress range from 0 (perfect fit) to 1 (worst possible fit). The aim of MDS analysis is to find a map of the variables that minimizes the s stress for a given number of dimensions.35 The number of dimensions can be likened to the number of latent underlying factors in the dataset. Therefore, when choosing the number of dimensions to represent the data, one must consider 1) the number of variables in the model; 2) the lack of fit (s stress value), given the number of dimensions; 3) an index of fit of the model (r2 value); and 4) interpretability of the dimensions.35 The first point addresses the fact that for each dimension of the data, there should be approximately 4 variables entered into the model. Therefore, for a 2D map, approximately 8 variables should be used. The second point addresses how well the MDS map actually fits the data. Stress values less than 0.15 typically are deemed acceptable. The third point addresses the variance accounted for within the model. As is the case with any regression analysis, one must consider the amount of variance accounted for. Typically, r2 values of 0.8 or higher are desirable. Finally, one must pick a solution based on the interpretability of the dimensions. Parsimony is crucial to interpreting the map of any given dataset.35
SPSS 20.0 statistical software (IBM, Chicago, IL) was used for all statistical processing.
Results
Behavioral task: habituation and test day 1.
During habituation (3 coin holders presented to the owl monkeys), neither RE (F1,18 = 0.27, P = 0.61) nor sex (F1,18 = 0.09, P = 0.96) led to a significant difference in the number of marshmallows retrieved.
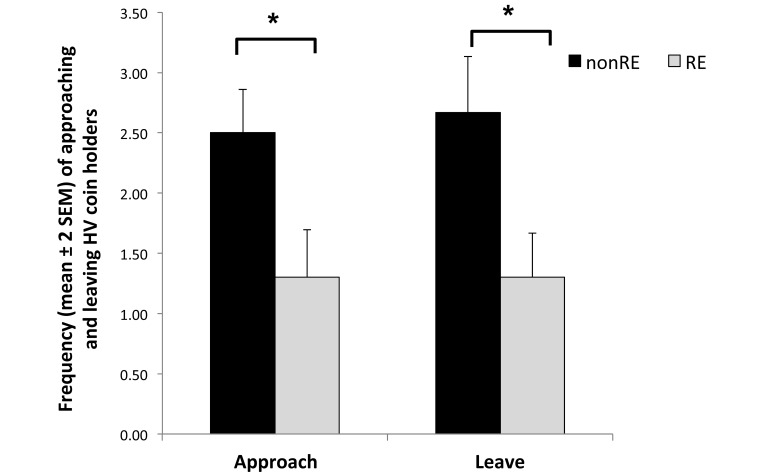
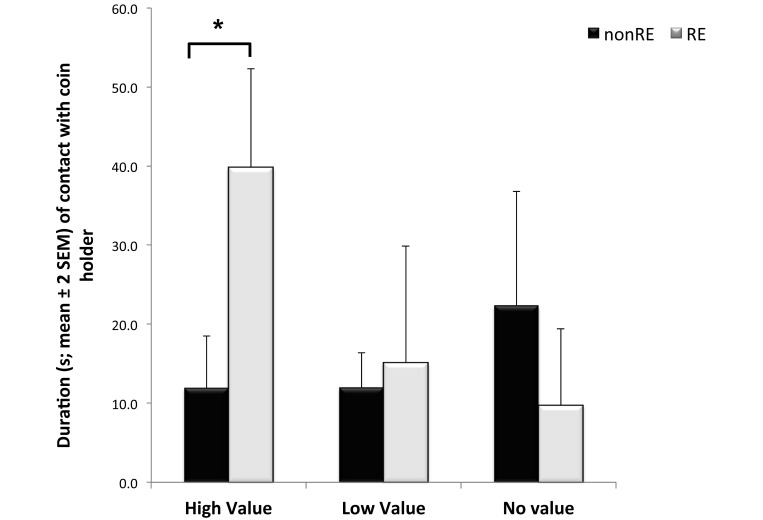
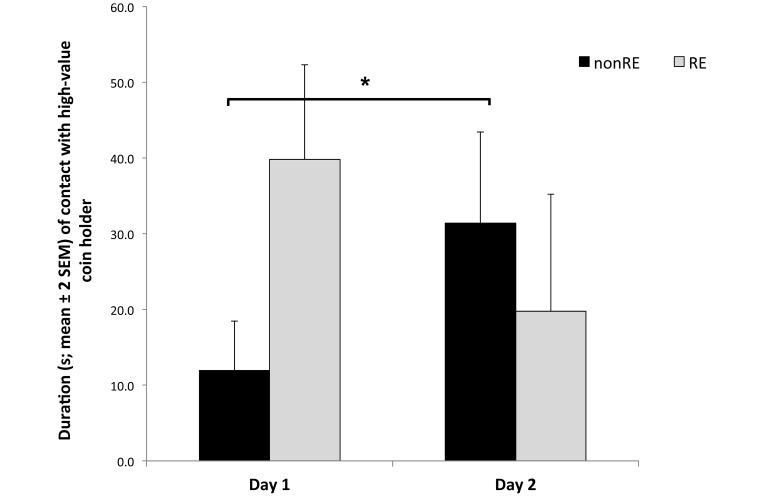
During the first day of testing (9 holders presented to the monkeys), no significant difference in the latency to approach the holders was observed between the 2 reproductive groups (F1,18 = 0.25, P = 0.62); however, the frequency of approaching the holders differed between RE and nonRE monkeys (F1,18 = 5.05, P = 0.036). Specifically, animals without RE approached and left the holders more often than did monkeys with RE (Figure 2). In addition, RE significantly affected the duration of contact with the coin holders, in that RE monkeys spent almost 4 times longer in contact with HV holders than did nonRE animals (F1,18 = 6.96, P = 0.016; Figure 3). The mean number of pieces of marshmallow retrieved and consumed was higher in RE animals (RE, 2.6 pieces; nonRE, 1.5 pieces; F1,18 = 14.04, P = 0.001). Duration of interaction with the other 2 sets of holders (LV and NV) did not differ according to RE. Finally, sex had no significant effect on any of the measured parameters (P > 0.45 in all cases); furthermore, no significant sex by RE interaction effects were found (P > 0.16 in all cases).
Figure 2.
Frequency of approaching and leaving high-value coin holders on days 1. Reproductive experience (RE) showed a significant (*, P < 0.05) effect during test day 1. Specifically, nonRE monkeys approached and left the coin holders more often than did RE animals.
Figure 3.
Duration of contact with the 3 kinds of coin holders (no value, no marshmallow reward present; low value, a single piece of marshmallow present; high value, 2 pieces of marshmallow present). RE showed a significant (*, P < 0.05) effect for high value holders. Specifically, RE monkeys maintained contact with high-value coin holders almost 4 times as long as did nonRE animals.
Behavioral task: test day 2.
In the second day of testing (9 holders presented to the animals for the third time), no effects of RE or sex were noted (P > 0.21 in all cases). The significant difference in the mean number of pieces of marshmallow retrieved and consumed disappeared (RE, 2.4 pieces; nonRE, 2.5 pieces; F1,18 = 0.96, P = 0.76).
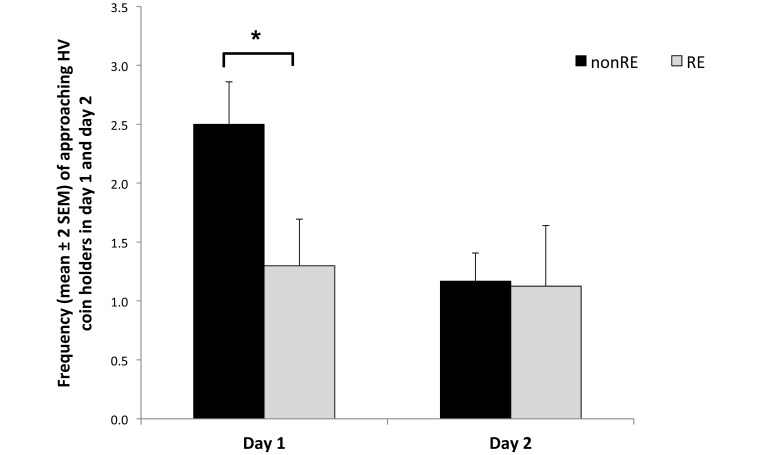
Regarding changes in scores from test day 1 to 2, the latency to approach the coin holders did not change between days (F1,18 = 2.27, P = 0.14), nor did it change by RE (F1,18 = 1.75, P = 0.20). The overall frequency of approaching the holders did not change by day (F1,18 = 0.69, P = 0.42) or RE (F1,18 = 1.17, P = 0.29). Specifically, the frequency of approach and leaving the holders decreased for nonRE monkeys and remained at the same levels for RE animals (Figure 4).
Figure 4.
Frequency of approaching coin holders on days 1 and 2. The frequency of approaching the holders decreased (*, P < 0.05) for nonRE monkeys and remained at the same level for RE animals.
RE significantly affected duration of contact from day 1 to day 2 for HV holders (F1,18 = 6.63, P = 0.018), but there was no significant interaction effect between RE and test day (F1,18 = 0.16, P = 0.69) or between day and sex (F1,18 = 0.63, P = 0.42). Specifically, the duration of contact increased between days 1 and 2 for nonRE monkeys but not RE animals (Figure 5). As a result, during the second test day, RE did not influence contact duration with the HV holders (P = 0.76).
Figure 5.
Duration of contact with high-value (HV) coin holders on days 1 and 2. Reproductive experience (RE) showed a significant effect for HV holders. Specifically, nonRE monkeys significantly (*, P < 0.05) increased the duration of contact between days 1 and 2, whereas that of RE animals did not differ significantly between the 2 d.
The duration of contact with the other 2 sets of holders (LV and NV) did not change by day nor was a day × RE interaction effect observed (P > 0.58 in all cases).
Physiologic assessment.
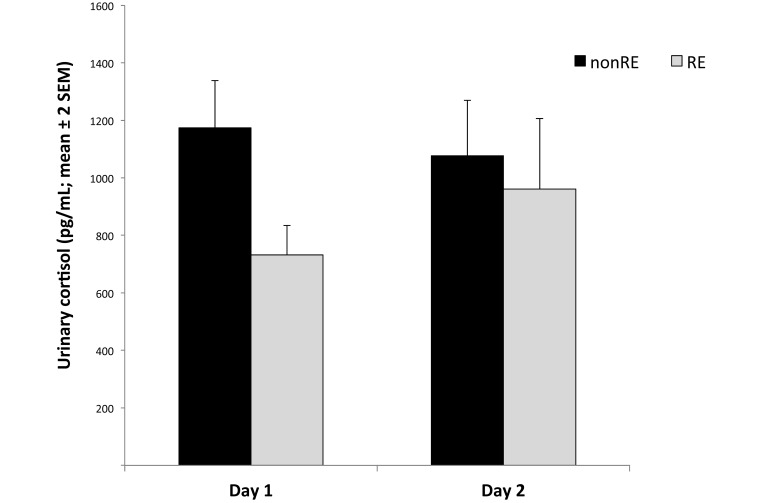
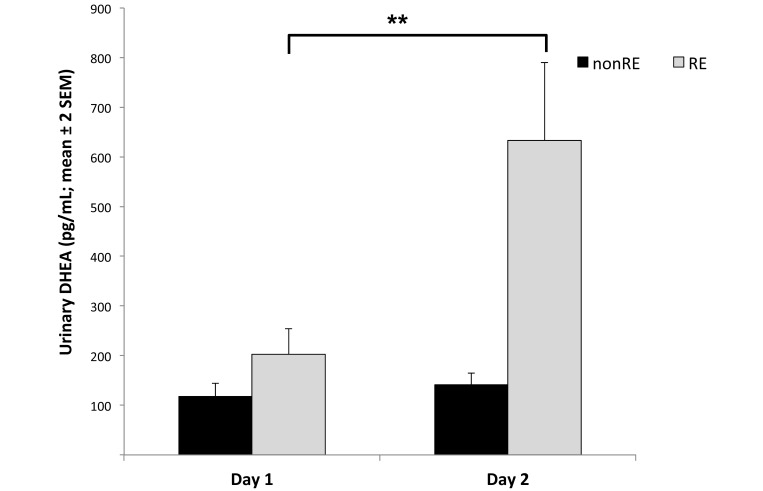
Cortisol urinary metabolites did not change significantly between before habituation and after test day 1 (F1,18 = 2.61, P = 0.12; Figure 6), whereas concentrations of DHEA urinary metabolites significantly increased between these time points (F1,18 = 14.6, P = 0.001). In addition, significant day × RE interaction effect was found (F1,18 = 11.7, P = 0.003). Specifically, DHEA levels significantly increased from day 1 to 2 in RE monkeys but not in nonRE animals (Figure 7). There was no significant day × sex interaction effect (F1,18 = 0.04, P = 0.84).
Figure 6.
Urinary cortisol concentrations did not change after the test. Reproductive experience (RE) did not show a significant effect on cortisol concentration.
Figure 7.
Urinary concentrations of DHEA metabolites on test day 1 were increased from baseline levels collected before habituation. Specifically, RE animals tripled (†, P < 0.01) their DHEA levels, whereas nonRE animals did not show a significant increase.
The DHEA:cortisol ratio (an index of physiologic resiliency) was higher in RE animals compared with nonRE monkeys (F1,18 = 26.3, P < 0.001).
Urinary cortisol and DHEA metabolites did not show any significant differences by sex.
Behavioral and physiologic correlates.
During test day 1, the DHEA:cortisol ratio was negatively correlated with the frequency of approaching the holders (r = – 0.48, P = 0.025) and positively correlated with the duration of contact with the HV holders (r = 0.41, P = 0.049). Considered separately, cortisol and DHEA levels at baseline and on test day 1 were not significantly correlated with behavior (P > 0.08 in all cases). No significant correlation between physiologic values and behaviors during test day 2 were noted.
MDS analysis.
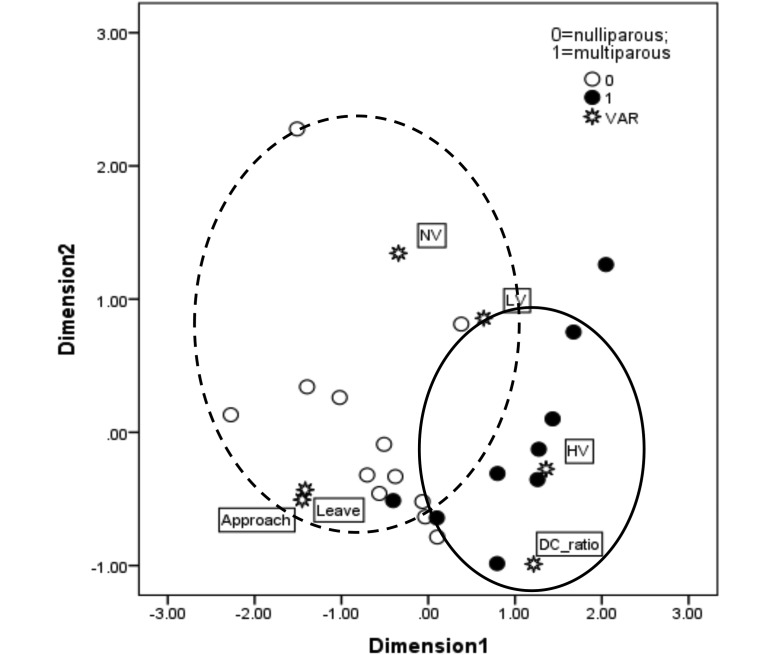
MDS analysis generated a map of association for behavioral measures and hormonal levels on the basis of RE (Figure 8).
Figure 8.
The stress value for the MDS analysis was 0.18, with R2 = 0.80, thus indicating that the map was not particularly accurate due to the low number of subjects. Nevertheless, 2 major clusters were clearly identified (circles). One cluster included most of the nonRE animals and was characterized by high frequencies of leaving and approaching the coin holders and by more time spent on no-value (NV) or low-value (LV) holders. The other cluster grouped together most of the RE monkeys and was characterized by high DHEA:cortisol ratios (DC_ratio) and prolonged contact with high-value (HV) holders. Dimensions in the multidimensional scaling analysis are scaled linear combinations of the original variables and represent the distance (correlation) among points (variables [VAR] and subjects) in the map. The distances among objects in the 2 dimensions are a measure of the overall association among variables.
The stress value for MDS was 0.18, with R2 = 0.80, thus indicating that the map was not particularly accurate, due to the low number of subjects. Nevertheless, 2 major clusters were clearly identified by the analysis. One cluster included most of the nonRE animals and was characterized by high frequencies of leaving and approaching the coin holders and by increased time spent interacting with NV and LV holders. The other cluster grouped together most of the RE monkeys and was characterized by high DHEA :cortisol ratios and high duration of contact with HV holders. Considering that MDS analysis offers an integrated view of the independent association among the variables included in the model, together with the relative position of each subject,35 the generated map was highly consistent with the univariate results we obtained from the experiments.
Discussion
The results from the current study support our hypothesis that RE influences ancillary behavioral and physiologic parental characteristics in owl monkeys, thus confirming previous results in other species.46,72 We consider 2 of our findings to be of particular interest are: 1) experienced parents had higher DHEA:cortisol ratios after exposure to habituation training and the first day of testing; and 2) according to their contact duration with the HV stimuli, RE owl monkeys exhibited 4-fold more efficient foraging strategies than did nonRE mating pairs during the foraging test. This difference disappeared in the second day of testing. Therefore, RE monkeys, which spent more time in contact with HV holders, displayed a more efficient foraging strategy than did the nonRE animals, which failed to demonstrate a preference for the HV holders, although nonRE animals caught up rather quickly to their RE counterparts.
Considering that the ratio between DHEA and cortisol has been found to be a reliable index of neuroprotection in both animal and human studies,5,26,31,44,73,74 the high DHEA:cortisol ratio in RE owl monkeys after habituation training indicates the likelihood of increased neuroprotection in these pairs. Recent evidence suggests that increased activation of the hypothalamic–pituitary–adrenal axis facilitates learning in demanding environments,32,33,74 therefore it is plausible to speculate that heightened DHEA:cortisol ratios may have shifted the allostatic load (that is, the physiologic consequences of chronic exposure to stress) to maintain efficiency during habituation training.32,48,52 Specifically, adrenal stress hormones may yield memory-enhancing effects when released acutely after learning; however, during prolonged exposure, stress hormones often result in compromised memory processes.32,48 Increasing the efficiency of the stress response associated with parenting, perhaps mediated by modified DHEA release, may result in adaptations such as more effective foraging strategies, as observed in the current study.
Interestingly, we found no significant effects of sex on either the neuroendocrine or behavioral responses to the paradigm, regardless of the RE group. In previous studies involving on rodents, parental experience influenced ancillary parental responses (including cognitive efficiency) and diminished fear responsiveness in both male and female subjects.5,21,41,43,45,63 Our current results also support prior results from monogamous New World monkeys, which indicated no sex-associated effects on physiologic and metabolic activity.10 We speculate that ancillary parental responses are similar even for species in which a large proportion of the parental care is performed by males.1,4,16 A plausible alternative hypothesis is that both members of the mating pair influence each other's behavioral and physiologic activity, thus explaining the similarities between male and female owl subjects.
From an evolutionary point of view, increased neural and behavioral plasticity may play a role in preparing new fathers to respond to the newborns, especially considering that paternal brains are less prepared to respond to offspring than are maternal brains.3,25,39,68 Paternal investment is a very demanding and highly organized process and, in primates, has evolved most exclusively in monogamous New World monkeys, in which the litters are composed of rapidly growing offspring and in which females can experience a highly fertile postpartum ovulation.23,56 Although owl monkeys and titi monkeys typically have single offspring, they face metabolic challenges due to the small body size of the adults and the rapid growth rate of offspring.51 Prior research suggests that offspring undergo a behavioral separation distress after separation from their father that persists into adulthood.62 In this situation, the male's extensive contribution to infant care appears essential in the development and survival of the offspring.20 If this is indeed the case, it is logical to hypothesize that evolutionary mechanisms necessary to prepare fathers for their highly demanding paternal role extend to ancillary parental behavioral and physiologic responses, as occurs in maternal mammals. Although several studies have reported a clear relationship between RE and the quality of parental behaviors in biparental primate species,6-8,81 this current study is the first time (to our knowledge) that adaptive modifications in emotional and cognitive responses have been linked directly to RE in male owl monkeys. These findings are supported by previous research demonstrating the effects of fatherhood on brain morphology in marmosets, indicating that first-time and experienced marmoset fathers exhibited enhanced density of dendritic spines on pyramidal neurons in the prefrontal cortex, compared with that in nonfathers.38
The role of glucocorticoids in regulating paternal behavior in biparental primate species has been a subject of debate. Early studies reported evidence of the involvement of glucocorticoids in the expression of paternal behavior; in particular, cortisol decreased, as did other sex steroids hormones (including testosterone and estrogens), in males after the birth of offspring.59 This decrease in steroid hormones, coupled with increases in prolactin and vasopressin levels,65,66,75,82 may serve as a mechanism that facilitates the transition from an aversive response to the offspring to the onset of nurturing paternal care. Recent studies, however, have not confirmed the involvement of glucocorticoids in the paternal behavior of marmosets.14 Even though the results of the current study suggest that glucocorticoids did not differ between the 2 reproductive groups, RE influenced relative DHEA levels in both male and female parental owl monkeys.
Although our data are correlational in nature and share the limitations of all observational studies, another clue of the mediating effect of physiologic and endocrine modifications on the efficiency of behavioral responses in the paradigm is that the time spent in contact with HV holders was positively correlated with the DHEA:cortisol ratio. The MDS analysis provided additional support of parenting-mediated effects in the foraging task. Not only did this multivariate analysis confirm a distinct separation between RE and nonRE in terms of the behavioral and physiologic responses to the experimental paradigm, it also clearly indicated the independent association between the index of potential neuroprotection (that is, the DHEA:cortisol ratio) and time spent with the HV holders. Therefore, the MDS map provided supplementary information in support of the overall conclusion of this and other investigations that parental experience may alter neural and physiologic central mechanisms linked to more efficient and adaptive responses of males and females in challenging environments.39
The limited control of animal selection in the Aotus population at the DuMond Conservancy may have been a mitigating factor in the current study. Coupled with this issue, the likelihood that mating pairs influenced each other during the cognitive task may have prompted the lack of a sex-specific effect by RE. In future studies with access to larger populations of animals, it would be interesting to assess only one subject of each pair. Another methodologic challenge in the current study was the high individual variability in age, RE, and individual history of the animals. Although we used analysis of covariance to address this important issue, we cannot completely rule age out as a confounding variable, given the small sample size. Even with these limitations, this colony represents a valuable opportunity to evaluate owl monkeys in particular and biparental primates in general in a controlled, yet naturalistic, environment.76,77
In light of the behavioral and hormonal differences between the reproductive groups in the present study, we conclude that RE significantly modifies both the male's and female's behavioral and hormonal repertoire in response to a challenging cognitive paradigm in owl monkeys. To our knowledge, this report provides the first evidence of RE-influenced parental adaptations in owl monkeys. These findings suggest that the prior research indicating modifications in ancillary parental responses adaptive for the successful care of offspring in rodents extends to other species. This information also is critical for future biomedical research, given that RE can affect the behavioral characteristics of these animals.
Acknowledgments
This research was funded or supported by The Schapiro Undergraduate Research Fellowship program, The Laughton Fellowship, the DuMond Conservancy, and the Psychology Department at Randolph–Macon College.
References
- 1.Almond RE, Ziegler TE, Snowdon CT. 2008. Changes in prolactin and glucocorticoid levels in cotton-top tamarin fathers during their mate's pregnancy: the effect of infants and paternal experience. Am J Primatol 70:560–565. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MV, Rutherford MD. 2012. Cognitive reorganization during pregnancy and the postpartum period: an evolutionary perspective. Evol Psychol 10:659–687. [PubMed] [Google Scholar]
- 3.Babb PL, Fernandez-Duque E, Schurr TG. 2010. AVPR1A sequence variation in monogamous owl monkeys (Aotus azarai) and its implications for the evolution of platyrrhine social behavior. J Mol Evol 71:279–297. [DOI] [PubMed] [Google Scholar]
- 4.Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. 2007. Neural correlates of pair-bonding in a monogamous primate. Brain Res 1184:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. 2011. Paternal experience and stress responses in California mice (Peromyscus californicus). Comp Med 61:20–30. [PMC free article] [PubMed] [Google Scholar]
- 6.Bardi M, French JA, Ramirez SM, Brent L. 2004. The role of the endocrine system in baboon maternal behavior. Biol Psychiatry 55:724–732. [DOI] [PubMed] [Google Scholar]
- 7.Bardi M, Petto AJ. 2002. Parental failure in captive common marmosets (Callithrix jacchus): a comparison with tamarins. Folia Primatol (Basel) 73:46–48. [DOI] [PubMed] [Google Scholar]
- 8.Bardi M, Petto AJ, Lee-Parritz DE. 2001. Parental failure in captive cotton-top tamarins (Saguinus oedipus). Am J Primatol 54:159–169. [DOI] [PubMed] [Google Scholar]
- 9.Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. 2003. Peripartum cortisol levels and mother–infant interactions in Japanese macaques. Am J Phys Anthropol 120:298–304. [DOI] [PubMed] [Google Scholar]
- 10.Boere V, Pinheiro EC, de Oliveira e Silva I, Paludo GR, Canale G, Pianta T, Welker A, Rocha-de-Moura RC. 2005. Comparison between sex and age class on some physiological, thermal, and hematological indices of the cerrado marmoset (Callithrix penicillata). J Med Primatol 34:156–162. [DOI] [PubMed] [Google Scholar]
- 11.Bridges RS. 2008. The neurobiology of the parental brain. San Diego (CA): Academic Press. [Google Scholar]
- 12.Brummelte S, Galea LA. 2010. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry 34:766–776. [DOI] [PubMed] [Google Scholar]
- 13.Brunton PJ, Russell JA. 2010. Endocrine induced changes in brain function during pregnancy. Brain Res 1364:198–215. [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh J, French JA. 2013. Postpartum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Horm Behav 63:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charney DS. 2004. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161:195–216. [DOI] [PubMed] [Google Scholar]
- 16.Curley JP, Mashoodh R, Champagne FA. 2011. Epigenetics and the origins of paternal effects. Horm Behav 59:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falk D. 2000. Primate diversity. New York (NY): WW Norton. [Google Scholar]
- 18.Fernandez-Duque E. 2007. Aotinae: social monogamy in the only nocturnal haplorines, p 139–154. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK. Primates in perspective. New York (NY): Oxford University Press. [Google Scholar]
- 19.Fernandez-Duque E, Juárez CP, Di Fiore A. 2008. Adult male replacement and subsequent infant care by male and siblings in socially monogamous owl monkeys (Aotus azarai). Primates 49:81–84. [DOI] [PubMed] [Google Scholar]
- 20.Fite JE, Patera KJ, French JA, Rukstalis M, Hopkins EC, Ross CN. 2005. Opportunistic mothers: female marmosets (Callithrix kuhlii) reduce their investment in offspring when they have to and when they can. J Hum Evol 49:122–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franssen CL, Bardi M, Shea EA, Hampton JE, Franssen RA, Kinsley CH, Lambert KG. 2011. Fatherhood alters behavioural and neural responsiveness in a spatial task. J Neuroendocrinol 23:1177–1187. [DOI] [PubMed] [Google Scholar]
- 22.French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. 1996. Urinary steroid and gonadotropin excretion across the reproductive cycle in females Wied black tufted-ear marmosets (Callithrix kuhli). Am J Primatol 40:231–245. [DOI] [PubMed] [Google Scholar]
- 23.French JA, Fite JE, Ross CN. 2008. Family life in marmosets: causes and consequences of variation in offspring care, p 461–479. In: Bridges RS. Neurobiology of the parental brain. New York (NY): Elsevier. [Google Scholar]
- 24.French JA, Koban T, Rukstalis M, Ramirez SM, Bardi M, Brent L. 2004. Excretion of urinary steroids in pre- and postpartum female baboons. Gen Comp Endocrinol 137:69–77. [DOI] [PubMed] [Google Scholar]
- 25.Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. 2008. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol 62:247–260. [DOI] [PubMed] [Google Scholar]
- 26.Goncharova ND, Vengerin AA, Chigarova OA. 2012. Repeated moderate stress stimulates the production of dehydroepiandrosterone sulfate (DHEAS) and reduces corticosteroid imbalance in old Macaca mulatta. Bull Exp Biol Med 153:750–753. [DOI] [PubMed] [Google Scholar]
- 27.Gudsnuk KM, Champagne FA. 2011. Epigenetic effects of early developmental experiences. Clin Perinatol 38:703–717. [DOI] [PubMed] [Google Scholar]
- 28.Izawa S, Sugaya N, Shirotsuki K, Yamada KC, Ogawa N, Ouchi Y, Nagano Y, Suzuki K, Nomura S. 2008. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biol Psychol 79:294–298. [DOI] [PubMed] [Google Scholar]
- 29.Jarcho MR, Mendoza SP, Bales KL. 2012. Hormonal and experiential predictors of infant survivorship and maternal behavior in a monogamous primate (Callicebus cupreus). Am J Primatol 74:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. 2011. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav 10:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon II, Stein LM, Bauer ME. 2010. Neuroendocrine and immunological correlates of chronic stress in ‘strictly healthy’ populations. Neuroimmunomodulation 17:9–18. [DOI] [PubMed] [Google Scholar]
- 32.Joëls M, Baram TZ. 2009. The neurosymphony of stress. Nat Rev Neurosci 10:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. 2004. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 7:221–231. [DOI] [PubMed] [Google Scholar]
- 34.Kaffman A, Meaney MJ. 2007. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry 48:224–244. [DOI] [PubMed] [Google Scholar]
- 35.Kemmler G, Holzner B, Kopp M, Dunser M, Greil R, Hahn E, Sperner-Unterweger B. 2002. Multidimensional scaling as a tool for analyzing quality of life data. Qual Life Res 11:223–233. [DOI] [PubMed] [Google Scholar]
- 36.Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. 2008. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav 37:43–56. [DOI] [PubMed] [Google Scholar]
- 37.Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. 1999. Motherhood improves learning and memory. Nature 402:137–138. [DOI] [PubMed] [Google Scholar]
- 38.Kozorovitskiy Y, Hughes M, Lee K, Gould E. 2006. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci 9:1094–1095. [DOI] [PubMed] [Google Scholar]
- 39.Lambert KG. 2012. The parental brain: transformations and adaptations. Physiol Behav 107:792–800. [DOI] [PubMed] [Google Scholar]
- 40.Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. 2005. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav 84:799–806. [DOI] [PubMed] [Google Scholar]
- 41.Lambert KG, Franssen CL, Bardi M, Hampton JE, Hainley L, Karsner S, Tu EB, Hyer MM, Crockett A, Baranova A, Ferguson T, Ferguson T, Kinsley CH. 2011. Characteristic neurobiological patterns differentiate paternal responsiveness in 2 Peromyscus species. Brain Behav Evol 77:159–175. [DOI] [PubMed] [Google Scholar]
- 42.Lambert KG, Franssen CL, Hampton JE, Rzucidlo AM, Hyer MM, True M, Kaufman C, Bardi M. 2013. Modeling paternal attentiveness: distressed pups evoke differential neurobiological and behavioral responses in paternal and nonpaternal mice. Neuroscience 234:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Lee AW, Brown RE. 2007. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in California mice (Peromyscus californicus). Physiol Behav 92:617–628. [DOI] [PubMed] [Google Scholar]
- 44.Lennartsson AK, Theorell T, Kushnir MM, Bergquist J, Jonsdottir IH. 2013. Perceived stress at work is associated with attenuated DHEAS response during acute psychosocial stress. Psychoneuroendocrinology 38:1650–1657. [DOI] [PubMed] [Google Scholar]
- 45.Leuner B, Glasper ER, Gould E. 2010. Parenting and plasticity. Trends Neurosci 33:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. 2005. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci 119:1084–1096. [DOI] [PubMed] [Google Scholar]
- 47.Macbeth AH, Luine VN. 2010. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev 34:452–467. [DOI] [PubMed] [Google Scholar]
- 48.Maclaughlin BW, Wang D, Noone AM, Liu N, Harazduk N, Lumpkin M, Haramati A, Saunders P, Dutton M, Amri H. 2011. Stress biomarkers in medical students participating in a mind body medicine skills program. Evid Based Complement Alternat Med 2011:950461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30:65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manson JE. 2008. Prenatal exposure to sex steroid hormones and behavioral–cognitive outcomes. Metabolism 57 Suppl 2:S16–S21. [DOI] [PubMed] [Google Scholar]
- 51.Mason WA, Mendoza SP. 1998. Generic aspects of primate attachments: parents, offspring, and mates. Psychoneuroendocrinology 23:765–778. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS. 2012. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA 109 Suppl 2:17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mock DW, Fujioka M. 1990. Monogamy and long-term pair bonding in vertebrates. Trends Ecol Evol 5:39–43. [DOI] [PubMed] [Google Scholar]
- 54.Morgan CA, Rasmusson A, Pietrzak RH, Coric V, Southwick SM. 2009. Relationships among plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans exposed to underwater navigation stress. Biol Psychiatry 66:334–340. [DOI] [PubMed] [Google Scholar]
- 55.Mota MT, Sousa MB. 2000. Prolactin levels of fathers and helpers related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol (Basel) 71:22–26. [DOI] [PubMed] [Google Scholar]
- 56.Mustoe AC, Jensen HA, French JA. 2012. Describing ovarian cycles, pregnancy characteristics, and the use of contraception in female white-faced marmosets, Callithrix geoffroyi. Am J Primatol 74:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Numan M, Insel TR. 2003. The neurobiology of parental behavior. New York (NY): Springer. [Google Scholar]
- 58.Numan M, Stolzenberg DS. 2009. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol 30:46–64. [DOI] [PubMed] [Google Scholar]
- 59.Nunes S, Fite JE, French JA. 2000. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii). Anim Behav 60:857–865. [DOI] [PubMed] [Google Scholar]
- 60.Pawluski JL, Barakauskas VE, Galea LA. 2010. Pregnancy decreases oestrogen receptor α expression and pyknosis, but not cell proliferation or survival, in the hippocampus. J Neuroendocrinol 22:248–257. [DOI] [PubMed] [Google Scholar]
- 61.Pinnock SB, Lazic SE, Wong HT, Wong IHW, Herbert J. 2009. Synergistic effects of dehydroepiandrosterone and fluoxetine on proliferation of progenitor cells in the dentate gyrus of the adult male rat. Neuroscience 158:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ragen BJ, Mendoza SP, Mason WA, Bales KL. 2012. Differences in titi monkey (Callicebus cupreus) social bonds affect arousal, affiliation, and response to reward. Am J Primatol 74:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rima BN, Bardi M, Friedenberg JM, Christon LM, Karelina KE, Lambert KG, Kinsley CH. 2009. Reproductive experience and the response of female Sprague–Dawley rats to fear and stress. Comp Med 59:437–443. [PMC free article] [PubMed] [Google Scholar]
- 64.Saltzman W, Abbott DH. 2011. Hormonal and behavioral responses to stress in lactating and non-lactating female common marmosets (Callithrix jacchus). Physiol Behav 104:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schradin C, Anzenberger G. 2004. Development of prolactin levels in marmoset males: from adult son to first-time father. Horm Behav 46:670–677. [DOI] [PubMed] [Google Scholar]
- 66.Schradin C, Reeder DM, Mendoza SP, Anzenberger G. 2003. Prolactin and paternal care: comparison of 3 species of monogamous New World monkeys (Callicebus cupreus, Callithrix jacchus, and Callimico goeldii). J Comp Psychol 117:166–175. [DOI] [PubMed] [Google Scholar]
- 67.Storey AE, Delahunty KM, McKay DW, Walsh CJ, Wilhelm SI. 2006. Social and hormonal bases of individual differences in the parental behaviour of birds and mammals. Can J Exp Psychol 60:237–245. [DOI] [PubMed] [Google Scholar]
- 68.Swain JE. 2011. The human parental brain: in vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry 35:1242–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tietz NW. 1976. Fundamentals of clinical chemistry. Philadelphia (PA): WB Saunders. [Google Scholar]
- 70.Ulmann L, Rodeau JL, Danoux L, Contet-Audonneau JL, Pauly G, Schlichter R. 2009. Dehydroepiandrosterone and neurotrophins favor axonal growth in a sensory neuron–keratinocyte coculture model. Neuroscience 159:514–525. [DOI] [PubMed] [Google Scholar]
- 71.van Heusden AM, Fauser BCJ. 2002. Residual ovarian activity during oral steroid contraception. Hum Reprod Update 8:345–358. [DOI] [PubMed] [Google Scholar]
- 72.Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. 2003. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav 79:373–381. [DOI] [PubMed] [Google Scholar]
- 73.Wemm S, Fanean A, Baker A, Blough ER, Mewaldt S, Bardi M. 2013. Problematic drinking and physiological responses among female college students. Alcohol 47:149–157. [DOI] [PubMed] [Google Scholar]
- 74.Wemm S, Koone T, Blough ER, Mewaldt S, Bardi M. 2010. The role of DHEA in relation to problem solving and academic performance. Biol Psychol 85:53–61. [DOI] [PubMed] [Google Scholar]
- 75.Woller MJ, Sosa ME, Chiang Y, Prudom SL, Keelty P, Moore JE, Ziegler TE. 2012. Differential hypothalamic secretion of neurocrines in male common marmosets: parental experience effects? J Neuroendocrinol 24:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolovich CK, Evans S, Green SM. 2010. Mated pairs of owl monkeys (Aotus nancymaae) exhibit sex differences in response to unfamiliar male and female conspecifics. Am J Primatol 72:942–950. [DOI] [PubMed] [Google Scholar]
- 77.Wolovich CK, Feged A, Evans S, Green SM. 2006. Social patterns of food sharing in monogamous owl monkeys. Am J Primatol 68:663–674. [DOI] [PubMed] [Google Scholar]
- 78.Workman JL, Barha CK, Galea LA. 2012. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 126:54–72. [DOI] [PubMed] [Google Scholar]
- 79.Wynne-Edwards KE. 2001. Hormonal changes in mammalian fathers. Horm Behav 40:139–145. [DOI] [PubMed] [Google Scholar]
- 80.Young LJ, Winslow JT, Nilsen R, Insel TR. 1997. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci 111:599–605. [DOI] [PubMed] [Google Scholar]
- 81.Zahed SR, Kurian AV, Snowdon CT. 2010. Social dynamics and individual plasticity of infant care behavior in cooperatively breeding cotton-top tamarins. Am J Primatol 72:296–306. [DOI] [PubMed] [Google Scholar]
- 82.Ziegler TE. 2000. Hormones associated with nonmaternal infant care: a review of mammalian and avian studies. Folia Primatol (Basel) 71:6–21. [DOI] [PubMed] [Google Scholar]