Abstract
Background
Prior estimates suggest that up to 40 % of the US general population (GP) report symptoms of gastroesophageal reflux disease (GERD). However, symptoms in the GP versus patients seeking care for gastrointestinal (GI) complaints have not been compared. We estimated the prevalence and severity of GERD symptoms in the GP versus GI patients, and identified predictors of GERD severity. We hypothesized that similar to functional GI disorders, psychosocial factors would predict symptom severity in GERD as much, or perhaps more, than care-seeking behavior alone.
Methods
We compared the prevalence of heartburn and regurgitation between a sample from the US GP and patients seeking GI specialty care. We compared GERD severity between groups using the NIH PROMIS® GERD scale. We then performed multivariable regression to identify predictors of GERD severity.
Results
There was no difference in the prevalence of heartburn between the GP and patient groups (59 vs. 59 %), but regurgitation was more common in patients versus GP (46 vs. 39 %; p = 0.004). In multivariable regression, having high visceral anxiety (p < 0.001) and being divorced or separated (p = 0.006) were associated with higher GERD severity.
Conclusions
More than half of a GP sample reports heartburn—higher than previous series and no different from GI patients. Although regurgitation was more prevalent in patients versus the GP, there was no difference in GERD severity between groups after adjusting for other factors; care seeking in GERD appears related to factors beyond symptoms, including visceral anxiety.
Keywords: Gastroesophageal reflux disease, Patient-reported outcomes, Symptoms
Introduction
Gastroesophageal reflux disease (GERD) is associated with impaired health-related quality of life and substantial resource utilization [1, 2]. The prevalence of reflux symptoms is steadily rising throughout the industrialized world [3]. An estimated 20–40 % of Western adult populations report chronic heartburn or regurgitation symptoms [4-7], although fewer meet formal diagnostic criteria for GERD [8]. Over nine million primary care visits are attributed to GERD annually; it remains the most common gastroenterology-related outpatient diagnosis [9]. GERD is associated with increased reports of restricted activity and missed work, imposing a financial burden for both healthcare systems and employers alike [10].
Although previous research evaluated GERD epidemiology in the general population (GP) [11] and patient population [12], respectively, no study has compared the prevalence or severity of GERD symptoms between these groups. Because care-seekers are a subset of the larger population, we might expect that patients have more frequent, severe, or bothersome symptoms than people in the GP. However, little is known about the differences between groups or what drives care-seeking behavior in the first place—not only for GERD symptoms, but for other chronic gastrointestinal (GI) symptoms as well.
Previous research has explored aspects of care seeking and resource utilization in GERD. For example, a French study compared patients with weekly versus less frequent symptoms and found that patients with weekly GERD perceived their symptoms to be more severe and had greater healthcare utilization [13]. Even subjects with infrequent GERD often experienced substantial impact on their daily activities and sought medical advice, suggesting that factors beyond symptom frequency may drive the GERD illness experience [13]. However, less is known about the role of psychosocial factors and care seeking in GERD. Although psychosocial factors are associated with healthcare seeking in irritable bowel syndrome (IBS) [14] and dyspepsia [15], their role in GERD remains unclear.
In this study, we sought to describe the prevalence and severity of GERD symptoms in a representative US GP sample versus a broad range of patients seeking GI sub-specialty care. Furthermore, we identified predictors of symptom severity and hypothesized that similar to functional GI disorders (FGIDs), psychosocial factors would predict symptom severity in GERD as much, or perhaps more, than care-seeking behavior alone.
Methods
Study Overview
To study the prevalence and severity of GERD symptoms in the GP and those seeking care for GI disorders, we conducted a cross-sectional online survey using items developed for the NIH Patient-Reported Outcome Measurement Information System (PROMIS®; www.nihPROMIS.org) [16, 17]. PROMIS is a federally supported NIH Roadmap Initiative that developed patient-reported outcome (PRO) measures across the breadth and depth of disease, including GI disorders. The PROMIS GI item banks cover 8 broad symptom categories, one of which is GERD [17, 18]. The PROMIS GERD items measure the frequency, severity, impact, and bother of cardinal GERD symptoms, including heartburn and regurgitation, using a seven-day recall period. The scales correlate significantly with both generic (e.g., Euro-QOL, SF12) and disease-targeted legacy instruments (e.g., Gastrointestinal Symptom Rating Scale [GSRS]) and demonstrate evidence of reliability [18]. In addition to NIH PROMIS items, we collected demographic and clinical information about each subject and administered the PROMIS Global Health items, the Visceral Sensitivity Index (VSI) [19, 20], SF-12® health survey, and the GI Symptoms Rating Scale (GSRS) [21].
Selection of Patients
We recruited a diverse group of participants from outpatient clinical practices and national cohorts seeking care at university, community, and Veteran Affairs institutions. We invited patients seeking care at these outpatient clinics for an active GI symptom of any kind, including, but not limited to, GERD symptoms. Our sample included patients with inflammatory bowel disease (IBD) seeking care at Cedars-Sinai Medical Center, a tertiary center in Los Angeles; patients with GI symptoms from systemic sclerosis seeking care at a specialty clinic at the University of Michigan; patients with FGIDs seeking care at a specialty clinic at the University of California Los Angeles; and patients with diverse GI conditions seeking care at a general GI clinic at the West Los Angeles Veterans Administration Medical Center. In addition, we partnered with the International Foundation for Functional Gastrointestinal Disorders (IFFGD) to survey a cohort of patients with diverse FGIDs enrolled in IFFGD mailing lists. All patients were invited to complete the confidential online survey instrument, administered by Survey Monkey software (www.surveymonkey.com). Patients without Internet access could request paper surveys sent to their home, or completed in clinic, as needed. Patients were excluded from participation if they failed to provide informed consent or if they had cognitive impairment that would interfere with participation.
Selection of Controls
In addition to GI patient recruitment, Cint® (www.cint.com), a survey research firm, recruited a sample of individuals representative of the GP in terms of gender, ethnicity, race, geographic location, and education level based on the 2010 census. Subjects were required to be 18 years of age or older and able to read English; there were no other exclusion criteria applied to the GP sample.
Measuring GERD Prevalence and Severity
We measured heartburn and regurgitation prevalence with items developed under the guidelines [22] of the NIH PROMIS consortium. Figure 1 shows the items and their scoring, each using a seven-day recall period. For the current study, we marked a respondent as positive for heartburn if they endorsed at least rare heartburn over the past week, and positive for regurgitation if they experienced at least one day of regurgitation over the past week.
Fig. 1.
NIH PROMIS® GERD Scale
In addition to measuring the prevalence of heartburn and regurgitation, we measured overall GERD symptom severity using the NIH PROMIS GERD scale [17, 18]. The NIH PROMIS GERD scale includes 13 items that assess cardinal GERD symptoms, including heartburn frequency, heartburn severity, heartburn bother, throatburn frequency, regurgitation frequency, regurgitation bother, “wet burp” frequency, and nighttime awakenings from regurgitation (see Fig. 1 for all items). These items were developed by the NIH PROMIS consortium based on 3 focus groups of 28 subjects with GERD, and were refined based on cognitive interviews.
The final 13-item GERD score was derived from a larger sample of 45 GERD items in the full NIH PROMIS item bank using a series of analyses to capture the most clinically valid and quantitatively efficient subset that would replicate the larger sample of items. As with all NIH PROMIS scales, the PROMIS GERD scale uses item response theory (IRT) to calculate scores on a T-metric [16, 18] ranging from 1 to 100 points, with 50 representing the mean score in the GP sample. Each 10-point interval corresponds with a standard deviation (SD) change from the mean. For example, a PROMIS GERD score of 60 is 1 SD above the population mean of 50. Validation of the PROMIS GERD scale revealed that is significantly correlates with the GSRS reflux subscale, VSI, EQ-5D preference-based score, and SF-12 physical and mental health component summary scores (PCS and MCS), and therefore has excellent construct validity (Table 1) [18].
Table 1.
Correlations between NIH PROMIS GERD score and legacy questionnaires, including the Visceral Sensitivity Index (VSI), GI Symptom Rating Scale (GSRS) reflux subscale, EQ-5D utility score, and SF 12 physical component score (PCS) and mental component score (MCS)
| PROMIS Score |
N Items |
VSI | GSRS reflux subscale |
EQ-5D | SF-36 PCS |
SF-36 MCS |
|---|---|---|---|---|---|---|
| GERD Scale |
13 | 0.5* | 0.73* | −0.22* | −0.41* | −0.47 |
p < 0.001
Statistical Analysis
We calculated descriptive statistics for demographic characteristics of the GP subjects and GI patients, including age, gender, race/ethnicity, education, marital status, and employment. We performed chi-square testing for binary variables, and Student’s t tests for continuous variables. Chi square was used to compare the prevalence of heartburn and regurgitation between the GP and GI groups. We then used ANOVA to compare PROMIS GERD scores among 3 groups: (1) GP sample; (2) all GI patients combined; and (3) the subgroup of GI patients specifically seeking care for GERD (in contrast to other GI conditions). Post hoc tests were used to examine the source of mean differences when the overall ANOVA F test was statistically significant.
We then performed multivariable linear regression analysis to identify correlates of GERD symptom severity, including patient status, underlying GI condition, and demographic characteristics. For these analyses, we used all subjects in both patient and GP samples. In all tests, we considered a P value of less than 0.05 significant. We performed all statistical analyses with SAS statistical software, version 9.2 (SAS Institute, Cary, NC). This study was approved by the institutional review boards of the West Los Angeles VA (PCC #0020), University of California at Los Angeles (IRB#11-003065.), Cedars-Sinai Medical Center (PRO00027093), and the University of Michigan (HUM00052942).
Results
Patient Characteristics and Descriptive Statistics
We recruited 707 patients and 1,107 GP to complete the online survey. Table 2 presents the demographic characteristics of both samples. There was no significant difference in age or gender, but there were significant differences in race/ethnicity, education, marital status, and employment status, as noted in the table. Of the 707 GI patients, 98 sought care specifically for GERD. Other prevalent diseases included combined FGIDs (N = 250), IBD (N = 212), and systemic sclerosis (N = 167).
Table 2.
Descriptive characteristics of general population versus gastrointestinal patient groups
| Variables | GP (N = 1,107) |
Patients (N = 707) |
GERD patients (N = 98) |
|---|---|---|---|
| Age in years*(mean ± SD) |
46 ± 16 | 48 ± 16 | 58 ± 12 |
| % Male* | 42 % | 39 % | 82 % |
| Race * | |||
| % White | 72 % | 56 % | 43 % |
| % Black | 12 % | 18 % | 31 % |
| % Latino | 11 % | 15 % | 21 % |
| Education * | |||
| % Less than high school |
4.5 % | 2.6 % | 3 % |
| % High school + | 60.5 % | 43 % | 65 % |
| % Advanced degree | 9.6 % | 20 % | 12 % |
| Marital status * | |||
| % Married | 46 % | 45 % | 30 % |
| % Divorced/separated | 20 % | 27 % | 46 % |
| Employment * | |||
| % Employed | 66 % | 54 % | 31 % |
| % Not working | 11.5 % | 9.5 % | 13 % |
| % Retired | 14 % | 17 % | 23 % |
| % Disabled | 8 % | 19 % | 33 % |
p < 0.05 for difference between patient and population sample
Prevalence and Severity of Heartburn and Regurgitation
There was no difference between the prevalence of heartburn in the past 7 days in the patient versus GP samples (59 vs. 59 %). However, regurgitation was more prevalent in patients than the GP in a bivariate analysis (46 vs. 39 %; p = 0.004).
Among those reporting any heartburn or regurgitation in the past week, we found no difference in PROMIS GERD scores between the symptom-reporting GP (N = 759) versus GI patients (N = 410) (mean 54.6 ± 8.6 and 54.1 ± 7.9, respectively). Among the subset of GI patients specifically seeking care for GERD (N = 98), severity was higher in the GERD versus non-GERD subjects (mean 58.6 ± 8.2 and 54.1 ± 7.9, respectively).
Correlates of GERD Symptom Severity
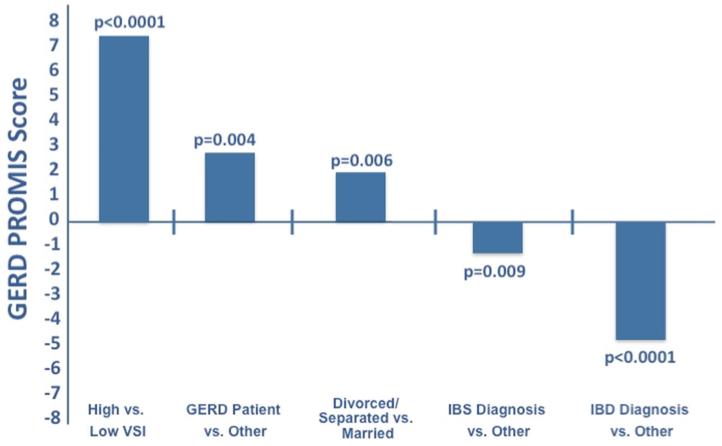
We performed multivariable regression adjusting for demographics, patient status, GI condition (among patients), and VSI scores. Figure 2 illustrates the adjusted analysis identifying correlates of GERD symptom severity. Using the median split of VSI scores, those with high visceral anxiety had higher adjusted GERD severity compared with those with low visceral anxiety (0.72 SD above mean, p < 0.001). Respondents who were divorced or separated also had higher severity compared with those who were married (0.18 SD above mean, p ≤ 0.006). Conversely, patients diagnosed with IBS or IBD who also reported GERD symptoms had a significantly lower GERD severity scores (0.25 SD below mean, and 0.53 SD below mean, respectively (p < 0.009 and p < 0.001)). Patients specifically seeking care for GERD had 0.25 SD higher severity than others (p = 0.004). All variables being equal, high visceral anxiety had a larger effect on symptom severity than being a GERD patient alone (z score scale 0.42 and 0.08, respectively).
Fig. 2.
Independent predictors of GERD symptom severity. This figure shows differences in NIH PROMIS GERD scores between those with versus without the variable of interest, after controlling for other factors. In this figure, the X-axis represents the mean score. Every 10 units on the PROMIS scale equal one standard deviation from the mean
Discussion
We found GERD symptoms were highly prevalent in the GP with over fifty percent reporting heartburn and one-third reporting regurgitation within the last week. Little is known, however, about the differences between GERD reporting in the GP versus GI care-seeking patients. We found that heartburn was equally prevalent in the two groups, while regurgitation was more common in the patient population. As expected, patients seeking care specifically for GERD had higher overall GERD symptom severity. However, visceral anxiety had the greatest impact on symptom severity. This suggests that factors other than symptoms, including GI-related cognitions or emotions, may drive care seeking for some patients with GERD symptoms.
Although psychosocial distress is often described in the context of FGIDs [23], such as IBS, data reveal that many GERD patients also experience concurrent psychological symptoms. For example, Baker and colleagues administered psychological assessments to GERD patients and compared them with age-matched controls. While most GERD patients had similar levels of psychological distress as controls, a subset had higher depression, somatization, and symptom-related distress [24]. Although research in FGIDs demonstrates that psychological distress is related to higher resource utilization and care seeking, this has not been studied in GERD.
In this study, we focused on visceral anxiety [19] as a potential factor associated with care seeking in GERD. Originally developed for use in FGIDs, visceral anxiety refers to a form of anxiety that contributes to distress related to GI symptoms in some patients [19, 20]. Visceral anxiety is marked by exaggerated emotional and behavioral responses arising from fear and concerns about GI sensations and their contexts [19, 20]. Symptom-related contexts include situations involving food and eating, such as restaurants or parties, or contexts that could trigger symptoms, such as sleep for some patients with GERD. GI symptom-specific anxiety includes hypervigilance, fear, worry, and avoidance of GI contexts.
In collaboration with our colleagues, members of our group previously developed a questionnaire to assess GI-specific anxiety—the Visceral Sensitivity Index (VSI), used in the current study [19, 20].Applying the VSI to diverse subjects with GERD symptoms, we found that those with higher visceral anxiety also had higher adjusted GERD severity versus those with lower visceral anxiety. Of note, the VSI does not contain any GERD-specific items and was originally developed for IBS rather than GERD; thus, correlations between VSI and GERD symptoms were not assured or necessarily expected. Nonetheless, the relationship between VSI and GERD severity was strong and independent (1.5 SD above the mean between high vs. low VSI; p < 0.0001).
Beyond VSI scores, demographic factors were also independently associated with higher GERD severity. Being divorced or separated was associated with higher GERD severity compared with those who are married. We are not aware of previous research identifying familial or social determinants of symptom severity in GERD, although research in FGIDs has shown that social support systems can impact symptom reporting and overall severity [25, 26]. Our study cannot explain the mechanism between marital status and GERD severity, although we conjecture that being divorced or separated is a surrogate for psychological distress, which itself may amplify symptom reporting in GERD [27] as may occur in FGIDs. Coupled with the finding that VSI scores determine GERD symptom reporting, these findings remind us to consider the full biopsychosocial context of an illness in lieu of focusing principally on symptom reporting [26]. Although this model is well established in IBS and other FGIDs, it is less established in GERD.
We also found that having concurrent IBS or IBD was a negative predictor of GERD severity among subjects reporting heartburn and regurgitation. Our study is not designed to unpack this relationship, but it may be possible that IBS and IBD patients are focused more on abdominal symptoms than GERD symptoms. In relation to chronic abdominal pain, bloating, diarrhea, or constipation, GERD symptoms might be relatively less troubling, and therefore rated at a lower severity in patients with active IBS or IBD. However, this remains untested and points to the importance of understanding the hierarchy of symptoms in patients with comorbid conditions.
Our results yielded a higher prevalence of heartburn and regurgitation symptoms in the GP than previously estimated for Western populations. It is difficult to compare the prevalence of GERD between studies as a consistent definition of the disease is lacking [28]. We specifically evaluated symptom prevalence rather than formal diagnoses of GERD, although we included a subgroup specifically seeking care for GERD. The studies that employed a similar definition of at least weekly heartburn or regurgitation found prevalence rates between 10 and 30 % [11, 29, 30], but these data were compiled between 10 and 20 years ago. El-Serag et al. [3] used these same studies in a regression analysis that revealed an increasing trend in the prevalence of GERD between 1982 and 2005 (p < 0.001) that appears to be accelerating; our results may simply reflect this epidemiological trend. Additional indirect evidence of risking GERD prevalence is reflected in the increase in complications of reflux disease over time [31, 32]. Higher body mass indices [33] and additional comorbidities may also be contributing factors although not specifically evaluated in our study.
The findings of our study must be interpreted in the context of the design. First, it is possible that bona fide GI patients exist among our randomly selected subjects from the GP. However, we would expect their prevalence to be in proportion to the GP, albeit the subset of the GP that participates in health-related surveys. In contrast, our patient group consisted only of GI care-seeking patients, with a subset specifically seeking care for GERD. Therefore, rare GI patients in the GP sample are unlikely to substantially alter the comparative results. Second, GERD patients presenting to subspecialty clinics may have higher visceral anxiety and symptom severity than patients in community-based clinics. Third, we were unable to capture specific details regarding therapeutic regimens, i.e., proton pump inhibitor (PPI) versus H2 blocker, although it is unclear whether adjusting for PPI exposure would modify the relationship between visceral anxiety and symptom severity. Moreover, many subjects presenting to our specialty clinics are already receiving PPI therapy from primary care. In fact, many patients have already failed PPI therapy, or only achieved partial response, suggesting the possibility of underlying functional heartburn in some patients; however, we do not have those data. Fourth, we did not investigate why patients in this sample reported more regurgitation than the general population. One explanation is that patients in this study may have experienced significant acid exposure given their likely poor response to PPI therapy in primary care. Fifth, we did not collect pH-metry or impedance data in our patient sample, and therefore cannot comment on mechanistic difference among patients or subgroups. Finally, as a cross-sectional study, we could only discuss associations between symptom severity, visceral anxiety, and demographic data rather than assert causation.
In conclusion, GERD symptoms are highly prevalent in the GP. While GERD symptom severity is highest among patients specifically seeking care for GERD (in contrast to the GP or other GI patients), visceral anxiety, marital and educational status, and GI comorbidities also play a significant role. The biopsychosocial model can be used in the clinical setting to help frame the GERD illness experience as in FGIDs. Our findings suggest that GERD patients may benefit from visceral anxiety screening to complement traditional medical therapy. Further prospective analysis is needed to confirm these findings in GERD and to test the incremental benefit of a biopsychosocial approach in GERD, similar to its documented benefit in FGIDs.
Acknowledgments
NIH/NIAMS U01 AR057936A, the National Institutes of Health through the NIH Roadmap for Medical Research Grant (AR052177). Puja Khanna was supported by Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant NIAMS 1 T32 AR053463 and ACR Research and Education Foundation Clinical Investigator Fellowship Award 2009_11. Dinesh Khanna was also supported by NIAMS K24 AR063120. Ron Hays was also supported by NIH/NIA Grants P30-AG028748 and P30-AG021684, and NCMHD Grant 2P20MD000182. Lin Chang was also supported by NIDDK P50 DK64539.
Abbreviations
- GI
Gastrointestinal
- PRO
Patient-reported outcome
- HRQOL
Health-related quality of life
- PROMIS
Patient-Reported Outcome Measurement Information System
Footnotes
Disclaimer The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veteran Affairs.
Conflict of interest Brennan Spiegel has received grant support from Ironwood, Amgen, and Shire Pharmaceuticals, and served as a consultant to Ironwood, Forest, and Takeda North America. Dinesh Khanna has served as consultant and/or received grant support from Actelion, Astra-Zeneca, Bayer, BMS, DIGNA, Genentech, Gilead, InterMune, Merck, Takeda, Savient, and United Therapeutics. Ron D. Hays has served as a consultant to Amgen, Allergan, Pfizer, and the Critical Path Institute. Gil Melmed has served as a consultant for Abbvie and Jannsen, is on the speaker’s bureau for Prometheus and Abbott, and has received research support from Pfizer. Lin Chang has served as a consultant to Ironwood, Forest, Prometheus, Salix, Takeda North America and has received grant support from Shire and Ironwood.
Contributor Information
Erica Cohen, Department of Gastroenterology, VA Greater Los Angeles Healthcare System, 11301 Wilshire Blvd, Bldg 115, Room 215, Los Angeles, CA 90073, USA; Department of Gastroenterology, Cedars-Sinai Medical Center, West Hollywood, CA, USA.
Roger Bolus, Division of Digestive Diseases, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; UCLA/VA Center for Outcomes Research and Education, Los Angeles, CA, USA.
Dinesh Khanna, Division of Rheumatology, University of Michigan, Ann Arbor, MI, USA.
Ron D. Hays, Division of General Internal Medicine and Health Services Research, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; Department of Health Services, UCLA School of Public Health, Los Angeles, CA, USA
Lin Chang, Center for Neurobiology of Stress, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; Division of Digestive Diseases, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Gil Y. Melmed, Department of Gastroenterology, Cedars-Sinai Medical Center, West Hollywood, CA, USA
Puja Khanna, Division of Rheumatology, University of Michigan, Ann Arbor, MI, USA.
Brennan Spiegel, Department of Gastroenterology, VA Greater Los Angeles Healthcare System, 11301 Wilshire Blvd, Bldg 115, Room 215, Los Angeles, CA 90073, USA; Division of Digestive Diseases, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; UCLA/VA Center for Outcomes Research and Education, Los Angeles, CA, USA.
References
- 1.Tack J, Becher A, Mulligan C, et al. Systematic review: the burden of disruptive gastro-oesophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther. 2012;35:1257–1266. doi: 10.1111/j.1365-2036.2012.05086.x. [DOI] [PubMed] [Google Scholar]
- 2.Becher A, El-Serag H. Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:618–627. doi: 10.1111/j.1365-2036.2011.04774.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 4.A Gallup survey on heartburn across America. The Gallup Organization, Inc.; Princeton, NJ: 1988. [Google Scholar]
- 5.Agreus L, Svardsudd K, Talley NJ, et al. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. Am J Gastroenterol. 2001;96:2905–2914. doi: 10.1111/j.1572-0241.2001.04680.x. [DOI] [PubMed] [Google Scholar]
- 6.Fedorak RN, Veldhuyzen van Zanten S, Bridges R. Canadian Digestive Health Foundation public impact series: gastroesophageal reflux disease in Canada: incidence, prevalence, and direct and indirect economic impact. Can J Gastroenterol. 2010;24:431–434. doi: 10.1155/2010/296584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank L, Kleinman L, Ganoczy D, et al. Upper gastrointestinal symptoms in North America: prevalence and relationship to healthcare utilization and quality of life. Dig Dis Sci. 2000;45:809–818. doi: 10.1023/a:1005468332122. [DOI] [PubMed] [Google Scholar]
- 8.Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179e1-3–1187e1-3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543–552. doi: 10.1016/s1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- 11.Locke GR, 3rd, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag H, Hill C, Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther. 2009;29:470–480. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 13.Bretagne JF, Honnorat C, Richard-Molard B, et al. Comparative study of characteristics and disease management between subjects with frequent and occasional gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther. 2006;23:607–616. doi: 10.1111/j.1365-2036.2006.02811.x. [DOI] [PubMed] [Google Scholar]
- 14.Solmaz M, Kavuk I, Sayar K. Psychological factors in the irritable bowel syndrome. Eur J Med Res. 2003;8:549–556. [PubMed] [Google Scholar]
- 15.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:158–167. doi: 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- 16.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel BM. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137–148. doi: 10.5056/jnm.2013.19.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel B, Hays RD, Bolus R, et al. Development of the NIH Patient Reported Outcomes Measurement Information System (PROMIS®) Gastrointestinal Symptom Scales. Am J Gastroenterol. 2014 doi: 10.1038/ajg.2014.237. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 20.Labus JS, Mayer EA, Chang L, et al. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 21.Revicki DA, Wood M, Wiklund I, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75–83. doi: 10.1023/a:1008841022998. [DOI] [PubMed] [Google Scholar]
- 22.DeWalt DA, Rothrock N, Yount S, et al. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45:S12–S21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel B, Khanna D, Bolus R, et al. Understanding gastrointestinal distress: a framework for clinical practice. Am J Gas-troenterol. 2011;106:380–385. doi: 10.1038/ajg.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker LH, Lieberman D, Oehlke M. Psychological distress in patients with gastroesophageal reflux disease. Am J Gastroenterol. 1995;90:1797–1803. [PubMed] [Google Scholar]
- 25.Wong RK, Drossman DA, Weinland SR, et al. Partner burden in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:151–155. doi: 10.1016/j.cgh.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel BM. Burden of illness in irritable bowel syndrome: looking beyond the patient. Clin Gastroenterol Hepatol. 2013;11:156–157. doi: 10.1016/j.cgh.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Naliboff BD, Mayer M, Fass R, et al. The effect of life stress on symptoms of heartburn. Psychosom Med. 2004;66:426–434. doi: 10.1097/01.psy.0000124756.37520.84. [DOI] [PubMed] [Google Scholar]
- 28.Delaney B. Prevalence and epidemiology of gastro-esophageal reflux disease. Aliment Pharmacol Ther. 2004;20:2–4. doi: 10.1111/j.1365-2036.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- 29.Talley NJ, Zinsmeister AR, Schleck CD, et al. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 30.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–1699. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 31.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 32.van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062–1066. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locke GR, 3rd, Talley NJ, Fett SL, et al. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–649. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]