Abstract
We describe a framework to help clinicians think about health-related quality of life in their gastrointestinal (GI) patients. We introduce “GI distress” as a clinically relevant concept and explain how it may result from physical symptoms, cognitions, and emotions. The GI distress framework suggests that providers should divide GI physical symptoms into four categories: pain, gas/bloat, altered defecation, and foregut symptoms. We describe how these physical symptoms can be amplified by maladaptive cognitions, including external locus of control, catastrophizing, and anticipation anxiety. We suggest determining the level of embarrassment from GI symptoms and asking about stigmatization. GI patients may also harbor emotional distress from their illness and may exhibit visceral anxiety marked by hypervigilance, fear, and avoidance of GI sensations. Look for signs of devitalization, indicated by inappropriate fatigue. When appropriate, screen for suicidal ideations. Finally, we provide a list of high-yield questions to screen for these maladaptive cognitions and emotions, and explain how the GI distress framework can be used in clinical practice.
Overview
Gastrointestinal (GI) illnesses can impair health-related quality of life (HRQOL) and lead to physical, mental, and social distress. This is obvious to anyone who suffers from chronic GI symptoms, or to any provider who cares for patients with digestive disorders. The purpose of this article is to introduce a framework to help clinicians think about HRQOL in their GI patients. It introduces “GI distress” as a concept with relevance in everyday clinical practice and describes how distress may result from a combination of GI physical symptoms, GI cognitions, and GI emotions. We describe a framework for thinking about GI distress and provide suggestions for how to incorporate the framework into practice.
Framework of GI distress
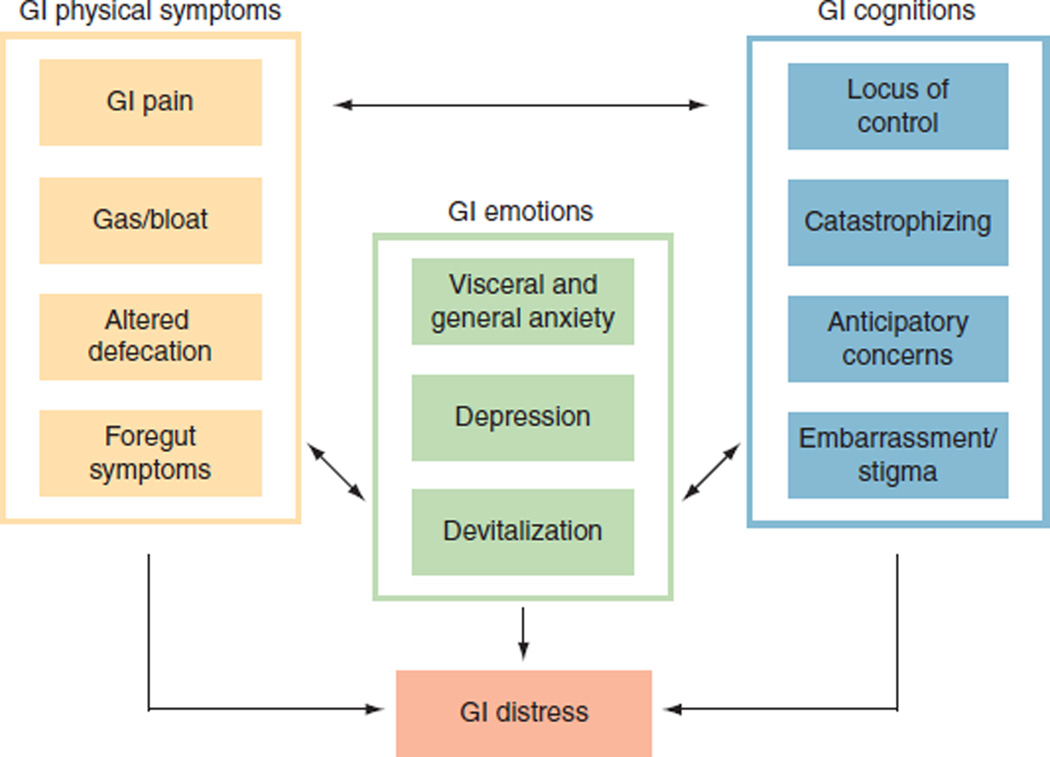
HRQOL encompasses three areas: physical health, psychological health, and social health (1,2). Traditional HRQOL questionnaires can summarize health in a single number, but these scores may not predict whether a patient will seek care. Patients typically seek care when they have reached a turning point of physical, emotional, or social distress. The concept of distress is appealing because it has a behavioral correlate (i.e., health-care seeking) and is not fixed on a scale (i.e., patients determine their own distress threshold). Figure 1 presents a suggested framework of GI distress with three main categories: GI physical symptoms, GI cognitions, and GI emotions. Throughout the text we provide guidance for clinical practice based on this evolving framework.
Figure 1.
Proposed framework of gastrointestinal (GI) distress. GI distress results from the combination of GI physical symptom severity, presence of maladaptive GI cognitions, and resulting GI emotions. Refer to the text for an explanation of each concept depicted in the figure.
GI physical symptoms
GI patients typically seek care because of physical symptoms, and, as GI providers, we are trained to interpret these symptoms to guide management. Although the variety of GI disorders is expansive, the alimentary tract is surprisingly efficient in its symptom expression. Figure 1 suggests that symptoms may fall into one of four basic groups: GI pain, gas/bloat, altered defecation, and foregut symptoms.
GI pain
Using irritable bowel syndrome (IBS) as a model, we have found that abdominal pain is multifaceted, and that some pain dimensions drive illness severity more than others (3). Specifically, we found that to fully understand the distress of abdominal pain, clinicians should measure various dimensions of pain, including severity (using the validated 0 (no pain) to 10 (worst imaginable pain) rating scale (refs. 4,5)), frequency, constancy (“Do you have pain all the time?” (ref. 3)), and predictability (“How well can you tell in advance when you will have pain?” (ref. 3)). This approach is consistent with guidance in somatic pain disorders that emphasizes the multidimensionality of pain (6).
Gas/bloat
We have found that GI patients often separate out bloating and gassiness as a unique symptom complex independent of other GI sensations. In patient cognitive interviews, we found that patients categorize bloating into two major categories: how bloating looks, and how bloating feels. Patients describe the look of bloating as “swollen,” “full of air,” or “distended,” and the feeling of bloating as tightness, pressure, gassiness, or heaviness. Although some patients describe bloating in terms of both look and feel, many select either one or the other category; it is useful to determine what patients mean by “bloating” because the vocabulary differs among individuals.
Altered defecation
Altered defecation includes symptoms of stool frequency and form. Diarrhea symptoms include frequent bowel movements, loose stools, and bowel urgency. We have found that patients consider bowel urgency to be multifaceted, and that clinicians should specifically ask about controllability and predictability of urgency (7). Constipation symptoms include infrequent bowel movements, hard stools, straining, incomplete evacuation, painful defecation, need for manual maneuvers, and perception of anorectal obstruction. Finally, altered defecation includes bowel incontinence and stool leakage. Leakage is often described as separate from incontinence; it may be useful to distinguish between these related concepts.
Foregut symptoms
There are at least four categories of specific foregut symptoms: difficulty swallowing, heartburn/reflux, nausea/vomiting, and dyspepsia. These categories include individual symptoms. For example, the dyspepsia category includes early satiety, postprandial fullness, and epigastric burning, among others, and “difficulty swallowing” encompasses dysphagia, odynophagia, and regurgitation. It may be useful to think about foregut symptoms using these clinically relevant categories.
Summary of clinical recommendations for understanding GI physical symptom distress
In order to understand the impact of GI physical symptoms on GI distress, consider grouping GI physical symptoms into at least four groups: pain, gas/dividing, altered defecation, and foregut. Ask about pain severity using a 0- to 10-point scale (4,5), and follow up with questions about frequency, constancy, and predictability of pain. Gas/bloat is typically a separate symptom complex; find out what patients mean by “bloating”—some refer to how it looks, others to how it feels. Patients consider bowel urgency to be multifaceted; ask about immediacy, controllability, and predictability to learn more. Foregut symptoms also come in clusters; it may be helpful to categorize foregut symptoms into one of four general groupings.
GI cognitions
The four GI physical symptom groups are ultimately processed and interpreted in the cerebral cortex (8,9). Our group and others have found neuroanatomical correlates of visceral symptom processing and have measured related cognitions of patients with GI disorders (10–18). In contrast to emotions, which capture how patients feel, cognitions describe what patients think or believe. Our framework includes four GI cognitions that have been culled from the literature (as cited throughout the text) and from our experience with patient cognitive interviews and focus groups: locus of control, catastrophizing, anticipatory concerns, and embarrassment/stigma. Table 1 provides sample questions to help screen for these cognitions in the clinical setting. The GI clinician should be on the lookout for outliers in these cognitions.
Table 1.
Sample questions to ask patients to screen for GI emotions and cognitions
| Sample screening questions (refs.) | |
|---|---|
| GI emotions | |
| Visceral anxiety | Are you constantly aware of the feelings you have in your belly? (33,34) Do you get anxious when you go to a new restaurant? (33,34) Do you always look for a bathroom when you first enter a place you haven’t been before? (33,34) Do you begin to worry and feel anxious as soon as you feel GI discomfort? (33,34) |
| Depression | Can you still enjoy the things you used to enjoy? (40,41) Can you laugh and see the funny side of things? (40) Do you feel as if you are slowed down? (40) Do you have difficulty thinking or concentrating? (41) |
| Devitalization | Do you sometimes feel that your body is like a battery that is losing its power? (42) Do you feel worn out? (43) Do you wake up already feeling exhausted? (42) Do you feel weak all over? (42) |
| GI cognitions | |
| Locus of control | How much control do you think you have over your GI symptoms? (32) Do you believe that, no matter what you do, you are going to get sick? (44) Do you think that your GI symptoms are meant to be? (44) Do you think that most things that affect your health happen to you by accident? (44) |
| Catastrophizing | Do you think there is something seriously wrong with your body? (23,45) Do you feel like you can’t keep the GI symptoms out of your mind? (22) Do you think that your GI symptoms will never end? (22) Do you feel like your GI symptoms are overwhelming? |
| Anticipatory concerns | Do you think that your GI symptoms will get worse? (22) Do you think that your GI symptoms could flare up at any time without notice? (27) Do you think that your GI symptoms might affect your life expectancy? (46) Do you feel like you need to plan your day out in order to pursue normal activities because of your GI symptoms? |
| Embarrassment/stigma | Do you keep your GI symptoms hidden from people because they would treat you differently if they knew? (47) Do you feel like you can’t be as open about your GI symptoms as you’d like to be? (47) Do you think that people would pass you over or limit your opportunities if they knew about your GI problems? (47) Do you think that people do not take your GI symptoms seriously? (47) |
These items are culled and adapted from existing questionnaires and have been selected as potentially useful questions to bear in mind during clinical encounters. For more information about the source questionnaires and their validation, see the references cited in the table.
Locus of control
“Locus of control” describes the extent to which individuals believe they can control events that affect them (19). Some individuals have an internal locus of control, where they believe events are a consequence of their own behavior; others have an external locus of control, where they believe events are driven by others, fate, or chance. Locus of control has been studied in various GI disorders, including Crohn’s disease, constipation, and IBS. Hobbis and colleagues found that external locus may propagate illness and contribute to health-care-seeking behavior in functional GI disorders (19). Fletcher and colleagues found that many patients with inflammatory bowel disease (IBD) control their symptoms by centralizing their locus of control, gaining mastery of their surroundings through relaxation techniques, and gaining knowledge about their disease (20). More recently, Lackner and colleagues found that IBS patients with internal locus are more likely to rapidly respond to cognitive-behavioral therapy than patients with external locus (21).
Catastrophizing
Catastrophizing is a form of maladaptive coping in which patients exaggerate the threat of illness as being worse than it actually is (9,22). Patients who catastrophize may believe their GI symptoms indicate impending demise, malignancy, or that “something is seriously wrong with my body” (23,24). Seres and colleagues found that catastrophizing is especially common in IBS (25). We also found that HRQOL in IBS is strongly related to disease-specific fears—more than to GI symptoms themselves (23). Lackner and Quigley demonstrated that patients with GI pain who worry excessively engage in more catastrophic thinking, have worse symptoms, and report more suffering (26).
Anticipatory concerns
Anticipatory concerns are prevalent in chronic GI disorders, particularly when symptoms wax and wane unpredictably. Drossman and colleagues found that few IBS patients know where, when, or by what a symptom flare is triggered (27). Patients often engage in advanced planning in order to pursue normal activities, such as knowing the locations of bathrooms, planning meals, and reducing participation in daily activities. IBD patients also express anticipatory concerns of impending disease flares (28).
Embarrassment and stigma
Embarrassment and stigma are common consequences of GI symptoms. In particular, diarrhea, incontinence, indigestion, and flatulence are often perceived as socially awkward and embarrassing symptoms (9). This can lead to avoidance of social encounters and, in some instances, social isolation. For example, Taft and colleagues found that 80% of IBD patients perceive stigma because of their illness (29). IBS patients frequently perceive stigma resulting from a lack of understanding by family, friends, and physicians regarding the health impact of IBS and the legitimacy of their emotions (27). We have found similar trends in other disorders marked by GI distress, including scleroderma (30).
It is worth screening for these maladaptive cognitions because they can be identified and addressed through cognitive approaches. Although cognitive therapy is not just within the purview of psychotherapists, referral to mental-health professionals for behavioral therapy can be useful. Less intensive therapy can be deployed by referring motivated patients to cognitive-therapy workbooks (such as the manual developed by Lackner (31)), or referring patients to support groups that explore maladaptive cognitions and provide social support. The ultimate goals are to identify and modify these cognitions. These may be achieved through teaching coping mechanisms and relaxation skills, developing a greater sense of self-efficacy, and allowing patients to recognize their own limitations.
Summary of clinical recommendations for understanding GI cognitive distress
In addition to measuring and treating GI physical symptoms, it is important to screen for maladaptive cognitions that might amplify those symptoms. Find out how much control patients think they have over their illness. Screen for external locus of control by asking, “How much control do you feel you have over your symptoms?” (32). Look for signs of catastrophizing; as a screen, ask, “Do you think your symptoms mean there is something seriously wrong with your body?” (23). Screen for evidence of anticipatory concerns, determine the level of embarrassment, and ask about stigmatization. While these cognitions may be common, they may become overwhelming in some patients when left unopposed and may undercut treatment success in even the most “organic” of diseases.
GI emotions
The framework suggests that GI emotions may amplify GI symptoms and cognitions (9). It encompasses three emotions: visceral anxiety, depression, and devitalization. Table 1 provides sample questions to help screen for these emotions in the clinical setting.
Visceral anxiety
Visceral anxiety is a form of anxiety that contributes to GI symptoms in some patients (33,34). Visceral anxiety is marked by exaggerated emotional and behavioral responses arising from fear of GI sensations and their contexts (33,34). Symptom-related contexts include situations involving food and eating, such as restaurants, parties, and other locations where bathrooms are difficult to reach. GI-specific anxiety includes hypervigilance, fear, worry, and avoidance of GI contexts. On the basis of this theory, members of our group have developed and tested a questionnaire to assess GI-specific anxiety—the Visceral Sensitivity Index (VSI) (33,34). Table 1 includes some questions from the VSI to assist in identifying visceral anxiety in clinical practice.
Generalized anxiety and depression
Generalized anxiety and depression are comorbid in many patients with chronic GI disorders (9,35,36). Although these emotions are usually not etiologic for GI illness, they may amplify the physical symptom experience and contribute to overall GI distress. In fact, chronic abdominal pain syndromes may increase the risk of suicidal behavior (37)—a feature also described in nonvisceral pain syndromes.
Devitalization
Left unopposed, GI physical symptoms may lead to devitalization, or “vital exhaustion,” which is inappropriate fatigue in response to chronic physical or emotional stress (38). Neuroscientists refer to the wear and tear from mounting stress as “allostatic load” (39). With allostatic overload, patients become overwhelmed, develop increased behavioral and physiological reactivity, and reach a state of vital exhaustion marked by fatigue, diminished motivation, sleep deprivation, and overeating. Clinicians can screen for vital exhaustion by measuring the degree to which patients “tire easily,” “feel low in energy,” “feel worn out,” and have a low sexual drive.
Summary of clinical recommendations for understanding GI emotional distress
Patients with GI physical symptoms often harbor emotional distress from their illness. GI patients may exhibit signs of visceral anxiety, a form of anxiety marked by hypervigilance, fear, worry, and avoidance of GI sensations. Look for signs of devitalization, marked by inappropriate fatigue. And, when appropriate, screen for suicidal ideations.
Clinical significance of the framework
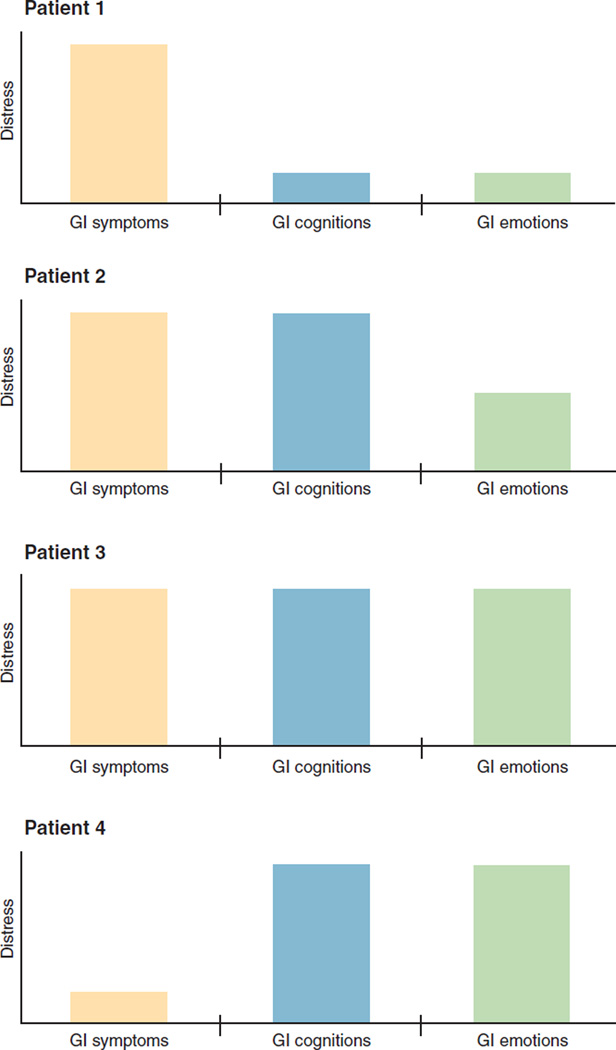
When evaluating a patient with GI physical symptoms, it may be useful to calibrate whether patients are “more gut than brain” or “more brain than gut” in their illness expression (24). Figure 2 demonstrates four sample profiles that vary in the relative impact of GI physical symptoms, GI cognitions, and GI emotions.
Figure 2.
GI distress profiles in four hypothetical patients. The GI distress framework suggests that clinicians should evaluate GI symptoms, screen for maladaptive GI cognitions, and evaluate related emotions. The relative influence of these three factors varies from patient to patient. In Patient 1, symptoms are the driving impact on overall distress—not cognitions or emotions. This patient should receive directed therapies for visceral pathology. Patient 2 harbors severe symptoms but also maladaptive cognitions (e.g., “I have no control over my illness”; “there is something seriously wrong with my body”; “I feel stigmatized”). This patient should receive therapies for visceral pathology but should also have his or her cognitions identified and properly addressed. Patient 3 has a high symptom burden, along with high cognitive and emotional distress; treatment may include both medical and psychological therapy. Patient 4 has a relatively low symptom burden but a high cognitive and emotional burden; treatment should address maladaptive cognitions and emotional distress.
In the first patient depicted in Figure 2, illness expression is driven primarily by the GI symptoms as a consequence of visceral pathology. This patient may harbor comorbid GI cognitions and emotions, but the impact of these factors is lower than that of the physical symptoms themselves. This patient is “more gut than brain” in illness expression and is typically treated with therapies directed at underlying mechanisms of gut pathology. The second patient has severe GI symptoms as well but also has strong GI emotions generated by the symptoms; for example, the patient may feel depressed that recurrent pain and diarrhea have impacted daily functioning. This patient is both “brain and gut” in illness expression; treatment requires GI-directed therapies, but the patient may also benefit from concurrent centrally acting therapies when warranted. The third patient exhibits severe GI symptoms and emotions but also harbors maladaptive cognitions; for example, the patient may feel that the GI symptoms are intrusive and embarrassing and will never get better. This patient requires GI-directed therapies but may also benefit from both centrally acting therapies and cognitive-behavioral approaches. The last patient has GI symptoms that are less severe than those of the others but nonetheless has concurrent emotional distress and maladaptive cognitions. This patient is “more brain than gut” in illness expression; it would be a mistake to focus exclusively on gut-directed therapies without acknowledging and treating the extraintestinal drivers of distress.
The three categories in Figure 1 are not independent but are highly codependent. For example, if a patient has emotional distress generated from GI symptoms, treatment should focus on improving those symptoms; improvement of physical symptoms may alleviate emotional and cognitive distress more effectively than use of psychological therapies alone. We also acknowledge that it is untenable to work through the framework with each patient in a busy practice. The level of detail in the framework may be optimally suited for tertiary-care centers with complex patients. Nonetheless, even primary- and secondary-care physicians can use portions of the framework as needed; simply recognizing the components might help in assessing and managing patients and referring patients to other specialists if indicated. In short, the framework acknowledges that GI distress results from physical symptoms, cognitions, and emotions that are inexorably linked. The framework implies that treatment depends on the relative weight of each domain on overall GI distress and may facilitate a more patient-centric approach to providing care.
ACKNOWLEDGMENTS
The opinions and assertions contained herein are solely the views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs.
CONFLICT OF INTEREST
Financial support: National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant U01 AR057936A and the NIH through NIH Roadmap for Medical Research grant AR052177.
Potential competing interests: Drs Spiegel and Chang have served as advisors to Prometheus Laboratories and Ironwood Pharmaceuticals and have received grant support from Takeda North America, Rose Pharmaceuticals, and Prometheus Laboratories. Dr Chang has also served as a consultant to Salix, Movetis, GlaxoSmithKline, and Ocera. Dr D. Khanna has received grants from the Scleroderma Clinical Trial Consortium to develop a GIspecific instrument in scleroderma.
Footnotes
Guarantor of the article: Brennan M.R. Spiegel, MD, MSHS.
Specific author contributions: Brennan M.R. Spiegel: manuscript preparation, manuscript approval; Dinesh Khanna: manuscript review; Roger Bolus: manuscript preparation, manuscript review; Nikhil Agarwal: manuscript review; Puja Khanna, manuscript review; Lin Chang: manuscript preparation, manuscript review.
References
- 1.Eisen GM, Locke GR, 3rd, Provenzale D. Health-related quality of life: a primer for gastroenterologists. Am J Gastroenterol. 1999;94:2017–2021. doi: 10.1111/j.1572-0241.1999.01272.x. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel BM, Bolus R, Harris LA, et al. Characterizing abdominal pain in IBS: guidance for study inclusion criteria, outcome measurement and clinical practice. Aliment Pharmacol Ther. 2010;32:1192–1202. doi: 10.1111/j.1365-2036.2010.04443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale: results from the proof cohort. Aliment Pharmacol Ther. 2009;30:1159–1170. doi: 10.1111/j.1365-2036.2009.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Revicki DA, Chen WH, Harnam N, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–169. doi: 10.1016/j.pain.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel B, Harris L, Lucak S, et al. Digestive Disease Week. New Orleans, LA: 2010. May 1–5, Characterizing “bowel urgency”: guidance for outcome assessment in D-IBS. [Google Scholar]
- 8.Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology. 1997;112:2120–2137. doi: 10.1053/gast.1997.v112.agast972120. [DOI] [PubMed] [Google Scholar]
- 9.Creed F, Levy R, Bradley L, et al. Psychosocial aspects of functional gastrointestinal disorders. In: Drossman DA, Rome III, editors. The Functional Gastrointestinal Disorders. 3rd edn. McLean, VA: Degnon Associates; 2006. pp. 295–368. [Google Scholar]
- 10.Chang L, Berman S, Mayer EA, et al. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol. 2003;98:1354–1361. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- 11.Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Berman SM, Naliboff BD, Chang L, et al. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am J Gastroenterol. 2002;97:2791–2797. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- 14.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Jarcho JM, Chang L, Berman M, et al. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: a pilot study. Aliment Pharmacol Ther. 2008;28:344–352. doi: 10.1111/j.1365-2036.2008.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seminowicz DA, Labus JS, Bueller JA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48.e2–57.e2. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blankstein U, Chen J, Diamant NE, et al. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Hobbis IC, Turpin G, Read NW. Abnormal illness behaviour and locus of control in patients with functional bowel disorders. Br J Health Psychol. 2003;8:393–408. doi: 10.1348/135910703770238266. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher PC, Schneider MA, Van Ravenswaay V, et al. I am doing the best that I can!: living with inflammatory bowel disease and/or irritable bowel syndrome (part II) Clin Nurse Spec. 2008;22:278–285. doi: 10.1097/01.NUR.0000325382.99717.ac. [DOI] [PubMed] [Google Scholar]
- 21.Lackner JM, Gudleski GD, Keefer L, et al. Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2010;8:426–432. doi: 10.1016/j.cgh.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel BM, Gralnek IM, Bolus R, et al. Clinical determinants of healthrelated quality of life in patients with irritable bowel syndrome. Arch Intern Med. 2004;164:1773–1780. doi: 10.1001/archinte.164.16.1773. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel B, Strickland A, Naliboff BD, et al. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103:2536–2543. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seres G, Kovacs Z, Kovacs A, et al. Different associations of health related quality of life with pain, psychological distress and coping strategies in patients with irritable bowel syndrome and inflammatory bowel disorder. J Clin Psychol Med Settings. 2008;15:287–295. doi: 10.1007/s10880-008-9132-9. [DOI] [PubMed] [Google Scholar]
- 26.Lackner JM, Quigley BM. Pain catastrophizing mediates the relationship between worry and pain suffering in patients with irritable bowel syndrome. Behav Res Ther. 2005;43:943–957. doi: 10.1016/j.brat.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Drossman DA, Chang L, Schneck S, et al. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig Dis Sci. 2009;54:1532–1541. doi: 10.1007/s10620-009-0792-6. [DOI] [PubMed] [Google Scholar]
- 28.Irvine EJ. Review article: Patients’ fears and unmet needs in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):54–59. doi: 10.1111/j.1365-2036.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- 29.Taft TH, Keefer L, Leonhard C, et al. Impact of perceived stigma on inflammatory bowel disease patient outcomes. Inflamm Bowel Dis. 2009;15:1224–1232. doi: 10.1002/ibd.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna D, Hays RD, Park GS, et al. Development of a preliminary scleroderma gastrointestinal tract 1.0 quality of life instrument. Arthritis Rheum. 2007;57:1280–1286. doi: 10.1002/art.22987. [DOI] [PubMed] [Google Scholar]
- 31.Lackner JM. Controlling IBS the Drug-Free Way: A 10-Step Plan for Symptom Relief. New York: Stewart, Tabori & Chang; 2007. [Google Scholar]
- 32.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 33.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 34.Labus JS, Mayer EA, Chang L, et al. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 36.Bodukam V, Hays RD, Maranian P, et al. Association of gastrointestinal involvement and depressive symptoms in patients with systemic sclerosis. Rheumatology (Oxford) 2010 doi: 10.1093/rheumatology/keq296. e-pub ahead of print 30 September 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel B, Schoenfeld P, Naliboff B. Systematic review: the prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- 38.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 39.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- 40.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Health Disorders. 4th edn. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- 42.Kopp MS, Falger PR, Appels A, et al. Depressive symptomatology and vital exhaustion are differentially related to behavioral risk factors for coronary artery disease. Psychosom Med. 1998;60:752–758. doi: 10.1097/00006842-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 44.Wallston BS, Wallston KA, Kaplan GD, et al. Development and validation of the health locus of control (HLC) scale. J Consult Clin Psychol. 1976;44:580–585. doi: 10.1037//0022-006x.44.4.580. [DOI] [PubMed] [Google Scholar]
- 45.Derogatis L. Administration, Scoring and Procedures Manual–II. Minneapolis, MN: National Computer Systems; 1983. SCL-90RR. [Google Scholar]
- 46.Spiegel BM, Bolus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology. 2007;46:113–121. doi: 10.1002/hep.21692. [DOI] [PubMed] [Google Scholar]
- 47.Jones MP, Keefer L, Bratten J, et al. Development and initial validation of a measure of perceived stigma in irritable bowel syndrome. Psychol Health Med. 2009;14:367–374. doi: 10.1080/13548500902865956. [DOI] [PubMed] [Google Scholar]