Abstract
This is a qualitative–quantitative study based on hospital records of female patients of reproductive age, presenting sexual dysfunction, and treated with 250 mg Tribulus terrestris extract (1 tablet thrice daily for 90 days). Safety monitoring included vital signs, physical examination, laboratory tests, and occurrence of adverse events. Efficacy analysis included results of the Female Sexual Function Index (FSFI), dehydroepiandrosterone (DHEA) levels together with total and free testosterone, and the patient and physician assessments. There was a statistically significant improvement in total FSFI scores (P < 0.0001) post-treatment, with improvement among 106 (88.33%) subjects. There was a statistically significant (P < 0.0001) increase in the level of DHEA, while the levels of both serum testosterone (P = 0.284) and free testosterone decreased (P < 0.0001). Most adverse events recorded were related to the gastrointestinal tract. Physical examination showed no significant changes post-treatment. Based on the results, it is concluded that the T. terrestris extract is safe and effective in the treatment of female sexual dysfunction.
Keywords: Tribulus terrestris, female sexual dysfunction, female sexual function index
Introduction
Sexual dysfunction in women is characterized by persistent and recurrent problems in sexual response or desire, which cause affliction and affect their relationship with their partner.1,2 Sexual dysfunctions may occur at any age and affect roughly 40% of women at some point in their lifetime, with 12% of women reporting afflictive sexual problems.3
There are different types of sexual dysfunctions, characterized as (1) sexual interest/arousal disorder; (2) orgasmic disorder; and (3) genitopelvic pain/penetration disorder.4 It is important to explain to the patient that transient sexual problems are common; however, if the problem persists over months or causes affliction to the patient or their partner, then the cause of the dysfunction should be investigated.5,6
There are several risk factors that may contribute to the emergence of female sexual dysfunction. These include the patient’s well-being; important events in romantic relationships; partner’s sexual problems; gynecological factors including childbirth, hysterectomy, vaginal pain, or pelvic pain; urinary tract alterations; and drug side effects (use of beta blockers, anti-depressant, or anti-psychotic drugs).7–11
Several therapeutic alternatives are used in the treatment of female sexual dysfunction; however, it is important to identify any possible contributing physical and emotional factors before beginning treatment.9,11,12 Therapeutic approaches include stress management and management of any relationship problems, treatment of vaginal dryness, dyspareunia, and investigation of possible drug side effects.10,11 The use of testosterone in the form of gels, creams, ointments, or in oral form is not recommended for pre-menopausal women, and carry the risk of side effects in addition to containing very high doses of testosterone.13–16 The use of medications for erectile dysfunction has proven to be largely ineffective.9,15,17 Dehydroepiandrosterone (DHEA) has been shown to be effective in improving sexual interest and satisfaction among women with adrenal insufficiency; however, the benefit of supplementation in women with normal adrenal function has not been confirmed.18–20
In this study, we evaluated the use of Tribulus terrestris extract to treat female sexual dysfunction. T. terrestris is a plant belonging to the Zygophyllacea family, with many common names including caltrop, cat’s head, and bindii. The leaves and roots are used for medicinal purposes in traditional medicine in India and China and were also in use in ancient Greece.21
Objectives
The primary study objective was to evaluate the efficacy of T. terrestris extract in female sexual dysfunction. The secondary objective was to evaluate the safety of the extract.
Material and Methods
This was a qualitative–quantitative study performed at Hospital das Clínicas de Teresópolis (Teresópolis—RJ, Brazil). Subjects were included based on inclusion and exclusion criteria, followed by hospital records verification. Inclusion criteria for female patients are that they should be of reproductive age (over 18 years old), with a clinical presentation of sexual dysfunction, who were treated with tablets containing 250 mg T. terrestris extract (brand name Androsten®) at the dose of 1 tablet three times per day for a period of 90 days. The study protocol was evaluated and approved by the Fundação Educacional Serra dos Órgãos Ethical Committee (approval no. 172.071). The research complied with the principles of the Declaration of Helsinki. All subjects gave their written, informed consent to participate in the research.
Exclusion criteria were pregnant or breastfeeding women, patients presenting hypersensitivity to any component of the study medication, and any other diseases or conditions that, in the opinion of the investigator, exclude the patient from the study. All study data were recorded in the clinical research form, in which subjects were identified using 3-digit sequential numbers.
In addition to physical examination results (vital signs, weight, body mass index [BMI]), safety analysis included the following laboratory test results: complete blood count, amylase, glucose and fasting glucose, serum prolactin, follicle-stimulating hormone, leutenizing hormone, thyroid-stimulating hormone, serum potassium, blood urea nitrogen, serum creatinine, total and fractionated billirrubins, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase. Occurrence and severity of adverse events were also included in the safety analysis. The primary safety and tolerability measures included any changes in vital signs and physical examination in relation to pretreatment, any changes in clinical laboratory examinations in relation to pretreatment, and the occurrence of adverse events after the first dose of study medication. Any laboratory examinations out of reference range were recorded as adverse events. The secondary safety measure was the evaluation of overall tolerability of the study medication performed post-treatment by the study physician, using the same classifications of “Very Good”, “Good”, “Fair”, or “Poor” as were used for the overall efficacy assessment.
The primary efficacy analysis included results of the Female Sexual Function Index (FSFI), a validated self-report measure of female sexual function. This is a 19-item questionnaire that is subdivided into six domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. The scoring system followed the instructions given by the authors who developed and validated the questionnaire. The maximum possible score is 36 points and the minimum possible score is 2 points.22
Secondary efficacy measures included the Patient and Physician Assessments, in which both the subject and the physician rated the patient’s overall condition on a scale of 1–10 points, with “1” corresponding to the worst assessment and “10” the best. The study physician also evaluated the overall efficacy and safety of the study medication as “Very Good”, “Good”, “Fair”, or “Poor”. DHEA levels together with total and free testosterone were also included in the efficacy analysis (reference ranges: DHEA: 15–170 ng/mL; serum testosterone: 3–63 ng/dL; free testosterone: 2–45 pmol/mL).
At the end of the treatment period, subjects who completed the treatment cycle were asked to rate their willingness to continue treatment on a scale of 1 (very unwilling) to 10 (very willing).
The clinical research form was filled, coded, and the data were analyzed using GraphPad Prism version 5.1 software. Frequency tables were generated and central tendency measures were calculated (mean, median, mode). As appropriate, we used the Student’s t-test or the repeated-measures analysis of variance (ANOVA) for continuous variables and Fisher’s test or the χ2 test for categorical variables (α = 0.05). Results were compared between each assessment and throughout the study.
Results
A total of 144 subjects were included in the initial analysis. Mean patient age was 41.01 years (±7.07). Sixty subjects (41.67%) were married, while 55 (38.19%) were divorced, 22 (15.28%) were single, and 7 (4.86%) were widows. Ethnicity (self-reported) was as follows: Asian (n = 5), Caucasian (n = 60), Black (n = 26), and mulatto (n = 53). The mean height of the total subjects was 161 cm (±5.97).
The relevant medical history including use of contraceptives, reproductive, and menstrual information together with data on depression is summarized in Table 1.
Table 1.
Medical history.
| HISTORY | YES | NO |
|---|---|---|
| Previous use of contraceptives | 126 | 18 |
| Previous pregnancy | 74 | 70 |
| History of miscarriage or abortion | 26 | 118 |
| Intermenstrual bleeding | 25 | 119 |
| Amenorrhea | 9 | 135 |
| Dysmenorrhea | 81 | 63 |
| History of depression | 42 | 102 |
Note: Data are n.
Mean DHEA level at pretreatment was 57.83 ng/mL (±30.11), while there was a statistically significant (P < 0.0001) increase to 67.18 ng/mL post-treatment. Mean serum testosterone levels were 24.21 ng/dL (±13.17) at pretreatment, while at post-treatment, the levels decreased to a mean of 22.65 ng/dL (±11.58), although this change was not statistically significant (P = 0.284). There was a statistically significant reduction in mean free testosterone post-treatment, 7.74 pmol/L (±3.906), in relation to pretreatment values of 7.83 pmol/L (±4.67) (P < 0.0001).
FSFI scores improved among 106 (88.33%) subjects at the end of the treatment period, while 14 subjects (11.67%) had decreased total scores compared to pretreatment values (Fig. 1). There was a statistically significant improvement in the total FSFI score (t = 6.835; df = 119; P < 0.0001). Splitting the sample into “responders” and “non-responders” and defining improvement as ≥20% increase in FSFI score, a total of 37 patients (30.83%) can be classified as “responders”.
Figure 1.
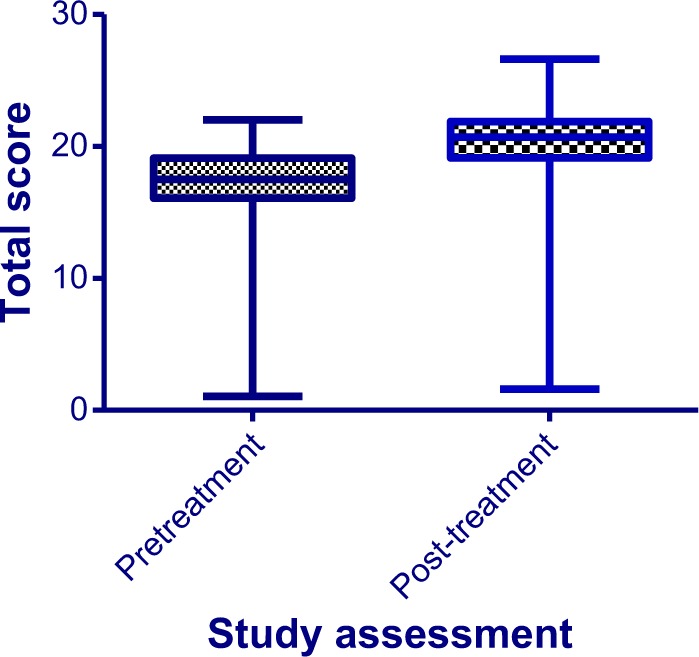
Mean total FSFI scores from pre- and post-treatment. There was a statistically significant improvement in the mean total FSFI score (t = 6.835; df = 119; P < 0.0001), from 16.57 (±4.49) at pretreatment to 19.93 (±3.89) at post-treatment.
Table 2 summarizes the FSFI scores showing both the total scores and those of the individual domains. Statistically significant (P < 0.005) improvements were seen in the following domains: desire, arousal, orgasm, and satisfaction. The most significant improvement was seen in the desire domain with an increase in the mean score of 1.84 points, with 85% of patients showing ≥20% score improvements in relation to pretreatment values.
Table 2.
FSFI scores.
| DOMAIN | MINIMUM SCORE | MAXIMUM SCORE | PRETREATMENT SCORE | POST-TREATMENT SCORE | STATISTICALLY SIGNIFICANT CHANGE |
|---|---|---|---|---|---|
| Desire | 1.2 | 6.0 | 2.51 | 4.35 | Yes (P < 0.0001) |
| Arousal | 0 | 6.0 | 2.43 | 3.09 | Yes (P < 0.0001) |
| Lubrication | 0 | 6.0 | 3.16 | 3.18 | No (P = 0.843) |
| Orgasm | 0 | 6.0 | 2.75 | 3.08 | Yes (P = 0.0014) |
| Satisfaction | 0.8 | 6.0 | 2.90 | 3.45 | Yes (P < 0.0001) |
| Pain | 0 | 6.0 | 2.82 | 2.77 | No (P = 0.67) |
| Total | 2.0 | 36.0 | 16.57 | 19.93 | Yes (P < 0.0001) |
Note: Data are n.
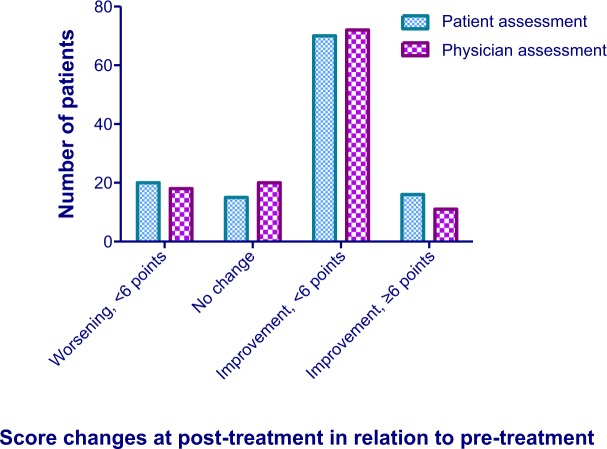
There were statistically significant improvements (P < 0.0001) in the scores of the Patient and Physician Assessments (Fig. 2). At pretreatment, no scores above 5 points on the 1–10 point scales were recorded in either assessment, while post-treatment, 36 patients had scores >6 points in the Patient Assessment and 25 patients had scores >6 points in the Physician Assessment.
Figure 2.
Patient and physician overall assessment scores from pre- and post-treatment. There were statistically significant (P < 0.0001) improvements in both patient and physician assessments post-treatment in relation to pretreatment values.
The adverse events reported during the treatment period are summarized in Table 3.
Table 3.
Adverse events.
| DIAGNOSIS | n | SEVERITY (n) | CAUSALITY: RELATION TO STUDY DRUG (n) |
|---|---|---|---|
| Abdominal discomfort | 6 | Moderate (4), Mild (2) | Likely (6) |
| Abdominal distension | 4 | Moderate (2), Mild (2) | Unknown (1), Likely (3) |
| Abdominal pain | 8 | Severe (4), Moderate (3), Mild (1) | Unknown (3), Likely (5) |
| Diarrhea | 2 | Mild (2) | Unknown (1), Likely (1) |
| Eructation | 3 | Mild (2), Moderate (1) | Unknown (1), Likely (2) |
| Flatulence | 1 | Moderate | Unknown |
| Gastric upset | 6 | Moderate (1), Brando (5) | Unknown (1), Likely (5) |
| Halitosis | 1 | Moderate | Likely |
| Headache | 4 | Moderate (2), Mild (2) | Unknown (3), Likely (1) |
| Insomnia | 4 | Severe (1), Moderate (1), Mild (2) | Unknown (3), Likely (1) |
| Irritability | 8 | Moderate (5), Mild (3) | Unknown (2), Likely (6) |
| Light sleep | 1 | Moderate | Unknown |
| Memory loss | 1 | Mild | Unknown |
| Mood swings | 1 | Mild | Unknown |
| Nausea | 2 | Mild | Likely (2) |
| Nightmares | 1 | Severe | Unknown |
| Oily skin | 2 | Moderate (1), Mild (1) | Unknown (2) |
| Polyuria | 1 | Moderate | Unknown |
| Pruritis | 1 | Moderate | Unknown |
Abbreviation: n, number of subjects.
Table 4 summarizes the results of the physical examination performed pre- and post-treatment. No significant changes were recorded post-treatment.
Table 4.
Physical examination.
| VARIABLE | PRETREATMENT RESULT | POST-TREATMENT RESULT | CHANGE FROM PRETREATMENT? |
|---|---|---|---|
| Weight (kg) | 64.51 (±9.81) | 64.30 (±9.46) | No (P = 0.7991) |
| BMI | 24.91 (±3.58) | 24.86 (±3.42) | No (P = 0.8795) |
| Systolic blood pressure (mmHg) | 120.7 (±7.34) | 120.5 (±7.70) | No (P = 0.7183) |
| Diastolic blood pressure (mmHg) | 78.2 (±9.2) | 77.96 (±9.58) | No (P = 0.6604) |
| Heart rate (bpm) | 66.56 (±5.97) | 66.69 (±5.93) | No (P = 0.3247) |
Note: Data are means (±SD).
Discussion
The results show that the treatment was effective in terms of improvement in the FSFI questionnaire, with the majority of patients showing improved post-treatment scores together with statistically significant improvements in the total score of the questionnaire as well as in most of the individual domain scores. Although side effects were reported, there were no reports of serious adverse effects during the treatment period. The safety evaluations including laboratory tests and physical examination results did not undergo significant alterations during the treatment period.
Overall, T. terrestris has a good safety profile; previously reported side effects include irritation of the gastric mucosa and gastric reflux. The side effects reported during the treatment period do reflect this information, with an overwhelming majority of side effects related to the gastrointestinal tract.23,24 However, these effects were transient and, as mentioned previously, no serious adverse effects were reported.
The assessment of the female patient presenting with sexual dysfunction should take into account a wide variety of factors, including an important emotional component. Interestingly, during pretreatment assessment, we observed a relatively high number of patients self-reporting depression during the assessment of medical history, a finding that reflects the data present in the literature.25,26
T. terrestris is often used in the treatment of infertility, decreased libido, and erectile dysfunction. It is also used among athletes to increase muscle resistance and improve performance in sports, although scientific evidence to support this effect is lacking.27–31 The active components of T. terrestris include the steroidal glycoside saponins furostanol and spirostanol, which are found in the leaves of the plant.27
Protodioscin is a chemical substance derived from the T. terrestris plant that has been confirmed to increase sexual desire and improve erections by means of conversion of protodioscin to DHEA.29,32 In animal studies, increased testosterone levels along with increased dehydrotestosterone and DHEA are suggestive of aphrodisiac activity.27–31 The aphrodisiac pro-erectile properties were co-related with a release of nitric oxide from nerve endings enervating the corpus cavernosum of the penis.24 However, the literature is lacking additional studies to confirm these effects.30,33 Indeed, our results showing a statistically significant increase in DHEA among the treated patients are interesting as this effect can also be observed among treated female subjects.
However, the lack of correlation between increasing DHEA levels with both free testosterone and serum testosterone levels is confounding. Contrary to expectations,34,35 mean testosterone levels decreased among the total treated subjects, despite the presence of a clinical response. It may be because the DHEA levels in women have a greater impact on FSFI domains in relation to testosterone, explaining our positive results. A larger, prospective, randomized, comparative study would likely provide further insight into the effect of T. terrestris extract on testosterone levels in women. It may also be of interest to examine the effects of different doses of T. terrestris extract in future study.
Conclusion
The co-occurrence of enhanced female sexual function and increased DHEA levels is suggestive of physiological alterations underlying clinical improvement following treatment. Our results strongly support the safety and effectiveness of T. terrestris extract in the treatment of female sexual dysfunction.
Acknowledgments
The authors would like to thank Daiane Bergamim, Flavia Dweck, and Renata Ribeiro Coutinho for their help with data verification, Silvia Maciel for CRF verification, and Oscar Roberto Guimarães for help with laboratory test data.
Footnotes
ACADEMIC EDITOR: Marlene von Friederichs-Fitzwater, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CRBG, RL, MG, LO. Analyzed the data: LO, AS. Wrote the first draft of the manuscript: MG, LO, CSA. Contributed to the writing of the manuscript: RL, CRBG, CSA, CPN, GFG. Agreed with manuscript results and conclusions: CRBG, RL, GFG, CSA, CPN, MG, LO, AS. Jointly developed the structure and arguments for the paper: CRBG, RL, LO. Made critical revisions and approved final version: CRBG, RL, GFG, CSA, CPN, MG, LO, AS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Bancroft J, Loftus J, Long JS. Distress about sex: a national survey of women in heterosexual relationships. Arch Sex Behav. 2003;32:193–208. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- 2.Basson R. Clinical practice. Sexual desire and arousal disorders in women. N Engl J Med. 2006;354:1497–1506. doi: 10.1056/NEJMcp050154. [DOI] [PubMed] [Google Scholar]
- 3.Kammerer-Doak D, Rogers RG. Female sexual function and dysfunction. Obstet Gynecol Clin North Am. 2008;35(2):169–183. doi: 10.1016/j.ogc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Shafer L. Sexual dysfunction. In: Carlson K, Eisenstat S, editors. Primary Care of Women. St. Louis: Mosby; p. 2002.p. 415. [Google Scholar]
- 6.Shifren JL, Monz BU, Russo PA, Segreti A, Johanes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 7.Zemishlany Z, Weizman A. The impact of mental illness on sexual dysfunction. Adv Psychosom Med. 2008;29:89–106. doi: 10.1159/000126626. [DOI] [PubMed] [Google Scholar]
- 8.Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:401–406. doi: 10.1007/s00192-006-0156-0. [DOI] [PubMed] [Google Scholar]
- 9.Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24–34. doi: 10.1111/j.1743-6109.2004.10105.x. [DOI] [PubMed] [Google Scholar]
- 10.Nathorst-Böös J, von Schoultz B. Psychological reactions and sexual life after hysterectomy with and without oophorectomy. Gynecol Obstet Invest. 1992;34:97–101. doi: 10.1159/000292735. [DOI] [PubMed] [Google Scholar]
- 11.Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2010;33(2):323–338. doi: 10.1016/j.psc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Basson R. Women’s sexual function and dysfunction: current uncertainties, future directions. Int J Impot Res. 2008;20:466–478. doi: 10.1038/ijir.2008.23. [DOI] [PubMed] [Google Scholar]
- 13.Carey JC. Pharmacological effects on sexual function. Obstet Gynecol Clin North Am. 2006;33:599–620. doi: 10.1016/j.ogc.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Cawood EH, Bancroft J. Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med. 1996;26:925–936. doi: 10.1017/s0033291700035261. [DOI] [PubMed] [Google Scholar]
- 15.Guidance for Industry . Female Sexual Dysfunction: Clinical Development of Drug Products for Treatment Draft Guidance. Rockville, MD: United States Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research; 2000. [Google Scholar]
- 16.Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- 17.Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction. J Am Med Assoc. 2008;300(4):395–404. doi: 10.1001/jama.300.4.395. [DOI] [PubMed] [Google Scholar]
- 18.Gurnell EM, Hunt PJ, Curran SE, et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93:400–409. doi: 10.1210/jc.2007-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt PJ, Gurnell EM, Huppert FA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85:4650–4656. doi: 10.1210/jcem.85.12.7022. [DOI] [PubMed] [Google Scholar]
- 20.Wierman ME, Basson R, Davis SR, et al. Androgen therapy in women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- 21.Antonio J, Uelmen J, Rodriguez R, Earnest C. The effects of Tribulus terrestris on body composition and exercise performance in resistance-trained males. Int J Sport Nutr Exerc Metab. 2000;10(2):208–215. doi: 10.1123/ijsnem.10.2.208. [DOI] [PubMed] [Google Scholar]
- 22.Rosen R, Brown C, Heiman J, et al. The Female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 23.Gauthaman K, Adaikan PG, Prasad RNV. Aphrodisiac properties of Tribulus terrestrisextract (protodioscin) in normal and castrated rats. Life Sci. 2002;71(12):1385–1396. doi: 10.1016/s0024-3205(02)01858-1. [DOI] [PubMed] [Google Scholar]
- 24.Neychev VK, Mitev VI. The aphrodisiac herb Tribulus terrestrisdoes not influence the androgen production in young men. J Ethnopharmacol. 2005;101:319–323. doi: 10.1016/j.jep.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369:512–525. doi: 10.1016/S0140-6736(07)60238-4. [DOI] [PubMed] [Google Scholar]
- 26.Longo DL, et al. Harrison’s Online. 18th ed. New York, NY: The McGraw-Hill Companies; 2012. [Accessed November 29, 2013]. Available at: http://www.accessmedicine.com/resourceTOC.aspx?resourceID=4. [Google Scholar]
- 27.Rowland DL, Tai W. A review of plant-derived and herbal approaches to the treatment of sexual dysfunctions. J Sex Marital Ther. 2003;29(3):185–205. doi: 10.1080/00926230390155096. [DOI] [PubMed] [Google Scholar]
- 28.Patel DK, Kumar R, Prasad SK, Hemalatha S. Pharmacologically screened aphrodisiac plant—a review of current scientific literature. Asian Pacific J Trop Biomed. 2011;1(1):S131–S138. [Google Scholar]
- 29.Mazaro-Costa R, Andersen ML, Hachul H, Tufik S. Medicinal plants as alternative treatments for female sexual dysfunction: utopian vision or possible treatment in climacteric women? J Sex Med. 2010;7(11):3695–3714. doi: 10.1111/j.1743-6109.2010.01987.x. [DOI] [PubMed] [Google Scholar]
- 30.Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of puncture vine (Tribulus terrestris) extract (protodioscin): an evaluation using a rat model. J Altern Complement Med. 2003;9(2):257–265. doi: 10.1089/10755530360623374. [DOI] [PubMed] [Google Scholar]
- 31.Adimoelja A. Phytochemicals and the breakthrough of traditional herbs in the management of sexual dysfunctions. Int J Androl. 2000;23(S2):82–84. doi: 10.1046/j.1365-2605.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- 32.Do J, Choi S, Choi J, Hyun JS. Effects and mechanism of action of a Tribulus terrestrisextract on penile erection. Korean J Urol. 2013;54(3):183–188. doi: 10.4111/kju.2013.54.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tharakan B, Manyam BV. Botanical therapies in sexual dysfunction. Phytother Res. 2005;6:457–463. doi: 10.1002/ptr.1634. [DOI] [PubMed] [Google Scholar]
- 34.van der Stege JG, Groen H, van Zadelhoff SJ, et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008;15:23–31. doi: 10.1097/gme.0b013e3180f6108c. [DOI] [PubMed] [Google Scholar]
- 35.Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91–96. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]