Abstract
Intensive statin therapy is a central component of secondary prevention after acute myocardial infarction (AMI), particularly among high-risk patients, such as those with diabetes mellitus (DM). However, the frequency and predictors of intensive statin therapy use after AMI among patients with DM have not been described. We examined patterns of intensive statin therapy use (defined as a statin with expected LDL-C lowering of >50%) at discharge among AMI patients with known DM enrolled in a 24-site US registry. Predictors of intensive statin therapy use were evaluated using multivariable hierarchical Poisson regression models. Among 1300 patients with DM after AMI, 22% were prescribed intensive statin therapy at hospital discharge. In multivariable models, ST-elevation AMI (RR 1.48, 95% CI 1.29–1.70), insurance for medications (RR 1.28, 95% CI 1.00–1.63) and higher LDL-C levels (RR 1.05 per 1 mg/dL, 95% CI 1.02–1.07) were independent predictors of intensive statin therapy whereas higher GRACE scores were associated with lower rates of intensive statin therapy (RR 0.94 per 10 points; 95% CI 0.91–0.98). In conclusion, only 1 in 5 patients with DM were prescribed intensive statin therapy at discharge after an AMI. Predictors of intensive statin therapy use suggest important opportunities to improve quality of care in this patient population.
Key terms: statins, myocardial infarction, diabetes mellitus
INTRODUCTION
Patients with DM experience higher rates of mortality and recurrent events following AMI than those without DM.1–4 Aggressive secondary prevention strategies are, therefore, critical in this patient population and supported by contemporary practice guidelines.5 A key component of secondary prevention following AMI is intensive statin therapy that has been shown to be superior to moderate statin treatment in reducing morbidity and mortality after AMI.6–10 Despite these data, recent analyses from the U.S. reveal that only ~38% of AMI patients are discharged on intensive statin therapy.11 Patients with DM represent one of the highest risk subgroups of AMI patients and thus have the most potential to benefit from aggressive secondary prevention efforts; however, the frequency and predictors of intensive statin therapy among patients with DM are unknown. Addressing this knowledge gap could identify an important opportunity for quality improvement efforts to support aggressive treatment in those most likely to benefit.
METHODS
Details of the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study, including the study design, patient selection, site characteristics, informed consent, appropriate treatment of research subjects and follow-up assessments, have been previously published.12 Between April 2005 and December 2008, patients from 24 U.S. hospitals were enrolled into the TRIUMPH registry. Patients were required to have biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI, such as prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes during the initial 24 hours of admission. For this analysis, only patients with established DM were included, which was defined as a chart-documented history of DM or taking any glucose lowering medication on admission.
Baseline sociodemographic and clinical data were obtained through chart abstraction and a detailed structured interview within 24 to 72 hours of admission. Lipid-lowering medications (type and dose) were documented at admission and hospital discharge. Statins prescribed at discharge were categorized as intensive (expected low-density lipoprotein cholesterol [LDL-C] lowering of >50%;13 i.e., atorvastatin 80 mg or rosuvastatin ≥20 mg daily)11 or moderate (all other statins). As a sensitivity analysis, given the new cholesterol guidelines,14 we also considered atorvastatin 40mg as intensive statin therapy (estimated LDL-C lowering of 45–50%). In addition, as a second sensitivity analysis, to account for hospitals with restrictive formularies, we excluded the 4 hospitals with limited access to intensive statins. Patients with documented allergies or contraindications to statin therapy were excluded from the analysis. Institutional Review Board approval was obtained at each participating hospital and informed consent was obtained from all patients for baseline and follow-up assessments.
The demographic and clinical characteristics of patients with DM who did and who did not receive intensive statin therapy at discharge after AMI were compared using chi-square test for categorical variables and t-tests for continuous variables. We used hierarchical modified Poisson regression with robust standard errors to examine the factors associated with prescription of intensive statin therapy at hospital discharge because our primary metric of interest (frequency of intensive statin prescription) was not rare, to avoid an overestimation of effect sizes, as could result from using logistic regression. Covariates included in the multivariable model were selected a priori based on clinical judgment and included sociodemographics (age, sex, race, marital status, prescription drug insurance), clinical features (history of smoking, body mass index), characteristics of the qualifying AMI event (ST-elevation [STEMI], Global Registry of Acute Coronary Events [GRACE] score—an assessment of the severity of AMI where higher scores indicate a higher risk of mortality15), severity of DM (DM duration, class of DM therapy [diet vs. oral medications only vs. any insulin therapy], HbA1c level ≥7%, and LDL-C level. Participating center was entered as a random effect to account for clustering of patients within hospitals. Site variability in the rates of intensive statin therapy was evaluated using median rate ratios, which estimates the relative difference in risk ratios of two hypothetically identical patients for being discharged on intensive statin therapy at two different sites. All analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05.
RESULTS
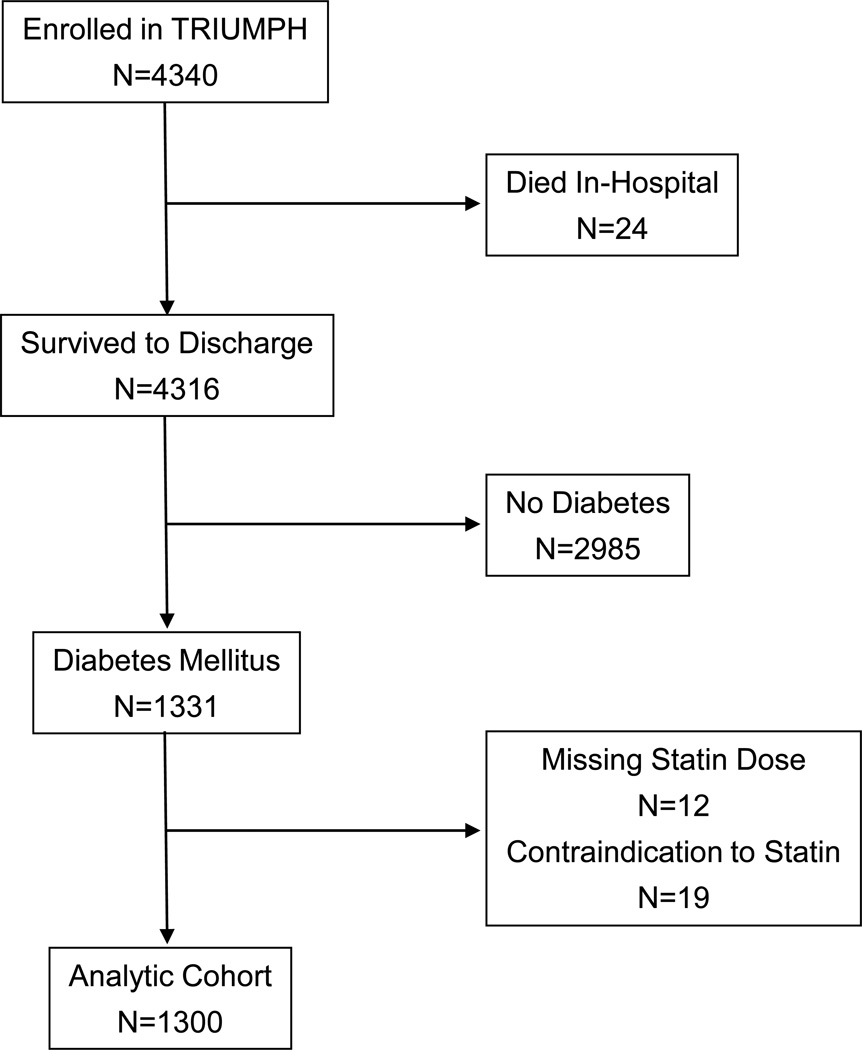
Of the 4340 patients enrolled in TRIUMPH, 4316 survived to hospital discharge, of whom 1331 (31%) had an established diagnosis of DM at admission. Statin dose was not available for 12 patients with DM, and 19 had a documented contraindication to statins, which resulted in an analytic population of 1300 patients (Figure 1). The mean age of the population was 61 years, 59% were men, and 58% were Caucasian (Table 1). One-third of patients presented with a STEMI, and 66% underwent invasive management of their AMI. The mean duration of DM was 12 years, mean HbA1c was 8.3%, and 32% were on insulin at admission (Table 2).
Figure 1.
Flowchart of analytic cohort
Table 1.
Baseline Demographic and Clinical Characteristics
| Intensive Statin Prescribed | ||||
|---|---|---|---|---|
| Variable | All Patients n=1300 |
Yes n=280 |
No n=1020 |
p value |
| Age (years) | 61±12 | 59±11 | 61±12 | 0.077 |
| White race | 58% | 62% | 57% | 0.185 |
| Men | 59% | 60% | 58% | 0.715 |
| Married | 48% | 54% | 47% | 0.026 |
| Medication insurance coverage | 72% | 80% | 70% | <0.001 |
| Hypertension | 84% | 81% | 85% | 0.131 |
| Current smoker | 29% | 29% | 28% | 0.870 |
| Prior coronary bypass surgery | 18% | 16% | 18% | 0.381 |
| Prior angioplasty | 25% | 26% | 25% | 0.602 |
| Prior myocardial infarction | 28% | 29% | 28% | 0.760 |
| Prior congestive heart failure | 15% | 11% | 16% | 0.023 |
| Chronic lung disease | 8.5% | 7.1% | 8.8% | 0.371 |
| Prior stroke | 7.0% | 8.6% | 6.6% | 0.245 |
| Body mass index (kg/m2) | 31.6±7.2 | 31.9±8.0 | 31.5±6.9 | 0.400 |
| ST-elevation AMI | 32% | 46% | 27% | <0.001 |
| GRACE score | 108±30 | 102±28 | 109±30 | <0.001 |
| Left ventricular dysfunctiona | 21% | 20% | 22% | 0.492 |
| In-hospital coronary angiogram | 89% | 94% | 88% | 0.005 |
| In-hospital angioplasty | 56% | 68% | 52% | <0.001 |
| In-hospital coronary bypass surgery | 10.9% | 5.0% | 12.5% | <0.001 |
Moderate or severe dysfunction, defined as ejection fraction <40%
AMI, acute myocardial infarction; GRACE, Global Registry of Acute Coronary Events.
Table 2.
Diabetes and Lipid Characteristics
| Intensive Statin Prescribed | ||||
|---|---|---|---|---|
| Variable | All Patients n=1300 |
Yes n=280 |
No n=1020 |
p value |
| DM duration (years) | 12±11 | 12±11 | 12±11 | 0.271 |
| DM treatments | 0.758 | |||
| Diet only | 29% | 31% | 29% | |
| Oral medications only | 39% | 38% | 39% | |
| Insulin | 32% | 31% | 32% | |
| Low-density lipoprotein cholesterol (mg/dL) | 100±45 | 110±53 | 97±42 | <0.001 |
| Hemoglobin A1c | 8.3±2.6 | 8.7±3.7 | 8.1±2.2 | 0.007 |
| Hemoglobin A1c ≥ 7% | 66% | 68% | 65% | 0.324 |
| Statin on arrival | 49% | 52% | 48% | 0.202 |
DM, diabetes mellitus
Among the 1300 patients with DM who were hospitalized with an AMI, 1138 (88%) were discharged on a statin at any dose but only 280 (22%) were prescribed intensive statin therapy. In sensitivity analyses, when 40 mg of atorvastatin was considered intensive statin therapy, and additional 117 (9%) of patients were considered as receiving intensive statins, for an overall rate of intensive statin prescription of 31%. In a second sensitivity analysis, when the 4 sites with restrictive formularies were excluded from the analysis, the overall rate of intensive statin prescription was 26%.
Compared with those not discharged on intensive statins, DM patients discharged on intensive statin therapy were more likely to have prescription medication insurance coverage, less often had a history of congestive heart failure, and were more likely to have presented with a STEMI (Table 1). Patients discharged on intensive statin therapy had higher HbA1c levels and higher LDL-C levels (Table 2), although DM duration and glucose-lowering treatments were similar between groups.
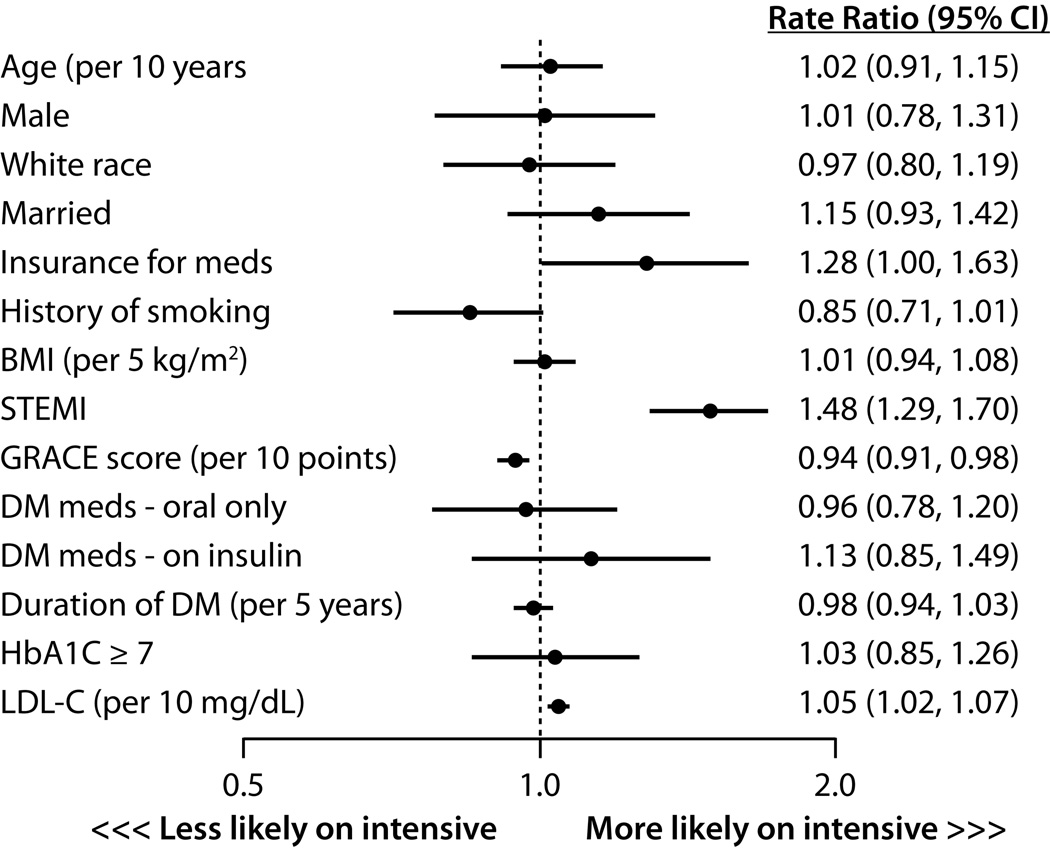
In the hierarchical, multivariable model, patients who presented with a STEMI were 48% more likely to be discharged on intensive statin therapy (95% CI 1.29–1.70; Figure 2). Other factors independently associated with a higher rate of intensive statin therapy at discharge were insurance for prescription medications and higher LDL-C levels. Paradoxically, higher GRACE scores were associated with a lower rate of intensive statin therapy. None of the DM severity measures were significantly associated with frequency of discharge prescription of intensive statin therapy, including DM duration, insulin treatment, or HbA1c level ≥7%.
Figure 2.
Predictors of intensive statin therapy prescription at discharge after an acute myocardial infarction
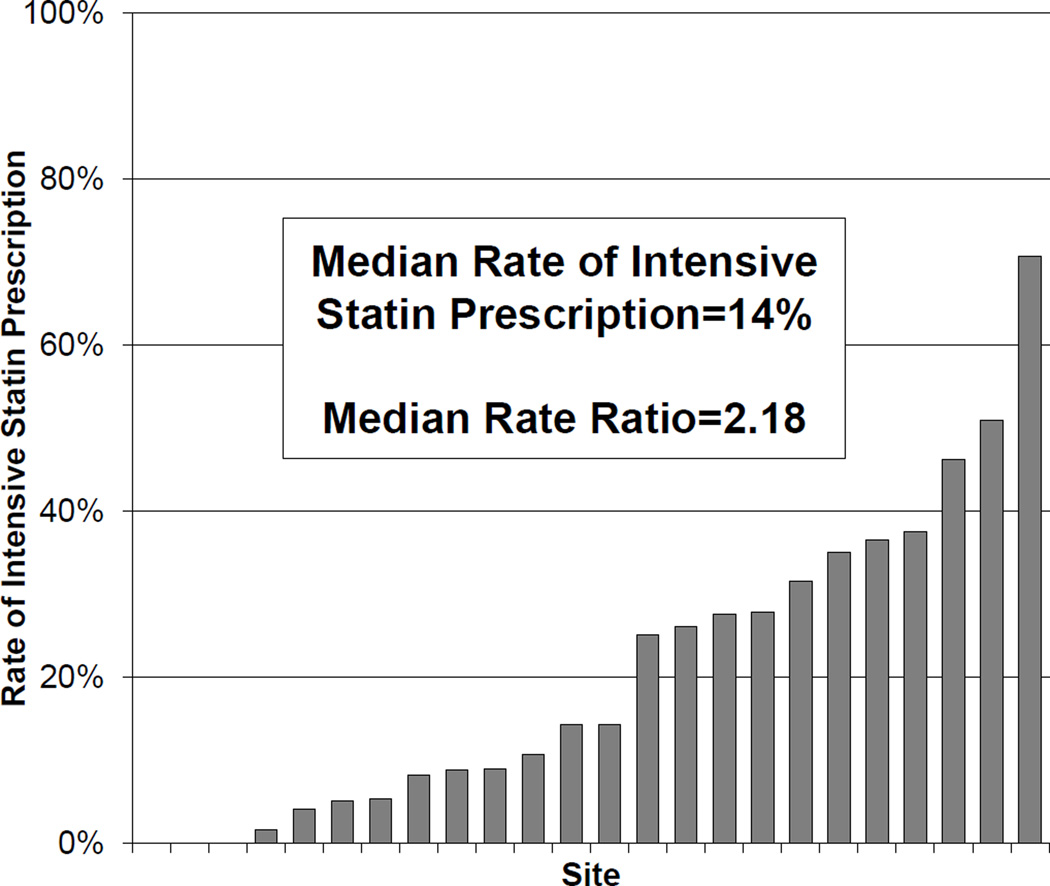
Among the 24 hospitals in TRIUMPH, the unadjusted rates of intensive statin therapy at discharge ranged from 0% to 67%, with a median rate of 14% (Figure 3). In the hierarchical multivariable model that adjusted for patient factors, the median rate ratio was 2.18 (95% CI 1.75–3.61), indicating that 2 identical patients had more than 2-fold difference in the likelihood of being discharged on intensive statin therapy when presenting to 2 random hospitals..
Figure 3.
Site variation in rates of prescription of intensive statin therapy
DISCUSSION
In a large, multicenter registry of AMI patients, we found that only 1 in 5 patients with DM were discharged on intensive statin therapy after an AMI. Interestingly, none of the factors independently associated with intensive statin prescription (higher LDL-C levels, STEMI on presentation and medication insurance) have previously been found to predict a worse prognosis after AMI or be associated with greater benefit from intensive statin therapy in patients with DM.16 In contrast, patients who were at higher risk of post-AMI death (i.e., higher GRACE scores) paradoxically received lower rates of intensive statin therapy. Furthermore, neither longer duration of DM nor treatment with insulin—factors known to be associated with worse prognosis in this patient population–17–19 were associated with intensive statin therapy. Finally, we observed a substantial variation in the use of intensive statins across sites, even after adjusting for patient factors. This constellation of findings suggests a substantial underuse of high intensity statins—a therapy known to improve outcomes in high-risk patients—and highlights an important opportunity for quality improvement in this high-risk patient group.
Prescription of statin therapy at discharge is a Class 1(A) guideline-recommended therapy for all patients after AMI regardless of baseline LDL-C level or other patient characteristics20 and is a core performance measure for quality AMI care.21 Furthermore, the more recently published lipid treatment guidelines recommended intensive statins to patients after AMI,14 based on the randomized trials that demonstrated a greater reduction in post-MI morbidity and mortality with intensive vs. moderate.6,10 One could certainly argue that all AMI patients are at sufficiently high risk for recurrent ischemic events to justify intensive statin therapy, as was recommended in the updated guidelines. However, at a minimum, the highest risk AMI patients should be receiving intensive statins, as they would be expected to have the greatest likelihood of benefit from aggressive secondary prevention.22
Despite the fact that DM patients represent a high-risk AMI cohort, with nearly double the rate of mortality and reinfarction compared with those without DM,23 we observed a low rate of intensive statin prescription at discharge in these patients. Instead, it appears that physicians preferentially risk-stratify patients based on the presence of ST-elevations and higher LDL-C levels—even though these factors have not been shown to be associated with a greater likelihood of mortality or a greater benefit with statins.24 In fact, pooled results from 2 trials of intensive vs. moderate statin therapy showed a slight trend toward a greater absolute and relative risk reduction with intensive statins among NSTEMI patients versus STEMI, although the interaction term was not significant.24 Interestingly, in the present analyses, GRACE scores—an objective estimation of risk of death within 6 months after discharge from AMI—were inversely related to intensive statin therapy. This suggests that the decision to prescribe intensive statins may be driven primarily by a subjective reaction to perceived severity of illness (i.e. STEMI) and lipid abnormalities (i.e. LDL-C level) rather than objective measures of risk (e.g. GRACE score, longer DM duration). We believe that these findings, along with our observation of substantial variation in intensive statin use across sites, should prompt efforts to improve systems of care delivery at hospitals providing treating AMI patients that promote matching the actual risk of the patient with the appropriate therapy.
One potential explanation for the low rates of intensive statin therapy among patients with DM could be related to recent concerns about the association of statin therapy with incident DM.25–26 However a meta-analysis of over 15,000 patients suggested that, while there was a modest hazard of incident DM with high-dose atorvastatin among patients at increased risk for DM, this risk was overshadowed by the cardiovascular protective effect of intensive statin therapy.27 Furthermore, among patients with established DM, a large meta-analysis showed a 9% absolute decrease in all-cause mortality among patients treated with statins and an even larger reduction in non-fatal cardiovascular events.28 Therefore, while there may be some apprehension about the impact of statins on glycemic control, the overall effect of intensive statin therapy on reducing cardiovascular morbidity and mortality among patients with DM should allay these concerns.
This study should be viewed in the light of the following potential limitations. First, we defined intensive statin therapy by an expected reduction in LDL-C of greater than 50% and not by a specific statin medication, as had been done in prior trials.6,10 This approach has been used in other observational studies11 and is also supported by the recent cholesterol guidelines.14 Second, although our study sample was derived from a large, multicenter AMI registry and the patient population was diverse and unselected, our analysis represents only a small subset of the hospitals in the U.S. and may not be generalizable to smaller and more rural hospitals. Third, patients with DM represent a high-risk cohort of patients after an AMI and thus would be expected to have the greatest benefit with aggressive secondary prevention efforts; however, no specific heterogeneity of treatment effect of intensive statin therapy by DM status has been proven. Fourth, although we were able to examine and identify several important patient-level predictors of the under use of intensive statin therapy in DM patients, it is not possible to definitively know the underlying motivations of the providers making these treatment decisions. Further qualitative research is needed to better understand these issues in greater depth. Finally, as TRIUMPH included a wide range of sites, including governmental and county hospitals, our results might have been impacted by formulary restrictions. However when we excluded sites with formularies that favored moderate statins from the analysis, the overall rate of intensive statin therapy remained low with substantial residual variability, suggesting that formulary restrictions were not the major limiting factor for intensive statin therapy.
ACKNOWLEDGMENTS
Sources of Funding: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): SCCOR Grant #P50HL077113-01.
MK: Research grants-American Heart Association, Genentech, Sanofi-Aventis, Gilead, Medtronic Minimed, Glucometrics; Consultation-Genentech, Gilead, F Hoffmann LaRoche, Medtronic Minimed, AstraZeneca. DKM: Consultation-Roche, Bristol Myer Squibb, Merck, Genentech, Janssen, Sanofi-Aventis, Genfit; Clinical trial leadership honoraria-AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, F. Hoffmann-La Roche Ltd, Genentech, Merck & Co., Orexigen Therapeutics, Takeda, Novo Nordisk. JAS: Research grants-NHLBI, AHA, ACCF, Gilead, Lilly, EvaHeart, Amorcyte. Consultation-United Healthcare, Genentech, Amgen; TMM: Research grants-VA Health Services Research and Development Career Development Award. SVA: Research grants: Significant-Genentech, Eli Lilly, Sanofi-Aventis, Gilead.
Footnotes
Financial Disclosures:
The other authors report no conflicts of interest.
References
- 1.Franklin K, Goldberg RJ, Spencer F, Klein W, Budaj A, Brieger D, Marre M, Steg PG, Gowda N, Gore JM Investigators G. Implications of diabetes in patients with acute coronary syndromes. The Global Registry of Acute Coronary Events. Arch Intern Med. 2004;164:1457–1463. doi: 10.1001/archinte.164.13.1457. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O'Connor CM, Diaz R, Belenkov YN, Varshavsky S, Leimberger JD, Velazquez EJ, Califf RM, Pfeffer MA. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004;110:1572–1578. doi: 10.1161/01.CIR.0000142047.28024.F2. [DOI] [PubMed] [Google Scholar]
- 3.Mehran R, Dangas GD, Kobayashi Y, Lansky AJ, Mintz GS, Aymong ED, Fahy M, Moses JW, Stone GW, Leon MB. Short- and long-term results after multivessel stenting in diabetic patients. J Am Coll Cardiol. 2004;43:1348–1354. doi: 10.1016/j.jacc.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 4.West NE, Ruygrok PN, Disco CM, Webster MW, Lindeboom WK, O'Neill WW, Mercado NF, Serruys PW. Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients. Circulation. 2004;109:867–873. doi: 10.1161/01.CIR.0000116750.63158.94. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA World Heart F, the Preventive Cardiovascular Nurses A. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Pravastatin or Atorvastatin E, Infection Therapy-Thrombolysis in Myocardial Infarction I. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 7.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 9.Study of the Effectiveness of Additional Reductions in C, Homocysteine Collaborative G. Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 11.Javed U, Deedwania PC, Bhatt DL, Cannon CP, Dai D, Hernandez AF, Peterson ED, Fonarow GC. Use of intensive lipid-lowering therapy in patients hospitalized with acute coronary syndrome: an analysis of 65,396 hospitalizations from 344 hospitals participating in Get With The Guidelines (GWTG) Am Heart J. 2010;160:1130–1136. doi: 10.1016/j.ahj.2010.08.041. 1136 e1131-1133. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong PH. Lack of therapeutic interchangeability of HMG-CoA reductase inhibitors. Ann Pharmacother. 2002;36:1907–1917. doi: 10.1345/aph.1C116. [DOI] [PubMed] [Google Scholar]
- 14.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 15.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA Investigators G. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 16.McGuire DK, Emanuelsson H, Granger CB, Magnus Ohman E, Moliterno DJ, White HD, Ardissino D, Box JW, Califf RM, Topol EJ. Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO-IIb study. GUSTO IIb Investigators. Eur Heart J. 2000;21:1750–1758. doi: 10.1053/euhj.2000.2317. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz HE. Insulin: potential negative consequences of early routine use in patients with type 2 diabetes. Diabetes Care. 2011;34(Suppl 2):S225–S230. doi: 10.2337/dc11-s225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandish S, Bailon O, Wyatt J, Smith J, Stevens A, Lujan M, Chilton R. Vasculotoxic effects of insulin and its role in atherosclerosis: what is the evidence? Curr Atheroscler Rep. 2011;13:123–128. doi: 10.1007/s11883-011-0165-4. [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Sullivan L, D'Agostino RB, Sr, Wilson PW Framingham Heart S. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27:704–708. doi: 10.2337/diacare.27.3.704. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 21.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118:2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 22.Chan PS, Nallamothu BK, Gurm HS, Hayward RA, Vijan S. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation. 2007;115:2398–2409. doi: 10.1161/CIRCULATIONAHA.106.667683. [DOI] [PubMed] [Google Scholar]
- 23.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SA, Cannon CP, Wiviott SD, de Lemos JA, Blazing MA, McCabe CH, Califf RM, Braunwald E. Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the Aggrastat to Zocor and Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 trials) Am J Cardiol. 2007;100:1047–1051. doi: 10.1016/j.amjcard.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 26.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 27.Waters DD, Ho JE, Boekholdt SM, Demicco DA, Kastelein JJ, Messig M, Breazna A, Pedersen TR. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61:148–152. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 28.Cholesterol Treatment Trialists C. Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]