Abstract
Parkinsonism is associated with changes in oscillatory activity patterns and increased synchronization of neurons in the basal ganglia and cortex in patients and animal models of Parkinson's disease, but the relationship between these changes and the severity of parkinsonian signs remains unclear. We examined this relationship by studying changes in local field potentials (LFPs) in the internal pallidal segment (GPi) and the subthalamic nucleus (STN), and in encephalographic signals (EEG) from the primarymotor cortex (M1) in Rhesus monkeys which were rendered progressively parkinsonian by repeated systemic injections of small doses of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Observations during wakefulness and sleep (defined by EEG and video records) were analyzed separately. The severity of parkinsonism correlated with increases in spectral power at frequencies below 15.5 Hz in M1 and GPi and reductions in spectral power at frequencies above 15.6 Hz with little change in STN. The severity of parkinsonism also correlated with increases in the coherence betweenM1 EEG and basal ganglia LFPs in the low frequency band. Levodopa treatment reduced low-frequency activity and increased high-frequency activity in all three areas, but did not affect coherence. The state of arousal also affected LFP and EEG signals in all three structures, particularly in the STN. These results suggest that parkinsonism-associated changes in alpha and low-beta band oscillatory activity can be detected early in the parkinsonian state in M1 and GPi. Interestingly, oscillations detectable in STN LFP signals (including oscillations in the beta-band) do not appear to correlate strongly with the severity of mild-to-moderate parkinsonism in these animals. Levodopa-induced changes in oscillatoryM1 EEG and basal ganglia LFP patterns do not necessarily represent a normalization of abnormalities caused by dopamine depletion.
Keywords: Parkinson's disease, Basal ganglia, Local field potential, Motor cortex, Sleep, Wakefulness
Introduction
Prominent beta-band (13–35 Hz) oscillations in local field potentials (LFPs) in the basal ganglia (Brown et al., 2001; Gatev et al., 2006; Hammond et al., 2007) are seen as a key feature of the pathophysiology of Parkinson's disease (PD). This notion is supported by the finding that beta-band oscillations in the subthalamic nucleus (STN) of PD patients are reduced by antiparkinsonian treatments, such as dopaminergic medications or high-frequency stimulation of the STN (Bronte-Stewart et al., 2009; Kuhn et al., 2008; Levy et al., 2002; Priori et al., 2004; Wingeier et al., 2006). Beta-band LFP oscillations also increase in the internal pallidal segment (GPi) (Brown et al., 2001; Cassidy et al., 2002), and abnormal delta and theta band activities have been found in electroencephalographic recordings (EEGs) from the primary motor cortex (M1) of parkinsonian patients (Neufeld et al., 1994; Serizawa et al., 2008; Soikkeli et al., 1991; but see Sebban et al., 1999).
Studies in animal models of PD are consistent with these observations in patients. Thus, the power of beta-band oscillations was found to be increased in basal ganglia LFPs in 6-hydroxydopamine treated (dopamine-depleted) rats (Avila et al., 2009; Mallet et al., 2008; Sharott et al., 2005), and in single neuron recordings in monkeys treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Bergman et al., 1994; Meissner et al., 2005; Wichmann et al., 1994). More recently, oscillatory activity patterns were also demonstrated in M1 neuron recordings in MPTP-treated primates (Pasquereau and Turner, 2011).
Because M1, STN, and GPi are anatomically connected (Mathai and Smith, 2011), the hypothesis has emerged that parkinsonism-related oscillations in these structures are linked. According to this view, dopamine depletion in PD triggers pathologic oscillatory patterns throughout the basal ganglia-thalamocortical network, eventually leading to the behavioral signs of parkinsonism. However, while there is strong evidence that abnormal oscillations occur in this network in the dopamine-depleted state, it is not clear whether the oscillations cause parkinsonism. While some evidence in PD patients suggests that beta band activity in the STN contributes to akinesia (Kühn et al., 2004;Williams et al., 2005), other studies failed to demonstrate a strong correlation between clinical impairments and STN beta-band activity at rest in these patients (Kuhn et al., 2009; Ray et al., 2008; Weinberger et al., 2006). Recordings in monkeys showed also that parkinsonism precedes changes in oscillatory single neuron activities in GPi (Leblois et al., 2007).
Because of this uncertainty, we examined the temporal relationship between the appearance of motor disabilities and the appearance of oscillatory LFP changes in STN and GPi, and M1 EEG in a progressive model of parkinsonism in primates. If increased low-frequency LFP or EEG oscillations truly cause parkinsonism, the onset of such changes would have to either coincide with or precede the appearance of parkinsonism. In order to avoid the confounding effects of parkinsonism-related changes of the state of arousal (Rye, 2006; Videnovic and Golombek, 2013), we focused our examination of parkinsonism-related oscillations on observations in the awake state. Finally, we examined the effect of antiparkinsonian levodopa treatment on oscillatory activity in STN, GPi andM1 in the fully developed parkinsonian state.
Materials and methods
General experimental strategy
We studied rhesus monkeys in the normal state, during the gradual development of parkinsonism induced by repeated small injections of MPTP, and again, when treated with antiparkinsonian doses of levodopa in the fully parkinsonian state. The animals were permanently implanted with EEG recording electrodes over theM1 cortex, as well as recording chambers that allowed us to record LFP signals in STN and GPi. Signals were recorded at weekly intervals, and then subjected to spectral analysis. The coherence between simultaneously recorded traces from different brain locations was also analyzed.
Animals, surgical procedures
Three adult rhesus monkeys (Macaca mulatta; 6–10 kg; monkeys A, B, and C; 1 male and 2 females) were used. The animals were pair housed with other monkeys, and had ad libitum access to food and water. All experiments were performed in accordance with the United States Public Health Service Policy on the humane care and use of laboratory animals, including the provisions of the “Guide for the Care and Use of Laboratory Animals” (Garber et al., 2011). All studies were approved by the Institutional Biosafety and Animal Care and Use Committees of Emory University.
The animals were first conditioned to accustom them to handling by the experimenter and to sitting in a primate chair. They then underwent aseptic surgery under isoflurane anesthesia (1–3%) for placement of epidural bone screw electrodes and two stainless steel recording chambers (Crist Instruments, Hagerstown, MD; inner chamber diameter 16 mm), positioned stereotactically and embedded, along with a stainless steel head holder, into an acrylic ‘cap’. Four epidural screw electrodes (diameter: 0.25 mm, length 0.4 mm) were inserted on each side of the skull bilaterally through small holes drilled in the skull over M1 for bipolar recordings. In this study, only recordings from the left side were used (ipsilateral to the basal ganglia recordings). Wires from the electrodes were soldered to a 9-pin connector that was also embedded in the acrylic. The chambers were directed at the pallidum and the STN on the animal's left side. The pallidal chamber was placed at a 40° angle from the vertical in the coronal plane (A = 11, L = 11, D = 2), and the STN chamber was positioned at a 36° angle anterior to the vertical in the sagittal plane (A=10, L=7, D=2). The animals were allowed to recover for one week after the surgery before recording and other procedures begun.
MPTP treatment
After the recordings in the normal state were completed, the animals were rendered progressively parkinsonian by weekly administration of small doses of MPTP (0.2–0.6 mg/kg i.m.).Monkey A received 21 injections (9.4 mg/kg total), monkey B received 26 injections (10.2 mg/kg total) and monkey C received 19 injections (6.0 mg/kg total). All three animals eventually reached comparable states of stable moderate parkinsonism (defined below). To assess the degree and stability of the MPTP-induced motor disability, the severity of parkinsonism was assessed weekly while the monkey was in an observation cage equipped with infrared beams, allowing us to continuously observe the animal and to automatically measure its body movements. In these sessions we also scored the motor impairment in terms of ten aspects of motor function (bradykinesia, freezing, extremity posture, trunk posture, action tremor, the frequency of arm and leg movements, finger dexterity, home cage activity, and balance). Each was scored on a 0 to 3 scale (maximal score 30). The maximal severity of parkinsonism in any animal in this study was 15, corresponding to moderately severe parkinsonism. For statistical comparisons, we binned the range of scores into three groups: stage 1 (scores between 0 and 5), stage 2 (scores between 6 and 10), and stage 3 (scores between 11 and 15). For each monkey, several recordings in each of the 3 stages of parkinsonism were available (Table 1).
Table 1.
Total number of recording sessions across different stages of parkinsonism (as defined in the Methods), and numbers of ‘wakefulness’ or ‘sleep’ periods within these recordings (each period lasted 10 s).
| Monkey | Baseline | Stage 1 | Stage 2 | Stage 3 | |
|---|---|---|---|---|---|
| A | Number of recording sessions | 3 | 10 | 3 | 2 |
| Number of wakefulness periods | 54 | 950 | 249 | 143 | |
| Number of sleep periods | 40 | 10 | 10 | 10 | |
| B | Number of recording sessions | 12 | 6 | 13 | 17 |
| Number of wakefulness periods | 627 | 389 | 769 | 994 | |
| Number of sleep periods | 109 | 44 | 31 | 68 | |
| C | Number of recording sessions | 8 | 4 | 4 | 5 |
| Number of wakefulness periods | 202 | 192 | 259 | 349 | |
| Number of sleep periods | 209 | 106 | 94 | 23 |
Electrophysiological mapping
All recordings were made while the animal sat in a primate chair with its head immobilized but its body and limbs free to move. The locations of STN and GPi were mapped by extracellular electrophysiological recording with tungsten microelectrodes (FHC, Bowdoinham, ME; Z = 0.5–1.0 MΩ at 1 kHz). The dura was pierced with a guide tube and the electrode lowered into the brain with a microdrive (MO-95B; Narishige, Tokyo, Japan). The locations and borders of STN and GPi (in chamber coordinates) were defined with single unit extracellular recordings. These signals were amplified (DAM-80 amplifier; WPI, Sarasota, FL), filtered (400–6000 Hz; model 3700 filter; Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DL1540; Yokogawa, Tokyo, Japan), and made audible via an audio amplifier. Neurons in the STN were identifiable by their typical activity pattern, which differed from that of the more dorsal zona incerta (low neuronal activity), and that of the more ventrally located substantia nigra pars reticulata (Starr et al., 2000). GPi neurons were identified by their location ventromedial to the external pallidal segment, and by their continuous high frequency discharge (DeLong, 1971).
After delineating STN and GPi, the sensorimotor portion of each nucleus was targeted for LFP recordings. LFPs were recorded with bipolar concentric electrodes (SNEX-100 X 120 mm; outer diameter, 250 µm; inter-contact separation, 500 µm; impedance, 25–35 kΩ; Rhodes Medical Instruments Inc., Tujunga, CA). On each recording day, one of these electrodes was placed in the STN and one in the GPi for simultaneous recordings from the two structures. Beginning 10 min after the insertion of the electrodes, LFPs were recorded continuously for at least 10 min. Video images of the animal's face and M1-EEG signals were recorded at the same time. LFP and EEG signals were amplified, band-pass filtered (0.3–3 kHz), sampled at 1 kHz, and stored on a computer disk with a data acquisition interface (Power 1401; CED, Cambridge, UK) and commercial software (Spike2, CED). Recordings were made weekly, before, during, and at least 5 days after each of the series of MPTP injections.
Drug treatment
We assessed the effect of systemic levodopa treatment on behavior and electrical signals in monkeys B and C, after they developed stablev stage 3 parkinsonism, by administering levodopa and the dopa-decarboxylase inhibitor benserazide (both from Sigma-Aldrich, St. Louis, MO) in a 4:1mixture (i.m.).Monkey B received 30 mg/kg levodopa, and monkey C 20 mg/kg. For each animal, we first determined the latency and peak of the levodopa effect on spontaneous movement in the observation cage described above. After a 20-minute baseline observation period, the monkeys received levodopa/benserazide, followed by continuous monitoring for 100 additional minutes. During these monitoring periods involuntary movements (chorea or dystonia) were not observed. The effects of levodopa on LFP and EEG signals were examined in separate sessions with the animal seated in the primate chair (as described above). After a baseline recording of at least 30 min, the monkey received the injection, and recording was continued for 2 additional hours.
Individual levodopa injection sessions were separated by at least 2 days. We carried out 8 levodopa injections in monkey B (4 for behavioral observation and 4 for electrophysiological recordings), and 6 in monkey C (3 for behavioral observation and 3 for electrophysiological recordings). In addition, 4 control injections (saline) were carried out in monkey C (2 for behavioral assessments, 2 for electrophysiological recordings).
Termination of the experiment
After completion of the experiment, the animals were killed with an overdose of pentobarbital sodium (100 mg/kg, i.v.) and transcardially perfused with cold oxygenated Ringer's solution, followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in a phosphate buffer (PB) solution. After perfusion, the brains were removed from the skull, cut coronally into 10 mm thick blocks, and post-fixed overnight in 4% paraformaldehyde. The blocks were then cut into 60 µm-thick coronal sections using a vibrating microtome. Sections were stained withMAP2 to verify electrode position or immunostained for tyrosine hydroxylase (TH) to assess the degree of loss of dopaminergic fibers in the striatum and dopaminergic neurons in the substantia nigra pars compacta (SNc) (Supplementary Figs. 1 and 2).
Data analysis
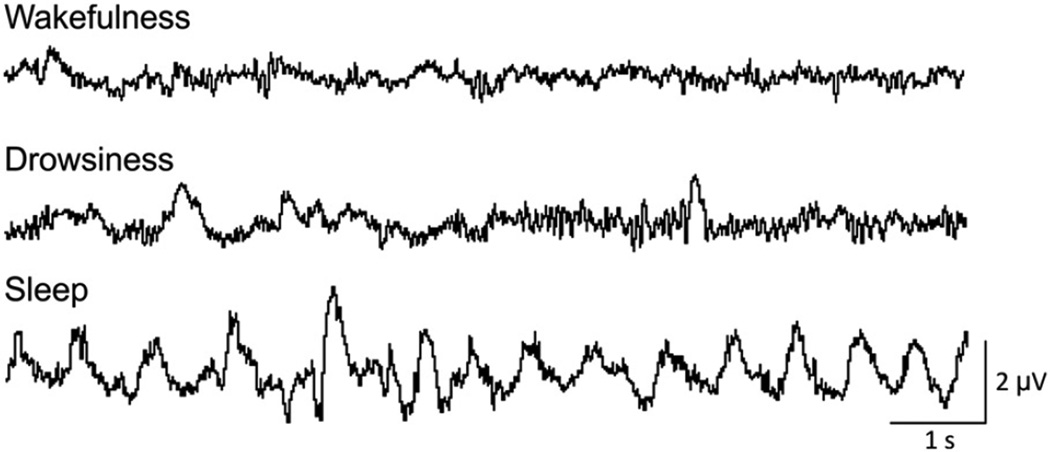
Scoring of the state of arousal was manually performed off-line on consecutive 10 s epochs of face video and EEG data, using the “sleep score” Spike2 script (CED). Each epoch was scored as representing wakefulness, drowsiness, or sleep (Fig. 1). The animal was classified as being “awake” if it was attentive to its surroundings with eyes open, and showed low-amplitude EEG. Closing of the eyes, with increased lower frequency activity in the EEG indicated “drowsiness”. “Sleep” episodes were defined as periods during which the monkey was quiet with its eyes closed while the EEG exhibited high amplitude slow activity (Barraud et al., 2009; Hsieh et al., 2008). Table 1 shows the numbers of wakefulness, drowsiness, and sleep epochs in our recording sessions. The tabulated data do not necessarily reflect the sleep disturbances that can be found in MPTP-treated monkeys under more natural conditions (Barraud et al., 2009). Since the level of arousal strongly alters basal ganglia LFPs and M1 EEG signals and may confound the analyses of parkinsonism-related changes (Adler et al., 2010; Salih et al., 2009; Stefani et al., 2006), we restricted the subsequent analysis to segments recorded while the monkeys were awake (exceptions to this are noted in the text).
Fig. 1.
Examples of M1 EEG recordings in monkey B, of 10 second epochs scored as representing wakefulness, drowsiness, or sleep. Calibration bars apply to all traces.
Spectral analysis
All subsequent data analysis steps were done off-line with custom written MATLAB scripts (MATLAB version 7.6, The Math works, Natick, MA, USA). After importing data into the Matlab environment, the identified epochs of wakefulness were extracted from each data record and concatenated to create a single file representing the awake stage on a given day. Simultaneously recorded EEG and LFP recordings from STN and GPi were band-pass-filtered using 4th order Butterworth filtering (3–70 Hz). The high-pass filtering with a cutoff at 3 Hz served to remove movement artifacts. Line noise artifacts were removed using a 60-Hz 4th order Butterworth notch filter. Power spectral density was computed using the Welch technique, with Hamming windowing, and a fast Fourier transform segment length of 256 samples with no overlap, resulting in a final spectral resolution of 3.9 Hz across the 3.9–62.5 Hz range. To reduce variability of the absolute power between recording days, we normalized the data to the total power of the respective recording session. The variability between days may have been caused by changes in the characteristics of the recording electrodes or of the tissue surrounding them. In principal, day-to-day differences in the amplitude of LFP signals from GPi or STN may also have been caused by differences in electrode placement. To minimize this source of variability, the electrodes were very carefully positioned from day to day to be in the same locations. We did not detect any major shifts in the anatomical electrode position on histologic analysis (Supplementary Fig. 1) however ANOVAs performed on absolute power values failed to detect reliable changes (in at least 2 monkeys).
Analyses of the coherence and the phase relationships of pairs of signals from M1, STN, and GPi (M1–STN, M1–GPi, and STN–GPi) were carried out using the Matlab csd function (segment length of 256 samples, Hamming windowing with no overlap, 3.9 Hz resolution). To perform the coherence analysis on the same amount of data for each parkinsonian stage and each monkey (so that biases in the coherence estimates related to the duration of the analyzed data were avoided), we randomly selected 50 10 s-epochs of data representing wakefulness, 10 10 s-epochs of data representing sleep and 50 10 s-epochs of the ‘entire’ data set (containing sleep-and wakefulness periods), from each monkey and stage of parkinsonism.
Changes in power, coherence, and phase were analyzed for 5 frequency bands: 3.9–7.7 Hz, 7.8–15.5 Hz, 15.6–23.3 Hz, 23.4–35.1 Hz, and 35.2–62.5 Hz. These bands were chosen because preliminary analyses showed that specific spectral changes occurred in these bands. “Wakefulness” data recorded at different grades of severity of parkinsonism were compared using two-way ANOVAs and Holm–Sidak post-hoc testing, using a significance criterion of p < 0.05.
The same method was used to assess the effects of the state of arousal on parkinsonism. Recordings made at baseline and in stage 3 of parkinsonism were compared, for epochs in which the monkeys were awake, for epochs when they were asleep, and for the entire data set (including all states of arousal, i.e., wakefulness, drowsiness, and sleep). Data were compared between baseline and stage 3 using a Mann–Whitney rank sum test, using a significance criterion of p < 0.05. The Z-score of changes in stage 3 was obtained by using the mean and standard deviation of the pre-MPTP baseline recording (Figs. 4 and 6).
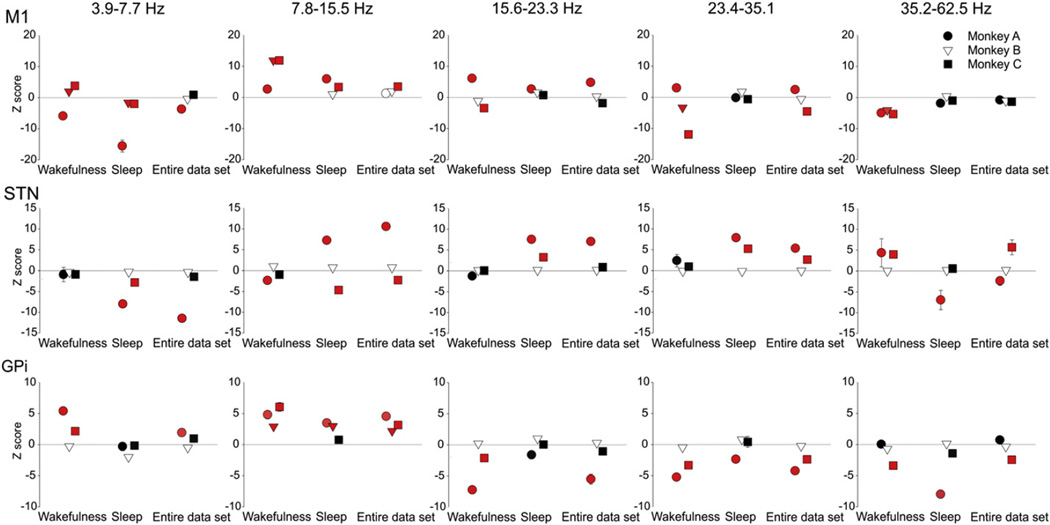
Fig. 4.
Z-scored changes between baseline and stage 3 parkinsonism in spectral power of M1 EEGs, and STN or GPi LFPs, analyzed separately for wakefulness, sleep, or the entire data set (encompassing wakefulness, drowsiness and sleep). Red symbols correspond to significant comparisons. p < 0.05, compared to normal state, 2-way ANOVA with post-hoc Holm–Sidak testing.
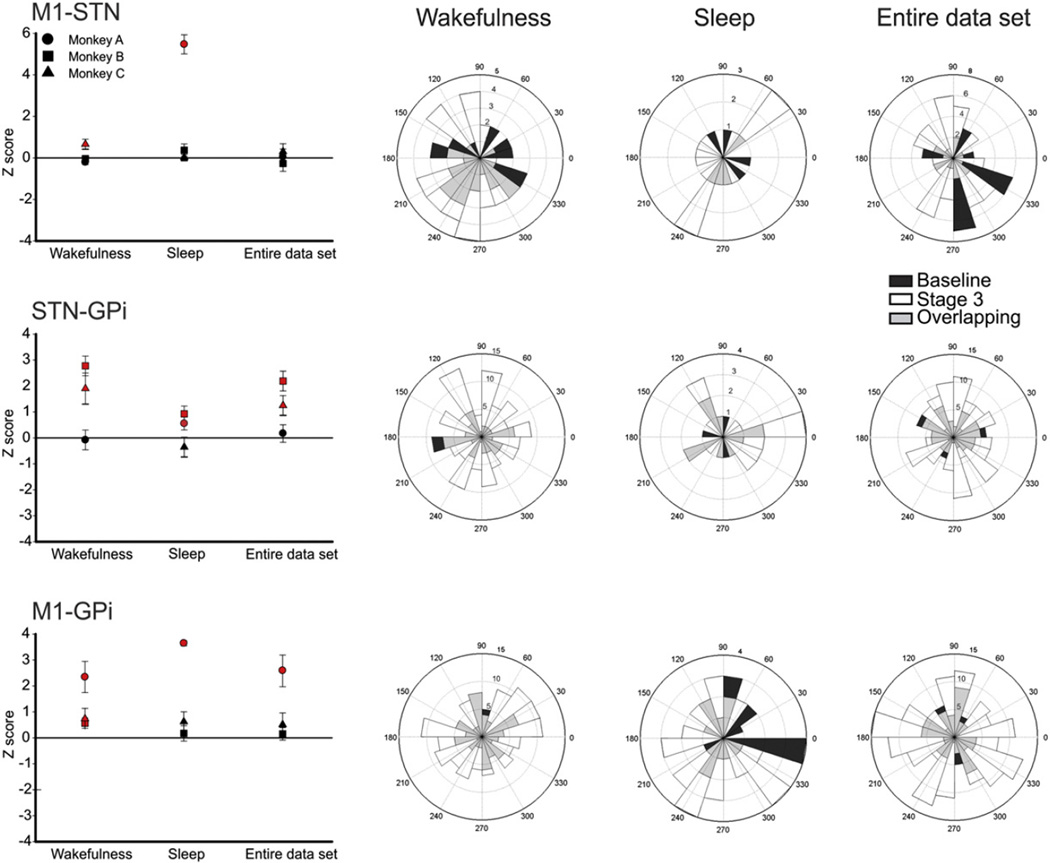
Fig. 6.
Comparison of M1–STN, STN–GPi, and M1–GPi coherence and phase angles in the 7.8–15.5 Hz range between baseline and stage 3 of parkinsonism across different states of arousal. The plots on the left show Z-scored coherence values for the 3 monkeys. Significant values (p < 0.05, comparisons between controls and stage 3 recordings) are indicated in red. The plots on the right are polar representation of preferred phase angles for the three signal pairs, at baseline and in stage 3 of parkinsonism across different states of arousal. For the phase representation, the data from all three animals were combined. The phase angles are shown in degrees. The numbers on the polar lines correspond to the number of the recording session in which the respective phase angle was found; phase angles measured in pre-MPTP baseline recordings are shown in white, those measured in stage 3 parkinsonism are shown in black. The gray markers correspond to the sessions in which baseline and stage 3 parkinsonism shared the same preferred phase angle (‘Overlapping’). Coherence and phase comparisons were done separately for each monkey using a Mann–Whitney rank sum test (p < 0.05), using recordings from the baseline period and from stage 3 of parkinsonism.
Comparison between baseline coherence during periods of wakefulness and sleep from the same recording sessions was carried out with a Wilcoxon signed rank test for paired data, using a significance criterion of p < 0.05 (Supplementary Fig. 3).
Analysis of relationship between signal changes and the state of parkinsonism
Data are expressed as mean± SEM. Because of differences between monkeys, statistics were done separately for each animal. For periods of wakefulness, the relationship between changes in oscillatory activities/ coherence and the stage of parkinsonism was assessed with two-way ANOVAs, with the stage of parkinsonism (Baseline, Stage 1, Stage 2, Stage 3), and frequency bands (3.9–7.7 Hz, 7.8–15.5 Hz, 15.6–23.3 Hz, 23.4–35.1 Hz, 35.2–62.5 Hz) as factors, and Holm–Sidak post-hoc testing, using a significance criterion of p < 0.05.
The relationship between the raw motor score (not the stage of parkinsonism) and the value of the spectral power in each band was analyzed with a Pearson correlation test (the results are shown in Table 2).
Table 2.
Correlation between raw parkinsonism scores and spectral power in separate spectral bands in M1 EEG, and STN or GPi LFPs.
| Monkey | 3.9–7.7 Hz | 7.8–15.5 Hz | 15.6–23.3 Hz | 23.4–35.1 Hz | 35.2–62.5 Hz | |
|---|---|---|---|---|---|---|
| M1 | A | −0.71** | 0.13 | 0.73*** | 0.42 | −0.47 |
| B | 0.55*** | 0.82*** | −0.42** | −0.82*** | −0.79*** | |
| C | 0.80*** | 0.88*** | −0.77*** | −0.88*** | −0.83*** | |
| STN | A | −0.14 | −0.21 | 0.00 | 0.09 | 0.31 |
| B | −0.25 | 0.57*** | 0.11 | 0.02 | −0.01 | |
| C | −0.28 | −0.42 | −0.02 | 0.28 | 0.59* | |
| GPi | A | 0.46 | 0.71*** | −0.68** | −0.78*** | −0.10 |
| B | −0.9 | 0.62*** | 0.15 | −0.29* | −0.41** | |
| C | 0.68*** | 0.70*** | −0.68*** | −0.77*** | −0.59** |
Shown are coefficients of correlation, calculated separately for each band. The significance of results was tested with Pearson's test
indicates p < 0.05,
indicates p < 0.01,
indicates p < 0.001.
To analyze the data from the levodopa and saline injections and to visualize spectral power changes over time, Welch's periodogram and coherence plots were constructed from consecutive 10-min windows of data without overlap (same techniques as above). Using the mean and standard deviation of the pre-injection baseline recording, we calculated the Z-score of changes after injection for each injection experiment. For each monkey we then averaged the Z-scores obtained at specific 10-minute post-injection intervals across all recording days.
Results
Behavioral effects of MPTP
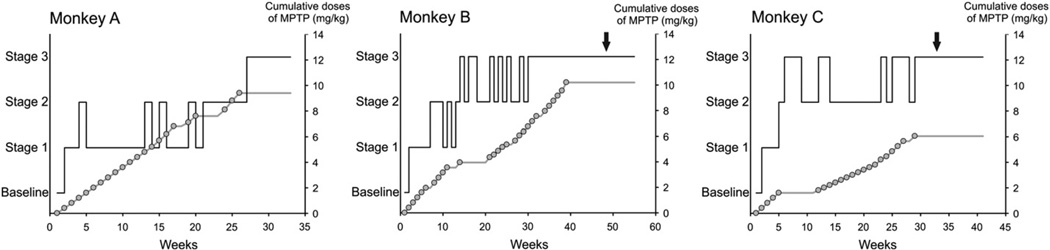
All animals were treated until they reached stage 3 parkinsonism. The progression of parkinsonian motor signs differed between monkeys (Fig. 2). All monkeys showed a rapid initial response to MPTP treatment, exhibiting at least mild parkinsonism after 1 or 2 injections of the toxin. Subsequent progression involved worsening of existing motor signs, rather than the development of new ones. All monkeys developed marked bradykinesia, arm and leg akinesia, and impairment in trunk posture. At the end of the MPTP treatment, monkeys A, B, and C reached parkinsonism rating scores of 15, 14 and 13, respectively, and no recovery was seen after this point. As expected, postmortem TH staining revealed a substantial loss of dopamine in striatum and SNc in all three animals (Supplementary Fig. 2), similar to that reported in previous papers (Galvan et al., 2011).
Fig. 2.
Temporal progression of the development of parkinsonism induced by MPTP injections, based on weekly evaluations. The solid black line shows the stage of parkinsonism. The cumulative dose of MPTP is shown as a gray line with open circles, where each circle corresponds to a single MPTP injection (0.2–0.6 mg/kg). The arrow indicates the start of levodopa testing (only done in monkeys B and C).
Power spectral analysis
Changes related to the severity of parkinsonism
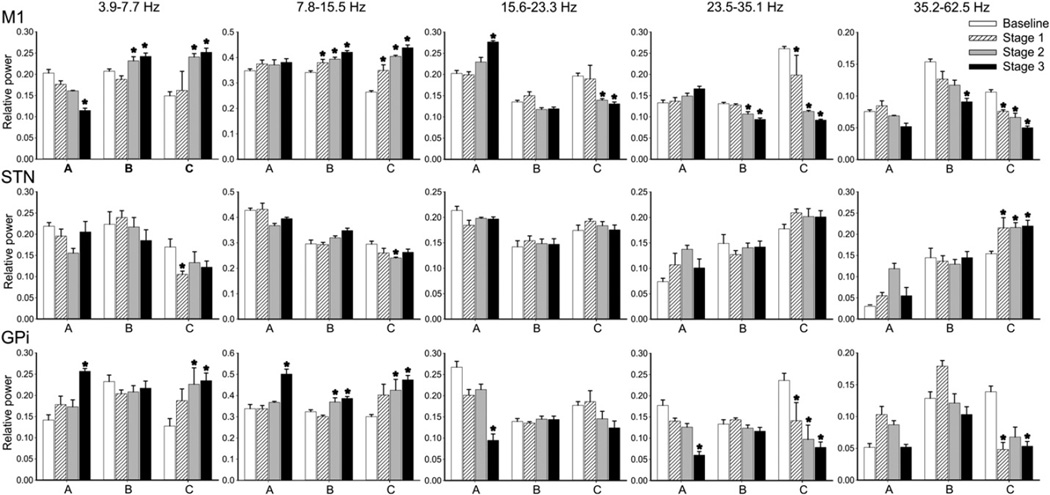
We examined the development of oscillatory changes in EEG and LFP signals separately for each animal (Fig. 3).
Fig. 3.
(2-column image) Relative spectral power (mean ± SEM) ofM1, STN and GPi signals (EEG, LFPs) across different stages of parkinsonism in monkeys A, B, and C. * indicates p < 0.05, compared to baseline; 2-way ANOVA with post-hoc Holm–Sidak testing.
In the M1 EEG signals, we found significant increases in power in the 3.9–7.7 Hz and 7.8–15.5 Hz range in monkeys B and C. In the 7.8– 15.5 Hz range, the increase was statistically significant in stage 1. In monkey A there was also a slight increase of power in the 7.8–15.5 Hz range, but this was not statistically significant. In contrast, power in the 23.4–35.1 Hz and 35.2–62.5 Hz frequency range decreased in monkeys B and C (Fig. 3). The increase in power at low frequencies (below 15.5 Hz) and the decrease in power at frequencies above 15.6 Hz were correlated with the progression of parkinsonian signs (Fig. 3 and Table 2).
There were no consistent parkinsonism-related changes seen in the STN (Fig. 3 and Table 2). The only significant change in oscillatory power in STN LFPs occurred in monkey C, with a decrease in frequencies below 15.5 Hz, and an increase in the 35.2–62.5 Hz band.
Spectral power in GPi LFPs increased in the 7.8–15.5 Hz range in monkeys B and C in stage 2, and in all monkeys in stage 3. In addition, we found a decrease in power in the 23.4–35.1 Hz frequency range in all monkeys, which was statistically significant in monkeys A and C. In monkey C, the decrease was already significant in stage 1, while it did not become significant in the other animal until stage 3. For all monkeys, the increase of power in the 7.8–15.5 Hz range and the decrease in the 23.4–35.1 Hz frequency range were significantly correlated with the motor score (Fig. 3 and Table 2).
Influence of the state of arousal
To study the influence of the state of arousal on the power spectral composition of signals across the stages of the disease, we compared episodes of wakefulness, episodes of sleep, and the entire data set (including wakefulness, drowsiness, and sleep). It would have been interesting to carry out this analysis for all periods of the experiment, but the amount of sleep data was not sufficient in stages 1 and 2 of parkinsonism, so that we compared only data collected at baseline and in stage 3 parkinsonism.
The increases in the 7.8–15.5 Hz band in M1 EEG, and in GPi LFPs, were seen regardless of the stage of arousal (Fig. 4). In addition, power spectral increases in the STN LFPs were found in the sleep segments (or when the entire record was analyzed) in the 15.6–23.3 Hz and the 23.4–35.1 Hz bands in monkeys A and C. The STN LFP changes were not seen when the monkey was awake.
Coherence analysis
Changes related to parkinsonism
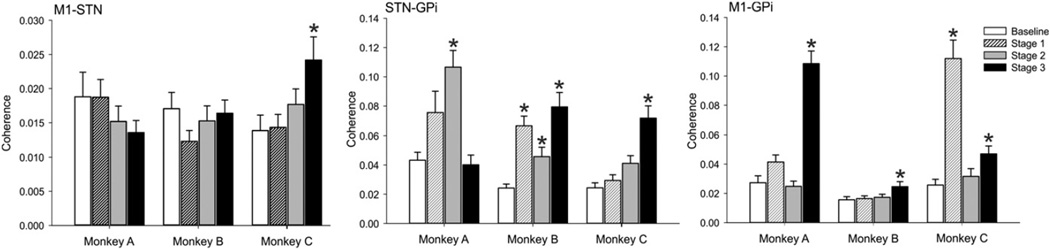
To study changes in the coherence of signals recorded in the different structures, we carried out a two-way ANOVA across the stages of parkinsonism and the 5 frequency bands. In signals from the wakefulness epochs, there were no consistent changes across all monkeys in stage 1 parkinsonism (see data from one of the frequency bands in Fig. 5), although increases in coherence in individual bands appeared in stages 2 and 3 of parkinsonism.
Fig. 5.
Coherence between M1 and STN, STN and GPi, and M1 and GPi signals in the 7.8–15.5 Hz frequency range across different stages of parkinsonism during periods of wakefulness, in monkeys A, B and C. * indicates p < 0.05 compared to baseline; two-way ANOVA with post-hoc Holm–Sidak testing.
In the 3.9–7.7 Hz frequency range, the coherence for STN–GPi pair of signals was increased for monkeys B and C, and increased for all three animals in the 7.8–15.5 Hz range (Fig. 5). In the 15.6–23.3 Hz range there was also an increase, reaching significance in monkeys B and C.
For the 7.8–15.5 Hz frequency range, the coherence in the M1-GPi pair of signals was statistically increased in the three animals, in stage 3 parkinsonism.
Changes in coherence between the different signals in the 23.4– 35.1 Hz and the 35.2–62.5 Hz frequency ranges were only seen in individual animals, and were therefore considered to be of questionable relevance.
We also calculated Pearson correlation coefficients for the individual signal pairs, using the raw parkinsonism scores (not the stages as defined above). In this analysis, the STN–GPi coherence in the 3.9–7.7 Hz band correlated with the worsening of parkinsonism for the 3 monkeys (Pearson correlation for monkeys A, B and C, respectively: R = 0.14 [p < 0.05], R=0.31 [p < 0.001] and R=0.32 [p < 0.001]), and for monkeys Band C in the 7.8–15.5Hz range (R=0.24 [p< 0.001] and R=0.31[p < 0.001] respectively). For the 15.6–23.3 Hz range, the STN–GPi coherence correlated with the worsening of parkinsonism in all 3monkeys (R=0.14 [p > 0.05], R=0.26 [p < 0.001] and R=0.18 [p < 0.001], respectively).
The M1–GPi coherence was significantly correlated with parkinsonism in monkeys A and B in the 7.8–15.5 Hz range (R=0.60 [p < 0.001] and R = 0.17 [p < 0.05], respectively).
There were no consistent changes in the analysis of phase angles for any of the frequency bands (see also Fig. 6).
Influence of the state of wakefulness
This analysis was carried out only on data from control periods and stage 3 parkinsonism (see above). As expected, the baseline M1–STN andM1–GPi coherence was stronger at low frequencies (<15.5 Hz) during sleep than during periods of wakefulness (see supplementary Fig. 3). The STN–GPi coherence was not affected by the state of arousal at baseline.
Compared with the baseline coherence values, stage 3 parkinsonism was associated with a higher STN–GPi and M1–GPi coherence in the 7.8–15.5 Hz range during wakefulness, without a change in phase angles (see above, and Fig. 6). The increase in STN–GPi coherence was also found when the comparison was performed using the entire data set (without regard for the state of arousal), and when the analysis was restricted to data from the sleep epochs. The greater coherence in theM1–GPi pair of signals found for the 3monkeyswas not consistently seen when epochs of sleep were included in the analysis.
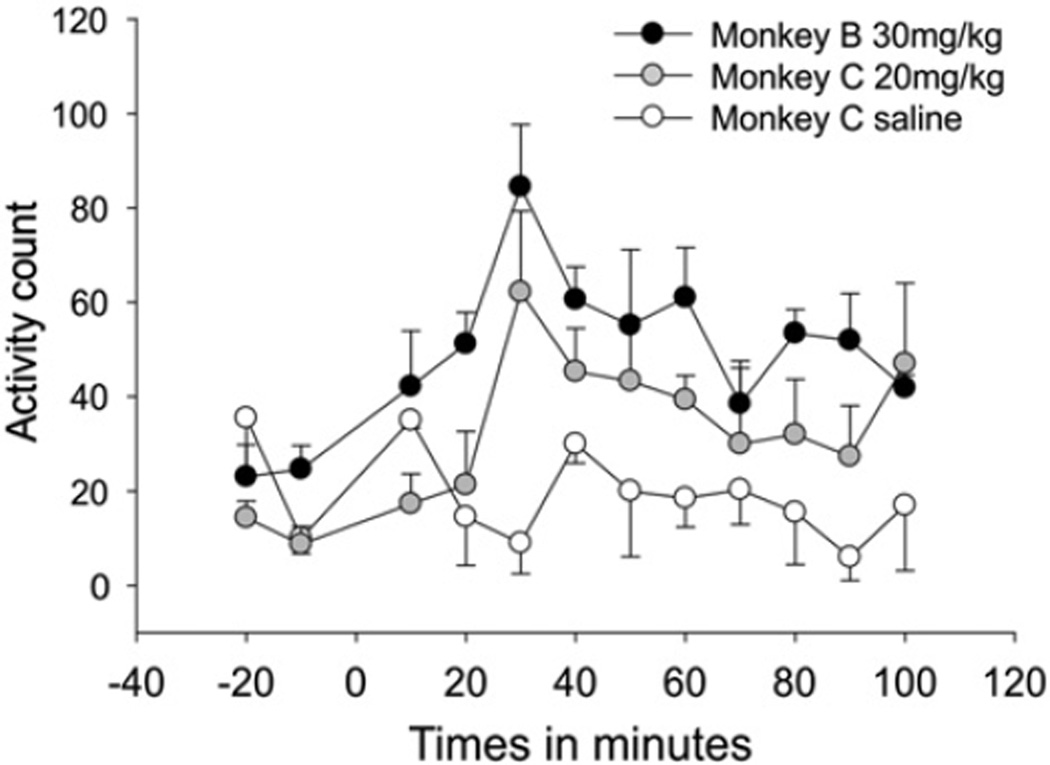
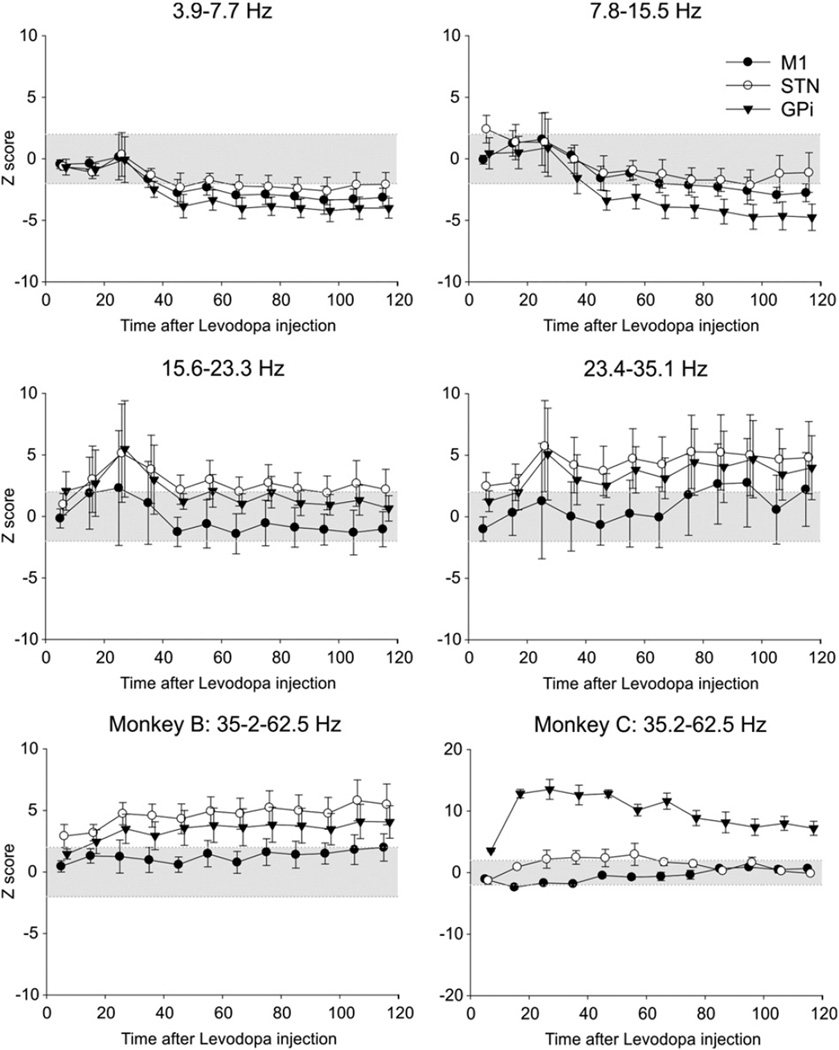
Effects of levodopa treatment
Treatment with levodopa increased each animal's locomotor activity (Fig. 7), peaking 30min after the drug administration, and persisting beyond the end of the observation time (100 min). Saline injections (only done in monkey C) did not alter locomotion. As shown in the Fig. 8, levodopa injections induced a gradual decrease in spectral power in STN– and GPi–LFPs and EEG signals from M1 for frequencies below 15.5 Hz, and a gradual increase in power at higher frequencies, above 23.4 Hz (see also Supplementary Fig. 4). The changes were more prominent in GPi then in M1 or STN. The increases at the higher frequencies (23.4–62.5 Hz) for STN and GPi started about 30min after the injection, and lasted until the end of the recording. Saline injections had no effect. Levodopa or saline injections did not significantly alter coherence for the M1–STN, STN–GPi and M1–GPi signal pairs (not shown).
Fig. 7.
Motor activity, as measured by counting infrared beam breaks in an activity monitoring cage, after levodopa injection (monkeys B and C) or saline injection (monkey C). Injections were given at time 0. Each data point represents the mean ± SEM of 3 experiments.
Fig. 8.
Summary of levodopa-induced changes in spectral power inM1 EEG, and STN– and GPi–LFPs. Injections were given at time 0 and changes (calculated for 10 min increments) are expressed as Z-score values, compared to the respective baselines. The figure shows data from monkey B in all but the last subplot. Changes were similar between monkeys, except for the 35.2–62.5 Hz range which is shown separately for monkeys B and C. Shown are results of an analysis of M1 EEG signals (filled circles, mean ± SEM), as well as LFPs from the STN (open circles) and GPi (triangles). The area marked in gray corresponds to Z-score values of ±2.
Discussion
This study confirmed that the development of parkinsonism is associated with increases in low frequency (7.8–15.5 Hz) spectral components and reductions in high frequency (>15.6 Hz) spectral power in basal ganglia LFP signals and M1 EEG signals, although such changes were not found in all animals. We also found an increase in the cortico-basal ganglia coherence in the low frequency range. The spectral changes in GPi and M1 were correlated with the severity of parkinsonism, with cortical signal changes appearing earlier than those in GPi. Interestingly, the distribution of spectral power in the STN LFP signals remained the same throughout the progression of parkinsonism. The magnitude of the changes in cortex and basal ganglia was dependent on the state of wakefulness.
Levodopa treatment reversed the spectral changes associated with parkinsonism, but did not affect the cortico-basal ganglia coherence. Levodopa treatment also reduced low-frequency oscillations in STN LFPs, despite the absence of pathological oscillations in the untreated parkinsonian state.
Parkinsonism-related changes in spectral power
We found that parkinsonism-related changes in the distribution of spectral power differed among M1, GPi, and STN. Our finding of a strong relationship between the severity of parkinsonism and spectral power of M1 EEG at low frequencies is in general agreement with previous studies that documented such increases in patients with PD (Pugnetti et al., 2010; Serizawa et al., 2008; Soikkeli et al., 1991). Motor scores were also correlated with increased low frequency power in GPi, but this became statistically significant only when the monkeys reached at least stage 2 of parkinsonism. This finding is reminiscent of previous findings in monkeys in which single cell oscillatory discharge in the pallidum was only detectable in moderate to severe parkinsonism (Leblois et al., 2007).
Many reports have shown that LFPs recorded in the STN of parkinsonian patients contain prominent low-frequency oscillations (mostly in the beta band) and low gamma-band power, and that both changes are reversible with levodopa treatment or high-frequency stimulation (Brown et al., 2001; Giannicola et al., 2010). This has led to the hypothesis that beta-band oscillations in the basal ganglia, and particularly in the STN, in some way cause or contribute to parkinsonism. However, in our study, abnormal oscillatory activity was not consistently found in the STN. Together with the aforementioned findings in the GPi recordings, this result suggests that a detectable level of oscillatory LFP activity in STN and GPi is, in fact, neither necessary nor sufficient for early signs of parkinsonism, including obvious bradykinesia and akinesia. This hypothesis is also supported by some of the studies in patients with PD which did not find a clear correlation between beta band power and the severity of parkinsonism under resting conditions (Weinberger et al., 2006). Likewise, neuroleptic-induced catalepsy was not associated with exaggerated beta band activity in STN or cortex in rodents (Mallet et al., 2008), and there were no changes found in M1 and SNr activity at rest in studies in 6-OHDA treated rats (Brazhnik et al., 2012). However, recent human studies have found significant correlations between motor score and aspects of beta band activity in the STN, using the complexity (Chen et al., 2010) or the stability of STN LFP signals (Little et al., 2012).
Even in the absence of such parkinsonism-related changes in spectral power in the STN, levodopa treatment reduced the power in low-frequency bands and increased it in the high-frequency bands. Levodopa treatment thus does not seem to specifically counteract or normalize pathologic spectral patterns in STN LFPs, but appears to more generally decrease power in lower frequencies and enhance power in higher frequencies in these signals. It is not clear whether these spectral changes were causally related to the reversal of parkinsonism in these animals.
The findings that abnormal oscillations in M1 EEG signals preceded those in the basal ganglia signals may suggest that abnormal cortical activity developed before that in the basal ganglia (see also Goldberg et al., 2002). However, this interpretation must be considered with caution, as the cortical signals tend to be larger and more robust than basal ganglia LFP signals (because of the laminar organization of cortex that contrasts with the non-laminar architecture of the basal ganglia), and may thus allow earlier detection of oscillations. Of course, such structural differences would not explain differences between pallidal and STN LFP signals.
Contrasting with the prominent parkinsonism-associated beta-band changes in basal ganglia LFPs in patients (Levy et al., 2002), most spectral changes in our study occurred in the 7.8–15.5 Hz band. A similar spectral distribution was also identified in previous single-cell recording studies in MPTP-treated monkeys (see, e.g., Bergman et al., 1994; Leblois et al., 2007; Moran et al., 2012; Soares et al., 2004). Based on such findings it has been suggested that the 8–15 Hz frequency range in monkeys may be a subset of, or equivalent to, the beta band in humans (Stein and Bar-Gad, 2013).
Parkinsonism-related changes in coherence in the cortico-basal ganglia network
Studies in PD patients (Shimamoto et al., 2013;Williams et al., 2002) and 6-ODHA treated rats (Magill et al., 2000, 2001; Sharott et al., 2005) demonstrated an increase in the coherence of oscillatory activities between cortex and the basal ganglia in parkinsonian individuals. Further, imaging studies in PD patients showed an increase of the functional connectivity between M1 and STN (Baudrexel et al., 2011), and STN single unit activity was found to be synchronized with M1 LFPs (Shimamoto et al., 2013). Our previous studies in MPTP-treated monkeys also demonstrated that cortico-basal ganglia interactions intensify in moderately parkinsonian animals (Gatev and Wichmann, 2009). In the present study, we found a correlation between the severity of (untreated) parkinsonism and coherence between GPi and STN or M1 in the 7.8–15.5 Hz and in the 15.6–23.3 Hz frequency range. The parkinsonism-related increase of coherence was not reversed by antiparkinsonian doses of levodopa, reminiscent of the results of a previous study in which dopaminergic medication had no significant effect on the cortico-subthalamic interaction (Litvak et al., 2011). This suggests that the observed coherence changes are associated with, but are not causal of, parkinsonism, and that they may not be primarily dopaminergic in origin. It is possible that these coherence changes result instead from other changes in the basal ganglia, thalamus, or cortex that are known to accompany parkinsonism in human PD and in MPTP-treated primates, such as changes in striatal spine density, loss of the thalamostriatal system, or changes in non-dopaminergic transmitter systems, such as the noradrenergic or serotonergic systems (Villalba and Smith, 2011; Villalba et al., 2009). Such changes would not be expected to be reversible by acute stimulation of dopamine receptors.
Interaction between the state of arousal and oscillatory activity in parkinsonism
Many studies have demonstrated that reduced dopamine transmission is associated with reduced arousal. Thus, changes in alertness are present in most parkinsonian patients (Najafi et al., 2013; Rye et al., 2000), in patients treated with dopamine receptor antagonists (Bliwise et al., 2012), and in MPTP-treated monkeys (Barraud et al., 2009). Because oscillatory and non-oscillatory neuronal activities in the basal ganglia are strongly affected by the state of arousal under normal conditions (Brown et al., 2001; DeLong, 1969; Lazarus et al., 2012; Salih et al., 2009; Stefani et al., 2006), it was important to examine whether some of the oscillatory changes that are attributed to ‘parkinsonism’ reflect concomitant changes in arousal instead. We found that the parkinsonism-related changes in M1 and GPi were similar across the studied stages of arousal, while parkinsonism-related changes in the STN were less prominent when the animal was fully awake than during other states (see also Najafi et al., 2013; Rye et al., 2000). This underscores the importance of taking the state of arousal of the subject into account when interpreting the relationship between oscillatory activity patterns in the basal ganglia and parkinsonian motor signs.
Potential study limitations
The relatively small number of animals is a limitation of this (and many other) non-human primate studies. To mitigate the inter-subject variability statistical analysis was performed individually for each monkey, and results considered of questionable importance if they occurred only in a single animal.
Another limitation in this study is that the animal's motoric state was not strictly controlled during the recordings. The animal's spontaneous movements were reduced after MPTP treatment, and this, by itself, may have altered cortical and basal ganglia activity patterns during the recording sessions. In addition, to control the damage introduced through probe insertions, LFP recordings were performed at single sites in STN and GPi in each animal. This may have lowered the sensitivity of our recording method, because previous studies have shown that the spatial distribution of oscillatory activities in the basal ganglia may be heterogeneous (de Solages et al., 2011; Kuhn et al., 2005; Levy et al., 2002). It may also be a limitation that the parkinsonism scores were recorded in temporal proximity to the recording sessions (1 day), but not during the LFP/EEG recordings themselves. We have no reason to believe, however, that the animal's parkinsonism was different between the observation and recording sessions. Finally, we acknowledge that the presented findings do not fully explore all possible changes in oscillatory measures of cortical and subcortical signals. For instance, recent studies suggest that it may be fruitful to study the interaction and relations between different frequency bands in the pathophysiology of parkinsonism (de Hemptinne et al., 2013; Lopez-Azcarate et al., 2010).
Conclusion
The presented results challenge the view that the motor signs of parkinsonism develop because of strong oscillatory synchrony reflected in spectral changes in LFP signals in the basal ganglia network. We found that different nodes of the cortico-basal ganglia network react differently to MPTP. It is particularly interesting that parkinsonism appears to be more strongly associated with detectable changes in oscillatory power in GPi and M1, than with changes in the STN, and that some of the levodopa-induced changes in oscillations are not a simple reversal of parkinsonian oscillation patterns. Finally, our results suggest that commonly seen oscillatory changes may be influenced by concomitant changes in arousal.
Supplementary Material
Acknowledgments
The authors thank Dr. Olivier Darbin for his work in the initial phases of the experiment, and Dr. Adriana Galvan for critically reading the manuscript. This study was supported by grants from the NIH/NINDS (R01-NS054976 and P50-NS071669) as well as an infrastructure grant from the NIH to the Yerkes Center (P51-RR165, now P51-OD11132).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2014.04.004.
Footnotes
The authors declare no competing financial interests.
References
- Adler A, Joshua M, Rivlin-Etzion M, Mitelman R, Marmor O, Prut Y, Bergman H. Neurons in both pallidal segments change their firing properties similarly prior to closure of the eyes. J. Neurophysiol. 2010;103:346–359. doi: 10.1152/jn.00765.2009. [DOI] [PubMed] [Google Scholar]
- Avila I, Parr-Brownlie LC, Brazhnik E, Castaneda E, Bergstrom DA, Walters JR. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp. Neurol. 2009;221:307–319. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate model. Exp. Neurol. 2009;219:574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, Steinmetz H, Deichmann R, Roeper J, Hilker R. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage. 2011;55:1728–1738. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Trotti LM, Wilson AG, Greer SA, Wood-Siverio C, Juncos JJ, Factor SA, Freeman A, Rye DB. Daytime alertness in Parkinson's disease: potentially dose-dependent, divergent effects by drug class. Mov. Disord. 2012;27:1118–1124. doi: 10.1002/mds.25082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J. Neurosci. 2012;32:7869–7880. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp. Neurol. 2009;215:20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J. Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Chen CC, Hsu YT, Chan HL, Chiou SM, Tu PH, Lee ST, Tsai CH, Lu CS, Brown P. Complexity of subthalamic 13–35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson's disease. Exp. Neurol. 2010;224:234–240. doi: 10.1016/j.expneurol.2010.03.015. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solages C, Hill BC, Yu H, Henderson JM, Bronte-Stewart H. Maximal subthalamic beta hypersynchrony of the local field potential in Parkinson's disease is located in the central region of the nucleus. J. Neurol. Neurosurg. Psychiatry. 2011;82:1387–1389. doi: 10.1136/jnnp.2010.223107. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons in the monkey during movement and sleep. Physiologist. 1969;12:207. [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J. Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and pharmacological modulation of GABA-B receptors in the globus pallidus of parkinsonian monkeys. Exp. Neurol. 2011;229:429–439. doi: 10.1016/j.expneurol.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Barbee R, Bielitzki J, Clayton L, Donovan J, Hendriksen C, Kohn D, Lipman N, Locke P, Melcher J, Quimby F, Turner P, Wood G, Wurbel H. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [Google Scholar]
- Gatev P, Wichmann T. Interactions between cortical rhythms and spiking activity of single basal ganglia neurons in the normal and parkinsonian state. Cereb. Cortex. 2009;19:1330–1344. doi: 10.1093/cercor/bhn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov. Disord. 2006;21:1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- Giannicola G, Marceglia S, Rossi L, Mrakic-Sposta S, Rampini P, Tamma F, Cogiamanian F, Barbieri S, Priori A. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson's disease. Exp. Neurol. 2010;226:120–127. doi: 10.1016/j.expneurol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J. Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep. 2008;31:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp. Neurol. 2005;194:212–220. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(Pt 4):735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J. Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp. Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Huang ZL, Lu J, Urade Y, Chen JF. How do the basal ganglia regulate sleep-wake behavior? Trends Neurosci. 2012;35:723–732. doi: 10.1016/j.tins.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur. J. Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Kuhn AA, Brown P. beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp. Neurol. 2012;236:383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Lopez-Azcarate J, Tainta M, Rodriguez-Oroz MC, Valencia M, Gonzalez R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J. Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. J. Neurosci. 2000;20:820–833. doi: 10.1523/JNEUROSCI.20-02-00820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Smith Y. The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front. Syst. Neurosci. 2011;5:64. doi: 10.3389/fnsys.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, Boraud T. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372–2382. doi: 10.1093/brain/awh616. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiol. Dis. 2012;45:583–590. doi: 10.1016/j.nbd.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Najafi MR, Chitsaz A, Askarian Z, Najafi MA. Quality of sleep in patients with Parkinson's disease. Int. J. Prev. Med. 2013;4:S229–S233. [PMC free article] [PubMed] [Google Scholar]
- Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD. EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia. 1994;5:23–28. doi: 10.1159/000106690. [DOI] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb. Cortex. 2011;21:1362–1378. doi: 10.1093/cercor/bhq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp. Neurol. 2004;189:369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Pugnetti L, Baglio F, Farina E, Alberoni M, Calabrese E, Gambini A, Di Bella E, Garegnani M, Deleonardis L, Nemni R. EEG evidence of posterior cortical disconnection in PD and related dementias. Int. J. Neurosci. 2010;120:88–98. doi: 10.3109/00207450903436346. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp. Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Rye DB. Excessive daytime sleepiness and unintended sleep in Parkinson's disease. Curr. Neurol. Neurosci. Rep. 2006;6:169–176. doi: 10.1007/s11910-996-0041-8. [DOI] [PubMed] [Google Scholar]
- Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson's disease. J. Sleep Res. 2000;9:63–69. doi: 10.1046/j.1365-2869.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- Salih F, Sharott A, Khatami R, Trottenberg T, Schneider G, Kupsch A, Brown P, Grosse P. Functional connectivity between motor cortex and globus pallidus in human non-REM sleep. J. Physiol. 2009;587:1071–1086. doi: 10.1113/jphysiol.2008.164327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban C, Zhang XQ, Tesolin-Decros B, Millan MJ, Spedding M. Changes in EEG spectral power in the prefrontal cortex of conscious rats elicited by drugs interacting with dopaminergic and noradrenergic transmission. Br. J. Pharmacol. 1999;128:1045–1054. doi: 10.1038/sj.bjp.0702894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa K, Kamei S, Morita A, Hara M, Mizutani T, Yoshihashi H, Yamaguchi M, Takeshita J, Hirayanagi K. Comparison of quantitative EEGs between Parkinson disease and age-adjusted normal controls. J. Clin. Neurophysiol. 2008;25:361–366. doi: 10.1097/WNP.0b013e31818f50de. [DOI] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson's disease. J. Neurosci. 2013;33:7220–7233. doi: 10.1523/JNEUROSCI.4676-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J. Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soikkeli R, Partanen J, Soininen H, Paakkonen A, Riekkinen P., Sr Slowing of EEG in Parkinson's disease. Electroencephalogr. Clin. Neurophysiol. 1991;79:159–165. doi: 10.1016/0013-4694(91)90134-p. [DOI] [PubMed] [Google Scholar]
- Starr PA, Subramanian T, Bakay RA, Wichmann T. Electrophysiological localization of the substantia nigra in the parkinsonian nonhuman primate. J. Neurosurg. 2000;93:704–710. doi: 10.3171/jns.2000.93.4.0704. [DOI] [PubMed] [Google Scholar]
- Stefani A, Galati S, Peppe A, Bassi A, Pierantozzi M, Hainsworth AH, Bernardi G, Orlacchio A, Stanzione P, Mazzone P. Spontaneous sleep modulates the firing pattern of parkinsonian subthalamic nucleus. Exp. Brain Res. 2006;168:277–280. doi: 10.1007/s00221-005-0175-y. [DOI] [PubMed] [Google Scholar]
- Stein E, Bar-Gad I. beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp. Neurol. 2013;245:52–59. doi: 10.1016/j.expneurol.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson's disease. Exp. Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J. Comp. Neurol. 2011;519:989–1005. doi: 10.1002/cne.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp. Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J. Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Williams D, Kühn A, Kupsch A, Tijssen M, van Bruggen G, Speelman H, Hotton G, Loukas C, Brown P. The relationship between oscillatory activity and motor reaction time in the parkinsonian subthalamic nucleus. Eur J Neurosci. 2005;21(1):249–258. doi: 10.1111/j.1460-9568.2004.03817.x. [DOI] [PubMed] [Google Scholar]
- Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM. Intraoperative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson's disease. Exp. Neurol. 2006;197:244–251. doi: 10.1016/j.expneurol.2005.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.