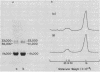
Abstract
Phycoerythrins of several species of the higher red alga Callithamnion show virtually identical spectra, typical of R-phycoerythrins, with absorption maxima at 565, 539, and 497 nanometers. One species, Callithamnion roseum, produces a phycoerythrin lacking the peak at 539 nanometers. Comparison of a “typical” R-phycoerythrin from Callithamnion byssoides with the “atypical” phycoerythrin of C. roseum shows that both proteins carry 35 bilins per native molecule of 240,000 daltons; however, C. byssoides phycoerythrin carries 27.6 phycoerythrobilin and 7.3 phycourobilin groups, whereas C. roseum phycoerythrin carries 24.1 phycoerythrobilin and 10.9 phycourobilin groups. These differences in the relative amounts of the bilin prosthetic groups account in large measure for the differences between the absorption spectra of the native proteins. The ratio of phycoerythrobilin to phycourobilin in C. roseum phycoerythrin can be modulated by varying the light intensity during growth.
Data on the physical, immunological and spectroscopic properties of Callithamnion phycoerythrins indicate that the variation in the relative number of the two bilin prosthetic groups does not affect significantly the conformation of the biliprotein.
Full text
PDF
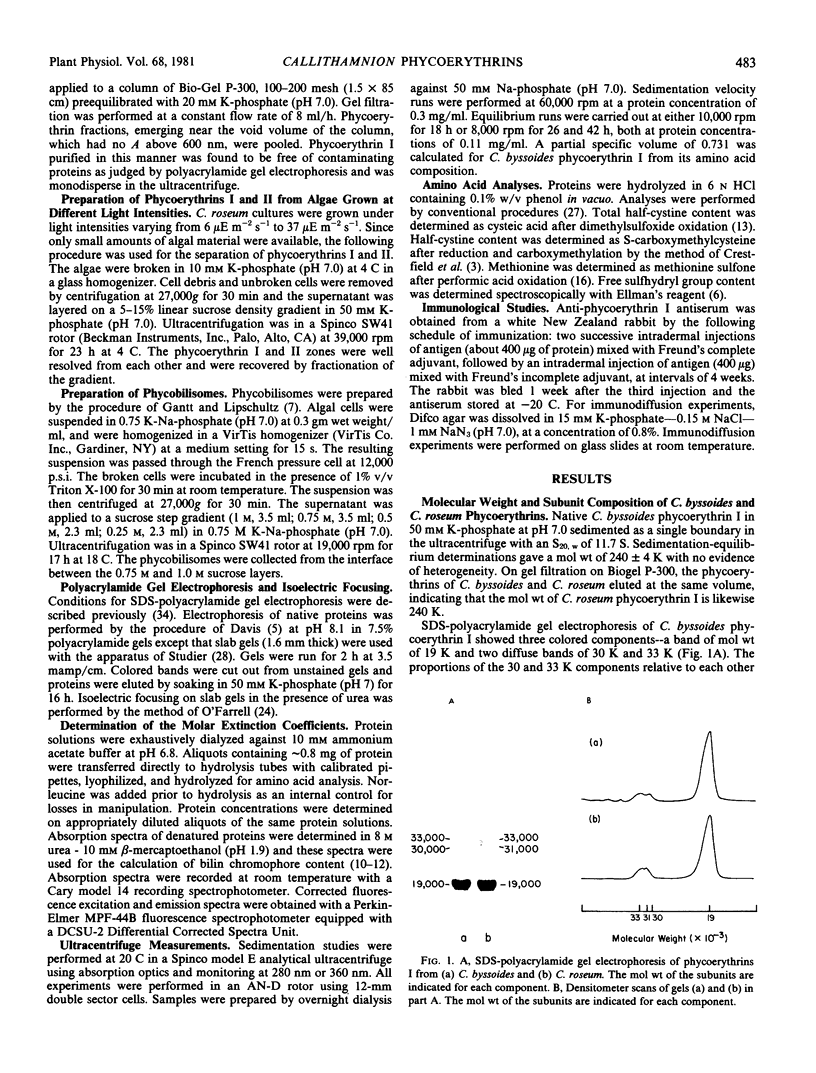
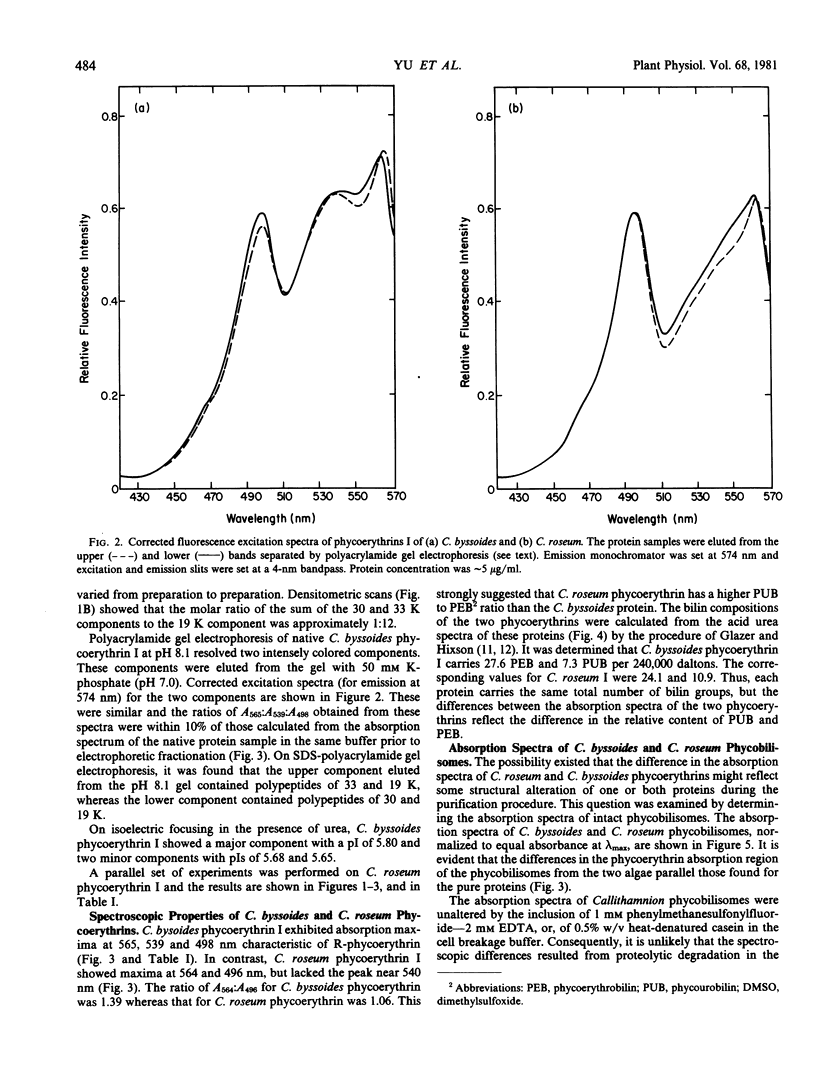
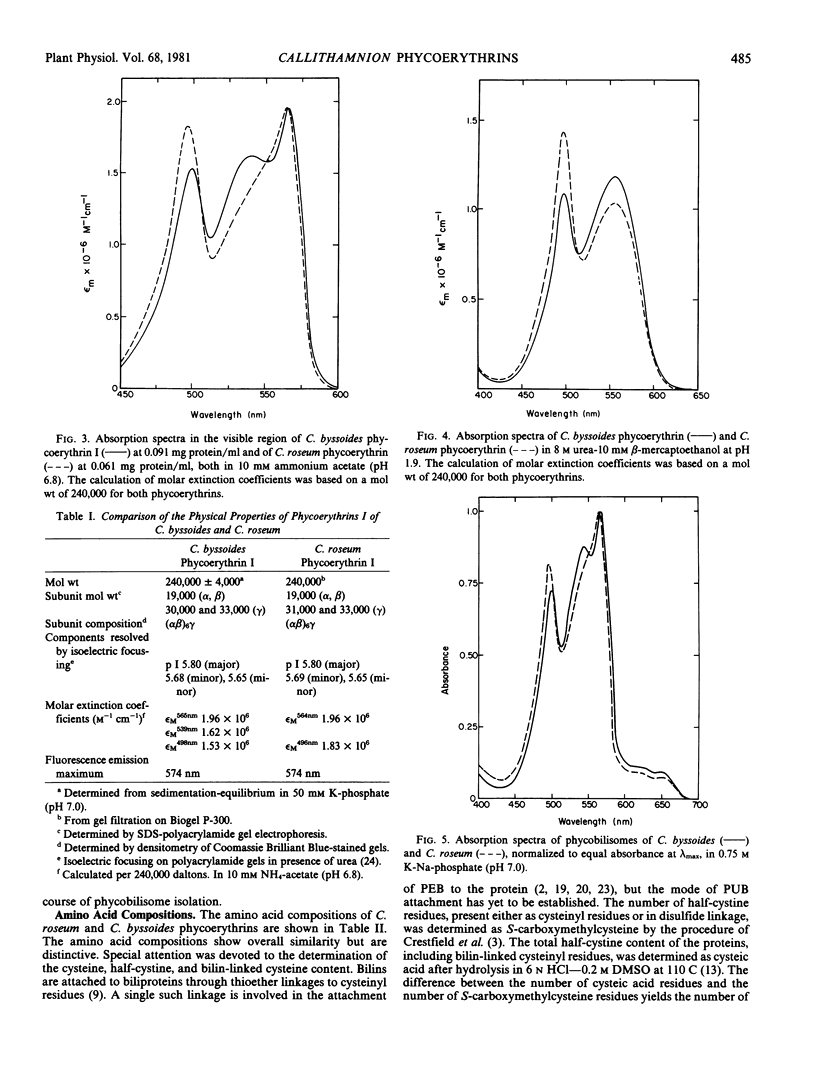

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant D. A., Hixson C. S., Glazer A. N. Structural studies on phycobiliproteins III. Comparison of bilin-containing peptides from the beta subunits of C-phycocyanin, R-phycocyanin, and phycoerythrocyanin. J Biol Chem. 1978 Jan 10;253(1):220–225. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Teale F. W. Number and distribution of chromophore types in native phycobiliproteins. Photochem Photobiol. 1970 Aug;12(2):99–117. doi: 10.1111/j.1751-1097.1970.tb06044.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum: pigment analysis. Biochemistry. 1974 Jul 2;13(14):2960–2966. doi: 10.1021/bi00711a027. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Fang S. Formation of hybrid proteins form the and subunits of phycocyanins of unicellular and filamentous blue-green algae. J Biol Chem. 1973 Jan 25;248(2):663–671. [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S. Characterization of R-phycocyanin. Chromophore content of R-phycocyanin and C-phycoerythrin. J Biol Chem. 1975 Jul 25;250(14):5487–5495. [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S., DeLange R. J. Determination of the number of thioether-linked cysteine residues in cytochromes c and phycobiliproteins. Anal Biochem. 1979 Jan 15;92(2):489–496. doi: 10.1016/0003-2697(79)90689-4. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S. Subunit structure and chromophore composition of rhodophytan phycoerythrins. Porphyridium cruentum B-phycoerythrin and b-phycoerythrin. J Biol Chem. 1977 Jan 10;252(1):32–42. [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W. B-Phycoerythrin from Rhodella violacea: characterization of two isoproteins. Arch Microbiol. 1975 Aug 28;104(3):255–261. doi: 10.1007/BF00447334. [DOI] [PubMed] [Google Scholar]
- Köst-Reyes E., Köst H. P. The protein-chromophore bond in B phycoerythrin from Porphyridium cruentum. Radiosulfur labeling experiments. Eur J Biochem. 1979 Dec;102(1):83–91. doi: 10.1111/j.1432-1033.1979.tb06265.x. [DOI] [PubMed] [Google Scholar]
- Muckle G., Otto J., Rüdiger W. On the linkages between chromophore and protein in biliproteins, VII. Amino acid sequence in the chromophore regions of C-phycoerythrin from Pseudanabaena W 1173 and Phormidium persicinum. Hoppe Seylers Z Physiol Chem. 1978 Mar;359(3):345–355. [PubMed] [Google Scholar]
- Mörschel E., Wehrmeyer W., Koller K. P. Biliprotein assembly in the disc-shaped phycobilisomes of Rhodella violacea. Electron microscopical and biochemical analysis of B-phycoerythrin and B-phycoerythrin--C-phycocyanin aggregates. Eur J Cell Biol. 1980 Aug;21(3):319–327. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Ellman's reagent: 5,5'-dithiobis(2-nitrobenzoic acid)--a reexamination. Anal Biochem. 1979 Apr 1;94(1):75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Wieczorek G. A., Turner B. C. Preparation of calcium phosphate for protein chromatography. Anal Biochem. 1965 Dec;13(3):402–404. doi: 10.1016/0003-2697(65)90332-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Teale F. W., Dale R. E. Isolation and spectral characterization of phycobiliproteins. Biochem J. 1970 Jan;116(2):161–169. doi: 10.1042/bj1160161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]
- van der Velde H. H. The natural occurrence in red algae of two phycoerythrins with different molecular weights and spectral properties. Biochim Biophys Acta. 1973 Apr 20;303(2):246–257. doi: 10.1016/0005-2795(73)90354-1. [DOI] [PubMed] [Google Scholar]