Abstract
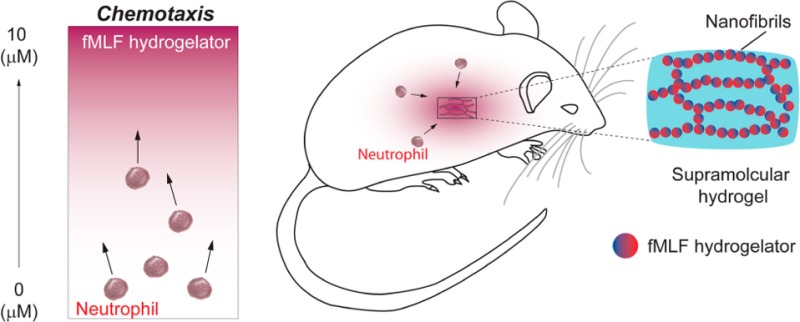
Most immunomodulatory materials (e.g., vaccine adjuvants such as alum) modulate adaptive immunity, and yet little effort has focused on developing materials to regulate innate immunity, which get mentioned only when inflammation affects the biocompatibility of biomaterials. Traditionally considered as short-lived effector cells from innate immunity primarily for the clearance of invading microorganisms without specificity, neutrophils exhibit a key role in launching and shaping the immune response. Here we show that the incorporation of unnatural amino acids into a well-known chemoattractant—N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLF)—offers a facile approach to create a de novo, multifunctional chemoattractant that self-assembles to form supramolecular nanofibrils and hydrogels. This de novo chemoattractant not only exhibits preserved cross-species chemoattractant activity to human and murine neutrophils, but also effectively resists proteolysis. Thus, its hydrogel, in vivo, releases the chemoattractant and attracts neutrophils to the desired location in a sustainable manner. As a novel and general approach to generate a new class of biomaterials for modulating innate immunity, this work offers a prolonged acute inflammation model for developing various new applications.
Upon the breach of the host physical barrier by intruding microorganisms, neutrophils, among all the leukocytes, are the first to influx into focus for bacterial invasion for host defense.1 Neutrophils used to be considered to function exclusively as the effector cells in the innate phase of immune response. However, the old view has been challenged since a growing body of evidence has shown that neutrophils play a crucial role in framing immune response, both innate and adaptive immunity.2 For example, neutrophils are found to have a B cell-helper neutrophil population in the splenic marginal zone, and these neutrophils can activate marginal zone B cells to secrete immunoglobulins against T cell-independent antigens.3
The efficient recruitment of neutrophils depends on many signals, including N-formyl peptides, chemokines, complement components, and leukotrienes.1 As byproducts of protein translation in the invading bacteria, N-formyl peptides form molecular gradients originating from the bacteria in the infected tissue, and the gradients of N-formyl peptides signal neutrophils to migrate (i.e., chemotaxis) toward their targets while overriding other minor signals, such as IL-8 and MIP-2.1 Proposed in 1965 and confirmed in 1984, fMLF represents the best-known N-formyl peptide and one of the most well-established chemoattractants for neutrophils (Figure 1).4 Having a well-defined molecular structure, fMLF offers an opportunity for chemical modifications and for precise control and accurate understanding of immunomodulation at the molecular level. Recognized for its potential as a useful reagent to induce acute inflammation in vivo, fMLF, in the form of aqueous solution, has been injected subcutaneously,5 intravenously,6 intraplantarly,7 intradermally,8 or just topically applied on the microvasculature9 to study the biology of neutrophils for various applications. Although the aqueous solution of fMLF is able to induce the accumulation of neutrophils, its effect is relatively weak and transient (2–6 h).10
Figure 1.
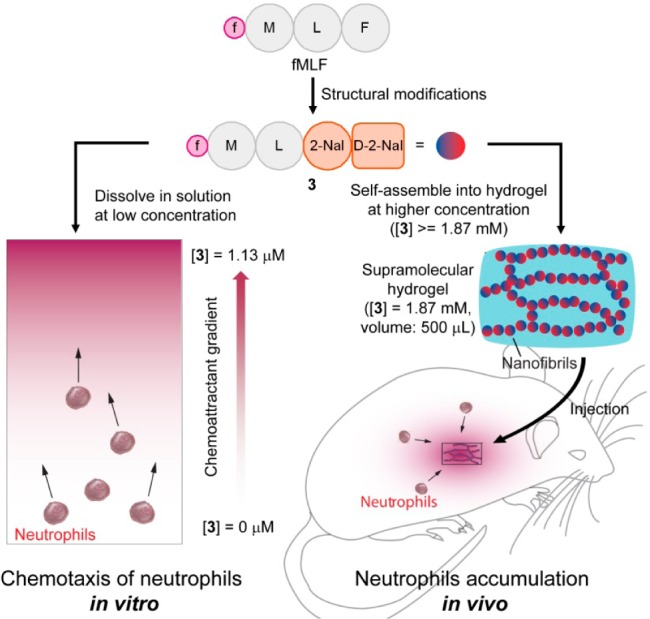
Illustration of conversion of fMLF to an fMLF-based hydrogelator (3) to induce chemotaxis of neutrophils in vitro and accumulation of neutrophils in vivo. In vitro assay: 3 induces chemotaxis of murine and human neutrophils at the minimum effective concentrations of 1.13 μM and 11.3 nM, respectively. In vivo assay: the hydrogel of 3 slowly releases 3 for attracting neutrophils to the location of the hydrogel (at the dosage of 0.935 μmol per mouse). Notation: f = formyl, M = l-methionyl residue, L = l-leucyl residue, F = l-phenylalaninyl residue, 2-Nal = l-3-(2-naphthyl)-alaninyl residue and d-2-Nal = d-3-(2-naphthyl)-alaninyl residue.
Instead of weak and transient acute inflammation, sometimes reagents that can elicit inflammation for a longer period are highly desired. However, the field of biomaterials concerning neutrophils and inflammation has been focused on suppressing inflammation as the foreign body response to biomaterials.11 Most recently, material scientists have started to pay attention to regulation of innate immunity and neutrophils, still focus on inflammation imaging12 and suppression.13 Demonstrated in a recent work, the intratumoral injection of fMLF solution every 2 days after the inoculation of tumor cells slows tumor growth in a xenograft tumor model.14 Similarly, the daily intratumoral injection of another chemoattractant, chemerin, decreases the tumor growth.15 In order to maintain a meaningful local concentration of chemoattractants, both studies required frequent intratumoral injections.14,15 Therefore, a formulation of chemoattractant (e.g., fMLF) for prolonged release not only acts as a useful tool to study the biology of neutrophils over long duration, but also holds promise for therapeutic applications, like cancer treatment. This potential has already led to the exploration of different formulations of fMLF,16−18 such as particles of fMLF in suspensions produced by sonication for studying neutrophil infiltration into pulmonary alveoli during murine pneumococcal pneumonia,16 physically encapsulated N-formyl peptides in poly(lactic-co-glycolic acid) (PLGA) microbeads for inducing chemotaxis of neutrophils,17 or human monocytes and monocyte-derived dendritic cells (DCs)18 in vitro. Based on the same idea of physical encapsulation by polymers, chemoattraction of regulatory T cells in vivo has been achieved by releasing CCL22 from PLGA particles.19 Despite this progress, heterogeneous suspensions of fMLF particles are far from ideal for in vivo applications due to differences between batches, and physical encapsulation using polymeric materials suffers from several limitations, such as burst release, low capacity for payload, and slow bioresorption of the polymeric materials, along with its inherent problem as mixtures of molecules with different molecular weight. These limitations demand the development of new approaches to attract neutrophils in vivo.
As an alternative, learning from nature, we chose to develop a biomimetic approach for sustained release of fMLF in vivo. Away from the dominant drug delivery idea of drug loading onto vehicles, such as biodegradable polymers, nature has provided a strategy, of which natural peptides and proteins self-assemble to form functional amyloids for sustained release.20 Certain hormones such as prolactin and growth hormone form amyloids for storage, which dissolve slowly as a way for sustained or regulated hormone secretion.21 This principle and mechanism have led to the development of supramolecular nanofibrils and hydrogels22 of bioactive molecules as “self-delivery drugs”.20 In fact, a hydrogel of lanreotide acetate (i.e., Somatuline Depot),23 based on this mechanism, has found clinical application for treating acromegaly. This success has stimulated the development of supramolecular hydrogels as a unique depot for controlled release.20,23−25 However, existing examples of using self-assembly of peptides as a releasing depot have almost all been discovered by accident. The self-assembly property was not included in the molecular design of the small molecular drug, which was discovered afterward. However, the real challenge for a “releasing depot” is how to modify existing bioactive molecules into derivatives with preserved bioactivity while gaining the new function of self-assembly into nanofibrils. Intrigued by the simplicity and effectiveness of the “self-delivery drugs”, also from the perspective of peptide engineering and peptide formulation, we hypothesized that, by rational chemical derivatization, fMLF-derived peptides would form supramolecular hydrogels without compromising the biological efficacy of fMLF, and the corresponding hydrogels would deliver long-term efficacy for local accumulation of neutrophils by sustained release of the chemotactic hydrogelators (Figure 1).
To demonstrate the concept illustrated in Figure 1, we designed, synthesized, and evaluated several fMLF-derived molecules; and we obtained N-formyl-l-methionyl-l-leucyl-l-3-(2-naphthyl)-alaninyl-d-3-(2-naphthyl)-alanine (i.e., fMet-Leu-(2-Nal)-(D-2-Nal) shown as 3 in Figure 1). In addition to behaving as a hydrogelator, 3 exhibits three advantageous features: ability to form a hydrogel efficiently (minimum gelation concentration (MGC) = 0.125% w/v in DPBS buffer), enhanced stability against proteolysis, and preserved activity to both mouse and human neutrophils. Moreover, the hydrogel of 3, in the murine peritonitis model, stimulates a much longer-lasting pro-inflammatory phase than fMLF solution does, and exhibits a 2 orders of magnitude increase in neutrophil accumulation compared to that of fMLF solution. This work, for the first time, not only offers a facile approach to convert chemoattractants into hydrogels with exceptional biostability and tailored activity for controlled accumulation of neutrophils in vivo, but also validates the concept of the supramolecular hydrogelators acting as chemoattractants for homing cells in a sustainable manner. Besides being a useful tool to study the biology of innate immunity, this prolonged inflammation model holds promise for various potential therapeutic applications26 (e.g., inhibiting tumor growth,14,15 decreasing mortality caused by lethal sepsis after microbial infection,27 and acting as a basis for vaccine adjuvants28). Furthermore, the same principle can apply to the design of molecular hydrogels of formyl peptide receptor (FPR) antagonists, which finds direct applications in controlling pain caused by microbial infection.29
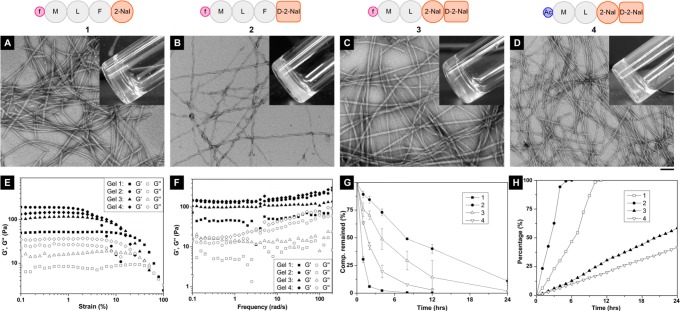
Figure 2 shows the schematic representation of the fMLF-derived peptides that self-assemble in water and form hydrogels and their characterizations (e.g., TEM, rheological properties, proteolytic stability, and the release profiles). The chemical structures are shown in Supporting Information Figure S1. Based on the structure of fMLF, we mainly modified fMLF at the C-terminus.30 Since the naphthyl group promotes formation of hydrogels from small peptides,23,24 we connected an unnatural amino acid with a naphthyl group (2-Nal) to fMLF to obtain fMLF-(2-Nal) (1). 1 self-assembles in Dulbecco’s Phosphate-Buffered Saline (DPBS) buffer to form a supramolecular hydrogel at a concentration of 0.2 w/v% (Figure 2A, inset). Transmission electron microscopy (TEM) reveals the network of nanofibrils (around 16 nm in diameter) (Figure 2A) in the hydrogel of 1 (0.2 w/v%), which shows storage modulus (G′) of around 50 Pa and critical strain of about 10.0% (Figure 2E,F). As shown in Figure 2G, incubation with a powerful protease (e.g., proteinase K) causes more than 90% of 1 to undergo hydrolysis in the first 2 h. The release of 1 from its hydrogel finishes in about 8 h (Figure 2H).
Figure 2.
Characterization of fMLF-derived hydrogelators (1, 2, 3) and a control peptide (4) and the hydrogels. (A–D) Typical TEM images of negatively stained fibrils of (A) the hydrogels of 1 (0.20 w/v%), (B) 2 (0.40 w/v%), (C) 3 (0.125 w/v%), and (D) 4 (0.075 w/v%), respectively, with the molecular representation on top (all hydrogels are at pH = 7.4 in DPBS buffer; the scale bar is 100 nm; notation: Ac = acetyl; inset: the optical images of the hydrogels). (E) Strain sweep and (F) frequency sweep of the hydrogels with the same concentrations as the hydrogels prepared for optical images and TEM. (G) Digestion of 1, 2, and 3, respectively, in a 3.5 mL HEPES buffer solution of 1.4 mg (0.4 mg/mL) by adding 2.8 μL of proteinase K solution at 37 °C. (H) Release profiles of the monomers from the hydrogels of 1, 2, 3, and 4 (0.4 w/v%) at 37 °C.
To improve the stability of the hydrogelator against proteolytic enzymes, we replaced the fourth l-amino acid residue to the corresponding d-amino acid residue31 to obtain a new peptide, fMLF-(D-2-Nal) (2), which still forms a hydrogel (Figure 2B, inset) but at a higher concentration (MGC: 0.4 w/v%) than that of 1. As shown in the TEM images, the nanofibrils in the hydrogel of 2 have diameters around 22 nm (Figure 2B). Rheology measurement shows the storage modulus and the critical strain of the hydrogel of 2 to be around 200 Pa and about 1.0%, respectively (Figure 2E,F). The incorporation of a d-amino acid, indeed, substantially enhances proteolytic stability of 2 in comparison with that of 1. For example, in the presence of proteinase K, more than 80% and 40% of 2 remains after 2 h and after 12 h, respectively (Figure 2G). The hydrogel of 2 collapses within the first 4 h and completely dissolves after 6 h in DPBS buffer at 37 °C (Figure 2H), suggesting the relatively loose molecular packing in the hydrogel of 2.
Therefore, to obtain an fMLF derivative that has better gelation properties, we changed the third amino acid residue on 2 from Phe to 2-Nal, which gave the peptide fML-(2-Nal)-(D-2-Nal) (3). This simple change boosts the intermolecular aromatic–aromatic interaction that promotes molecular self-assembly in water for hydrogelation, so 3 exhibits excellent gelation properties with an MGC of 0.125 w/v% (Figure 2C, inset). The hydrogel prepared at the concentration of 0.125 w/v% has nanofibrils with diameters around 18 nm (Figure 2C), storage modulus around 100 Pa, and critical strain around 2.0% (Figure 2E,F). Not only does 3 maintain resistance to the proteinase K proteolysis (Figure 2G), but the improved gelation property also results in a longer sustained release of 3 in vitro. The stability of 3 is much higher than that of fMLF itself.32 The hydrogel of 3 releases about 60% of 3 after 24 h incubation at 37 °C (Figure 2H).
The importance of the formyl group for the activity of the N-formyl peptides has been well documented, and the replacement of the formyl group by the acetyl group in fMLF causes a 1000 to 10 000 fold activity drop.33 Therefore, we also synthesized a control molecule of 3, AcML-(2-Nal)-(D-2-Nal) (4). It forms a hydrogel (Figure 2D, inset) at a low concentration (MGC = 0.075 w/v%). The hydrogel of 4 contains nanofibrils with diameters around 17 nm (Figure 2D) and has a storage modulus around 140 Pa and a critical strain around 2.0% (Figure 2E,F). 4 is less stable against proteolytic digestion by proteinase K than 3. Matching with its excellent gelation property, the hydrogel of 4 also gradually releases 4 (with 40% release at the first 24 h) (Figure 2H).
These four hydrogels, prepared at the MGCs of the hydrogelators, exhibit comparable storage moduli (G′), loss moduli (G″), and critical strains (Figure 2E,F). Together with the facts that the moduli of these hydrogels depend little on the frequency and the nanofibrils serving as the matrices of the hydrogels have close diameters (16–22 nm), these results suggest that the hydrogels of fMLF-based molecules share similar morphological and rheological properties. In addition, the MGC values of the hydrogelators correlate well with the release profiles of the hydrogelators. For example, the order of the hydrogelators having MGCs from high to low is 2, 1, 3, and 4, which is the same as the order of the hydrogelator release rates (Figure 2H). This trend suggests that molecular engineering of the hydrogelators to control the MGC values should be effective and useful for tailoring the release profiles of the chemoattractants.
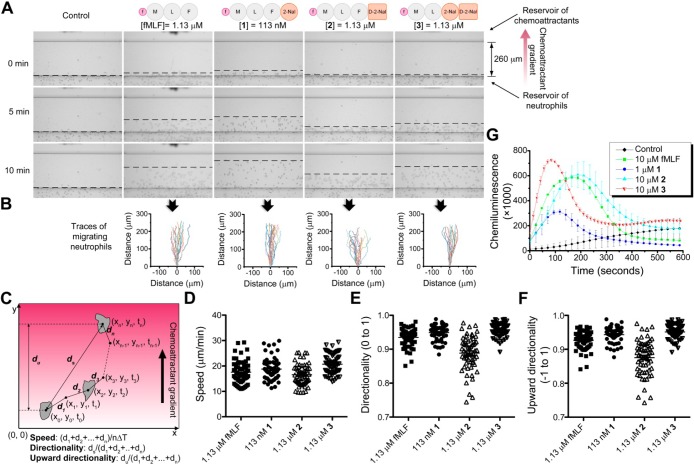
After confirming that the fMLF derivatives act as hydrogelators and exhibit proteolytic stability, we determined their activity to murine neutrophils by measuring chemotaxis and ROS production. Figure 3A shows the frames of the chemotaxis (performed on the EZ-TAXIScan34) induced by different molecules at 0, 5, and 10 min to determine the minimum effective concentrations of the fMLF derivatives. The recorded frames of the first 20 min serve as traces of cell migration (Figure 3B), which, being analyzed by the algorithm shown in Figure 3C, provide three important parameters for neutrophil chemotaxis: migration speed (Figure 3D), directionality (0 to 1) (Figure 3E), and upward directionality (−1 to 1) (Figure 3F) to characterize, respectively, how fast the neutrophils move, how straight they migrate, and how faithfully they follow the gradients of the chemoattractants. Chemoattractants also induce the production of reactive oxygen species (ROS) by the NADPH oxidase assembled on the plasma membrane and phagosome.1 Thereby, the quantification of ROS production using isoluminol also indicates the activity of the chemoattractants. According to Figure 3A–G, at its minimum effective concentration (1.13 μM), which is about 1000 times lower than the minimum gelation concentration, 3 not only induces chemotaxis of murine neutrophils as effectively as fMLF (i.e., the almost identical migration speed (Figure 3D) and directionality (Figure 3E), and slightly better upward directionality (Figure 3F)), but also exhibits slightly more potent activity for generation of ROS than that of fMLF (Figure 3G). The reason for the faster ROS production activated by 3 is currently unknown, which might be related to its interaction with formyl peptide receptors (FPRs). Interestingly, 1, at 113 nM, exhibits similar chemotactic activities as that of fMLF at 1.13 μM (Figure 3A), but generates only half as much ROS as fMLF (Figure 3G). 2 exhibits drops in the chemotactic activity and the ROS production when compared with fMLF, which matches the previously reported results that the d enantiomer is a less active chemoattractant than the l enantiomer.354 failed to induced the chemotaxis even at 113 μM, 100 fold of the minimum effective concentration of 3 (Supporting Information Figure S2), which matches the 1000 to 10 000 fold activity drop reported in the literature.33 Therefore, 4 is considered to have no chemotactic activity, and the hydrogel of 4 can be used as a control for the hydrogel of 3.
Figure 3.
Induction of chemotaxis and ROS production of murine neutrophils by fMLF-derived hydrogelators (1, 2, and 3) in vitro. (A) Snapshots of chemotaxis of murine neutrophils at 0, 5, and 10 min induced by the gradient of fMLF; 1, 2, and 3 performed on EZ-TAXIScan,34 with the blank control. (B) Traces of 20 typical migrating neutrophils corresponding to different chemoattractants in the first 20 min. (C) Scheme and formulas for the calculation of the migration parameters. (D) Migration speed, (E) directionality, and (F) upward directionality of the murine neutrophils during chemotaxis from three independent samples, and each sample has 20 cell traces. (G) ROS production in the neutrophils (5 × 105) after stimulation with DMSO (0.1 v/v%, as the negative control), fMLF (10 μM), 1 (1 μM), 2 (10 μM), and 3 (10 μM).
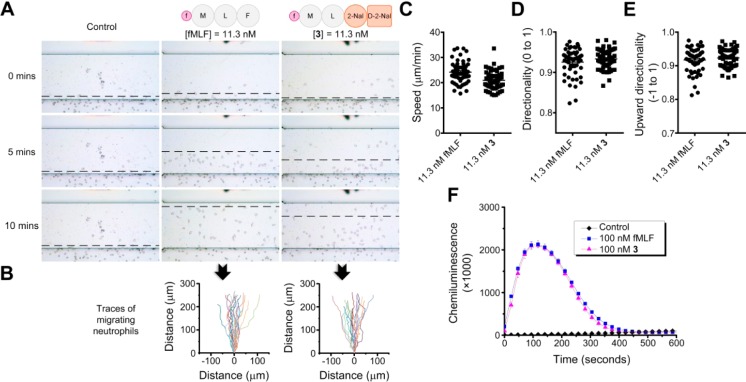
These results clearly show that 3 fulfills all three criteria of the molecular design: excellent gelation property for the purpose of sustained release, fair stability against proteolysis, and well preserved chemotactic activity to murine neutrophils. Encouraged by the in vitro activity of 3 to attract murine neutrophils, we also investigated the activity of 3 on human neutrophils. As shown in Figure 4A, the minimum effective concentration of fMLF to human neutrophils is 11.3 nM determined by a 10-fold serial dilution, which is 100 times lower than the minimum effective concentration of fMLF to murine neutrophils (1.13 μM). This result agrees with the observation that fMLF’s activity to murine FPR is 100 to 10 000 fold less than to human and rabbit FPRs.36 Moreover, 3, at 11.3 nM, exhibits the same effectiveness as fMLF to human neutrophils, as evidenced by the indistinguishable migration traces (Figure 4B), similar migration parameters (Figure 4C,D,E), and essentially identical ROS production (Figure 4F). Although the binding pockets of the mouse and human FPRs might be quite different, which is suggested by fMLF’s significantly different activity to FPRs,36 the well preserved chemotactic activity of 3 to both mouse and human neutrophils indicates that 3 maintains binding to both human and murine FPRs. How the modification can satisfy the binding to the two seemingly quite different pockets is surprisingly interesting and certainly deserves further exploration.
Figure 4.
Induction of chemotaxis and ROS production of human neutrophils by 3 in vitro. (A) Snapshots of chemotaxis of purified neutrophils from healthy human donors at 0, 5, and 10 min induced by fMLF, 3, and PBS (as the control) performed on EZ-TAXIScan. (B) Traces of 20 typical migrating neutrophils corresponding to different chemoattractants. (C) Migration speed, (D) directionality, and (E) upward directionality of the human neutrophils during chemotaxis from three independent samples, and each sample has 20 cell migration traces. (F) ROS production in the neutrophils (5 × 105) after the addition of fMLF (100 nM), 3 (100 nM), and PBS (as the blank control).
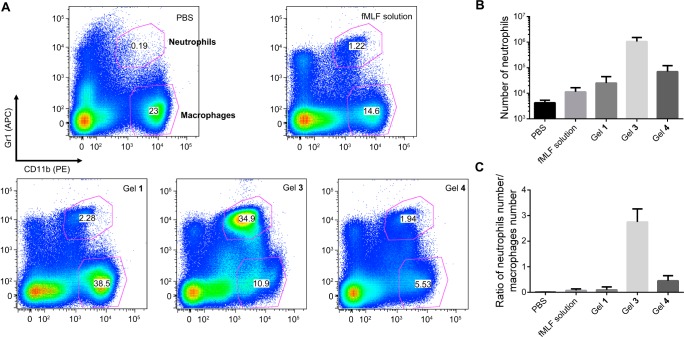
After successfully demonstrating the in vitro properties (i.e., gelation property for sustained release, stability, and the chemotactic activity to both mouse and human neutrophils) of 3, we used an in vivo murine model to determine whether the hydrogel of 3 achieves a longer proinflammatory effect for attracting neutrophils than the solution of fMLF. We collected peritoneal lavages for flow cytometry 24 h after the injection of DPBS solution, fMLF solution, and the gels of 1, 3, and 4 into mice, respectively. In the representative flow cytometry plots (Figure 5A) using the markers Gr1 and CD11b, the double positive (Gr1+CD11b+) corresponds to neutrophils and the Gr1–CD11b+ are monocytes macrophages. The acute inflammation starts with the rapid influx of neutrophils and switches to monocyte-derived inflammatory macrophages, both from the blood. Although it is not clear cut, the initial influx with a high percentage of neutrophils is considered the pro-inflammatory phase and the later stage with dominant macrophages is the resolution phase. Therefore, the ratio of neutrophils to macrophages is the indicator of the inflammation phase.37 As shown in Figure 5A, while the effect of the solution of fMLF almost disappears 24 h after the injection, the hydrogel of 3, having the same amount of N-formyl peptides as the fMLF solution, still attracts high counts of neutrophils. According to the quantification (Figure 5B), the neutrophil number attracted by the hydrogel of 3 is 2 orders of magnitude higher than that of the solution of fMLF. Moreover, the ratio of the number of neutrophils vs the number of macrophages (Figure 5C) suggests that the inflammation of the peritoneum (peritonitis) induced by the hydrogel of 3 is in a much earlier phase than the solution of fMLF, which is in the resolution phase of the inflammation. Although the hydrogel of 4 also results in stronger accumulation of neutrophils than the solution of fMLF does, the total number of neutrophils is still 1 order of magnitude lower than that induced by the hydrogel of 3 (Figure 5B). In addition, the ratio of neutrophils to macrophages (Figure 5C) clearly shows that the inflammation induced by the hydrogel of 4 is also close to the resolution phase. These results agree with the chemotactic activity of the hydrogelators 3 and 4, and suggest the accumulation of neutrophils caused by the hydrogel of 4 probably due to the inflammatory response toward stable foreign materials.
Figure 5.
Hydrogels stimulate prolonged accumulation of murine neutrophils in vivo. (A) Representative flow cytometry plots showing the neutrophils (Gr1+CD11b+) and the monocytes and macrophages (Gr1−CD11b+) from the cells collected from the peritoneal lavage of wild-type mice 24 h after receiving the intraperitoneal injections (IP injections) of 500 μL of PBS (as the control), the solution of fMLF, and the hydrogels of 1, 3, and 4 containing 0.935 μmol of peptides. (B) The number of neutrophils and (C) the ratio of the number of neutrophils vs the number of macrophages according to the FACS quantification from three independent experiments.
As the other control, the hydrogel of 1 results in similar results as the solution of fMLF: low total number of neutrophils and low ratio of neutrophils to macrophages 24 h after the injections (Figure 5B and C). Although 1 is roughly 10 times more chemotactically active than 3 (Figure 3), the hydrogel of 1 releases more rapidly than the hydrogel of 3, and 1 is less stable than 3. Therefore, the stronger accumulation of neutrophils induced by the hydrogel of 3 than by the hydrogel of 1 likely not only is due to the inflammatory response to stable foreign materials (as the case of the hydrogel of 4), but also originates from the sustained release of more stable chemoattractive hydrogelators. This result suggests that it is feasible to modulate inflammation by controlling the rheological properties of the hydrogels, the release profiles, and the stabilities of the hydrogelators.
Because neutrophils, as short-lived cells,1 have a half-life of 1.5 h in the circulation in mice, the accumulation of neutrophils in this murine model is different from the other cell accumulations where the attracted cells remain alive. Therefore, the results, shown in Figure 5, could not be interpreted as the explosive accumulation of neutrophils induced in the first couple of hours all at once and then no activity to follow. Instead, the results from 24 h strongly support that the accumulation of neutrophils is due to a constant attraction by the sustained release of the hydrogelators from the hydrogel of 3.
Forty-eight hours after injections (Supporting Information Figure S3), the number of neutrophils attracted by the hydrogel of 3 is 1.1 ± 0.3 × 104 and the ratio of neutrophils to macrophages is 0.05 ± 0.03, which shows that the peritonitis induced by the hydrogel of 3 also moves into the resolution phase, suggesting that the controlled release exhausts between 24 and 48 h. This might be related to the watery environment of the peritoneum due to peritoneal fluid.
In summary, this study illustrates the evolution of multifunctional molecular hydrogelators for attracting neutrophils in vitro and in vivo. As the new class of “self-delivery” biomaterials, supramolecular hydrogels are only in their infancy for immunomodulation, with some initial but exciting exploration on vaccines for adaptive immunity.38 Our work is the first example of using molecular self-assembly for the construction of immunomodulatory materials for innate immunity. The self-assembled hydrogel of fMLF-derived peptides, as a unique tool, can be very useful to researchers who need to induce sustained innate immune recruitment, which had been unavailable before. Furthermore, this hydrogel also holds therapeutic potential that has yet to be explored. More broadly, besides fMLF, there are many small biological peptides, playing essential roles in diverse biological functions. However, our preliminary study on the attracting neutrophils to the tumor sites on a B16–F10 melanoma model indicates that the peritumoral injection of the gel of 3 or the solution of fMLF gas has little effect on the growth of the tumor in rat. This result suggests more extensive study on this observation is needed. The concept illustrated in this work along with other work shows the potential to modify those peptides to form supramolecular hydrogels without compromising the bioactivity,39 and the insight of molecular design gained from this work can be very useful for the further development of immunomodulating hydrogels. The general concept of using supramolecular hydrogel of bioactive small molecules as a “self-delivery” system, thus, provides a novel approach to therapeutics and an attractive and validated alternative to the traditional drug delivery system.
Acknowledgments
The authors acknowledge the National Institute of Health (CA142746, B. X.; HL085100, AI076471, HL092020 and GM076084, H. R. L.), Kenneth Rainin Foundation (B. X.), and Chinese Council Scholarship (J. L. and J. S.). We thank the Brandeis EM facility for help on EM experiment, Isaac M. Chiu for helpful discussions on the manuscript, and Subhanjan Mondal, Chunbao Guo, and Hongmei Li for their help on mice experiments.
Supporting Information Available
Experimental details of synthesis, hydrogelation, biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
Jiro Sakai, Department of Veterinary Medicine, University of Cambridge, Madingley Road, Cambridge, CB3 0ES, United Kingdom.
Author Contributions
Fan Zhao and Jingyu Li contributed equally to this work.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kolaczkowska E.; Kubes P. (2013) Nat. Rev. Immunol. 13, 159. [DOI] [PubMed] [Google Scholar]
- Nathan C. (2006) Nat. Rev. Immunol. 6, 173. [DOI] [PubMed] [Google Scholar]; Mantovani A.; Cassatella M. A.; Costantini C.; Jaillon S. (2011) Nat. Rev. Immunol. 11, 519. [DOI] [PubMed] [Google Scholar]
- Puga I.; Cols M.; Barra C. M.; He B.; Cassis L.; Gentile M.; Comerma L.; Chorny A.; Shan M.; Xu W.; Magri G.; Knowles D. M.; Tam W.; Chiu A.; Bussel J. B.; Serrano S.; Lorente J. A.; Bellosillo B.; Lloreta J.; Juanpere N.; Alameda F.; Baro T.; de Heredia C. D.; Toran N.; Catala A.; Torrebadell M.; Fortuny C.; Cusi V.; Carreras C.; Diaz G. A.; Blander J. M.; Farber C.-M.; Silvestri G.; Cunningham-Rundles C.; Calvillo M.; Dufour C.; Notarangelo L. D.; Lougaris V.; Plebani A.; Casanova J.-L.; Ganal S. C.; Diefenbach A.; Arostegui J. I.; Juan M.; Yague J.; Mahlaoui N.; Donadieu J.; Chen K.; Cerutti A. (2012) Nat. Immunol. 13, 170.22197976 [Google Scholar]
- Ye R. D.; Boulay F.; Wang J. M.; Dahlgren C.; Gerard C.; Parmentier M.; Serhan C. N.; Murphy P. M. (2009) Pharmacol. Rev. 61, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.-L.; Lee E. J.; Murphy P. M. (1999) J. Exp. Med. 189, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagels M.; Chambers J.; Arfors K.; Hugli T. (1995) Blood 85, 2900. [PubMed] [Google Scholar]
- Rittner H. L.; Hackel D.; Voigt P.; Mousa S.; Stolz A.; Labuz D.; Schäfer M.; Schaefer M.; Stein C.; Brack A. (2009) PLoS Pathog. 5, e1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D.; Nagy J. A.; Pyne K.; Dvorak H. F.; Dvorak A. M. (1998) J. Exp. Med. 187, 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T.; Katori M. (1992) J. Leukocyte Biol. 52, 337. [DOI] [PubMed] [Google Scholar]
- Colditz I. G.; Movat H. Z. (1984) J. Immunol. 133, 2169. [PubMed] [Google Scholar]
- Williams D. F. (2008) Biomaterials 29, 2941. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wong R.; Khalidov I.; Wang A. Y.; Leelawattanachai J.; Wang Y.; Jin M. M. (2011) Biomaterials 32, 7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Li J.; Cho J.; Malik A. B. (2014) Nat. Nano. 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Khayat A.; Cheng H. S.; Graves D. T. (1997) Lab. Invest. 76, 579. [PubMed] [Google Scholar]
- Pachynski R. K.; Zabel B. A.; Kohrt H. E.; Tejeda N. M.; Monnier J.; Swanson C. D.; Holzer A. K.; Gentles A. J.; Sperinde G. V.; Edalati A.; Hadeiba H. A.; Alizadeh A. A.; Butcher E. C. (2012) J. Exp. Med. 209, 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J.-F.; Fortin A.; Bergeron Y.; Dumas M.-C.; Champagne M.-E.; Bergeron M. G. (2007) Infect. Immun. 75, 5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress H.; Park J.-G.; Mejean C. O.; Forster J. D.; Park J.; Walse S. S.; Zhang Y.; Wu D.; Weiner O. D.; Fahmy T. M.; Dufresne E. R. (2009) Nat. Methods 6, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Jain S.; Benjamin Larman H.; Gonzalez S.; Irvine D. J. (2005) Biomaterials 26, 5048. [DOI] [PubMed] [Google Scholar]
- Jhunjhunwala S.; Raimondi G.; Glowacki A. J.; Hall S. J.; Maskarinec D.; Thorne S. H.; Thomson A. W.; Little S. R. (2012) Adv. Mater. 24, 4735. [DOI] [PMC free article] [PubMed] [Google Scholar]; Glowacki A. J.; Yoshizawa S.; Jhunjhunwala S.; Vieira A. E.; Garlet G. P.; Sfeir C.; Little S. R. (2013) Proc. Natl. Acad. Sci. U. S. A. 110, 18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.; Ma M. L.; Xu B. (2009) Chem. Soc. Rev. 38, 883. [DOI] [PubMed] [Google Scholar]
- Maji S. K.; Perrin M. H.; Sawaya M. R.; Jessberger S.; Vadodaria K.; Rissman R. A.; Singru P. S.; Nilsson K. P. R.; Simon R.; Schubert D.; Eisenberg D.; Rivier J.; Sawchenko P.; Vale W.; Riek R. (2009) Science 325, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estroff L. A.; Hamilton A. D. (2004) Chem. Rev. 104, 1201. [DOI] [PubMed] [Google Scholar]; Aida T.; Meijer E. W.; Stupp S. I. (2012) Science 335, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]; Branco M. C.; Sigano D. M.; Schneider J. P. (2011) Curr. Opin. Chem. Biol. 15, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif-Cheikh R.; Bismuth F.; Torres M. L.; Alloza R.; Bosch M. T.; Montes M.; Fuster E.; Valles J.; Cordero J. A.; Peraire C.; Obach R.; Antonijoan R. (1998) Proc. Int. Symp. Control. Rel. Bioact. Mater. 25, 798. [Google Scholar]
- Maji S. K.; Schubert D.; Rivier C.; Lee S.; Rivier J. E.; Riek R. (2008) PLoS Biol. 6, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula P. K.; Cruikshank G. A.; Karp J. M.; John G. (2009) Biomaterials 30, 383. [DOI] [PubMed] [Google Scholar]
- Dufton N.; Perretti M. (2010) Pharmacol. Ther. 127, 175. [DOI] [PubMed] [Google Scholar]; Gavins F. N. E. (2010) Trends Pharmacol. Sci. 31, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. D.; Kim Y.-K.; Lee H. Y.; Kim Y.-S.; Jeon S. G.; Baek S.-H.; Song D.-K.; Ryu S. H.; Bae Y.-S. (2010) J. Immunol. 185, 4302. [DOI] [PubMed] [Google Scholar]
- Kurosaka K.; Chen Q.; Yarovinsky F.; Oppenheim J. J.; Yang D. (2005) J. Immunol. 174, 6257. [DOI] [PubMed] [Google Scholar]
- Chiu I. M.; Heesters B. A.; Ghasemlou N.; Von Hehn C. A.; Zhao F.; Tran J.; Wainger B.; Strominger A.; Muralidharan S.; Horswill A. R.; Wardenburg J. B.; Hwang S. W.; Carroll M. C.; Woolf C. J. (2013) Nature 501, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A.; Fay S. P.; Seligmann B. E.; Freer R. J.; Muthukumaraswamy N.; Mueller H. (1990) Biochemistry 29, 313. [DOI] [PubMed] [Google Scholar]; Freer R. J.; Day A. R.; Muthukumaraswamy N.; Pinon D.; Wu A.; Showell H. J.; Becker E. L. (1982) Biochemistry 21, 257. [DOI] [PubMed] [Google Scholar]
- Powell M.; Stewart T.; Otvos L.; Urge L.; Gaeta F.; Sette A.; Arrhenius T.; Thomson D.; Soda K.; Colon S. (1993) Pharm. Res. 10, 1268. [DOI] [PubMed] [Google Scholar]
- Durr M. C.; Kristian S. A.; Otto M.; Matteoli G.; Margolis P. S.; Trias J.; van Kessel K. P.; van Strijp J. A.; Bohn E.; Landmann R.; Peschel A. (2006) Cell Microbiol. 8, 207. [DOI] [PubMed] [Google Scholar]
- Marasco W. A.; Showell H. J.; Freer R. J.; Becker E. L. (1982) J. Immunol. 128, 956. [PubMed] [Google Scholar]; Freer R. J.; Day A. R.; Radding J. A.; Schiffmann E.; Aswanikumar S.; Showell H. J.; Becker E. L. (1980) Biochemistry 19, 2404. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S.; Nomura Y.; Nitta N.; Akiyama S.; Tamatani T.; Goshoh Y.; Yoshida T.; Sato T.; Kikuchi Y. (2003) J. Immunol. Methods 282, 1. [DOI] [PubMed] [Google Scholar]
- Vyas J. M.; Shawar S. M.; Rodgers J. R.; Cook R. G.; Rich R. R. (1992) J. Immunol. 149, 3605. [PubMed] [Google Scholar]; Aswanikumar S.; Schiffmann E.; Corcoran B. A.; Pert C. B.; Morell J. L.; Gross E. (1978) Biochem. Biophys. Res. Commun. 80, 464. [DOI] [PubMed] [Google Scholar]
- Gao J. L.; Murphy P. M. (1993) J. Biol. Chem. 268, 25395. [PubMed] [Google Scholar]
- Nathan C. (2002) Nature 420, 846. [DOI] [PubMed] [Google Scholar]
- Rudra J. S.; Tian Y. F.; Jung J. P.; Collier J. H. (2010) Proc. Natl. Acad. Sci. U. S. A. 107, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.; Zhou J.; Guvench O.; Sangiorgi F. O.; Li X.; Zhou N.; Xu B. (2014) Bioconjugate Chem. 25, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xing B. G.; Yu C. W.; Chow K. H.; Ho P. L.; Fu D. G.; Xu B. (2002) J. Am. Chem. Soc. 124, 14846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.