Abstract
The membrane of anthocyanin containing Hippeatrum petal vacuoles was examined for protein and enzyme content after purification by equilibrium density centrifugation. Light scattering, protein, and a Mg2+-dependent nucleotide specific ATPase were associated with membrane having a density of 1.08 to 1.12 grams per cubic centimeter. A small amount of acid phosphatase was also present in this region of the gradient, but this activity peaked at about 1.12 grams per cubic centimeter. A component of yeast tonoplast, α-mannosidase, was not significantly present. UDP-glucose, anthocyanidin-3-O-glucosyltransferase, thought to be a cytosol enzyme in Hippeastrum, was absent from tonoplast of vacuoles isolated by osmotic shock in 0.2 molar K2HPO4 or 0.35 molar mannitol. Vacuolar acid phosphatase was insensitive to ethylenediaminetetraacetate but was 80% inhibited by 10 millimolar KF, while ATPase was inactivated by 2 millimolar ethylenediaminetetraacetate and only 50% inhibited by 10 millimolar KF. Five major and about 9 minor polypeptides were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of membrane protein on 5 to 30 and 6 to 16% gradient gels.
Full text
PDF
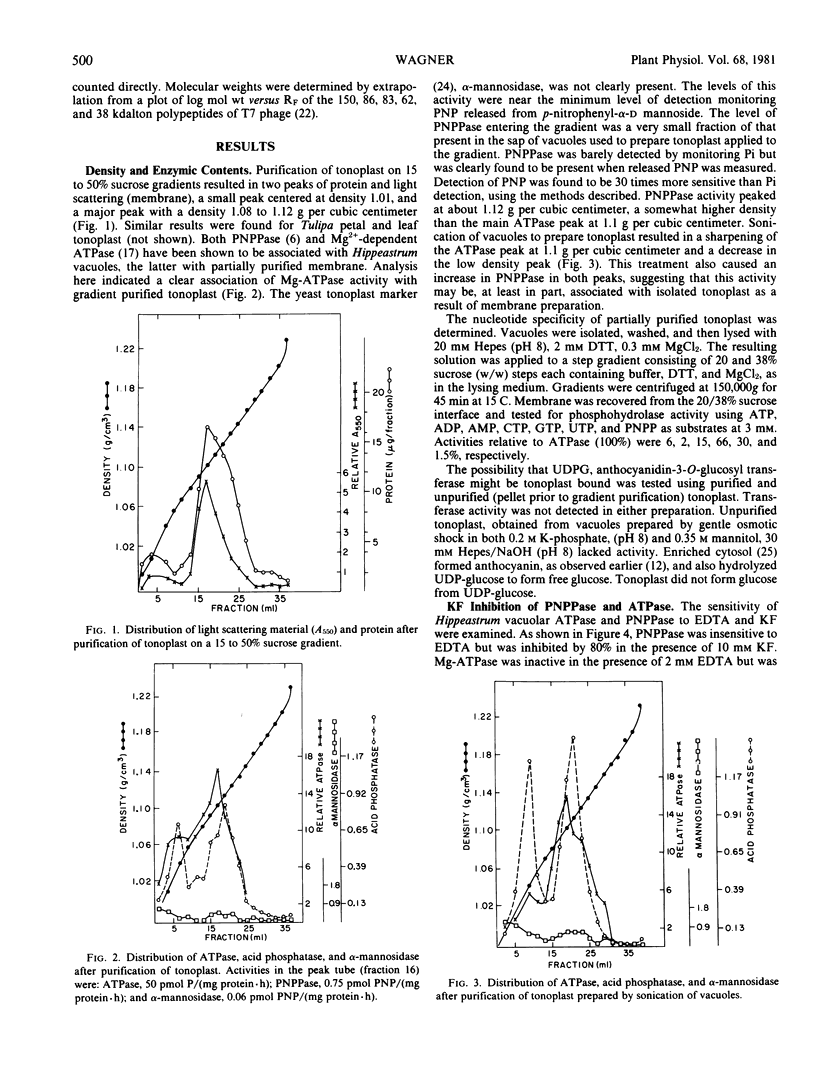
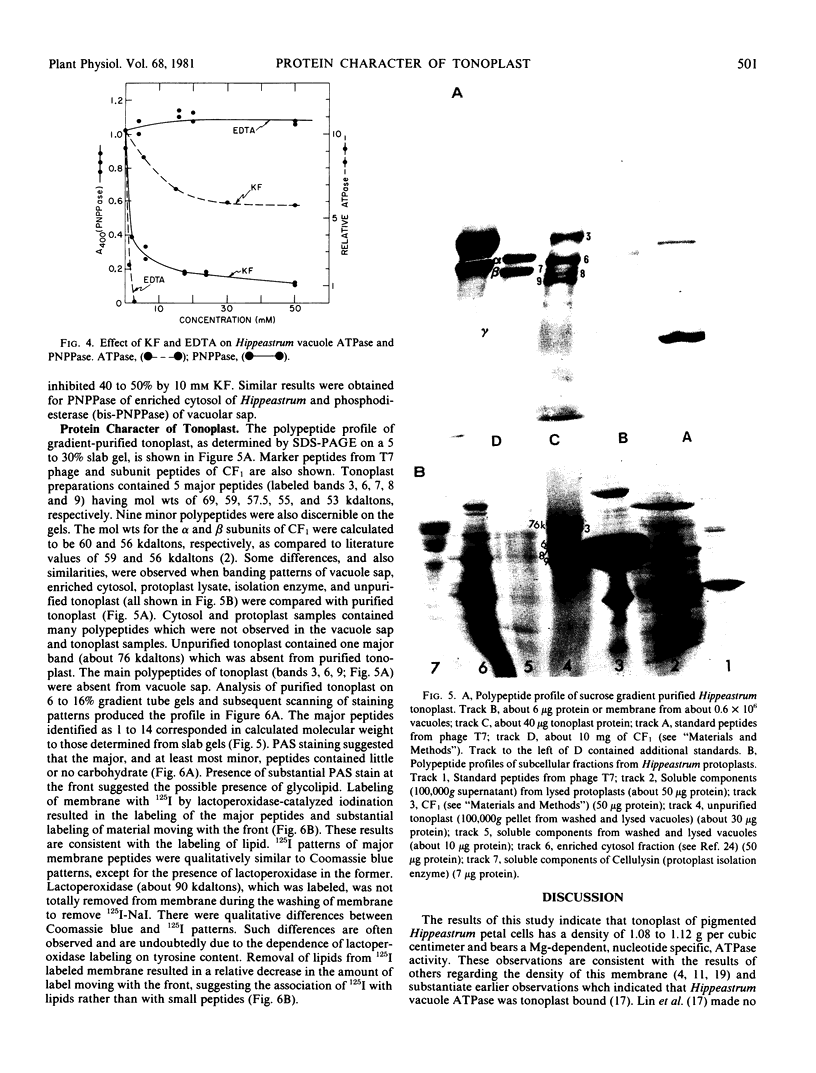
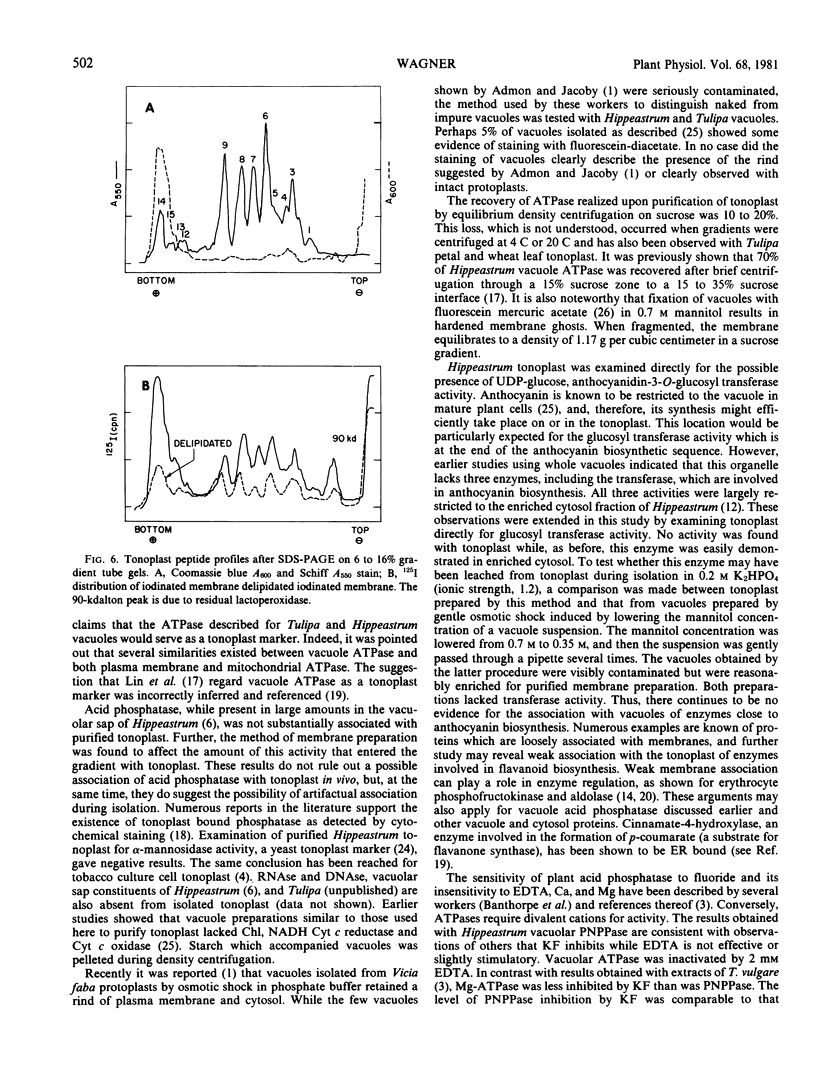

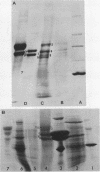
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admon A., Jacoby B. Assessment of cytoplasmic contaminations in isolated vacuole preparations. Plant Physiol. 1980 Jan;65(1):85–87. doi: 10.1104/pp.65.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltscheffsky H., Baltscheffsky M. Electron transport phosphorylation. Annu Rev Biochem. 1974;43(0):871–897. doi: 10.1146/annurev.bi.43.070174.004255. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J. S. The identification of F actin of the pollen tube and protoplast of Amaryllis belladonna. Exp Cell Res. 1974 Oct;88(2):435–439. doi: 10.1016/0014-4827(74)90269-9. [DOI] [PubMed] [Google Scholar]
- DeRosier D., Mandelkow E., Silliman A. Structure of actin-containing filaments from two types of non-muscle cells. J Mol Biol. 1977 Jul 15;113(4):679–695. doi: 10.1016/0022-2836(77)90230-3. [DOI] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Michaeli D. Direct evidence for a sugar transport mechanism in isolated vacuoles. Plant Physiol. 1979 Jul;64(1):61–64. doi: 10.1104/pp.64.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Huber C. T., Morrison M. Heterogeneity of the outer membrane of mitochondria. Biochemistry. 1973 Oct 9;12(21):4274–4282. doi: 10.1021/bi00745a036. [DOI] [PubMed] [Google Scholar]
- Karadsheh N. S., Uyeda K. Changes in allosteric properties of phosphofructokinase bound to erythrocyte membranes. J Biol Chem. 1977 Nov 10;252(21):7418–7420. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Strapazon E., Steck T. L. Interaction of the aldolase and the membrane of human erythrocytes. Biochemistry. 1977 Jun 28;16(13):2966–2971. doi: 10.1021/bi00632a025. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Hesse H., Edelmann K. Quantitative determination of coupling factor CF1 of chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):202–210. doi: 10.1016/0005-2728(73)90135-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Lin P. S. A critical evaluation of plasma membrane fractionation. Biochim Biophys Acta. 1973 Nov;300(3):211–254. doi: 10.1016/0304-4157(73)90005-1. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]