Abstract
Worldwide the prevalence of smoking among people living with HIV/AIDS is elevated compared to the general population. This probably reflects the cluster of individual characteristics that have shared risk factors for HIV infection and smoking. A cross-sectional study, enrolling a convenience sample from a Brazilian HIV clinical cohort was conducted to evaluate the prevalence of tobacco smoking and the factors associated with current smoking and abstinence. A total of 2,775 HIV-infected individuals were interviewed: 46.2% have never smoked, 29.9% were current smokers and 23.9% were former smokers. Current smokers had a higher prevalence of alcohol and illicit drug use when compared to the other two groups. A higher proportion of heterosexual individuals were former smokers or never smokers while among men who have sex with men (MSM) a higher proportion were current smokers. Former smokers had been more frequently diagnosed with high blood pressure, diabetes mellitus, cardiovascular diseases and depression, while for current smokers lung diseases were more frequent. Former smokers and current smokers were more likely to have had any hospital admission (42.0% and 41.2%, respectively) than participants who never smoked (33.5%) (p<0.001). Multivariate model results showed that current smokers (versus never smokers) were more likely to be less educated, to report the use of alcohol, crack and cocaine and to present clinical comorbidities. Former smokers (versus current smokers) were more likely to be older, to have smoked for a shorter amount of time and to have smoked >31 cigarettes/day. MSM (compared to heterosexuals) and cocaine users (versus non-users) had lower odds of being former smokers. Considering our results, smoking cessation interventions should be tailored to younger individuals, MSM and substance users.
Introduction
The prevalence of smoking among people living with HIV/AIDS is elevated compared to the general population in both high income countries [1]–[6] and low or middle income countries (LMIC) [7]–[11], although up to 70% of smokers live in LMIC [12]. This probably reflects the cluster of individual characteristics that have shared risk factors for HIV infection and smoking (e.g. younger age, low education level, low socioeconomic status, illicit drug and alcohol use), rather than being a causal relationship [6], [13], [14].
Smoking is a well-established risk factor for several comorbid conditions among HIV-infected individuals, including: pulmonary infectious diseases, non AIDS-defining cancers (e.g. lung cancer), cardiovascular diseases (CVD) and tuberculosis [15]–[23]. Some authors have highlighted significant association between nicotine dependence, depression and combined antiretroviral therapy (cART) poor-adherence [11], [24], [25], but it increases all-cause mortality, even after controlling for CD4+ T-cell count and HIV viral load [5], [15]–[23], [26]–[28]. Additionally, it may limit the effectiveness of cART by promoting HIV-1 gene expression [29]. Recent findings suggest that HIV-infected smokers report a desire to quit but may have substantial difficulties in the process. It is believed that these difficulties may be strengthened by comorbid psychiatric conditions and social support networks comprised mainly by other smokers [30]–[33].
In Brazil, after several regulatory policies, the prevalence of smoking in the general population has declined and it is estimated at 14.8% among the general population [34]. However, very limited data is available on the prevalence of smoking among individual living with HIV/AIDS in the country [7]. The understanding of factors associated with smoking and smoking cessation is crucial to plan targeted interventions for these individuals. The aim of this study is to describe the prevalence of tobacco smoking as well as the factors associated with current smoking and abstinence in a sample of HIV-infected individuals under care at the Instituto de Pesquisa Clínica Evandro Chagas/FIOCRUZ (IPEC) clinical cohort.
Materials and Methods
This was a cross-sectional study that enrolled a convenience sample selected within the population of the IPEC/FIOCRUZ clinical cohort that had at least one clinical appointment between January 01, 2011 and July 31, 2013.
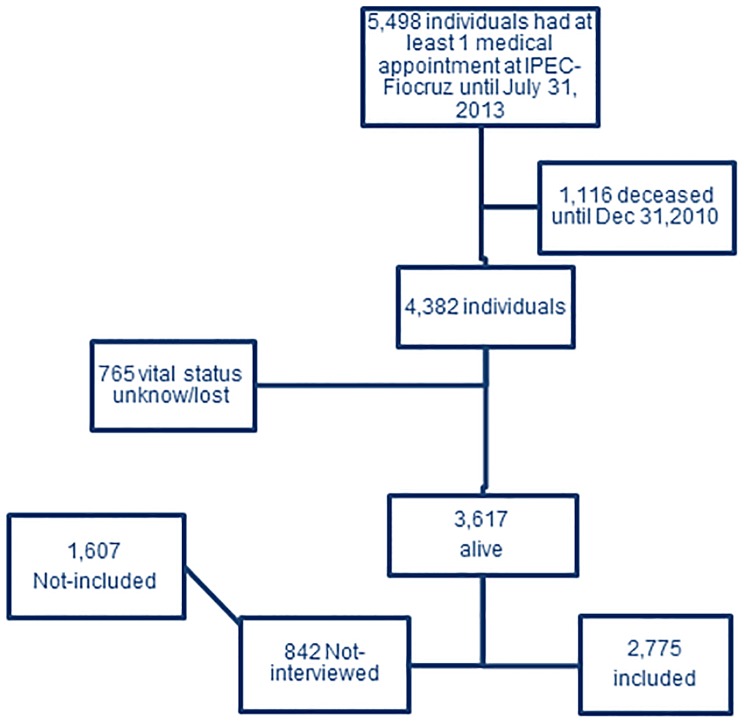
Details of the cohort procedures, definitions and results have been described in previous publications [35]–[37]. Between 1986 and July 31, 2013, 5,498 HIV-infected individuals had at least one clinical appointment at IPEC. Among those, 1,116 were deceased and 765 had unknown vital status or were lost by December 31, 2010. In total, 3,617 patients were alive and potentially eligible for the cross-sectional interview that started in January 01, 2011 (Fig. 1).
Figure 1. Study Population.
Participants
To be included in the cohort a participant must be HIV-infected, 18 years of age or older, and must have signed an informed consent. For this cross-sectional study, cohort participants were invited to participate either when attending a routine appointment or were reached by phone using a structured phone interview.
Measures and definitions
Trained nurses administered standardized questionnaire assessing tobacco consumption in interviews that took place in the patients' regular clinical appointments or by phone calls. The questionnaire was comprised by the following questions: 1) Have you ever smoked cigarettes or any other tobacco products in any moment of your life? (If No, stop the questionnaire; if Yes: age of smoking initiation in years); 2) Do you currently smoke cigarette or any other tobacco product? (Yes or No: If No, age when quit smoking in years); and, 3) How many cigarettes do you currently smoke or used to smoke per day?”. Individuals were grouped as 1) never smoked; 2) current smoker (smoking or in abstinence for less than twelve months) and 3) former smokers (cessation for more than twelve months).The mean number of smoked cigarettes/day was categorized into less than 10; 11–20; 21–30 and 31 or more [38].
Data collected during these interviews were linked with the cohort clinical database using the identification number attributed to each patient. This observational, longitudinal, clinical database was established in 1998 (when all patients seen from 1986 to 1998 were retrospectively included) and is updated for all patients receiving HIV and specialty care (i.e. cardiology, endocrinology, ophthalmology, dermatology, gastroenterology, gynecology and proctology) at the clinic. It is a comprehensive database containing demographic, clinical, and therapeutic information abstracted from the medical records of patients. Prescription of antiretrovirals (drug, dosage, and usage) is documented by the medical provider and support staff in the medical records. Trained abstractors record this information onto standardized forms for processing with regular updates to the database.
Data obtained from the clinical database included socio-demographic, behavioral, clinical and therapeutic information, as follows:
Race/ethnicity: self-reported and categorized into white and non-white.
Education: categorized according to the number of years of schooling into lower or equal/higher than 9 years (equivalent to lower and higher than high school).
Heavy alcohol use: patient's lifetime self-report ingestion of more than 20 drinks/week (men) or 14 drinks/week (women).
Heavy cocaine or crack use was defined based on a patients' lifetime self-report of weekly to daily use of snorted or smoked cocaine.
HIV exposition category was categorized as heterosexual, men who have sex with men (MSM), injection drug use (IDU) and other/unknown.
Comorbidities were defined as clinical diagnosis registered in patient's chart during cohort follow-up. High blood pressure, diabetes (DM), chronic obstructive pulmonary disease (COPD), tuberculosis, pneumonia and non-AIDS-related cancer were evaluated. Cardiovascular disease (CVD) was considered as cardiac arrhythmia, heart failure, coronary disease, ischemic heart disease, peripheral vascular insufficiency and stroke. A positive report of treatment for depression was considered as a proxy for depression diagnosis.
Hospital admission was defined as any hospitalization up to questionnaire administration.
Nadir CD4 cell count was defined as the lowest CD4+ T cell count (cells/mm3) measured during follow-up, categorized in lower or equal/higher than 200 cells/mm3.
Combined antiretroviral treatment (cART) was defined as the use of at least three antiretroviral drugs: two nucleoside reverse transcriptase inhibitor (NRTI) and either one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitor (NNRTI), and categorized into ever and never used cART.
Data analysis
Descriptive analysis and proportions of current smokers, former smokers and never smokers were performed. Chi-square tests and Fisher exact tests were used for categorical variables, and Kruskal-Wallis was used for the asymmetric continuous variable.
Univariate analyses were conducted to compare 1) never smokers with current smokers and; 2) current smokers with former smokers. Two logistic regression models were used to identify independent variables associated with current smoking and smoking abstinence. After univariate analysis, covariates with p-values <0.20 were selected and entered in the initial multivariate model. Covariates with the highest p-values in the analysis were sequentially removed. Variables with statistical significance at 5% (p<0.05) and those that were considered as confounders (e.g., when removed, a change equal to or higher than 10% in the odds ratio of any other variable of the model was observed) remained in the final model. The softwares R 3.0.2 (R Foundation for Statistical Computing: R. 2013) and IBM SPSS version 20 were used to generate all the analyses.
Ethics
IPEC-FIOCRUZ institutional review board has approved this study and all study participants have signed an informed consent form.
Results
A total of 2,775 (76.7%) of individuals under active follow-up responded the questionnaire; 1,281 have never smoked (46.2%), 830 (29.9%) were current smokers and 664 (23.9%) were former smokers.
Table 1 shows the characteristics of the sample stratified by smoking status. The majority of participants were male (65.4%) and the overall male-female ratio was 1.89∶1. This ratio is similar for former smokers and those who never smoked (1.67∶1 and 1.61∶1) but higher for current smokers (2.70∶1) (p<0.001). Former smokers were on average 5 years older than the other two groups.
Table 1. Demographic and clinical characteristics stratified by smoking status, HIV-infected individuals under care at IPEC, 2011–13.
| Current smoker 830(29.9%) | Former smoker 664(23.9%) | Never smoked 1281(46.2%) | p-value* | |
| Male | 606(73.0) | 416(62.5) | 792(61.8) | <0.001 |
| Age | <0.001 | |||
| <30 years | 161(19.4) | 77(11.6) | 224(17.5) | |
| 31–40 years | 249(30.0) | 132(19.9) | 463(36.1) | |
| 41–50 years | 257(31.0) | 240(36.1) | 391(30.5) | |
| 51 years or more | 163(19.6) | 215(32.4) | 203(15.8) | |
| Schooling | ||||
| Less than 9 nine years | 440(53.1) | 329(49.7) | 580(45.4) | 0.002 |
| 9 years or more | 388(46.9) | 333(50.3) | 698(54.6) | |
| White | 447(54.1) | 370(55.8) | 659(51.7) | 0.21 |
| Heavy alcohol use | 137(16.5) | 74(11.1) | 92(7.2) | <0.001 |
| Cocaine inhaled | 162(19.5) | 47(7.1) | 67(5.2) | <0.001 |
| Crack use | 28(3.4) | 5(0.8) | 2(0.2) | <0.001 ** |
| Time of tobacco use in years(min-max)) | 23(0–67) | 12(0–52) | - | <0.001*** |
| Cigarettes per day | - | <0.001 | ||
| <10 | 415(50.4) | 380(57.7) | - | |
| 11–20 | 305(37.1) | 171(25.9) | - | |
| 21–30 | 37(4.5) | 14(2.1) | - | |
| 31 or more | 66(8.0) | 94(14.3) | - | |
| HIV exposure category | <0.001 | |||
| Heterosexuals | 349(42.0) | 360(54.2) | 681(53.2) | |
| MSM | 278(33.5) | 156(23.5) | 319(24.9) | |
| IDU | 23(2.8) | 11(1.7) | 9(0.7) | |
| Other/unknown | 180(21.7) | 137(20.6) | 272(21.2) | |
| Clinical comorbidities | ||||
| High blood pressure | 208(25.1) | 249(37.5) | 338(26.4) | <0.001 |
| DM | 79(9.5) | 106(16.0) | 147(11.5) | 0.001 |
| CVD | 61(7.3) | 63(9.5) | 54(4.2) | <0.001 |
| COPD | 80(9.6) | 54(8.1) | 65(5.1) | <0.001 |
| TB | 249(29.9) | 160(24.1) | 256(20.0) | <0.001 |
| Pneumonia | 248(30.0) | 198(29.8) | 288(22.5) | <0.001 |
| Non-AIDS defining cancer | 18(2.2) | 25(3.8) | 45(3.5) | 0.12 |
| Depression treatment | 243(29.3) | 203(30.6) | 295(23.0) | 0.001 |
| Hospital admission | 342(41.2) | 279(42.0) | 429(33.5) | <0.001 |
| cART | 757(91.2) | 630(94.9) | 1161(90.6) | 0.004 |
| Nadir CD4+ T cell (cells/mm3) | 0.364 | |||
| < = 200 | 434(52.4) | 365(55.0) | 659(51.6) | |
| >200 | 395(47.6) | 299(45.0) | 618(48.4) |
*Chi-square,
**Fischer exact test,
***Kruskal-Wallis.
Current smokers had a higher prevalence of alcohol and illicit drug use when compared to the other two groups. When compared to former smokers, current smokers had a longer time of tobacco use (12 years IQR: 0–52 and 23 years IQR: 0–67, respectively; p<0.001) and were more likely to smoke >10 cigarettes/day (42.3% and 49.6%, respectively).
A higher proportion of heterosexual individuals were former smokers or never smokers while among MSM a higher proportion were current smokers (p<0.001).
Former smokers have been diagnosed more frequently with high blood pressure (37.5%; p<0.001), DM (16.0%; p = 0.001), CVD (9.5%; p<0.001) and depression (30.6%; p = 0.001), while for current smokers COPD (9.6%; p<0.001), TB (29.9%; p<0.001) and pneumonia (30.0%) were more frequent. Former smokers and current smokers were more likely to have any hospital admission (42.0% and 41.2%, respectively) than participants who never smoked (33.5%) (p<0.001).
At the first logistic regression model, individuals who were current smokers, compared to never smokers, were more likely to report the use of crack cocaine (AOR 7.49, CI 95%1.69–33.13), inhaled cocaine (AOR 3.42; IC 95% 2.46–4.75) and alcohol (AOR 1.71; CI 95%1.26–2.34). They were less likely to have more than 9 years of education (AOR 0.69; IC95% 0.57–0.84). Clinical comorbidities as CVD, COPD, TB and depression were independently associated with current smoking, as depicted in Table 2.
Table 2. Factors associated with current smoking compared to those who never smoked, HIV-infected patients under care at IPEC, 2011–2013.
| Crude OR (CI95%) | p-value | Adjusted OR(CI95%) | |
| Male | 1.67(1.38–2.02) | <0.001 | - |
| Age | |||
| <30 years | 1 | 0.013 | 1 |
| 31–40 years | 0.75(0.58–0.96) | 0.025 | 0.69(0.52–0.90) |
| 41–50 years | 0.91(0.71–1.18) | 0.495 | 0.82(0.62–1.08) |
| 51 years or more | 1.12(0.84–1.49) | 0.452 | 1.08(0.79–1.47) |
| Schooling | 0.002 | ||
| Less than 9 nine years | 1 | 1 | |
| 9 years or more | 0.73(0.61–0.87) | 0.69(0.57–0.84) | |
| Non-white | 0.63(0.16–2.46) | 0.484 | - |
| HIV exposition category | 0.001 | ||
| Heterosexuals | 1 | 1 | |
| MSM | 1.70(1.38–2.09) | 2.36(1.87–2.98) | |
| IDU | 4.99(2.28–10.89) | 2.89(1.24–6.67) | |
| Other/unknown | 1.29(1.03–1.62) | 1.37(1.07–1.75) | |
| Heavy alcohol use | 2.55(1.93–3.38) | <0.001 | 1.71(1.26–2.34) |
| Cocaine inhaled | 4.39(3.26–5.93) | <0.001 | 3.42(2.46–4.75) |
| Crack use | 22.33(5.30–93.98) | <0.001 | 7.49(1.69–33.13) |
| Clinical comorbidities | |||
| High blood pressure | 0.93(0.76–1.14) | 0.496 | - |
| DM | 0.81(0.61–1.08) | 0.153 | - |
| Cardiovascular disease | 1.80(1.24–2.63) | 0.002 | 1.67(1.11–2.50) |
| COPD | 1.99(1.42–2.80) | <0.001 | 1.99(1.39–2.86) |
| TB | 1.71(1.39–2.09) | <0.001 | 1.45(1.17–1.81) |
| Pneumonia | 1.48(1.21–1.80) | <0.001 | - |
| cancer | 0.61(0.35–1.06) | 0.07 | 0.46(0.25–0.83) |
| Depression | 1.38(1.13–1.69) | <0.001 | 1.28(1.03–1.59) |
| Lifetime Hospital admission | 1.39(1.16–1.67) | <0.001 | - |
| cART | 0.93(0.69–1.27) | 0.665 | - |
| Nadir CD4+ T cell (cells/mm3) | 0.737 | - | |
| < = 200 | 1 | ||
| >200 | 0.97(0.81–1.16) |
Variables with p-values <0.20 entered in the initial multivariate model. Those with statistical significance at 5% (p<0.05) or confounders remained in the final model.
Regarding smoking cessation, the second logistic model showed that former smoker were more likely to be older, to have smoked for a shorter period of time and to have smoked >31 cigarettes/day than current smokers. MSM had a lower odds of being former smoker (AOR 0.51; CI 95% 0.36–0.71) compared to heterosexuals. Cocaine users (AOR 0.37; CI 95% 0.23–0.58) also had lower odds of being former smoker when compared to non-users (Table 3).
Table 3. Factors associated with smoking cessation (former smokers) compared to current smoking, HIV-infected patients under care at IPEC, 2011–2013.
| Crude OR(CI 95%) | p-value | Adjusted OR(CI95%) | |
| Male | 0.62(0.49–0.77) | <0.001 | - |
| Age | |||
| <30 years | 1 | 1 | |
| 31–40 years | 1.11(0.79–1.56) | 0.557 | 3.73(2.39–5.81) |
| 41–50 years | 1.95(1.41–2.7) | <0.001 | 33.8(19.7–57.9) |
| 51 years or more | 2.76(1.96–3.87) | <0.001 | 351.7(163.5–756.6) |
| Schooling | 0.186 | - | |
| Less than 9 nine years | 1 | - | |
| 9 years or more | 1.15(0.93–1.41) | - | |
| Non-White | 0.40(0.04–3.88) | 0.585** | - |
| Smoking time (years) | 0.93(0.92–0.94) | <0.001*** | 0.8(0.78–0.82) |
| Cigarettes per day | |||
| <10 | 1 | 1 | |
| 11–20 | 0.61(0.48–0.77) | <0.001 | 1.17(0.78–1.62) |
| 21–30 | 0.41(0.22–0.48) | 0.006 | 0.78(0.32–1.86) |
| 31 or more | 1.55(1.01–2.19) | 0.012 | 4.23(2.61–6.87) |
| HIV exposition category | <0.001 | ||
| Heterosexuals | 1 | 1 | |
| MSM | 0.54(0.43–0.69) | 0.51(0.36–0.71) | |
| IDU | 0.46(0.22–0.96) | 1.69(0.60–4.07) | |
| Other/unknown | 0.74(0.56–0.96) | 0.61(0.41–0.89) | |
| Heavy alcohol use | 1.58(1.16–2.13) | 0.003 | - |
| Cocaine inhaled | 0.31(0.22–0.44) | <0.001 | 0.37(0.23–0.58) |
| Crack use | 0.22(0.08–0.57) | <0.001 | - |
| Clinical comorbidities | |||
| High blood pressure | 1.79(1.44–2.24) | <0.001 | 1.84(1.30–2.59) |
| DM | 1.81(1.32–2.46) | <0.001 | 1.57(0.98–2.51) |
| CVD | 1.32(0.91–1.91) | 0.138 | - |
| COPD | 0.83(0.58–1.19) | 0.310 | - |
| TB | 0.74(0.59–0.94) | 0.012 | - |
| Pneumonia | 0.99(0.79–1.24) | 0.940 | - |
| cancer | 1.76(0.95–3.26) | 0.068 | - |
| Depression treatment | 1.06(0.85–1.33) | 0.587 | - |
| Lifetime Hospital admission | 1.03(0.84–1.27) | 0.751 | - |
| cART | 1.79(1.17–2.72) | 0.005 | - |
| Nadir CD4+ T cell (cells/mm3) | 0.313 | - | |
| < = 200 | 1 | - | |
| >200 | 0.9(0.73–1.11) | - |
**Fischer exact test; ***Kruskal-Wallis. Variables with p-values <0.20 entered in the initial multivariate model. Those with statistical significance at 5% (p<0.05) or confounders remained in the final model.
Discussion
Our study provides insight on tobacco smoking use and factors associated with current smoking and abstinence in a sample selected from a large clinical cohort in Rio de Janeiro, Brazil. The observed prevalence of current smoking (29.9%) was similar to another HIV cohort from Recife, Brazil (28.9%) [7], so far the only information available in the country. Compared to international HIV-cohorts from high [1], [13], [39]–[41] and low [11], [17], [42], [43] income settings (prevalence ranging from 40–67% and 46–47%, respectively), the prevalence found herein was considerably lower. It was also lower than the prevalence found at the SMART clinical trial (40.5%), which included 5472 HIV-infected patients from 33 countries [5]. This overall lower prevalence of current smokers might be reflecting the successful public policies against tobacco adopted during the last decades in Brazil that led to an overall decrease of smoking in the Brazilian population [34].
Nevertheless, as reported in other settings [11], [13], [26], [44], the smoking prevalence found for this study population was twice the prevalence of tobacco use among the Brazilian general population (14.8%) [36]. One third of individuals in our study are still smoking and addressing this behavior must be a priority in the care provision package offered to them. Given widespread cART use, mortality due to AIDS-related causes has decreased [45], [46] while the proportion of non-AIDS related causes of death have increased [36]. In fact, a Danish study has shown that among HIV smoking individuals the number of years lost due to tobacco use was twice the number of years lost by HIV. The authors have estimated that life expectancy of a 35 years old HIV-infected individual who smokes is 62.6 years while for a never-smoker HIV-infected individual, it was estimated in 78.9 years [47], pointing out the significant burden posed by smoking in this population.
Our results show that current smokers were mostly men, with lower education level, higher prevalence of alcohol and drug use and higher prevalence of respiratory disease, findings that are in accordance with the international literature [7], [10], [44], [48]. However, there were no significant differences among the three groups regarding non-AIDS related cancers, contrary to previous studies [5]. This result is most likely related to survival bias, since cancer is a well-known cause of death among HIV-infected individuals [49]. Also, differently from other studies [5], the nadir CD4 was similar among the groups. One could speculate that this lack of difference is related to the late entry in treatment, which is still common in our country [50], [51].
Current smokers, compared to never smokers (Table 2), were more likely to have used other drugs such as alcohol and cocaine and to have less than 9 years of education. The clustering of multiple psychosocial problems among HIV-infected smokers has been described in other studies [6], [30], [31], [44] and may represent a challenge for programs aiming at smoking cessation in this population. Additionally, current smoking was more likely among already stigmatized populations such as MSM [52], [53] and IDU [54], adding challenges to smoking related prevention efforts.
Current smokers had a higher chance of presenting CVD when compared to those who never smoked, which could be expected since tobacco is a well-established risk factor for CVD overall and among the HIV-infected population [15], [19], [20], [55]. Moreover, a Tuberculosis (TB) diagnosis was also more likely for them. This is extremely important in our settings because TB is the main cause of death for HIV-infected individuals in Brazil [36] and prevalence of TB in Rio de Janeiro city is high [56]. Tobacco use was already associated with mortality in tuberculosis patients [57]–[59], as well as a predictor for non-adherence to anti-TB drugs [60], but the impact of interactions between smoking and these diseases on the mortality of HIV-infected individuals under care in Brazil must be better understood in future studies.
Considering the overall individuals who had ever smoked (n = 1,492), 44.5% (664/1492) have succeed on quitting at some point in their lives. Former smokers were more likely than current smokers to be older maybe because most smokers who achieve abstinence need many tries before definitely quitting [61], [62]. They were also more likely to have smoked for a shorter period of time, which may be related to a less severe dependence [63], although a higher number of cigarettes per day was also associated with abstinence. The same psychosocial variables associated to current smoking (being an MSM and using cocaine), were barriers to smoking cessation, as also described by Shirley et al [33]. These findings reinforce the need of targeting interventions to young people and MSM, as well as the need to address the abuse/dependence of other substances. The appropriate interventions, however, are not well defined yet. A growing number of studies are being conducted to address smoking among HIV-infected individuals [64]–[67], but results are still preliminary and larger clinical trials are warranted in this area.
Our unadjusted results also showed that former smokers were more likely to be using cART than current smokers and those who never smoked (Table 1). We believe one possible reason for this finding is the higher prevalence of older patients (>40 years, 68.5%) among former smokers, since the association of smoking with cART was no longer present in the adjusted logistic model (Table 3). A previous analysis of the cohort has indicated that older patients in follow-up were infected while younger and are aging with HIV [35]. As a consequence, a higher proportion of them have started to use cART during follow up. Another plausible explanation would be that patients under cART may receive more systematic counseling to quit smoking, as this procedure has been progressively incorporated into good clinical practice for patients with HIV/AIDS.
The prevention of non-communicable diseases among HIV/AIDS patients is a major concern as a larger proportion of individuals with HIV are living longer and facing the double challenge of HIV infection, with its required lifelong treatment, and the increasing burden of chronic non-communicable diseases associated with tobacco use, such as cancer and cardiovascular diseases [68].
This study has noteworthy limitations. First we did not have a standardized instrument to measure the severity of smoking use, which was marginally inferred by the number of cigarettes and length of smoking. Second, as measures were self-reported and no biochemical verification was made, individuals were prone to memory bias, especially the former smokers, and social desirability bias. Memory bias may have overestimated the number of cigarettes smoked among those who quit. Social desirability bias may have underestimated overall prevalence of smoking as it can be understood as an unapproved behavior. Third, the findings cannot be generalized to entire IPEC clinical cohort due to differences among population enrolled during the 25 years of follow –up, the effect of cART over mortality, possible cohort effects and the non-probabilistic characteristic of the sample selected for the cross-sectional study (S1 Table). Fourth, although the questions used to capture smoking behavior were simple, we did not capture the information of how many individuals answered them face-to-face or by phone. The impact of the two data collection methods may or may not have influenced participants' answers. In addition, our sample included participants who were and who were not on cART, and cART was not associated to smoking or smoking cessation. Future studies should also evaluate how cART effects – both objective (e.g., viral load suppression) and subjective (e.g. quality of life) - are related to smoking/abstinence among individuals under antiretroviral therapy. Finally, data were collected from HIV-infected patients at routine clinic visits (and by telephone). Smoking rates in this population may differ from that not engaged in care, limiting data generalization.
In conclusion, these results suggest that individuals living with HIV/AIDS in our cohort are vulnerable to tobacco use. Smoking cessation interventions must be urgently incorporated into the package of care provided to the most vulnerable populations such as MSM, the less educated and drug users.
Supporting Information
Characteristics of cohort individuals who were included vs. not included in the cross-sectional tobacco study.
(PDF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Fuster M, Estrada V, Fernandez-Pinilla MC, Fuentes-Ferrer ME, Tellez MJ, et al. (2009) Smoking cessation in HIV patients: rate of success and associated factors. HIV Med 10:614–619. [DOI] [PubMed] [Google Scholar]
- 2. Webb MS, Vanable PA, Carey MP, Blair DC (2007) Cigarette smoking among HIV+ men and women: examining health, substance use, and psychosocial correlates across the smoking spectrum. J Behav Med 30:371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (2010) Vital signs: current cigarette smoking among adults aged>or = 18 years – United States, 2009. MMWR Morb Mortal Wkly Rep 59:1135–1140. [PubMed] [Google Scholar]
- 4. Lifson AR, Lando HA (2012) Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep 9:223–230. [DOI] [PubMed] [Google Scholar]
- 5. Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, et al. (2010) Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health 100:1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pacek LR, Latkin C, Crum RM, Stuart EA, Knowlton AR (2013) Current Cigarette Smoking Among HIV-Positive Current and Former Drug Users: Associations with Individual and Social Characteristics. AIDS Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batista J, Militao de Albuquerque Mde F, Ximenes RA, Miranda-Filho Dde B, Lacerda de Melo HR, et al. (2013) Prevalence and socioeconomic factors associated with smoking in people living with HIV by sex, in Recife, Brazil. Rev Bras Epidemiol 16:432–443. [DOI] [PubMed] [Google Scholar]
- 8. Waweru P, Anderson R, Steel H, Venter WD, Murdoch D, et al. (2013) The prevalence of smoking and the knowledge of smoking hazards and smoking cessation strategies among HIV- positive patients in Johannesburg, South Africa. S Afr Med J 103:858–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar SR, Swaminathan S, Flanigan T, Mayer KH, Niaura R (2009) HIV & smoking in India. Indian J Med Res 130:15–22. [PubMed] [Google Scholar]
- 10. Munyati SS, Redzo N, Dauya E, Matambo R, Makamure B, et al. (2006) Human immunodeficiency virus, smoking and self-rated health in Harare, Zimbabwe. Int J Tuberc Lung Dis 10:1279–1285. [PubMed] [Google Scholar]
- 11. Jaquet A, Ekouevi DK, Aboubakrine M, Bashi J, Messou E, et al. (2009) Tobacco use and its determinants in HIV-infected patients on antiretroviral therapy in West African countries. Int J Tuberc Lung Dis 13:1433–1439. [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (2007) The Union against tuberculosis and lung diseases. A WHO/The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics. Available: http://www.who.int/tobacco/resources/publications/tb_tobac_ monograph.pdf. Accessed 2014 May 28.
- 13. Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, et al. (2007) Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDS 21:458–468. [DOI] [PubMed] [Google Scholar]
- 14. Vidrine DJ, Arduino RC, Lazev AB, Gritz ER (2006) A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 20:253–260. [DOI] [PubMed] [Google Scholar]
- 15. Lewden C, Salmon D, Morlat P, Bevilacqua S, Jougla E, et al. (2005) Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol 34:121–130. [DOI] [PubMed] [Google Scholar]
- 16. Collins RL, Kanouse DE, Gifford AL, Senterfitt JW, Schuster MA, et al. (2001) Changes in health-promoting behavior following diagnosis with HIV: prevalence and correlates in a national probability sample. Health Psychol 20:351–360. [PubMed] [Google Scholar]
- 17. Gritz ER, Vidrine DJ, Lazev AB, Amick BC III, Arduino RC (2004) Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res 6:71–77. [DOI] [PubMed] [Google Scholar]
- 18. Mamary EM, Bahrs D, Martinez S (2002) Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDS 16:39–42. [DOI] [PubMed] [Google Scholar]
- 19. Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, et al. (2000) Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis 31:808–812. [DOI] [PubMed] [Google Scholar]
- 20. Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, et al. (2003) Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 37:292–298. [DOI] [PubMed] [Google Scholar]
- 21. Arcavi L, Benowitz NL (2004) Cigarette smoking and infection. Arch Intern Med 164:2206–2216. [DOI] [PubMed] [Google Scholar]
- 22. Ehrlich RI, White N, Norman R, Laubscher R, Steyn K, et al. (2004) Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis 8:369–376. [PubMed] [Google Scholar]
- 23. Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, et al. (2002) Selected major risk factors and global and regional burden of disease. Lancet 360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 24. Peretti-Watel P, Spire B, Lert F, Obadia Y. Group V (2006) Drug use patterns and adherence to treatment among HIV-positive patients: evidence from a large sample of French outpatients (ANRS-EN12-VESPA 2003). Drug Alcohol Depend 82 Suppl 1: S71–79. [DOI] [PubMed] [Google Scholar]
- 25. Webb MS, Vanable PA, Carey MP, Blair DC (2009) Medication adherence in HIV-infected smokers: the mediating role of depressive symptoms. AIDS Educ Prev 21:94–105. [DOI] [PubMed] [Google Scholar]
- 26. Shirley DK, Kaner RJ, Glesby MJ (2013) Effects of smoking on non-AIDS-related morbidity in HIV-infected patients. Clin Infect Dis 57:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Wijk JP, Cabezas MC (2012) Hypertriglyceridemia, Metabolic Syndrome, and Cardiovascular Disease in HIV-Infected Patients: Effects of Antiretroviral Therapy and Adipose Tissue Distribution. Int J Vasc Med 2012:201027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benard A, Mercie P, Alioum A, Bonnet F, Lazaro E, et al. (2010) Bacterial pneumonia among HIV-infected patients: decreased risk after tobacco smoking cessation. ANRS CO3 Aquitaine Cohort, 2000–2007. PLoS One 5:e8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feldman DN, Feldman JG, Greenblatt R, Anastos K, Pearce L, et al. (2009) CYP1A1 genotype modifies the impact of smoking on effectiveness of HAART among women. AIDS Educ Prev 21:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, et al. (2009) Characteristics of HIV-positive cigarette smokers: a sample of smokers facing multiple challenges. AIDS Educ Prev 21:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds NR (2009) Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev 21:106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nahvi S, Cooperman NA (2009) Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev 21:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shirley DK, Kesari RK, Glesby MJ (2013) Factors Associated with Smoking in HIV-Infected Patients and Potential Barriers to Cessation. AIDS Patient Care STDS 27:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malta DC, Iser BP, Sa NN, Yokota RT, Moura L, et al. (2013) Trends in tobacco consumption from 2006 to 2011 in Brazilian capitals according to the VIGITEL survey. Cad Saude Publica 29:812–822. [PubMed] [Google Scholar]
- 35. Torres TS, Cardoso SW, Velasque Lde S, Marins LM, Oliveira MS, et al. (2013) Aging with HIV: an overview of an urban cohort in Rio de Janeiro (Brazil) across decades of life. Braz J Infect Dis 17:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grinsztejn B, Luz PM, Pacheco AG, Santos DV, Velasque L, et al. (2013) Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One 8:e59768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribeiro SR, Luz PM, Campos DP, Moreira RI, Coelho L, et al. (2014) Incidence and determinants of severe morbidity among HIV-infected patients from Rio de Janeiro, Brazil, 2000–2010. Antivir Ther [DOI] [PubMed] [Google Scholar]
- 38. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 39. Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE (2010) Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav 14:824–835. [DOI] [PubMed] [Google Scholar]
- 40. Pines H, Koutsky L, Buskin S (2011) Cigarette smoking and mortality among HIV-infected individuals in Seattle, Washington (1996–2008). AIDS Behav 15:243–251. [DOI] [PubMed] [Google Scholar]
- 41. Oka F, Naito T, Oike M, Saita M, Inui A, et al. (2013) Influence of smoking on HIV infection among HIV-infected Japanese men. J Infect Chemother 19:542–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amiya RM, Poudel KC, Poudel-Tandukar K, Kobayashi J, Pandey BD, et al. (2011) Physicians are a key to encouraging cessation of smoking among people living with HIV/AIDS: a cross-sectional study in the Kathmandu Valley, Nepal. BMC Public Health 11:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Louwagie GM, Ayo-Yusuf OA (2013) Tobacco use patterns in tuberculosis patients with high rates of human immunodeficiency virus co-infection in South Africa. BMC Public Health 13:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Cleirigh C, Valentine SE, Pinkston M, Herman D, Bedoya CA, et al. (2014) The Unique Challenges Facing HIV-Positive Patients Who Smoke Cigarettes: HIV Viremia, Art Adherence, Engagement in HIV care, and Concurrent Substance Use. AIDS Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakhaee F, Black D, Wand H, McDonald A, Law M (2009) Changes in mortality following HIV and AIDS and estimation of the number of people living with diagnosed HIV/AIDS in Australia, 1981–2003. Sex Health 6:129–134. [DOI] [PubMed] [Google Scholar]
- 46. Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, et al. (2006) Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 43:27–34. [DOI] [PubMed] [Google Scholar]
- 47. Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, et al. (2013) Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 56:727–734. [DOI] [PubMed] [Google Scholar]
- 48. Duval X, Baron G, Garelik D, Villes V, Dupre T, et al. (2008) Living with HIV, antiretroviral treatment experience and tobacco smoking: results from a multisite cross-sectional study. Antivir Ther 13:389–397. [PMC free article] [PubMed] [Google Scholar]
- 49. Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, et al. (2014) Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 384(9939): 241–248. 50. Souza-Jr PR, Szwarcwald CL, Castilho EA (2007) Delay in introducing antiretroviral therapy in patients infected by HIV in Brazil, 2003–2006. Clinics (Sao Paulo) 62:579–584. [Google Scholar]
- 50. Souza-Jr PR, Szwarcwald CL, Castilho EA (2007) Delay in introducing antiretroviral therapy in patients infected by HIV in Brazil, 2003–2006. Clinics (Sao Paulo) 62:579–584. [PubMed] [Google Scholar]
- 51. Grinsztejn B, Veloso VG, Friedman RK, Moreira RI, Luz PM, et al. (2009) Early mortality and cause of deaths in patients using HAART in Brazil and the United States. AIDS 23:2107–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mayer KH, Bekker LG, Stall R, Grulich AE, Colfax G, et al. (2012) Comprehensive clinical care for men who have sex with men: an integrated approach. Lancet 380:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robinson WT, Brown MC, Moody-Thomas S (2014) Smoking and experiences with tobacco cessation among men who have sex with men: New Orleans, 2011. AIDS Behav 18 Suppl 3: 324–332. [DOI] [PubMed] [Google Scholar]
- 54. Villanti A, German D, Sifakis F, Flynn C, Holtgrave D (2012) Smoking, HIV status, and HIV risk behaviors in a respondent-driven sample of injection drug users in Baltimore, Maryland: The BeSure Study. AIDS Educ Prev 24(2):132–47. [DOI] [PubMed] [Google Scholar]
- 55. Triant VA, Lee H, Hadigan C, Grinspoon SK (2007) Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ministerio da Saude do Brasil (2012) Boletim epidemiologico: Especial Tuberculose. Available: http://www.saude.rs.gov.br/upload/1337634001_Tuberculose-Boletim%20Epidemio.pdf. Accessed 2014 May 28.
- 57. Alavi-Naini R, Moghtaderi A, Metanat M, Mohammadi M, Zabetian M (2013) Factors associated with mortality in tuberculosis patients. J Res Med Sci 18:52–55. [PMC free article] [PubMed] [Google Scholar]
- 58. Berhe G, Enquselassie F, Aseffa A (2013) Assessment of risk factors for development of active pulmonary tuberculosis in northern part of Ethiopia: a matched case control study. Ethiop Med J 51:227–237. [PubMed] [Google Scholar]
- 59. Narasimhan P, Wood J, Macintyre CR, Mathai D (2013) Risk factors for tuberculosis. Pulm Med 2013:828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Naidoo P, Peltzer K, Louw J, Matseke G, McHunu G, et al. (2013) Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC Public Health 13:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, et al. (2014) Natural History of Attempts to Stop Smoking. Nicotine Tob Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goren A, Annunziata K, Schnoll RA, Suaya JA (2014) Smoking cessation and attempted cessation among adults in the United States. PLoS One 9:e93014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dale LC, Olsen DA, Patten CA, Schroeder DR, Croghan IT, et al. (1997) Predictors of smoking cessation among elderly smokers treated for nicotine dependence. Tob Control 6:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cropsey KL, Hendricks PS, Jardin B, Clark CB, Katiyar N, et al. (2013) A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non-treatment seeking smokers with HIV. Addict Behav 38:2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gritz ER, Danysh HE, Fletcher FE, Tami-Maury I, Fingeret MC, et al. (2013) Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis 57:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Humfleet GL, Hall SM, Delucchi KL, Dilley JW (2013) A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res 15:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Matthews AK, Conrad M, Kuhns L, Vargas M, King AC (2013) Project Exhale: preliminary evaluation of a tailored smoking cessation treatment for HIV-positive African American smokers. AIDS Patient Care STDS 27:22–32. [DOI] [PubMed] [Google Scholar]
- 68. Crabtree-Ramírez B, Del Río C, Grinsztejn B, Sierra-Madero J (2014) HIV and noncommunicable diseases (NCDs) in Latin America: a call for an integrated and comprehensive response. J Acquir Defic Syndr 67:S96–S98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of cohort individuals who were included vs. not included in the cross-sectional tobacco study.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.