Abstract
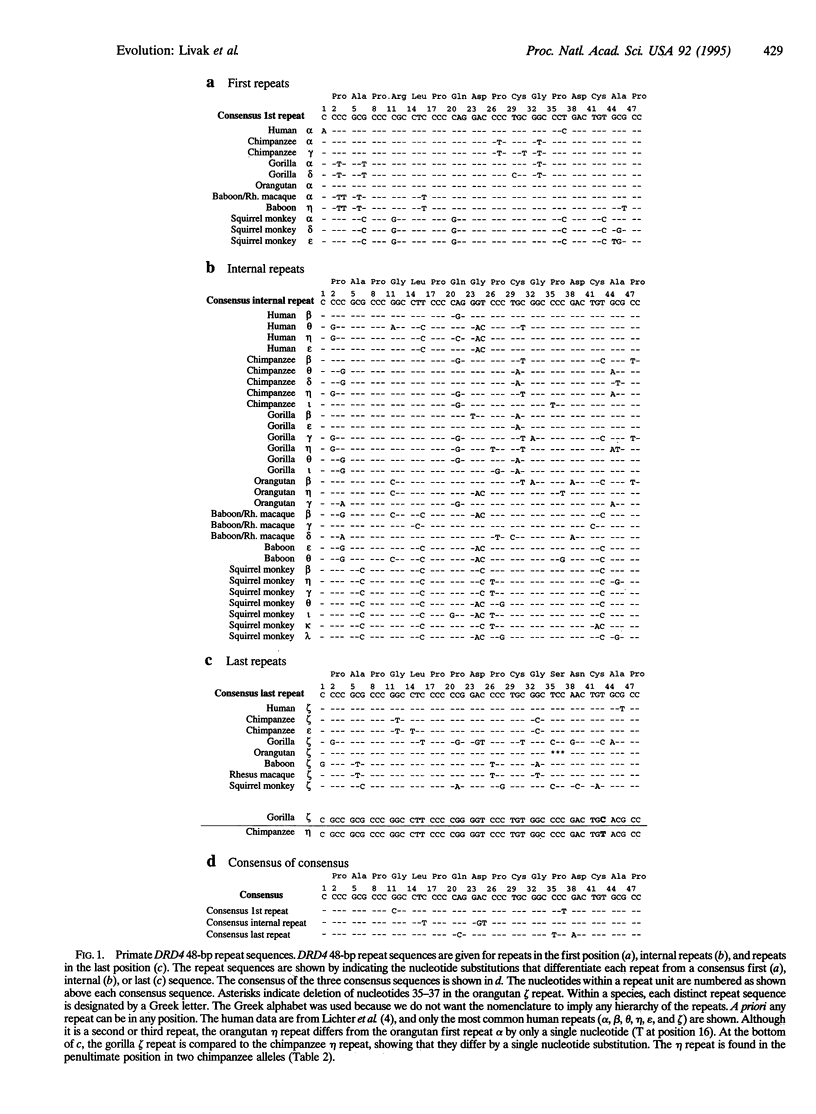
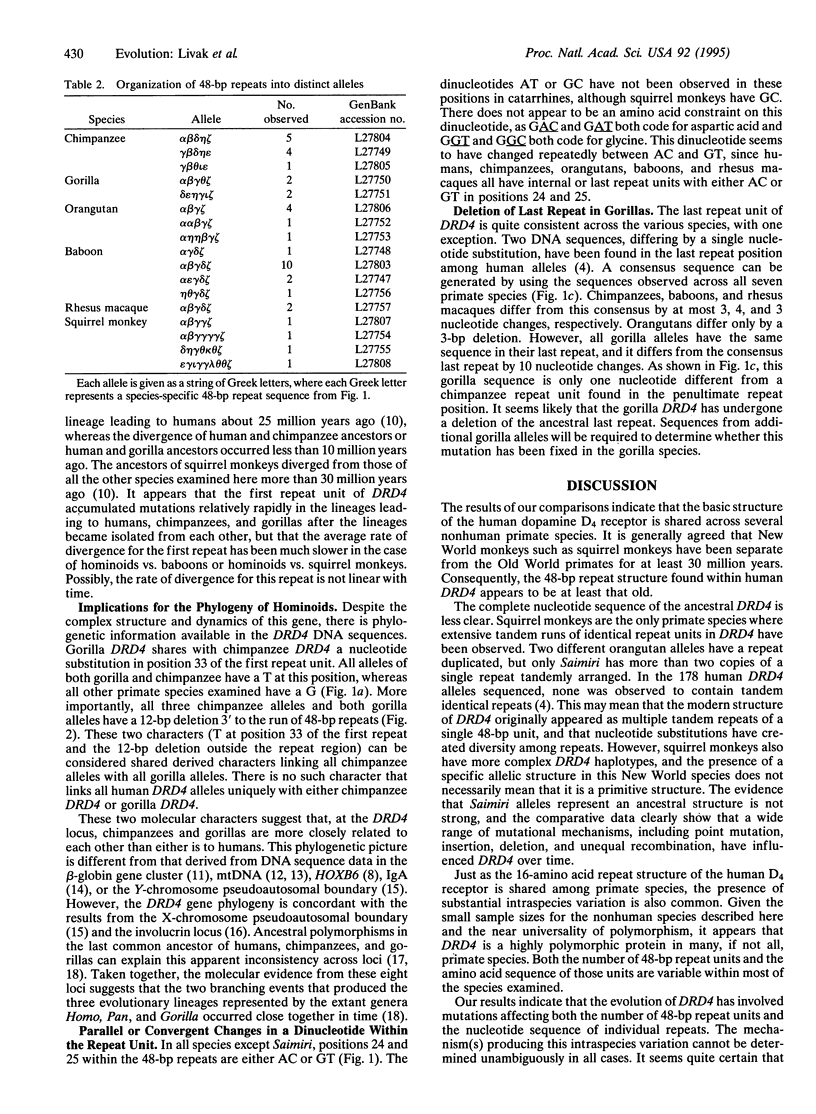
The dopamine D4 receptor is one of five receptors known to function in mammalian dopaminergic pathways. The DNA sequence of the human dopamine D4 receptor gene (DRD4) has previously been investigated in several populations and found to be highly polymorphic at both the DNA and amino acid levels, exhibiting at least 25 alleles. This variation results from differences in the number and DNA sequence of a 48-bp (16-amino acid) repeat unit in the coding region of DRD4. In the present study, DRD4 DNA sequence was examined in at least two individuals from each of five nonhuman primate species. All five species exhibit intraspecies variability, including both single nucleotide substitutions and variation in the number of 48-bp repeat units. No differences were found between the two alleles of one individual from a sixth nonhuman species. Within each species, all of the DRD4 alleles share species-specific features, indicating that while repeat-unit variation is nearly ubiquitous, ancestral variation has been lost and subsequently regenerated in each of the evolutionary lineages studied. Chimpanzees and gorillas share a unique 12-bp deletion in the coding region of DRD4, outside the repeat-unit segment of the gene. This suggest that the extant chimpanzee DRD4 is more closely related to the gorilla DRD4 than either is to the human DRD4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Civelli O., Bunzow J. R., Grandy D. K. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- Djian P., Green H. Vectorial expansion of the involucrin gene and the relatedness of the hominoids. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8447–8451. doi: 10.1073/pnas.86.21.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis N., Yen P., Neiswanger K., Shapiro L. J., Goodfellow P. N. Evolution of the pseudoautosomal boundary in Old World monkeys and great apes. Cell. 1990 Nov 30;63(5):977–986. doi: 10.1016/0092-8674(90)90501-5. [DOI] [PubMed] [Google Scholar]
- Horai S., Satta Y., Hayasaka K., Kondo R., Inoue T., Ishida T., Hayashi S., Takahata N. Man's place in Hominoidea revealed by mitochondrial DNA genealogy. J Mol Evol. 1992 Jul;35(1):32–43. doi: 10.1007/BF00160258. [DOI] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Tanabe H., Watanabe Y., Kurosaki K., Saitou N., Ueda S. Evolutionary rate of immunoglobulin alpha noncoding region is greater in hominoids than in Old World monkeys. Mol Biol Evol. 1991 Nov;8(6):743–752. doi: 10.1093/oxfordjournals.molbev.a040687. [DOI] [PubMed] [Google Scholar]
- Koop B. F., Tagle D. A., Goodman M., Slightom J. L. A molecular view of primate phylogeny and important systematic and evolutionary questions. Mol Biol Evol. 1989 Nov;6(6):580–612. doi: 10.1093/oxfordjournals.molbev.a040574. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Lichter J. B., Barr C. L., Kennedy J. L., Van Tol H. H., Kidd K. K., Livak K. J. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet. 1993 Jun;2(6):767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- Pamilo P., Nei M. Relationships between gene trees and species trees. Mol Biol Evol. 1988 Sep;5(5):568–583. doi: 10.1093/oxfordjournals.molbev.a040517. [DOI] [PubMed] [Google Scholar]
- Ruano G., Rogers J., Ferguson-Smith A. C., Kidd K. K. DNA sequence polymorphism within hominoid species exceeds the number of phylogenetically informative characters for a HOX2 locus. Mol Biol Evol. 1992 Jul;9(4):575–586. doi: 10.1093/oxfordjournals.molbev.a040743. [DOI] [PubMed] [Google Scholar]
- Ruvolo M., Disotell T. R., Allard M. W., Brown W. M., Honeycutt R. L. Resolution of the African hominoid trichotomy by use of a mitochondrial gene sequence. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1570–1574. doi: 10.1073/pnas.88.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Guan H. C., Van Tol H. H. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993 Sep 30;365(6445):441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Wu C. M., Guan H. C., Ohara K., Bunzow J. R., Civelli O., Kennedy J., Seeman P., Niznik H. B., Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992 Jul 9;358(6382):149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]



