IN THE LAST DECADE, THE STUDY OF AUTOPHAGY has intensified markedly as evidenced by the number of published articles, 3,762 from 2013 to 2014 compared with 136 from 2003 to 2004, and the advent of a journal entitled Autophagy. The literal translation of “autophagy” emanates from Greek language as “self-eating.” Autophagy is a dynamic, energy-dependent cellular process that responds to stresses with the ultimate purpose of self-preservation. The autophagic process is suited uniquely to the timely identification of surplus, unnecessary, or dysfunctional proteins and organelles; the engulfment and subsequent digestion of these molecules maintains cellular function and recycles reusable cytoplasmic constituents, especially in the form of amino acids. As such, insufficient or defective autophagy accumulates abnormal or dysfunctional proteins and organelles. In agreement with this context, growing evidence demonstrates that a number of diseases are associated directly with impaired autophagy. Notably, neurodegenerative diseases, cancer, infection, aging, and ischemia/reperfusion (I/R) injury, to name a few, all exhibit its correlation with autophagic defects.1-4 Thus, manipulation of this ubiquitous process may be an avenue for endogenous repair or cleanup of disturbed systems.
THE AUTOPHAGIC PROCESS
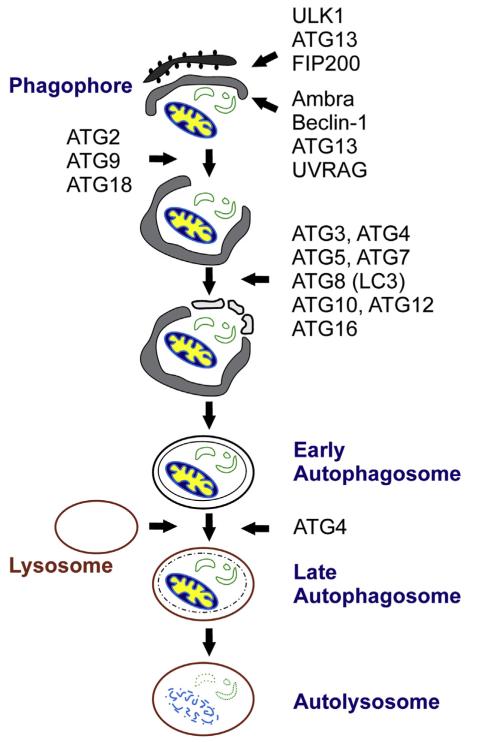
How does autophagy work? Autophagy involves the envelopment of cytoplasmic components, including proteins and organelles, in a double membrane structure called an autophagosome that later fuses with a lysosome to generate a single membrane structure called an autolysosome (Fig 1). This process is initiated through the expression of several ATG genes and the resultant proteins that are required for the initiation, elongation, and maturation of the autophagosome, fusion with a lysosome, and then degradation of the contents entrapped in the autolysosome. So far, >30 ATGs have been identified in mammals. Eighteen of these ATGs have been designated as core components, essential to autophagosome formation.1 Most of these core proteins aggregate at the phagophore assembly site that is thought to represent membrane budding of existing cellular organelles (endoplasmic reticulum, mitochondria, or Golgi). After phagophore formation (ULK1, ATG13, FIP200, Ambra, Beclin-1, ATG13, and UVRAG), the autophagy-related proteins guide the elongation of the double membrane structure that completely surrounds the soon to be catabolized proteins or organelles (ATG2, ATG3, ATG4, ATG5, ATG7, ATG8, ATG9, ATG10, ATG12, ATG16, and ATG18). Once the proteins or organelles are enveloped in the autophagosome and later entrapped in the autolysosome, autolysosomal cargos are degraded into amino acids by a variety of acidic enzymes in this single membrane structure.
Fig 1.
The initiation, elongation, and maturation of the autophagy process with the involved proteins.
Regulation of autophagy occurs via mammalian target of rapamycin (mTOR)-dependent and mTOR-independent pathways. mTOR has been identified as a central but not exclusive regulator of autophagy, where it serves as an essential sensor for nutrient repletion.1-4 A cell with a stable energy source has active mTOR signaling through its phosphorylation, leading to inhibition of autophagy. Conversely, a nutrient-depleted cell has decreased or inhibited mTOR activity, thus activating autophagy. As implied in the name of mTOR, rapamycin induces autophagy by inhibiting mTOR.
Much less is known about the relationship between autophagy and mTOR-independent signaling pathways; however, the phosphinositol signaling pathway, cyclic adenosine monophosphate–Epac–PLC–IP3, Ca2+–calpain–Gs α pathway, and ceramide and sphingolipid pathways have all been associated with regulation of autophagy.3
KEY AUTOPHAGIC PROTEINS
Cell starvation is an excellent model for the study of autophagy, because under nutrient-depleted conditions with little mTOR activity, the autophagic process is stimulated markedly. Although a host of proteins are involved in the formation of phagophores and autophagosomes, ATG12-5, ATG7, and Beclin-1, a mammalian ortholog of ATG6, represent key proteins that are necessary for the initiation of autophagy and the maturation of the autophagosomes.1-4 Importantly, even though many of these proteins function exclusively as autophagy regulators, Beclin-1 plays a pivotal role in determining which fate cells undergo, that is, autophagy or apoptosis.2 The antiapoptotic Bcl-2 family of proteins, including Bcl-2, Bcl-xL, and Mcl-1, can bind directly to Beclin-1. Because Beclin-1 is not only an essential initiator of autophagy but has a Bcl-2 homology 3 (BH3)-only domain, a direct interaction between the Beclin-1 and Bcl-2 family of proteins prevents Beclin-1 from assembling a phagophore, which in turn suppresses autophagy. In contrast, when the proapoptotic BH3-only proteins bind to antiapoptotic proteins, rather than Beclin-1, this autophagy initiator protein becomes free from BH3-only proteins and autophagy begins thereafter. This complex interplay is an essential determinant as to whether cells develop autophagy or apoptosis. Although controversies remain unsettled on autophagy-induced cell death, an improved understanding of autophagy demonstrates its primary prosurvival role and recognizes that either insufficient or impaired autophagy may give way to cell death.2
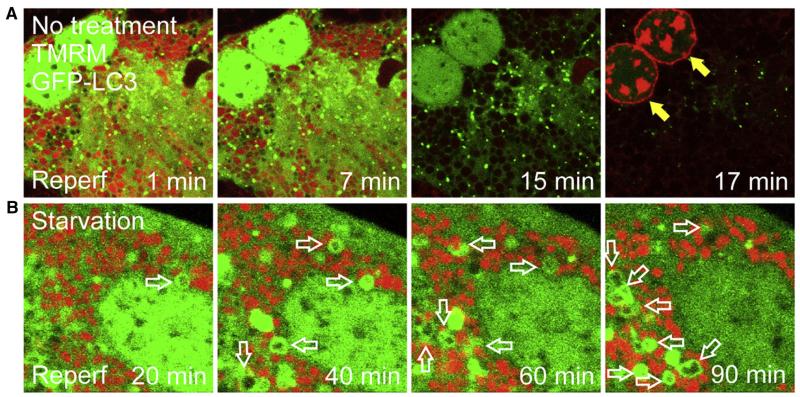
In addition to the important initiating proteins, the transition from autophagosome formation to a functioning autolysosome represents completion of the autophagic process. A key molecular marker signifying this transition is the conversion of microtubule-associated protein light chain 3 (LC3-I), a mammalian homolog of ATG8, to LC3-II. LC3-I, an inactive cytosolic ATG8, can be activated to LC3-II by conjugating with phosphati-dylethanolamine. In contrast with LC3-I, LC3-II localizes to the autophagosomes. This is why LC3 appears from diffuse to punctate stains after the induction of autophagy (Fig 2).1,2 Autophagy is a dynamic process between autophagosomal formation and autolysosomal clearance. Therefore, the measurement of the conversion rate from LC3-I to LC3-II and the degradation rate of LC3-II in the presence of lysosomal inhibitors, such as bafilomycin or chloroquine, represents autophagic flux, the gold standard for assessing a dynamic nature of the autophagic process.4
Fig 2.
Confocal microscopic images of sequestration of dysfunctional mitochondria by mitophagy. Hepatocytes expressing green fluorescing GFP-LC3 were subjected to ischemia/reperfusion with red fluorescing tetramethylrhodamine methylester (TMRM) and propidium iodide. (A) During the early phase of reperfusion (Reperf), the mitochondria in a control hepatocyte transiently repolarize. However, the mitochondria completely depolarize later and the cell loses viability thereafter (filled arrows). Note the lack of mitophagy onset. (B) Under the starvation condition, the hepatocyte contains green ring-like autophagosomes surrounding the mitochondria (empty arrows), indicative of mitophagy onset. The cell maintains the mitochondrial membrane potential and remains viable after reperfusion.
AUTOPHAGY IN THE LIVER
Autophagy in the liver has been relatively well studied, because multiple physiologic or pathologic conditions exhibit either increased or decreased autophagy. In addition, impairment of the autophagic process has a profound effect on liver function in alcoholism, drug toxicity, and I/R injury.5 The liver maintains an increased rate of basal autophagy compared with other organs, and, furthermore, hepatocytes are exquisitely sensitive to nutrient deprivation with a rapid increase in autophagy.5 This trait not only highlights the importance of autophagy to the liver, but also singles out hepatocytes as an ideal research tool. Selective autophagy, or the autophagic engulfment of discrete organelles or molecules such as mitochondria (mitophagy) or lipids (lipophagy), serves as integral machinery of hepatocellular homeostasis. Routine autophagy of the mitochondria and lipids permits the cellular recycling of necessary constituents to meet the anabolic needs of the hepatocyte. Additionally, lipophagy helps to modulate hepatocellular lipid stores more efficiently to respond to the energy demands of the liver and to protect the cells from lipid-mediated damage. Decreased levels of autophagy have been shown to contribute to lipid accumulation and subsequent development of nonalcoholic fatty liver disease.5 Similarly, decreased autophagy culminates in hepatocyte damage and fibrosis in alcoholic liver disease.5 Likewise, alterations in autophagy are evident in a number of other liver diseases, including hepatitis B and C, hepatocellular carcinoma, and inborn errors of metabolism.5
Although autophagy often becomes active at the early stage of numerous liver diseases as a consequence of an initial adaptive strategy, chronic or prolonged insults to the liver accompany the impairment of autophagy. One good example is I/R injury in the liver. The liver is an aerobic organ and innately vulnerable to low oxygen or ischemic stress. Paradoxically, a recovery of blood flow and normal physiologic pH during reperfusion worsens ischemic tissue damage, an event called reperfusion injury. I/R injury is a major obstacle to hepatectomy and liver transplantation.
During ischemia, a markedly decreased blood supply to the liver causes a temporary nutrient depletion or starvation in hepatocytes. Although starvation is a robust stimulus for autophagy in normal livers, hepatocytes in ischemic livers are unable to instigate autophagy owing to a shortage of adenosine triphosphate (ATP) as well as tissue acidosis. Autophagy is a highly energy-demanding, multistep process with involvement of numerous enzymes, each of which functions optimally at physiologic acid–base balance. In addition to stifled autophagy, ATP depletion after prolonged ischemia increases substantially cellular calcium concentrations resulting from the collapse of ATP-driven calcium pumps and secondary ion exchangers as well as blockade of electrogenic calcium uptake by the mitochondria. This ischemic calcium overloading, in turn, activates calpains (calcium-dependent proteases) that hydrolyze key autophagy proteins, particularly ATG7 and Beclin-1. Therefore, with the combination of ATP depletion, tissue acidosis, and loss of TAG, the formation of autophagosomes is impeded, and the autophagic flux becomes minimal during prolonged ischemia.
Reperfusion restores oxygen supply and recovers hepatocellular acid-base balance. Consequently, at the early phase of reperfusion, the mitochondria repolarize transiently and restore the proton motive force, leading to a partial recovery of ATP. Induction of autophagy eliminates dysfunctional proteins and organelles that were produced by ischemia. This clearing process, however, is short lived. As reperfusion proceeds, partially recovered ATP is eventually exhausted by a myriad of energy-requiring processes, including autophagy. Furthermore, a subset of the mitochondria accumulates calcium and reactive oxygen species in excess, resulting in the mitochondrial permeability transition. The opening of high-conductance, permeability transition pores in the mitochondrial inner membranes causes abruptly a nonselective diffusion of solutes, and the electron transport chains become uncoupled from ATP production. The mitochondria eventually lose the integrity of their barrier to permeability and release proapoptotic proteins and calcium into the cytosol, which further activates calpains and depletes cells of ATG7 and Beclin-1. At this time point, the capacity of autophagic clearance is surpassed by reperfusion-induced mitochondrial dysfunction. As a consequence, autophagy no longer removes dysfunctional mitochondria, abnormal mitochondria accumulate in excess, widespread onset of the mitochondrial permeability transition occurs, and ultimately, hepatocyte death ensues (Fig 2).
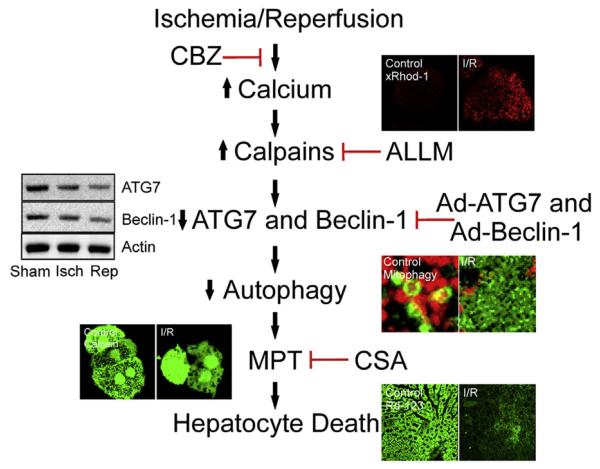
Strategies to augment autophagy include nutrient depletion or starvation, pharmacologic agents (such as rapamycin and carbamazepine, a Food and Drug Administration-approved anticonvulsant drug), or genetic overexpression of ATG7 or Beclin-1 before ischemia. In animal models, these regimens suppress the mitochondrial permeability transition onset and increases hepatocyte viability after I/R (Fig 3).6,7 Moreover, early endogenous upregulation of autophagy may lead to enhanced mitophagy and timely elimination of dysfunctional mitochondria that drive the cascade of I/R injury. Thus, impaired or insufficient autophagy is a causative event contributing to mitochondrial dysfunction and hepatocyte death after I/R. Future therapeutic strategies should aim for enhanced autophagy to limit I/R injury, especially in clinical scenarios like hepatic resection or transplantation.
Fig 3.
The proposed mechanism of ischemia/reperfusion (I/R) injury and therapeutic strategies. I/R causes calcium overloading, which consequently activates calpains. Carbamazepine (CBZ) or acetyl-Leu-Leu-methioninal (ALLM) suppresses calcium overloading and calpains, respectively. Calpain activation results in the degradation of ATG7 and Beclin-1, key autophagy proteins. Gene therapy such as adenoviral overexpression of ATG7 (Ad-ATG7) and Beclin-1 (Ad-Beclin-1) delays the depletion of autophagy proteins. As a consequence of ATG7 and Beclin-1 deprivation, autophagy becomes impaired or insufficient, leading to onset of mitochondrial permeability transition (MPT) and subsequent hepatocyte death. Cyclosporin A (CSA) is an MPT blocker.
SUMMARY
The recent excitement about autophagy research is justified; the importance of the pathobiology of this process is obvious. Clearly, functional autophagy is central to basic cellular housekeeping and recycling activity, maintenance of homeostasis, and injury response. An improved understanding of the autophagic process should lead to potentially innovative therapies with direct relevance to surgical diseases. Although autophagy has been one of nature’s closely guarded secrets until recently, an abundance of new findings provides substantial promise for therapeutic manipulation of this endogenous process. Stay tuned as we discover and use new therapeutics to take advantage of autophagy in the management of surgery patients.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases grant DK079879 and DK090115 (J.-S. Kim), National Institute on Aging AG028740 (J.-S. Kim), and the National Cancer Institute 5T32CA106493 (K.E. Behrns).
REFERENCES
- 1.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–31. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 4.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czaja MJ, Ding WX, Donohue TM, Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–58. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JS, Nitta T, Mohuczy D, O’Malley KA, Moldawer LL, Dunn WA, Jr, et al. Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725–36. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JS, Wang JH, Biel TG, et al. Carbamazepine suppresses calpain-mediated autophagy impairment after ischemia/reperfusion in mouse livers. Toxicol Appl Pharmacol. 2013;273:600–10. doi: 10.1016/j.taap.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]