Abstract
Cytopenias are key prognostic indicators of life-threatening infection, contributing to immunosuppression and mortality. Here we define a role for Caspase-1-dependent death, known as pyroptosis, in infection-induced cytopenias by studying inflammasome activation in hematopoietic progenitor cells. The NLRP1a inflammasome is expressed in hematopoietic progenitor cells and its activation triggers their pyroptotic death. Active NLRP1a induced a lethal systemic inflammatory disease that was driven by Caspase-1 and IL-1β but was independent of apoptosis-associated speck-like protein containing a CARD (ASC) and ameliorated by IL-18. Surprisingly, in the absence of IL-1β-driven inflammation, active NLRP1a triggered pyroptosis of hematopoietic progenitor cells resulting in leukopenia in the steady state. During periods of hematopoietic stress induced by chemotherapy or lymphocytic choriomeningitis virus (LCMV) infection, active NLRP1a caused prolonged cytopenia, bone marrow hypoplasia and immunosuppression. Conversely, NLRP1-deficient mice showed enhanced recovery from chemotherapy and LCMV infection, demonstrating that NLRP1 acts as a cellular sentinel to alert Caspase-1 to hematopoietic and infectious stress.
Keywords: inflammasome, NLRP1, IL-18, IL-1β, progenitor cells, pyroptosis, cytopenia, sepsis
INTRODUCTION
The production of IL-1β and IL-18 can be triggered by innate immune receptors such as NLRP3, which form inflammasome complexes to activate Caspase-1 following recognition of infection and host tissue damage (Martinon et al., 2009). However, the most ancient function for this family of innate immune receptors can be traced back to plant nucleotide-binding leucine-rich repeat (NB-LRR) proteins, where activating mutations cause cell death (Bernoux et al., 2011; Dietrich et al., 1994; Swiderski et al., 2009). Activation of mammalian nucleotide-binding oligomerisation domain, leucinerich repeat (NLR) proteins can also cause cell death (Fernandes-Alnemri et al., 2007; Fink et al., 2008), but the importance of Caspase-1 activation and pyroptosis in disease states is unclear. For example, it is known that NLRP3 activating mutations in humans can be effectively treated by neutralising IL-1β, suggesting that cell death induced by NLRP3 activation does not play a significant role in pathology (Lachmann et al., 2009). Similarly, the NLRP1b inflammasome is activated by anthrax lethal toxin to cause macrophage pyroptosis, but this does not play a role in anthrax sensitivity in vivo (Moayeri et al., 2003; Terra et al., 2010). Sepsis is commonly associated with a range of cytopenias including anemia, lymphopenia, neutropenia and thrombocytopenia however the etiological triggers for these conditions have not been elucidated. Here we report for the first time the physiological consequences of unrestrained NLRP1a activation in the absence of IL-1β-induced inflammation. We show that this results in pyroptosis of hematopoietic progenitor cells and compromises hematopoiesis during periods of hematopoietic and infectious stress.
NLRP1 expression is highly restricted to the hematopoietic cell compartment and its expression and function is guided by several regulatory mechanisms and putative protein interactions (Kummer et al., 2007). The sterol regulatory element binding protein-1a (SREBP-1a) basic helix-loop-helix leucine zipper transcription factor, differentially regulates the expression of inflammation-related genes including Nlrp1a in hematopoietic cells. It is hypothesised that Nlrp1a expression is regulated at an additional level via NFκB-responsive elements in the Srebp1a promoter, allowing the induction of Srebp1a and Nlrp1a during conditions of hematopoietic and infectious stress (Im et al., 2011). At a post-translational level, NLRP1 undergoes autocleavage at Ser1213 in the FIIND domain (ZU5- and UPA-like domains) which is required for its function (D'Osualdo et al., 2011; Finger et al., 2012; Levinsohn et al., 2012). NLRP1 function can also by regulated by several interacting partners. The pro-survival proteins Bcl-2 and Bcl-xL can bind NLRP1 and inhibit its activation and oligomerisation via interactions between the leucine-rich repeats (LRR) of NLRP1 and the loop regions of Bcl-2 and Bcl-xL (Bruey et al., 2007). This NLRP1-inhibitory activity is independent of the well-documented pro-survival functions of Bcl-2 and Bcl-xL and occurs prior to formation of the inflammasome complex.
To date, no studies have described the specific consequences of unrestrained pyroptosis in vivo, or NLRP1 inflammasome activation in the absence of infection. Here we show the effect of an activating mutation in NLRP1 as well as NLRP1 deficiency in response to hematopoietic stress induced by infection or chemotherapy. We demonstrate that this inflammasome activity induces IL-1β-dependent autoinflammation and IL-1β-independent deletion of hematopoietic progenitor cells.
RESULTS
Identification of NLRP1 mutant mice
Autoinflammatory disease and acute inflammatory diseases such as sepsis are commonly associated with neutrophilia. To identify genetic regulators of these conditions, we performed an N-ethyl-N-nitrosourea (ENU) mutagenesis screen for dominant mutations that cause neutrophilia in G1 mice and isolated a pedigree that was named Neut1 (Figure S1). The Neut1 mutation was genetically mapped via standard positional cloning techniques to a 700 kbp interval on chromosome 11 between 70.5 and 71.2 Mbp, and sequence analysis identified a point mutation in the gene encoding Nlrp1a, predicted to cause a glutamine-to-proline substitution at amino acid 593 (Nlrp1aQ593P). This region of the protein is likely to be a flexible linker region between the NACHT (nucleotide-binding, NB) and LRR domains, where activating mutations are found in other NB-LRR proteins in plants (Bendahmane et al., 2002; Moffett et al., 2002; Zhang et al., 2003).
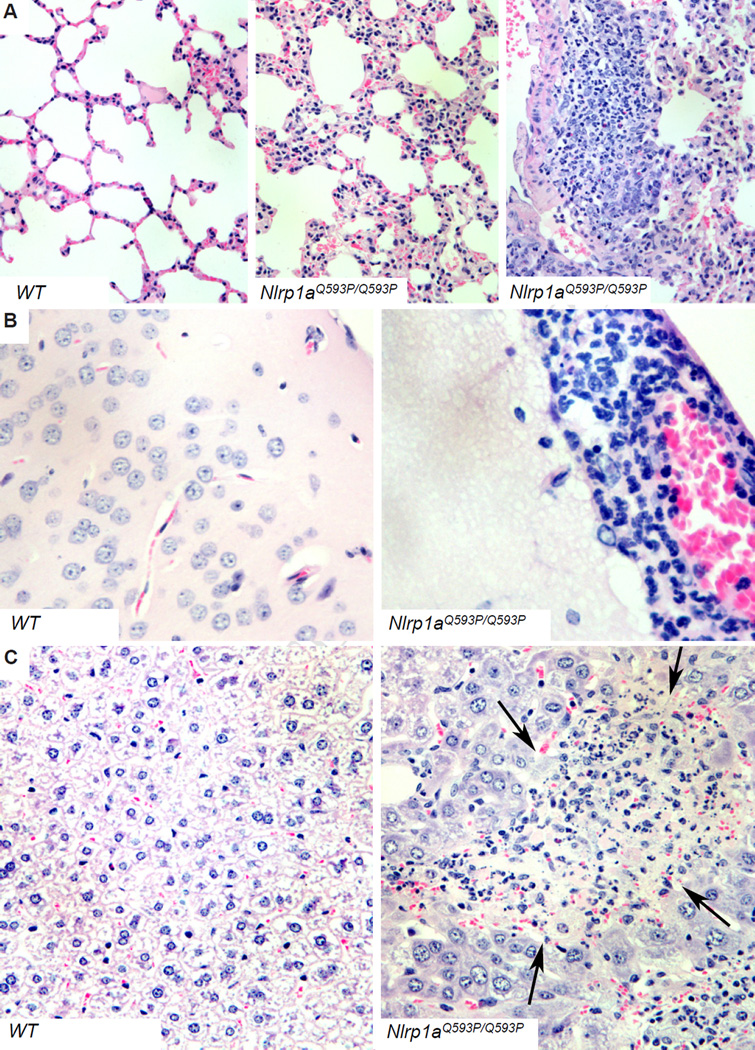
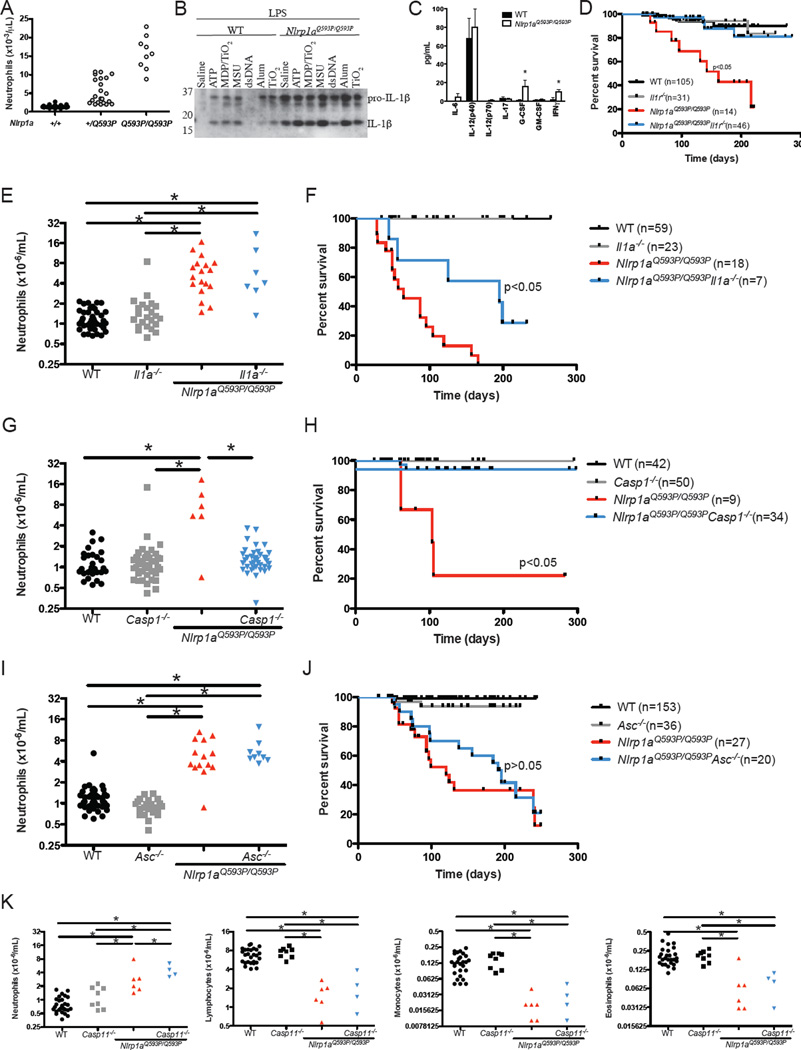
Nlrp1a+/Q593P congenic BALB/c mice were fertile and remained healthy to at least 8 months of age, despite histological evidence of a multi-organ neutrophilic inflammatory disease characterised by meningitis, hepatitis, pneumonitis, pancreatitis, pulmonary peri-arteritis, myocarditis and inflammatory bowel disease. In Nlrp1aQ593P/Q593P homozygotes, a similar but lethal condition developed by 3–5 months of age (Figure 1). Neutrophil counts in these animals were 15-fold higher than wild-type, and they exhibited lymphopenia and splenomegaly (Figure 2A and Table S1). The peritonitis in Nlrp1aQ593P/Q593P homozygotes was associated with a loss of peritoneal macrophages (Table S1). These data demonstrate that active NLRP1a drives a systemic inflammatory phenotype that can be distinguished from inflammatory disease induced by active NLRP3 (Brydges et al., 2009).
Figure 1. Inflammatory disease induced by the NLRP1aQ593P mutation.
Hematoxylin and eosin-stained tissues show pneumonitis (A), meningitis (B) and hepatitis (C).
Figure 2. Caspase-1 and IL-1β are major contributors to NLRP1aQ593P–induced disease.
(A) Effects of heterozygous and homozygous Nlrp1aQ593P mutations on peripheral blood neutrophil numbers in mice on a BALB/c background. (B) Nlrp1aQ593P/Q593P macrophages were treated with 2 ng/mL LPS for 4h before the inflammasome was activated with 5 mM ATP for 30 min or MDP complexed with TiO2, 100 µg/mL uric acid crystals (MSU), 7 µg/mL poly(dA:dT) (dsDNA) or alum for 15 h. IL-1β secretion was assessed by immunoblot using antibodies specific for the 35 kDa pro-IL-1β and the processed, bioactive 17 kDa IL-1β. (C) Cytokine levels were measured in the serum of wild-type and Nlrp1aQ593P/Q593P mice. *p<0.05, mean ± SD, n=3 independent samples. (D) Disease-free survival in Nlrp1aQ593P/Q593P mice deficient in the IL-1R. (E–J) Neutrophil numbers and disease-free survival in Nlrp1aQ593P/Q593P mice deficient in IL-1α, Caspase-1 or ASC. Neutrophil numbers in the peripheral blood were analysed at 7 weeks age. A significant change (p<0.05) in disease-free survival is evident between Nlrp1aQ593P/Q593P Il1a−/− and Nlrp1aQ593P/Q593P mice, as well as between Nlrp1aQ593P/Q593P Casp1−/− and Nlrp1aQ593P/Q593P mice. (K) Enumeration of neutrophils, lymphocytes, monocytes and eosinophils in Casp11−/− Nlrp1aQ593P/Q593P mice. *p<0.05.
Inflammatory disease in NLRP1 mutant mice is dependent on IL-1β and Caspase-1 but independent of ASC and Caspase-11
The semi-dominant nature of the Nlrp1aQ593P mutation suggested that the allele produces a constitutively active – or more easily activated – form of NLRP1a. We therefore conducted an analysis of inflammasome activity in bone marrow-derived macrophages. Cells were primed with LPS, stimulated with a range of inflammasome activators, and supernatants analysed for the 17 kDa processed form of IL-1β. In contrast to wild-type macrophages, LPS priming alone was sufficient to induce Nlrp1aQ593P/Q593P cells to secrete significant amounts of processed IL-1β whereas additional activation by ATP, MDP/TiO2, MSU or alum were required for IL-1β processing by wild-type macrophages (Figure 2B). The increased amount of secreted IL-1β from Nlrp1aQ593P/Q593P cells was not due to alterations in TLR4-induced activation of NFκB or MAP kinase activation and no changes in the short-term induction of pro-IL-1β was noted in cell lysates of Nlrp1aQ593P/Q593P macrophages (Figure S2). Serum IL-1β was below the limit of detection in the majority of Nlrp1aQ593P/Q593P mice, however G-CSF and IFNγ levels were elevated in Nlrp1aQ593P/Q593P mice (Figure 2C). To determine the contribution cytokines make to Nlrp1aQ593P-mediated disease, we firstly generated Nlrp1aQ593P/Q593P mice lacking either the interleukin-1 receptor (IL-1R) or IL-1α. Nlrp1aQ593P/Q593P Il1r−/− mice did not develop inflammatory disease (Figure 2D). In contrast, Nlrp1aQ593P/Q593P Il1a−/− mice developed neutrophilia and a lethal systemic inflammatory disease (Figure 2E and 2F), indicating that the phenotype of Nlrp1aQ593P/Q593P mice is largely attributable to the activity of IL-1β. Nlrp1aQ593P/Q593P Casp1−/− mice did not develop neutrophilia or inflammatory disease, and exhibited survival rates equivalent to wild-type counterparts (Figure 2G and 2H).
The NLRP3 and AIM2 inflammasome require the recruitment of the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), through a pyrin-pyrin domain interaction. The lack of a pyrin domain in mouse NLRP1a suggested that ASC was unlikely to interact or influence the phenotype of Nlrp1aQ593P/Q593P animals. As predicted, Nlrp1aQ593P/Q593PAsc−/− mice developed neutrophilia and lethal inflammatory disease at the same rate as Nlrp1aQ593P/Q593P littermate controls (Figure 2I and 2J), indicating that ASC is not required for NLRP1a-mediated responses in mice, distinguishing this from NLRP3- and AIM2-dependent models of inflammation.
It has been documented that the conventionally-derived Caspase-1 deficient mice used in these experiments are also genetically deficient in Caspase-11, a putative interacting partner for NLRP1 (Kayagaki et al., 2011; Martinon et al., 2002). To confirm that the inflammatory phenotype was a consequence of Caspase-1 activation and not Caspase-11 activation, we generated Nlrp1aQ593P/Q593PCasp11−/− mice. Deficiency of Caspase-11 did not prevent neutrophilia, lymphopenia, monocytopenia or the systemic inflammatory disease (Figure 2K and data not shown), indicating that NLRP1a predominately activates Caspase-1.
IL-18 negatively regulates NLRP1-induced inflammation
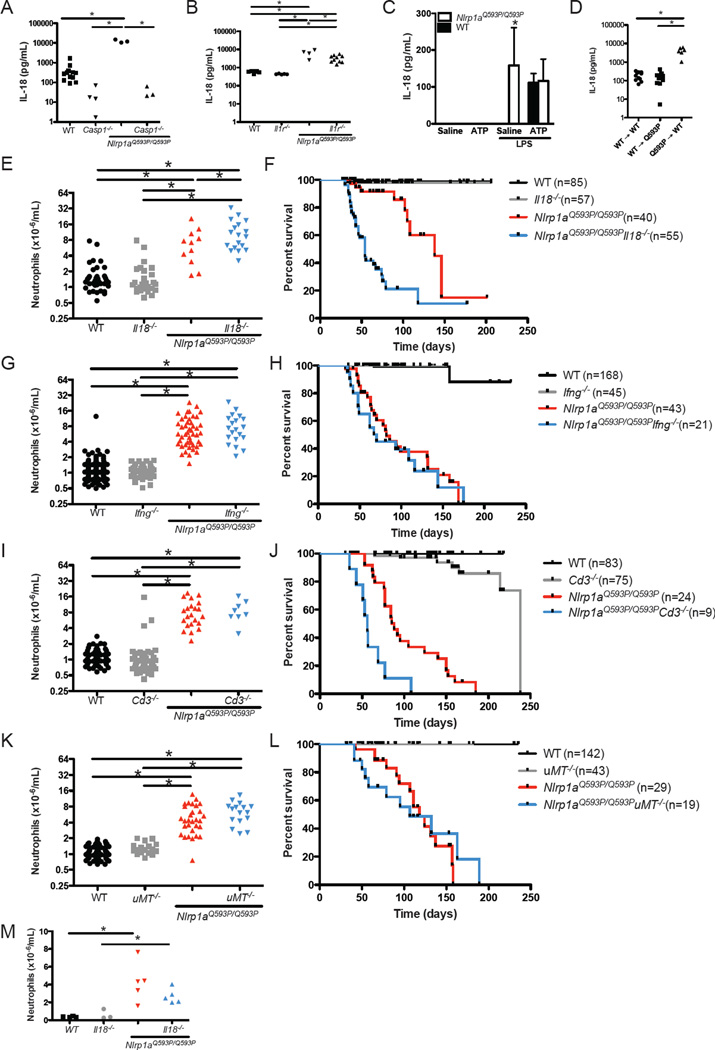
IL-18 was elevated 20-fold in the serum of Nlrp1aQ593P/Q593P mice compared to littermate controls (Figure 3A). This was Caspase-1-dependent and only modestly affected by loss of the IL-1 receptor (Figure 3A and 3B). Furthermore, Nlrp1aQ593P/Q593P macrophages produced high levels of IL-18 upon stimulation with LPS alone (Figure 3C). By reconstituting lethally-irradiated wild-type mice with Nlrp1aQ593P/Q593P bone marrow cells, we could show that the elevation in serum IL-18 in Nlrp1aQ593P/Q593P mice were due to defects in hematopoietic cells (Figure 3D). To examine the contribution of IL-18 to NLRP1aQ593P-mediated disease, we generated Nlrp1aQ593P/Q593P Il18−/− mice. Remarkably, deletion of IL-18 increased the number of neutrophils in the blood (Figure 3E), and greatly accelerated the onset of disease (Figure 3F). Nlrp1aQ593P/Q593P Il18−/− mice succumbed to disease at 5–10 weeks of age in contrast to Nlrp1aQ593P/Q593P littermate controls, in which disease was only apparent at 3–5 months of age (Figure 3F). This could not be attributed to IL-18 negatively regulating IL-1β production, as IL-18 pretreatment of dendritic cells or macrophages did not inhibit IL-1β production (Figure S3). Histological analysis revealed striking differences from littermate Nlrp1aQ593P/Q593P controls (Table 1). Myocarditis was more frequent, and likely contributed to the rapid deterioration of these animals (Figure 3F and Table 1). This is consistent with a proposed role for IL-18 in the prevention of myocardial necrosis and inflammatory cell infiltration during infection (Yoshida et al., 2002). Neutrophilic infiltration was not observed in the livers of Nlrp1aQ593P/Q593P Il18−/− mice, suggesting a potential proinflammatory role for IL-18 in this organ (Table 1).
Figure 3. IL-18 inhibits the lethal multiorgan inflammatory disease in Nlrp1aQ593P/Q593P mice.
(A, B) Serum IL-18 was measured by ELISA in wild-type, Casp1−/−, Nlrp1aQ593P/Q593P, Nlrp1aQ593P/Q593P Casp1−/−, Il1r−/− and Nlrp1aQ593P/Q593P Il1r−/− mice. (C) IL-18 production by Nlrp1aQ593P/Q593P macrophages following stimulation with 2 ng/mL LPS and 5mM ATP. (D) Serum IL-18 levels in lethally-irradiated mice after reconstitution with Nlrp1aQ593P/Q593P or wild-type bone marrow cells. (E–L) Neutrophil numbers in the peripheral blood of Nlrp1aQ593P/Q593P mice lacking IL-18 (E), IFNγ (G), T cells (I) or B cells (K). Survival of Nlrp1aQ593P/Q593P mice lacking IL-18 (F), IFNγ (H), T cells (J) or B cells (L). A significant change (p<0.05) in disease-free survival is evident between Nlrp1aQ593P/Q593P Il18−/− and Nlrp1aQ593P/Q593P mice, as well as between Nlrp1aQ593P/Q593P Cd3−/− and Nlrp1aQ593P/Q593P mice. (M) Neutrophil numbers in the peripheral blood of germ-free Nlrp1aQ593P/Q593P Il18−/− and Nlrp1aQ593P/Q593P mice. *p<0.05.
Table 1.
Regulators of pathology in Nlrp1aQ593P/Q593P mice
| Nlrp1aQ593P/Q593P | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue | Abnormality | WT | Il1r−/− | Il18−/− | Ifng−/− | Casp1−/− | Cd3−/− | |
| Liver | neutrophil foci | 0/8* | 4/7 | 0/6 | 0/9* | 7/9 | 0/13* | 7/7 |
| Lung | neutrophil infiltration | 0/8* | 6/7 | 0/6* | 9/9 | 9/9a | 0/13*c | 6/7 |
| Spleen | depleted follicles | 0/8* | 6/7 | 0/6* | 9/9 | 9/9 | 0/8* | 8/8 |
| excess granulocytes | 0/8* | 7/7 | 0/6* | 9/9 | 9/9 | 0/8* | 8/8 | |
| Bone Marrow | excess granulocytes | 0/8* | 7/7 | 1/5* | 9/9 | 7/7 | 0/9* | 2/2 |
| Brain | neutrophils in meninges | 0/8* | 5/6 | 0/3* | 8/8 | 9/9b | 0/13* | 5/7d |
| Gut | neutrophil invasion or granuloma | 0/9* | 6/7 | 0/5* | 8/9 | 6/9 | 1/13* | 6/8 |
| Heart | neutrophil infiltration | 0/8 | 1/4 | 0/6 | 9/9* | 5/9 | 0/13 | 5/7 |
| Skin | ulceration | 0/8 | 0/7 | 0/6 | 0/9 | 0/9 | 0/13 | 6/7* |
4/9 lungs with lymphoid foci
6/9 encephalitis
2/13 lungs with lymphoid infiltrates
3/7 with neutrophil foci in brain
p<0.05, Nlrp1aQ593P/Q593P vs. other genotypes
IL-18 does not negatively regulate NLRP1a-induced inflammatory disease via IFNγ
IL-18 was originally identified as a cytokine that could potently induce IFNγ in synergy with IL-12 (Okamura et al., 1995). We and others have shown previously that IFNγ can negatively regulate IL-1β production (Masters et al., 2010). We therefore hypothesised that IL-18 was regulating infection-induced systemic inflammation in NLRP1a mutant mice via IFNγ. Nlrp1aQ593P/Q593P Ifng−/− mice uniquely developed encephalitis, demonstrating a tissue-specific role for IFNγ in the negative regulation of NLRP1a-induced inflammation in the brain (Table 1). However, Nlrp1aQ593P/Q593P Ifng−/− mice did not phenocopy Nlrp1aQ593P/Q593P Il18−/− mice. Blood neutrophil numbers and survival curves in Nlrp1aQ593P/Q593P Ifng+/+ and Nlrp1aQ593P/Q593P Ifng−/− animals were indistinguishable (Figure 3G and 3H). Thus, IL-18 negatively regulates Nlrp1aQ593P/Q593P-induced systemic inflammation independently of IFNγ.
T cells negatively regulate cutaneous inflammatory disease in NLRP1a mutant mice
Guarda et al recently proposed a key role for T cells in the negative regulation of the NLRP1 inflammasome (Guarda et al., 2009). We tested this directly by examining the incidence of inflammatory disease and pathology in T cell-deficient Nlrp1aQ593P/Q593P Cd3−/− mice. No change in neutrophilia was evident (Figure 3I), but a modest reduction in disease-free survival was observed in Nlrp1aQ593P/Q593P Cd3−/− mice relative to Nlrp1aQ593P/Q593P littermate controls (Figure 3J) due to a severe cutaneous inflammatory disease characterised by neutrophil infiltration of the dermis (Table 1). This disease was not seen in Nlrp1aQ593P/Q593P Il18−/−, Nlrp1aQ593P/Q593P Ifng−/− or B cell-deficient Nlrp1aQ593P/Q593P μMT−/− mice (Figure 3K and 3L and Table 1). These data confirm a highly specific role for T cells in the negative regulation of NLRP1a-mediated inflammation in the skin, which could be due to the absence of regulatory T cells or the absence of CD40-induced regulation of the inflammasome, as proposed by Guarda et al (Guarda et al., 2009). However, neither the actions of IFNγ or T cells can explain the inhibitory effect of IL-18 on NLRP1aQ593P-mediated inflammatory disease.
Microbes contribute to systemic inflammatory disease in the absence of IL-18
To investigate the role of commensal organisms in the acute inflammatory disease in Nlrp1aQ593P/Q593P Il18−/− and Nlrp1aQ593P/Q593P mice, we rederived Nlrp1aQ593P/Q593P Il18−/− mice and littermate controls to a germ-free environment. Germ-free Nlrp1aQ593P/Q593P mice exhibited neutrophilia and myocarditis (Figure 3M and Figure S3) indicating that microbes are not required to initiate disease. However, germ-free Nlrp1aQ593P/Q593P Il18−/− mice were indistinguishable from littermate germ-free Nlrp1aQ593P/Q593P mice, such that at 10 weeks of age when 75% of conventionally-housed Nlrp1aQ593P/Q593P Il18−/− mice succumb to disease, no mortality was observed in germ-free Nlrp1aQ593P/Q593P Il18−/− mice. Moreover, no significant differences in neutrophil numbers were observed between Nlrp1aQ593P/Q593P Il18−/− and Nlrp1aQ593P/Q593P mice (Figure 3M). These data indicate that microbes accelerate the onset and the severity of the disease of Nlrp1aQ593P/Q593P mice.
NLRP1a activation induces deletion of hematopoietic progenitor cells
To examine the role of hematopoietic cells in Nlrp1aQ593P-driven disease, we transplanted wild-type or Nlrp1aQ593P/Q593P bone marrow into lethally irradiated wild-type or Nlrp1aQ593P/Q593P recipient mice. Nlrp1aQ593P/Q593P bone marrow induced neutrophilia, lymphopenia and inflammatory disease in wild-type recipients, demonstrating that the phenotype is intrinsic to hematopoietic cells (Figure 4A and data not shown). In the reciprocal experiment, neutrophilia and inflammatory disease in Nlrp1aQ593P/Q593P animals was ameliorated by transplantation of wild-type bone marrow. In some recipients of Nlrp1aQ593P/Q593P bone marrow, we observed a decrease in engraftment that correlated with a reduction in the severity of multiorgan inflammatory disease in the host (Figure 4B and 4C). This variable reconstitution efficiency raised the possibility that the Nlrp1aQ593P mutation might affect the function of hematopoietic stem and/or progenitor cells. Consistent with this, we found that NLRP1a was highly expressed in hematopoietic stem cells and progenitor cells of both myeloid and lymphoid origin (Figure 4D), and Nlrp1aQ593P/Q593P mice exhibited a reduction in the proportion of lineage− c-kit+ cells in the bone marrow that was dependent on Caspase-1 but independent of the IL-1 receptor (Figure 4E). We therefore performed competitive bone marrow transplants, injecting equal numbers of wild-type and Nlrp1aQ593P/Q593P bone marrow cells into lethally-irradiated wild-type recipient mice. Analysis at 8 weeks post-transplant demonstrated that Nlrp1aQ593P/Q593P hematopoietic stem and progenitor cells failed to compete with wild-type counterparts, indicating a cell-intrinsic defect (Figure 4F).
Figure 4. NLRP1a activation influences myelopoiesis.
(A) Transfer of Nlrp1aQ593P/Q593P bone marrow to lethally irradiated wild-type recipients results in neutrophilia and B and T lymphopenia. The transfer of wild-type bone marrow to lethally-irradiated Nlrp1aQ593P/Q593P mice is sufficient to restore normal hematopoiesis. (B,C) Reduced reconstitution efficiency of Nlrp1aQ593P/Q593P bone marrow in (B) the bone marrow and (C) peripheral blood of irradiated recipients 8 weeks post transplant. *p<0.05 by ANOVA and SNK. (D) Expression of Nlrp1a, Nlrp3, Asc and Actin in purified populations of hematopoietic stem cells (HSC), lin−c-kit+Sca1+ (LSK), common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP) and common lymphoid progenitors (CLP). (E) Decrease of lin−c-kit+ hematopoietic progenitor cells in the bone marrow of Nlrp1aQ593P/Q593P and Il1r−/−Nlrp1aQ593P/Q593P mice. (F) Contribution of wild-type and Nlrp1aQ593P/Q593P bone marrow to lethally-irradiated recipients, 8 weeks post-transplant. Equal numbers of wild-type and Nlrp1aQ593P/Q593P bone marrow cells were initially transplanted. (G,H) The differentiation of progenitor cells in blast colonies is altered by the Nlrp1aQ593P mutation through Caspase-1 activity, and occurs independently of IL-1β. Individual blast colonies from whole bone marrow cultures stimulated with SCF+IL-6 for 7 days were resuspended in agar and incubated for a further 7 days in the presence of M-CSF, GM-CSF or SCF+IL-3+Epo before colony types were enumerated. G: granulocyte; GM: granulocyte-macrophage; M:macrophage. (G) *p<0.05, mean ± SEM, Il1r−/−Nlrp1aQ593P/Q593P vs. Il1r−/−, n=14–16 independent recloned colonies from 2 independent experiments. (H) *p<0.05, mean ± SEM, n=19–20 independent recloned colonies from 2 independent experiments. (I) Enumeration of lymphocytes, neutrophils, platelets, monocytes, eosinophils and red blood cells in Il1r−/−Nlrp1aQ593P/Q593P mice. *p<0.05. (J) Caspase-1 activity in lin−c-kit+ bone marrow progenitor cells using FAM-YVAD-FMK Caspase-1 substrate. MFI: Mean fluorescence intensity. (K) IL-1R-independent but Caspase-1-dependent reduction in viability of Nlrp1aQ593P/Q593P lin−c-kit+ hematopoietic progenitor cells.
In the absence of the IL-1R (i.e in Nlrp1aQ593P/Q593P Il1r−/− mice), IL-1β-driven inflammatory disease does not develop, and emergency granulopoiesis is not engaged. We were therefore able to examine the frequency and differentiation of myeloid progenitor cells without the confounding effects of inflammatory disease in Nlrp1aQ593P/Q593P mice. We cultured bone marrow and spleen cells from healthy Nlrp1aQ593P/Q593P Il1r−/− mice in semi-solid agar. Table 2 demonstrates significant deficiencies in macrophage and neutrophil progenitor cells in bone marrow from Nlrp1aQ593P/Q593P Il1r−/− mice. To examine more primitive lineage-committed myeloid progenitor cells, we cultured bone marrow in semi-solid agar containing SCF+IL-6 for seven days, picked individual multipotent blast colonies, and re-cultured them in GM-CSF, M-CSF or SCF+IL-3+Epo for another seven days. Analysis of progenitor cells in Nlrp1aQ593P/Q593P Il1r−/− blast colonies revealed a deficit in macrophage progenitor cells and granulocyte-macrophage progenitor cells (Figure 4G). In Nlrp1aQ593P/Q593P blast colonies but not Nlrp1aQ593P/Q593P Casp1−/− blast colonies, there was a significant decrease in macrophage progenitor cells (Figure 4H) confirming that the loss of progenitor cells was a consequence of Caspase-1 activity. These findings indicate that Caspase-1 activation in myeloid progenitor cells can affect their differentiation to mature myeloid cells. Reductions in hematopoietic progenitor cell populations were reflected by significant deficiencies in lymphocytes, monocytes, eosinophils and platelets at steady state in healthy Nlrp1aQ593P/Q593P Il1r−/− animals (Figure 4I) and occurred independently of ASC (Figure S4).
Table 2.
The NLRP1aQ593P mutation reduces the frequency of myeloid progenitor cells in the bone marrow independently of the IL-1R
| Number of colonies | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Stimulus | Blast | Neutrophil | Granulocyte/Macrophage | Macrophage | Eosinophil | Megakaryocyte |
| Il1r−/− | GM-CSF | 24±8 | 7±2 | 33±17 | 5±5 | ||
| G-CSF | 15±8 | 0±0 | 0±0 | ||||
| M-CSF | 2±2 | 6±6 | 47±18 | ||||
| IL-3 | 4±2 | 27±9 | 11±6 | 23±14 | 3±3 | 7±8 | |
| SCF+IL-6 | 8±3 | 24±5 | 5±3 | 3±1 | 1±1 | 2±2 | |
| Saline | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | |
| Il1r−/−Nlrp1aQ593P/Q593P | GM-CSF | 13±10 | 3±2* | 8±7* | 1±0 | ||
| G-CSF | 7±2* | 0±0 | 0±0 | ||||
| M-CSF | 0±0* | 0±1 | 8±4* | ||||
| IL-3 | 2±2 | 9±8* | 3±2* | 4±2* | 1±1 | 4±3 | |
| SCF+IL-6 | 3±1* | 10±8* | 1±1* | 2±1* | 0±0 | 2±1 | |
| Saline | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | |
p<0.05, Mean ± SD, n=4–7 independent experiments. 2.5 × 104 bone marrow cells were cultured in the presence of 100 ng/ml murine SCF, 10 ng/mL rm IL-3,10 ng/mL rm GM-CSF, 10 ng/mL rm M-CSF or rh G-CSF in 0.3% agar/DME/FCS. After 7 days, cultures were fixed, stained and counted.
The cell intrinsic deficiency of progenitor cells in Nlrp1aQ593P/Q593P mice, which was rescued by deleting Caspase-1, strongly suggested that these cells were undergoing pyroptotic cell death. In agreement with this, we could detect increased Caspase-1 activity in hematopoietic progenitor populations (Figure 4J). Moreover, there was an increased rate of death of purified Nlrp1aQ593P/Q593P Il1r−/− hematopoietic progenitor cells cultured ex vivo (Figure 4K), which was not observed for Nlrp1aQ593P/Q593P Casp1−/− progenitor cells (Figure 4K). In Nlrp1aQ593P/Q593P animals but not Nlrp1aQ593P/Q593P Il1r−/− animals, an increase in granulocyte precursors was detected when bone marrow was cultured in semi-solid agar for 7 days in hematopoietic growth factors (Table S2). The IL-1β-dependent emergency granulopoiesis observed in Nlrp1aQ593P/Q593P mice drives granulocyte expansion but not lymphocyte or monocyte expansion. In these animals, the overwhelming granulopoietic stimulus driven by IL-1β and G-CSF (Figure 2C) likely outweighs the pyroptotic death of granulocyte progenitors caused by active NLRP1a. The result of NLRP1a activation is a net increase in mature neutrophils in the peripheral circulation, however deleting the IL-1 receptor removes the stimulus for emergency granulopoiesis, highlighting the pyroptotic defect in hematopoietic stem and progenitor cells.
Emergency hematopoiesis induced by chemotherapy is compromised by inflammasome activation in progenitor cells
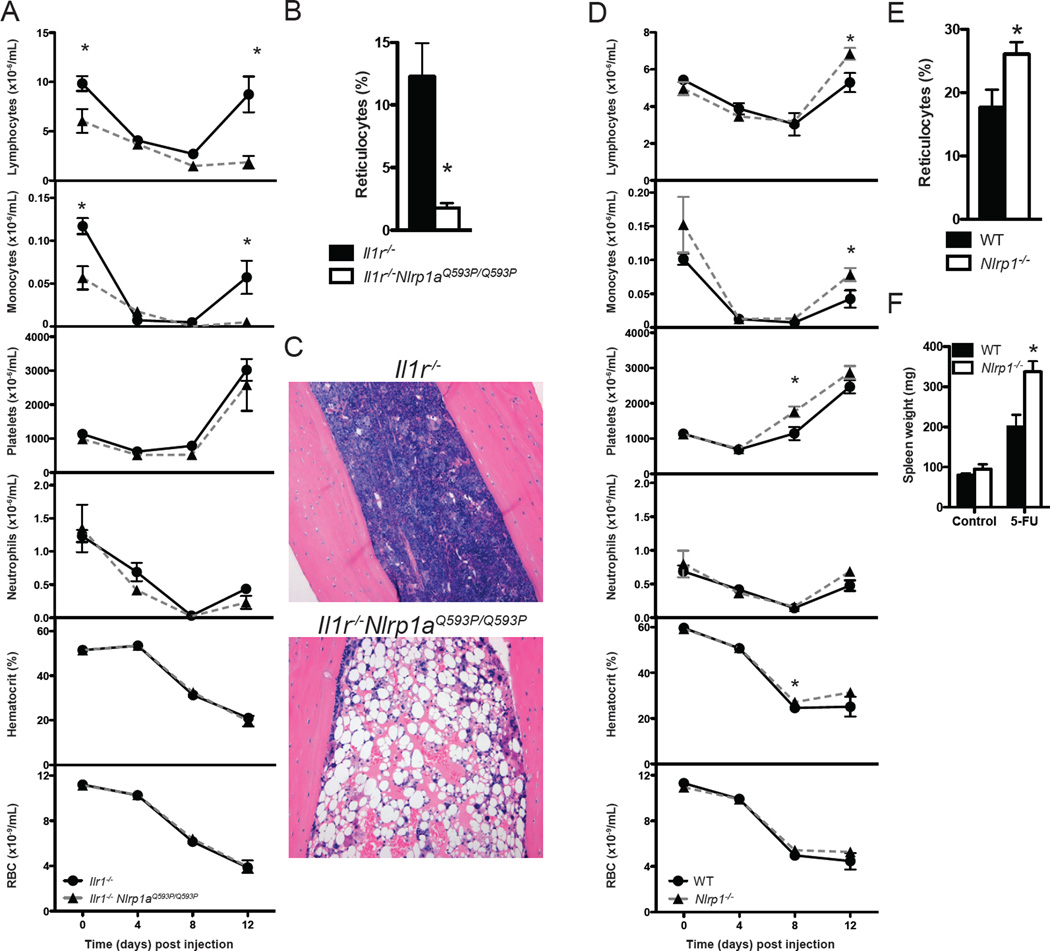
To evaluate the effect of active NLRP1a during hematopoietic stress, Nlrp1aQ593P/Q593P Il1r−/− mice (which do not develop autoinflammatory disease) were challenged with 5-fluorouracil. Strikingly, these mice succumbed shortly after the nadir of leukopenia at 12 days post-injection. They exhibited hypoplastic bone marrow, lymphopenia, monocytopenia and a deficit of reticulocytes supporting a functional deficiency in hematopoietic progenitor cells (Figure 5). No significant changes were detected in the number of bone marrow cells at steady state (Il1r−/−, 38±1 × 106 cells vs. Nlrp1aQ593P/Q593P Il1r−/−, 30±12 × 106 cells, per 2 femurs, n=3, mean±SD, p>0.05). To confirm the physiological relevance of data obtained from mice with active NLRP1a (Nlrp1aQ593P/Q593P), we generated mice homozygous for a targeted deletion of the entire Nlrp1 locus (Nlrp1a, Nlrp1b and Nlrp1c; Figure S5). NLRP1 deficiency improved recovery of the hematopoietic compartment following hemoablative chemotherapy, with modest increases in the numbers of platelets, lymphocytes, monocytes, reticulocytes and spleen weights compared to littermate controls (Figure 5D, 5E and 5F).
Figure 5. NLRP1a activation prevents recovery from hematopoietic stress.
(A) Leukopenia induced by 150 mg/kg 5-fluorouracil in Il1r−/−Nlrp1aQ593P/Q593P mice at Day 12 but not Il1r−/− littermate controls. *p<0.05, mean ± SEM, n=3–4 mice. (B) Reticulocyte numbers 12 days following challenge with 5-fluorouracil. *p<0.05, mean ± SEM, n=4 mice. (C) Hematoxylin and eosin staining of bone marrow of Il1r−/−Nlrp1aQ593P/Q593P and Il1r−/− mice 12 days after treatment with 5-FU. (D) The composition of blood following injection with 150 mg/kg 5-fluorouracil. *p<0.05, mean ± SEM, n=8–18 mice. (E) Reticulocyte numbers 12 days following challenge with 5-fluorouracil. *p<0.05, mean ± SEM, n=8–18 mice. (F) Spleen weight at steady state or 12 days post-injection with 5-FU. *p<0.05, mean ± SEM, n=3–18 mice.
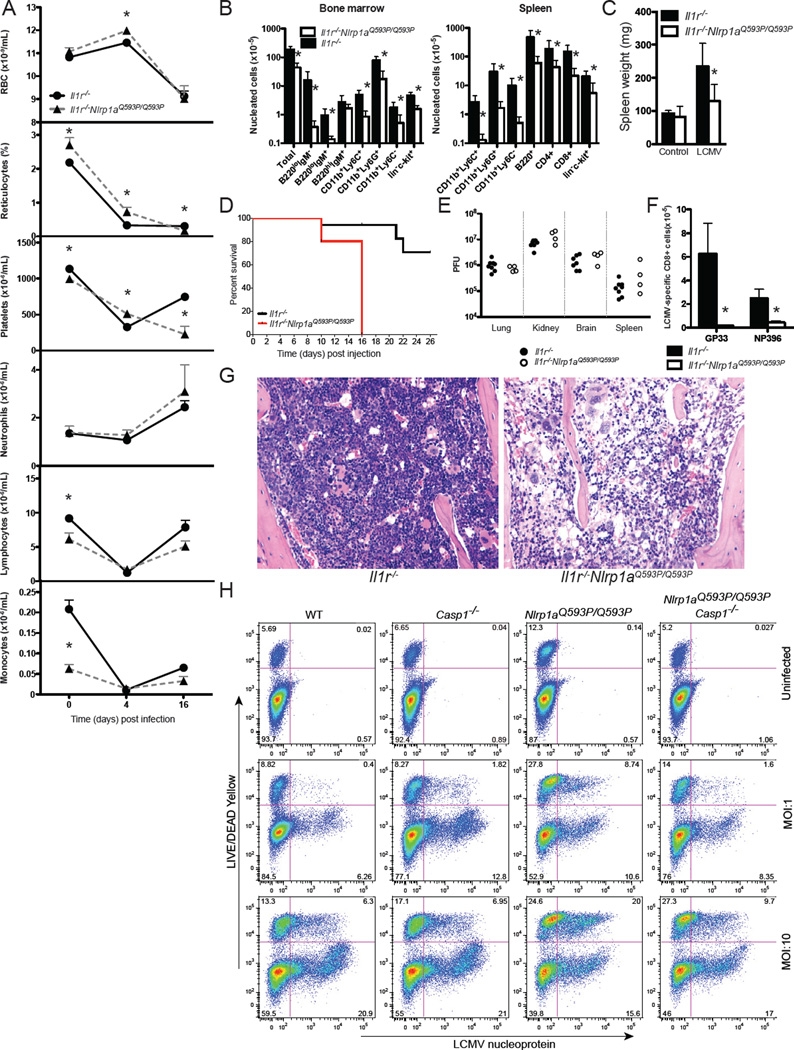
NLRP1a activation prolongs cytopenia following LCMV infection
To further examine the physiological consequences of NLRP1a activation in hematopoietic progenitor cells, and to investigate the relationship between inflammasome activation and cytopenia during infection, we utilised lymphocytic choriomeningitis virus (LCMV). LCMV infects bone marrow cells, reduces the frequency of hematopoietic progenitor cells by 50–90%, and induces pan-cytopenia (Binder et al., 1997). We hypothesised that deficiencies in hematopoietic progenitor cells, specifically megakaryocyte and erythroid progenitor cells, caused by NLRP1a activation would exacerbate these effects. Indeed, infection with LCMV caused thrombocytopenia, and severe bone marrow and splenic hypoplasia in Nlrp1aQ593P/Q593P Il1r−/− mice requiring euthanasia (Figure 6A, 6B, 6C, 6D and 6G). Despite functional defects in the hematopoietic system, no changes in viral titer were observed in Nlrp1aQ593P/Q593P Il1r−/− mice compared to Il1r−/− mice (Figure 6E). Tetramer-positive, LCMV-reactive CD8+ T cells failed to develop in Nlrp1aQ593P/Q593P Il1r−/− animals (Figure 6F), suggesting that NLRP1a activation may also impair adaptive immune responses. To test the ability of LCMV to directly infect hematopoietic progenitor cells in the bone marrow, we purified lin− c-kit+ bone marrow progenitor cells and infected them ex vivo with LCMV (Figure 6H). As we predicted, LCMV was able to directly infect lin− c-kit+ bone marrow progenitor cells as assessed by intracellular flow cytometry for LCMV nucleoprotein and a viral plaque assay (Figure 6H and data not shown). LCMV infection of progenitor cells increased their rate of cell death, as assessed by viability staining prior to fixation and permeabilisation for intracellular staining of LCMV nucleoprotein (Figure 6H). This cell death was increased in Nlrp1aQ593P/Q593P cells, and was dependent on Caspase-1 (Figure 6H). Taken together, these data demonstrate that systemic activation of NLRP1a can lead to a functional defect in hematopoietic progenitor cells and compromise hematopoiesis, generating severe cytopenia that contributes to immunosuppression.
Figure 6. LCMV infection induces prolonged cytopenia in NLRP1a mutant mice.
(A) Enumeration and analysis of red blood cells (RBC), reticulocytes, platelets (PLT), neutrophils, lymphocytes and monocytes in the blood of mice infected with LCMV docile. *p<0.05, mean ± SEM, n=4–15. (B) Nucleated bone marrow and spleen cells 16 days after infection. *p<0.05, mean ± SEM, n=4–8. (C) Spleen weight of infected mice 16 days after infection. *p<0.05, mean ± SEM, n=4–8. (D) Disease-free survival of Il1r−/−Nlrp1aQ593P/Q593P mice and littermate controls injected with 2 × 106 PFU LCMV. *p<0.05, n=5–17. (E) Viral titers in the lung, kidney, brain and spleen calculated by plaque assay. (F) Tetramer-positive, CD8+ T cells 16 days after infection. *p<0.05, Mean ± SEM, n=4–8. (G) Hematoxylin and eosin staining of bone marrow from mice 8 days after infection with LCMV. (H) Infection of purified lin−c-kit+ hematopoietic progenitor cells cultured in SCF+IL-3+Epo for 3 days. Viability was assessed using LIVE/DEAD yellow stain and virus was detected by intracellular staining for LCMV nucleoprotein. Representative data from triplicate wells of 5 independent experiments.
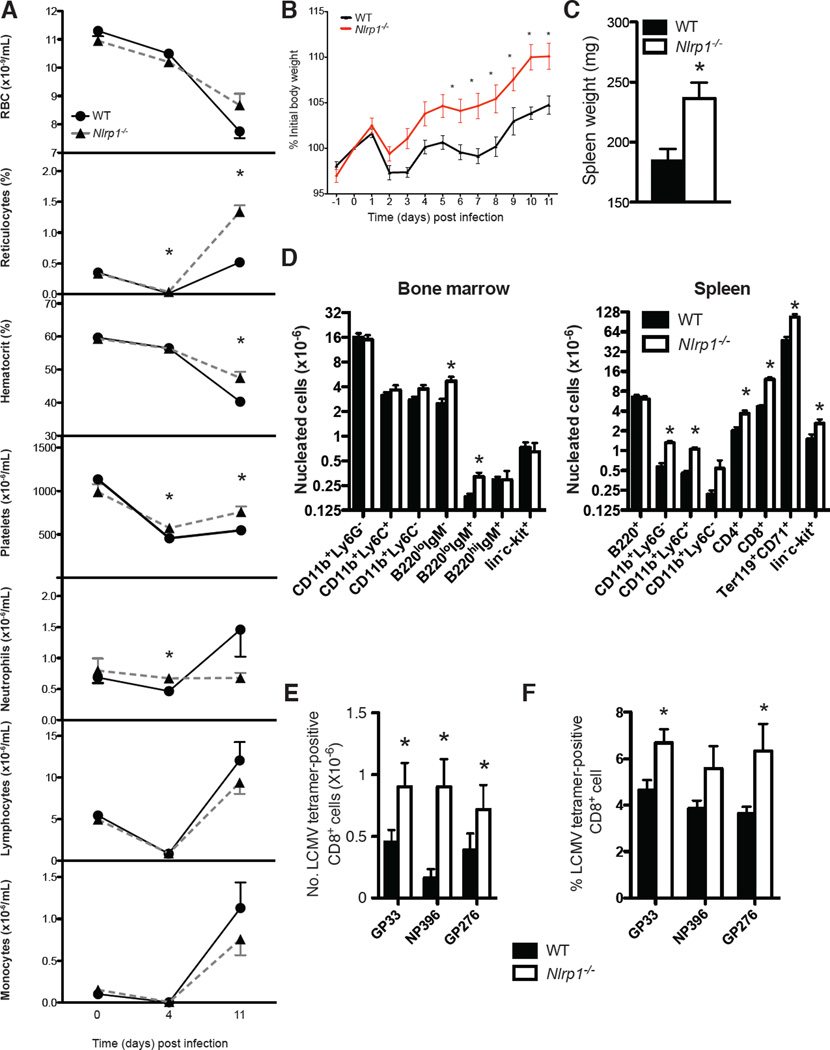
To confirm the relevance of data from LCMV-infected Nlrp1aQ593P/Q593P Il1r−/− animals, we examined the response of NLRP1-deficient mice to LCMV. NLRP1 deficiency impaired the induction of pan-cytopenia by two different strains of LCMV and improved recovery from infection (Figure 7 and Figure S6). NLRP1-deficient animals displayed increases in reticulocytes, platelet numbers and hematocrit in the peripheral blood, as well as increases in body weight, spleen weight, bone marrow B cell precursors, and myeloid, erythroid and lymphoid cells in the spleen relative to littermate controls (Figure 7A, 7B, 7C and 7D). In contrast to Nlrp1aQ593P/Q593P Il1r−/− animals, which display major deficiencies in hematopoietic stem and progenitor cells, Nlrp1−/− animals displayed increases in this population in the spleen following LCMV infection (Figure 7D). Moreover, the numbers of splenic tetramer-positive, LCMV-reactive CD8+ T cells were elevated in Nlrp1−/− animals (Figure 7E). To confirm a cell-intrinsic role for NLRP1 in T cells, we generated bone marrow chimeras containing equal numbers of wild-type and Nlrp1−/− bone marrow cells. Infection of these animals demonstrated enhanced development of Nlrp1−/−, tetramer-positive, LCMV-reactive CD8+ T cells (Figure 7F), showing that this effect on the adaptive immune response is cell-intrinsic.
Figure 7. NLRP1 deficiency enhances hematopoietic responses to LCMV.
(A) Peripheral blood parameters of LCMV-infected mice at steady state (day 0) and day 4 and 11 post-infection with LCMV (clone 13). *p<0.05, mean ± SEM, n=4–7. (B) Weight of mice after infection with LCMV. *p<0.05, mean ± SEM, n=6. (C) Spleen weight 12 days after LCMV infection. *p<0.05, mean ± SEM, n=6. (D) Total nucleated cells in the bone marrow and spleen 12 days after LCMV infection. *p<0.05, mean ± SEM, n=6. (E) Tetramer-positive (LCMV-specific) CD8+ T cells in the spleen 12 days post LCMV-infection. *p<0.05, mean ± SEM, n=6. (F) Tetramer-positive (LCMV-specific) CD8+ T cells in the spleen of bone marrow chimeras 12 days post LCMV-infection. Chimeric mice were generated by transplanting lethally-irradiated wild-type recipients with equal numbers of wild-type and Nlrp1−/− bone marrow cells. *p<0.05, Mean ± SEM, n=4.
DISCUSSION
This study demonstrates that NLRP1a can generate a functional Caspase-1-containing inflammasome in vivo, independently of ASC and Caspase-11, to drive an IL-1β-dependent inflammatory disease that is negatively regulated by IL-18. Most importantly, our research indicates cell-intrinsic roles for NLRP1a-induced pyroptosis in hematopoietic progenitor cells that prevents their proliferation and differentiation to mature cells. Unrestrained pyroptosis in hematopoietic stem and progenitor cells leads to leukopenia at steady state, and anemia and leukopenia during periods of hematopoietic stress induced by chemotherapy or infection.
Pyroptosis is reported to be an effective mechanism of clearance for intracellular bacteria. Here, we propose that the activity of the NLRP1a complex in hematopoietic progenitor cells may restrict their proliferation or enable the self-destruction of infected cells to limit dissemination of infection during their proliferation and differentiation to mature cells. Profound cytopenias accompanied by febrile neutropenia or more commonly neutrophilia, are commonly found in patients immediately following their diagnosis with sepsis (Venet et al., 2010). Our data raise the possibility that anemia, leukopenia and immunosuppression during viral infection or sepsis may be a result of inflammasome activity in hematopoietic progenitor cells. Transplantation of lethally-irradiated recipients with HSC and additional lineage-restricted progenitor cells, protects against lethal challenge with mouse cytomegalovirus, Aspergillus fumigatus or P. aeruginosa (Arber et al., 2003; BitMansour et al., 2002). Pseudomonas aeruginosa-induced lethal sepsis was attributed to a restriction of differentiation from HSCs to lineage-restricted myeloid progenitor cells due to defects in cell cycle regulators and transcription factors controlling myeloid cell differentiation including PU.1, C/EBPα, SKP2, p21cip1 and LRG47 (Rodriguez et al., 2009). Although no single mechanism is likely to account for the spectrum of defects observed in sepsis (a heterogenous disease contributed to by a range of genetic factors, pathogen virulence factors and comorbidities), the contribution of the hematopoietic progenitor cell compartment is likely to be critical for disease outcome. Here, we provide evidence that NLRP1a inflammasome activation in hematopoietic progenitor cells can contribute to cytopenias and adversely affect the outcome of severe infection or hematopoietic stress induced by chemotherapy.
Our findings suggest that NLRP1a acts as a cellular sentinel, poised for activation beyond an evolutionarily determined threshold. Based on studies of similar proteins in plants, this threshold is likely set, at least in part, by interactions between the LRRs and the NACHT domain (Bernoux et al., 2011; Dietrich et al., 1994; Swiderski et al., 2009). We hypothesise that the Q593P mutation in the linker region between the LRRs and the NACHT domains of NLRP1a destabilises the interaction between these two domains, reducing the threshold for activation of NLRP1a. We propose that this leads to an inflammasome that is more easily activated, thereby inducing the death of progenitor cells. This compromises hematopoiesis at the steady state, generating a comorbidity in NLRP1a mutant mice that severely compromises subsequent responses to hematopoietic stress induced by chemotherapy or infection. Our data in NLRP1-deficient mice are consistent with this mode of action of NLRP1. Eliminating this cellular sentinel does not appear to compromise hematopoiesis at steady state, however, following chemotherapy or infection, NLRP1-deficient mice have increased numbers of hematopoietic progenitor cells, a reduced period of cytopenia and recover more rapidly than control animals. In these scenarios, the removal of NLRP1 effectively increases the resistance of progenitor cells to hematopoietic stress. Future studies will be required to examine the role of NLRP1 regulators and NLRP1 function in other settings, where activation of NLRP1 may be essential for responses to infection and cellular stress. One context where NLRP1 is thought to be important is autoimmunity, with known human genetic associations to vitiligo, lupus, rheumatoid arthritis and celiac disease (Jin et al., 2007; Magitta et al., 2009; Pontillo et al., 2010; Pontillo et al., 2012). Activated NLRP1 could provide an inflammatory stimulus in these diseases, however our observation that impaired NLRP1 activation enhances antigen-specific T cell responses shows for the first time that T-cell mediated autoimmunity in humans could be promoted by a lack of NLRP1 function.
Our data also reveal a crucial role for IL-18 in responding to infection during inflammatory responses initiated by the NLRP1a inflammasome. When microbes are present, the loss of IL-18 greatly exacerbates lethal systemic inflammatory disease in NLRP1a mutant mice. IL-18 has a broad range of pro- and anti-inflammatory activities, driving the production of IFNγ, IL-13, IL-4, GM-CSF, IL-1β, IL-8 and TNFα (Arend et al., 2008). Targeting IL-18 reduces disease severity in collagen-induced arthritis (Plater-Zyberk et al., 2001), contact hypersensitivity reactions (Wang et al., 2002) and atherosclerotic lesion development (Mallat et al., 2001) but increases severity of DSS-induced colitis and enhances azoxymethane/DSS-induced adenocarcinoma formation (Salcedo et al., 2010; Zaki et al., 2010). IL-18 binding protein and a humanised IL-18 monoclonal antibody are in clinical trials for the treatment of rheumatoid arthritis, psoriasis and inflammatory bowel disease (Tak et al., 2006). Our data highlight unexpected risks that may be associated with neutralising IL-18, and suggest that these therapeutics should be used with caution when the mechanism of inflammatory disease is not well understood.
Our data on mutant NLRP1a are consistent with activating mutations in the same region of more ancient NB-LRR proteins in plants that also cause cell death (Bernoux et al., 2011; Dietrich et al., 1994; Swiderski et al., 2009). This conserved mechanism and function indicate a central role for pyroptosis in the innate immune response. Although this may be a protective mechanism for isolated progenitor cells, we show that systemic activation of Caspase-1 in the progenitor cell compartment compromises hematopoiesis. This suggests that inhibition of the NLRP1 inflammasome may alleviate the profound anemia, leukopenia and immunosuppression commonly found in septic patients and those undergoing chemotherapy.
METHODS
Mice
The Casp1−/−, Asc−/−, Il18−/−, Ifng−/−, Cd3−/−, uMT−/−, Il1a−/− and Il1r−/− mouse strains were generated on or had been backcrossed at least 10 generations with the C57BL/6 background. ENU mutagenesis was performed as described previously. All animal experiments complied with the regulatory standards of, and were approved by, the Walter and Eliza Hall Institute Animal Ethics Committee.
NLRP1 targeting
MICER plasmids (MHPP282m18 and MHPN379g03) containing DNA surrounding the NLRP1 locus were used for homologous recombination into the mouse genome and subsequent deletion of loxP-flanked DNA. Each construct was sequentially electroporated into mouse ES cells, followed by a further electroporation of a plasmid expressing Cre recombinase that deletes the entirety of Nlrp1c, Nlrp1b and exons 1–10 of Nlrp1a. BamHI probes were used to confirm correct M18 insertion and Cre-mediated recombination in ES cells by Southern blot analysis.
Hematology and flow cytometry
Automated cell counts were performed on blood collected from the retroorbital plexus into Microtainer tubes containing EDTA (Sarstedt), using an Advia 2120 hematological analyser (Siemens, Munich, Germany). Flow cytometric analysis of hematopoietic cells was performed using a BD Biosciences LSRFortessa cell analyser.
Histopathology
Organs were collected in 10% buffered formalin. Tissue sections were prepared from paraffin blocks and stained with hematoxylin and eosin or Masson’s Trichrome Stain.
Bone marrow chimeras
For hematopoietic reconstitution experiments, congenic Ly5.1-expressing BALB/c.SJL mice or Ly5.2-expressing Nlrp1aQ593P/Q593P BALB/c.SJL mice were reconstituted with 5 × 106 Ly5.2-expressing wild-type or Nlrp1aQ593P/Q593P BALB/c.SJL bone marrow cells. For some experiments, Ly5.1-expressing C57BL/6J mice were reconstituted with a mix of 2.5×106 Ly5.2-expressing C57BL/6J Nlrp1−/− bone marrow cells and 2.5×106 bone marrow cells from C57BL/6J ubiquitin-GFP mice (Schaefer et al., 2001). Recipient mice received two 5.5 Gy doses of irradiation given 3 h apart.
Isolation of bone marrow derived macrophages and dendritic cells
Macrophages were derived from 107 bone marrow cells in L929 cell conditioned medium for 5 days at 37°C/10%CO2. Dendritic cells were derived in media containing 20 ng/mL GM-CSF for 10 days.
Cytokine ELISA
Cytokines were measured by ELISA (eBioscience, CA, USA) or BioPlex (BioRad, CA, USA) according to the manafacturer’s instructions. IL-18 was analysed by sandwich ELISA using IL-18-specific antibodies (R&D Systems, MN, USA).
Progenitor cell analysis
Clonal cultures of hematopoietic cells were performed as described (Croker et al., 2004). For recloning assays, single colonies were removed at day 7 from cultures of bone marrow stimulated by 100 ng/mL SCF + 100 ng/mL IL-6 and resuspended in agar then added to plates containing 10 ng/mL GM-CSF, 10 ng/mL M-CSF or 100 ng/mL SCF + 10 ng/mL murine IL-3 + 4 U/mL Epo for a further 7 days. Cultures were then fixed, stained for acetylcholinesterase, Luxol Fast Blue and hematoxylin, and the cellular composition of each colony determined at 100 to 400× magnification. For analysis of progenitor cell viability, 1.5×104 lin− c-kit+ bone marrow cells were monitored for viability as described (Croker et al., 2011). For RNA expression analysis, Nlrp1a, Nlrp3, Asc and Actin was quantified by reverse-transcriptase PCR from 3.75–7.5 ng cDNA prepared from HSC (lin− c-kithi Sca-1hi IL-7R−), CMP (lin− Sca-1− c-kit+ CD34+ CD16/32lo), GMP (lin− Sca-1− c-kit+ CD34+ CD16/32hi) and CLP (lin− Sca-1+ c-kit+ IL-7R+). Caspase-1 activity was measured by flow cytometry after labelling lin− c-kit+ cells with a fluorescent inhibitor probe FAM-YVAD-FMK for Caspase-1 (ImmunoChemistry Technologies, Bloomington, MN, USA).
Immunoblotting
Cell lysates and supernatants were analysed by immunoblot using protocols described previously (Croker et al., 2004).
Lymphocytic choriomeningitis virus infection
Mice were infected by intravenous injection of 2 × 106 PFU LCMV docile or 3.6 × 105 PFU LCMV clone 13. Virus titres were determined using protocols described previously (Battegay et al., 1991). Staining of LCMV-specific T cells with tetramers was performed using protocols described previously (Pellegrini et al., 2009). For intracellular detection of LCMV nucleoprotein, purified lin− c-kit+ bone marrow progenitor cells were cultured in 100 ng/mL SCF + 10 ng/mL murine IL-3 + 4 U/mL Epo for 3 days, then cells were fixed in BD Biosciences Fixation and Permeabilisation Solution and permeabilised in BD Biosciences Perm/Wash buffer according to the manufacturer’s instructions, before staining with an antibody specific to LCMV nucleoprotein (clone VL4).
Statistics
Unless otherwise specified, data are presented as mean ± standard deviation (SD) or standard error of the mean (SEM). Comparisons were performed using a Student’s t test, Fisher’s exact test or ANOVA followed by a Student Newman Keuls test. Survival curves were analysed using a log-rank (Mantel-Cox) test.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Mifsud, L. DiRago and J. Corbin for excellent technical assistance, and E. Lanera, S. Ross, S. Guzzardi, S. Brown, E. Salt and M. Salzone for outstanding animal husbandry. We thank Vishva Dixit for Caspase-11-deficient mice. This work was supported by: Project Grants (637367, 1002426), a Program Grant (461219), Fellowships (B.A.C., D.J.H., W.S.A., S.L.M., B.T.K.) and an Independent Research Institutes Infrastructure Support Scheme Grant (361646) from the Australian National Health and Medical Research Council (NHMRC); Fellowships from the Australian Research Council (B.A.C., B.T.K.), the Sylvia and Charles Viertel Foundation (B.T.K.) and the Cancer Council of Victoria (D.M.); the Australian Cancer Research Fund, the Australian Phenomics Network, and a Victorian State Government Operational Infrastructure Support Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, Shizuru JA, Brown JM. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- Bendahmane A, Farnham G, Moffett P, Baulcombe DC. Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 2002;32:195–204. doi: 10.1046/j.1365-313x.2002.01413.x. [DOI] [PubMed] [Google Scholar]
- Bernoux M, Ve T, Williams S, Warren C, Hatters D, Valkov E, Zhang X, Ellis JG, Kobe B, Dodds PN. Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell host & microbe. 2011;9:200–211. doi: 10.1016/j.chom.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BitMansour A, Burns SM, Traver D, Akashi K, Contag CH, Weissman IL, Brown JM. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood. 2002;100:4660–4667. doi: 10.1182/blood-2002-05-1552. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- Croker BA, O'Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang JG, et al. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci U S A. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC. CARD8 and NLRP1 Undergo Autoproteolytic Processing through a ZU5-Like Domain. PLoS ONE. 2011;6:e27396. doi: 10.1371/journal.pone.0027396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL. Arabidopsis mutants simulating disease resistance response. Cell. 1994;77:565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell death and differentiation. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin J, Gough PJ. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012 doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, Raffatellu M, Osborne TF. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell metabolism. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, Wittkowski H, Bek S, Hartmann N, Bosset S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS pathogens. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magitta NF, Boe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, Undlien DE, Joner G, Njolstad PR, Kvien TK, et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison's disease and type 1 diabetes. Genes and immunity. 2009;10:120–124. doi: 10.1038/gene.2008.85. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–E45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Masters SL, Mielke LA, Cornish AL, Sutton CE, O'Donnell J, Cengia LH, Roberts AW, Wicks IP, Mills KH, Croker BA. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO reports. 2010;11:640–646. doi: 10.1038/embor.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett P, Farnham G, Peart J, Baulcombe DC. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 2002;21:4511–4519. doi: 10.1093/emboj/cdf453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C, Joosten LA, Helsen MM, Sattonnet-Roche P, Siegfried C, Alouani S, van De Loo FA, Graber P, Aloni S, Cirillo R, et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001;108:1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontillo A, Brandao L, Guimaraes R, Segat L, Araujo J, Crovella S. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity. 2010;43:583–589. doi: 10.3109/08916930903540432. [DOI] [PubMed] [Google Scholar]
- Pontillo A, Girardelli M, Kamada AJ, Pancotto JA, Donadi EA, Crovella S, Sandrin-Garcia P. Polimorphisms in inflammasome genes are involved in the predisposition to systemic lupus erythematosus. Autoimmunity. 2012;45:271–278. doi: 10.3109/08916934.2011.637532. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Chora A, Goumnerov B, Mumaw C, Goebel WS, Fernandez L, Baydoun H, HogenEsch H, Dombkowski DM, Karlewicz CA, et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114:4064–4076. doi: 10.1182/blood-2009-04-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O'HUigin C, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- Swiderski MR, Birker D, Jones JD. The TIR domain of TIR-NB-LRR resistance proteins is a signaling domain involved in cell death induction. Mol Plant Microbe Interact. 2009;22:157–165. doi: 10.1094/MPMI-22-2-0157. [DOI] [PubMed] [Google Scholar]
- Tak PP, Bacchi M, Bertolino M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. European journal of drug metabolism and pharmacokinetics. 2006;31:109–116. doi: 10.1007/BF03191127. [DOI] [PubMed] [Google Scholar]
- Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F, Davin F, Guignant C, Larue A, Cazalis MA, Darbon R, Allombert C, Mougin B, Malcus C, Poitevin-Later F, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34:358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- Wang B, Feliciani C, Howell BG, Freed I, Cai Q, Watanabe H, Sauder DN. Contribution of Langerhans cell-derived IL-18 to contact hypersensitivity. J Immunol. 2002;168:3303–3308. doi: 10.4049/jimmunol.168.7.3303. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kand T, Tanaka T, Yokoyama T, Kurimoto M, Tamura J, Kobayashi I. Interleukin-18 reduces expression of cardiac tumor necrosis factor-alpha and atrial natriuretic peptide in a murine model of viral myocarditis. Life Sci. 2002;70:1225–1234. doi: 10.1016/s0024-3205(01)01509-0. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goritschnig S, Dong X, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. The Plant cell. 2003;15:2636–2646. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.