Abstract
Premutation alleles in FMR1 can cause the late-onset neurodegenerative disorder, fragile X-associated tremor ataxia syndrome (FXTAS) and/or the fragile X-associated primary ovarian insufficiency in approximately 20% of heterozygotes. Heterozygotes of the FMR1 premutation have a higher incidence of immune mediated disorders such as autoimmune thyroid disorder, especially when accompanied by FXTAS motor signs. We describe the time course of symptoms of immune mediated disorders and the subsequent development of FXTAS in four women with an FMR1 CGG expansion, including three with the premutation and one with a gray zone expansion. These patients developed an immune mediated disorder followed by neurological symptoms that become consistent with FXTAS. In all patients we observed a pattern involving an initial appearance of disease symptoms – often after a period of heightened stress (depression, anxiety, divorce, general surgery) followed by the onset of tremor and/or ataxia. Immune mediated diseases are associated with the manifestations of FXTAS temporally, although further studies are needed to clarify this association. If a cause and effect relationship can be established, treatment of pre-existing immune mediated disorders may benefit patients with pathogenic FMR1 mutations.
Keywords: Autoimmune disease, FXTAS, Premutation, FMR1 gene, CGG repeat, RNA toxicity, Genetic Counseling
INTRODUCTION
The fragile X–associated tremor/ ataxia syndrome (FXTAS) is an adult-onset progressive neurodegenerative disorder that affects heterozygotes for the fragile X mental retardation 1 (FMR1) gene premutation (CGG expansion of 55-200 repeats). The FXTAS has also been described in some patients with a gray zone expansion (45-54 repeats) [Liu et al 2012; Hall et al 2011]. The symptoms of FXTAS include progressive intention tremor, cerebellar ataxia, autonomic dysfunction, peripheral neuropathy, psychiatric problems, executive function, and memory deficits followed by cognitive decline [Jaquemont et al., 2003; Coffey et al., 2008; Adams et al., 2007; Berry-Kravis et al., 2007]. Cranial MRI studies at the time of clinical presentation typically demonstrate white matter disease often in the middle cerebellar peduncles (MCP), the splenium of the corpus callosum and periventricular areas combined with atrophy of brain [Jacquemont et al., 2003; Coffey et al., 2008; Adams et al., 2007; Berry-Kravis et al., 2007; Apartis et al., 2012].
Elevated levels of the FMR1 mRNA, observed in premutation heterozygotes are the basic molecular pathology in FXTAS [Tassone et al., 2007]. The increased mRNA levels cause a gain-of-function toxicity to cells leading to the formation of intranuclear inclusions in neurons and astrocytes throughout the central nervous system (CNS) and subsequent sequestration of critical proteins necessary for normal cell function [Garcia-Arocena et al., 2010; Sellier et al., 2010, 2013; Hagerman et al., 2013]. Similar inclusions have been found in the peripheral nervous system and peripheral organs, including heart, testes, thyroid, adrenals and islet cells of Langerhans of those with FXTAS [Hunsaker et al., 2011]. Mitochondrial dysfunction also occurs in older premutation heterozygotes both with and without FXTAS [Ross-Inta et al., 2010; Napoli et al., 2011]. This cellular dysfunction is hypothesized to lead to neuronal cell death and white matter disease in the CNS [Adams et al., 2007; Greco et al., 2006; Hashimoto et al., 2011; Wang et al., 2013] associated with the clinical features of FXTAS.
The relative risk of immune-mediated disorders (IMDs) among female heterozygotes for the FMR1 premutation has been assessed compared to controls without the premutation. Autoimmune thyroid disorder and fibromyalgia [Coffey et al., 2008; Winarni et al., 2012] were found to be significantly increased in premutation women, particularly in those with FXTAS compared to age-matched controls. However, a study by Hunter et al. [2010] did not find an association between IMD and the premutation. There is evidence both for [Behm et al., 2012] and against fibromyalgia being an IMD [Schmidt-Wilcke et al., 2011; Üçeyler at al., 2011] but the cytokine profiles are disturbed in patients with fibromyalgia and such disturbances are common in those with the premutation because of miRNA dysfunction as described below [Sellier et al., 2013].
In the current clinical studies, the timing of the ages of onset of one or more IMDs and of medical, psychiatric and neurological problems were assessed in four women with an FMR1 CGG expansion. Each experienced periods of heightened stress, including anxiety, depression, surgery, and chemotherapy, which are hypothesized to act as triggers for the onset of their IMD symptoms and/or FXTAS. Due to this mixture of relatively ill-defined symptoms of anxiety, pain, fibromyalgia, and chronic fatigue syndrome, which are often considered to be functional, medical or psychiatric problems, it is sometimes difficult for physicians to understand the etiology and provide adequate treatment. In the current report, we describe the time course of the occurrence of IMDs, medical or psychiatric problems and FXTAS along with potential triggers, in an effort to understand the natural history of these illnesses in this population.
PATIENTS AND METHODS
The four patients in this study were recruited through the Fragile X Treatment and Research Center, MIND Institute at University of California, Davis Medical Center and who participated in our premutation studies. Each patient signed an informed consent approved by our UC Davis IRB. The medical assessment included a developmental, psychological, neurological, and medication history, and a detailed medical and neurological examination.
These patients were selected for reporting because they had a recent study visit and they were found to have an autoimmune dysfunction with symptoms of FXTAS. Each patient was considered to have an IMD if diagnosed and treated for such by a physician and the medical records were obtained. Molecular measures included CGG repeat size in FMR1 and activation ratio (AR), which expresses the percentage of cells that have the normal allele on the active X chromosome, as described [Tassone et al., 2008].
CLINICAL REPORTS
Patient1
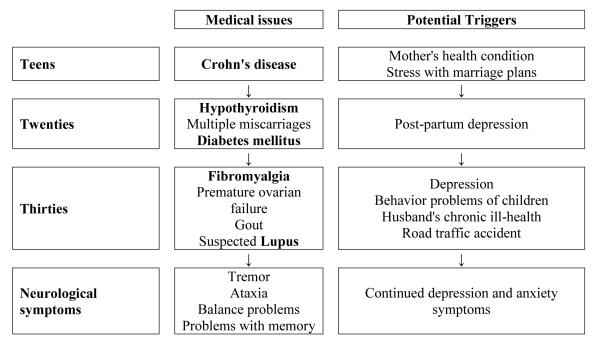
(Timeline 1) was a 42-year-old woman, carrying alleles of 30 and 102 CGG repeats and an AR of 0.54. Her maternal grandfather and mother were found to be heterozygotes of the premutation and both had FXTAS. The proband developed chronic abdominal pain, cramping and bloody diarrhea in her late teens, which was diagnosed as Crohn’s disease. At age 19, she was under stress due to her marriage plans and her mother’s ill health. After marriage she reportedly suffered from hypothyroidism and multiple miscarriages, four of which were documented in her twenties. She developed gestational diabetes at age 27, which persisted post pregnancy, also post-partum depression.
Timeline 1.
Time course of immune mediated symptoms followed by onset of neurological symptoms/FXTAS along with the potential triggers in a 42-year-old female heterozygote with 102 CGG repeats and AR of 0.54
In her 30’s, she suffered from widespread persistent muscle pain that was painful to touch leading to a diagnosis of fibromyalgia; cessation of menses, which was diagnosed as premature ovarian failure and a painful, swollen toe that was identified as gout. She developed a red malar rash, intermittent at first and then the rash persisted with sun exposure in her mid-30s and this was thought to be related to lupus by her rheumatologist. Her antiphospholipid antibody level was low to medium positive with an intermittent antinuclear antibody (ANA) positivity and a consistently increased C-reactive protein at age 40. Other stress involved her three children who were diagnosed with FXS as well as her husband’s chronic obstructive pulmonary disease requiring chronic oxygen therapy.
A hand tremor was first noticed at age 35. On examination, the tremor was subtle during finger-to-nose touching and there was a mild degree of ataxia with impaired tandem walking. This intention tremor worsened in her late thirties and interfered with handwriting and typing. After a car accident at age 37, her depression worsened, aggravated by the lack of child support. Her ataxia progressed with two to three falls per month and she subsequently developed problems with memory beginning at age 39. She was unable to tandem walk at age 42.
Her cranial MRI showed a mild degree of atrophy and mild periventricular white matter disease but she did not have the middle cerebellar peduncle (MCP) sign of white matter involvement at age 41. Her lumbar spine had minor spondylosis at several levels.
After an unsuccessful trial with venlafaxine and mirtazepine for depression and anxiety in her late 30s and early 40s, she experienced some relief from pain and depression with duloxetine. Her other medications include metformin and saxagliptin for diabetes, gabapentin, acetaminophen with codeine for pain, and colchicine for gout.
Patient 2
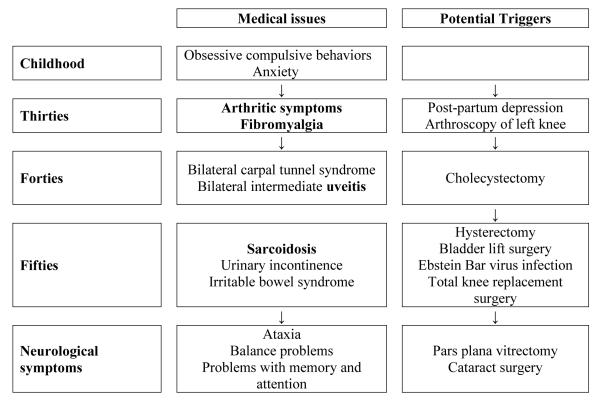
(Timeline 2) was a 58-year-old Caucasian woman, with FMR1 alleles of 31 and 100, 113 CGG repeats with an AR of 0.69. Her premutation was mosaic in repeat size with some cells having 100 repeats and other cells with 113 repeats. As a child, she reported that she was extremely shy with mildly obsessive-compulsive behavior and fingernail biting when anxious. She experienced post-partum depression after her first pregnancy at age 34 and this pregnancy was followed by development of arthritic symptoms. She complained of muscle pain and tenderness to touch, diagnosed as fibromyalgia at age 41; symptoms were alleviated by exercise.
Timeline 2.
Time course of immune mediated symptoms followed by onset of neurological symptoms/FXTAS along with the potential triggers in a 58-year-old female heterozygote with 100, 113 CGG repeats and AR of 0.69
She had arthroscopy of the left knee at age 41 for osteoarthritis, cholecystectomy at the age of 42 with general anesthesia. Neurological symptoms began 1 month after surgery. She had daily complaints of tingling and numbness in her hands associated with pain in her wrists that progressed to bilateral carpal tunnel syndrome. At age 48 years, she developed bilateral intermediate uveitis.
Symptoms of ataxia and difficulty with tandem walking were first seen in her early fifties and she also noticed intermittent resting and intention tremor in both arms. This was accompanied by urinary incontinence and development of irritable bowel syndrome. She underwent a hysterectomy and bladder lift procedure at age 53. She reported mild memory and attention problems after a total knee replacement surgery at age 55.
Viral titers for the Ebstein Barr virus were present at age 56, although the monospot test for infectious mononucleosis was non-reactive. She was evaluated for sarcoidosis based on her eye findings. A chest CT scan showed enlarged mediastinal lymph nodes that were non-caseating granulomas on biopsy, consistent with a diagnosis of sarcoidosis at age 56. She had no major pulmonary symptoms and her pulmonary functions were normal. She underwent a pars plana vitrectomy at age 56 and a cataract surgery at age 57, without change in vision post-surgery.
Her cranial MRI findings at age 50 showed extensive non-specific periventricular deep white matter hyperintensities subcortically at the gray/ white matter junctions of the frontal and parietal lobes. Additional white matter hyperintensities were identified in the periventricular regions of the frontal lobes.
Patient 3
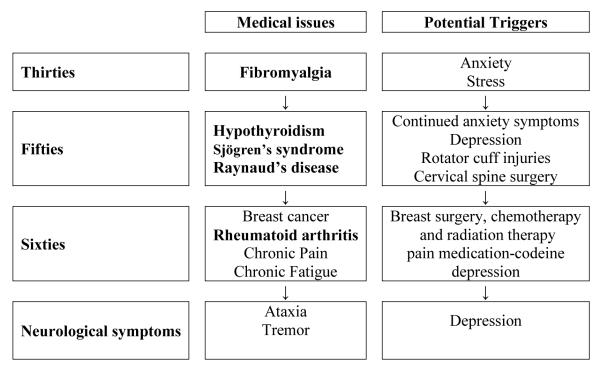
(Timeline 3) was a 71-year-old retired elementary school teacher with alleles of 26 and 60 CGG repeats, and an AR of 0.40. At age 35, she experienced muscle pain and soreness. She was diagnosed with hypothyroidism at age 50; age 59, she experienced worsening muscle pain, particularly in her arms, shoulders, back and legs, which was then diagnosed as fibromyalgia. She was ANA positive and was also diagnosed with Sjögren’s syndrome (with dry eyes and mouth and a positive SSA). She underwent surgery just prior to the diagnosis of fibromyalgia for a rotator cuff injury followed by cervical spine surgery for disc herniation. The fibromyalgia symptoms worsened after the surgeries.
Timeline 3.
Time course of immune mediated symptoms followed by onset of neurological symptoms/FXTAS along with the potential triggers in a 71-year-old female heterozygote in a 71-year-old female heterozygote with 60 CGG repeats and AR of 0.40
She experienced considerable stress at home in her 60s due to conflicts between her son with learning disabilities and her husband. She also had chronic fatigue and she could only perform her daily activities such as dressing by taking breaks in between tasks. Depression was diagnosed at age 64 and she experienced partial relief of her symptoms with sertraline. Due to her pain symptoms, amitriptyline was added to sertraline, which significantly relieved her chronic pain, chronic fatigue and fibromyalgia symptoms. She was also diagnosed with Raynaud’s disease, although her symptoms were mild since she lived in warm climate.
About two months after her two surgeries in her sixties, she experienced a mild degree of ataxia. Her ataxia was stable until the age of 68, when she was diagnosed with breast cancer and underwent surgery, chemotherapy and radiation therapy. After this treatment her chronic fatigue syndrome came back and her ataxia worsened dramatically. At age 68, she developed an intention tremor and problems with her memory, particularly word retrieval and her handwriting worsened. She had multiple falls at age 69 and she started using a cane. She developed arthritic pains in her fingers, dislocation of her left thumb with difficulty grasping objects and a significant right foot deformity. Her rheumatoid factor was positive. She experienced some relief from these pain symptoms with tramadol. She also had complaints of numbness and tingling in her feet and arms beginning at age 69.
She was on a trial with memantine for FXTAS and had noticed some improvements in her balance and memory. Her other medications included gabapentin for pain in her legs, anastrazole for her breast cancer follow-up, risedronate for osteoporosis, occasional fluticasone nasal spray for allergies, and multivitamins. She had a diagnosis of probable FXTAS.
Patient 4
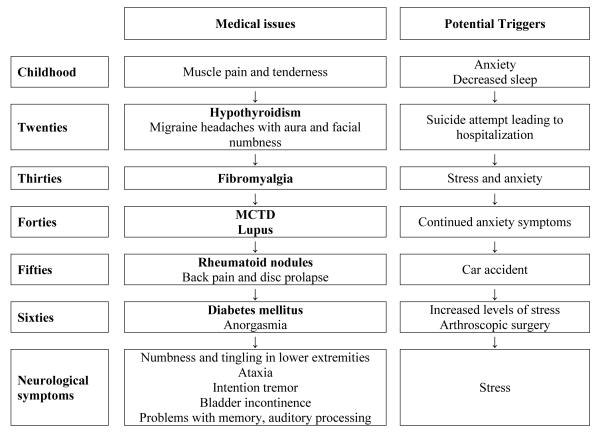
(Timeline 4) was a 69-year-old Caucasian woman with an FMR1 gray zone allele of 52 CGG repeats in addition to her normal allele of 32 repeats. She reported anxiety symptoms in her school years with decreased sleep during periods of stress and frequent nighttime awakenings. This was followed by muscle pain and tenderness. She also had a history of depression and a psychiatric hospitalization due to attempted suicide in her early 20’s. She reportedly developed hypothyroidism and migraine headaches requiring emergency room evaluation. This was associated with an aura (blurred central vision) and right-sided facial numbness. At age 35, her muscle aches and soreness worsened and she complained of increasing pain sensitivity even to light touch, after which a diagnosis of fibromyalgia was made.
Timeline 4.
Time course of immune mediated symptoms followed by onset of neurological symptoms/FXTAS along with the potential triggers in a 69-year-old female heterozygote with an FMR1 gray zone allele of 52 CGG repeats
She experienced symptoms of dry mouth, swallowing difficulties and joint pains at 42 years of age and had intermittent ANA and SSA positivity. She was diagnosed with a mixed connective tissue disease although lupus was suspected but was not diagnosed. She developed rheumatoid nodules on her feet and fingers with dislocation of her right thumb in her 50’s. She was in a car accident at age 58, which led to extreme back pain and a disc prolapse. She later developed Type II diabetes mellitus at age 60 years.
Problems with memory, auditory processing and visual short-term memory began at age 65, followed by ataxia with listing to one side. Intention tremor and bladder incontinence began at age 67 in addition to urinary urgency and frequency. She also experienced anorgasmia, beginning at age 67. During periods of stress, she noticed a significant decrease in her energy level. At age 69, she had arthroscopic surgery for a meniscal tear involving general anesthesia, a few weeks after which her tremor, handwriting and balance problems worsened. At the time of her clinical visit, she had intermittent numbness and tingling in her lower extremities and difficulty walking on a hard floor because of pain in her feet.
Her cranial MRI showed white matter hyperintensities in her insula and small areas scattered in the deep cortex with mild to moderate atrophy of the cerebral cortex and cerebellum, but no MCP sign. She met criteria for probable FXTAS although she had only a gray zone allele.
DISCUSSION
Initially, FXTAS was thought of principally as a CNS disorder with a primary motor component of an intention tremor and ataxia and concomitant white matter disease and global brain atrophy [Jacquemont et al., 2003; Adams et al., 2007; Brunberg et al., 2002]. However, it is now clear that FXTAS is much broader, with significant non-motor components, such as cognitive decline/ dementia and related neuropsychiatric involvement [Tassone et al., 2000; Seritan et al., 2008; Bourgeois et al., 2009] and peripheral nervous system involvement including neuropathy and autonomic dysfunction [Hunsaker et al., 2011]. Although fewer women than men will develop FXTAS, it appears that female heterozygotes with and without FXTAS, may have a greater tendency to develop certain types of medical disorders, particularly immune mediated dysfunction , hypertension, and fibromyalgia [Coffey et al., 2008; Winarni et al., 2012; Leehey et al., 2011]. The pathogenesis is likely related to a gain-of-function toxic effect from elevated levels of the expanded CGG-repeat FMR1 mRNA, which is present in all patients with the premutation [Tassone et al., 2000; Greco et al., 2002; Tassone et al., 2007]. This is thought to cause psychiatric problems such as depression and anxiety [Bourgeois et al., 2009; Roberts et al., 2009], fragile X-associated primary ovarian insufficiency (FXPOI) [Sullivan et al., 2005], and autonomic dysfunction [Coffey et al., 2008] along with FXTAS [Adams et al., 2007].
The expanded CGG repeat in the FMR1 mRNA in those with the premutation is thought to sequester proteins including KHDRBS1/Sam68 and DGCR8/Drosha that are important for diverse cellular functions, including maturation of miRNAs. As a consequence of their sequestration, miRNA dysregulation has been observed in those with the premutation [Sellier et al., 2013]. Given the role of miRNAs in the physiology of immune system including the fine-tuning of T cell reactivity to antigens and of antibody response [Lu L-F et al., 2009], it is possible that dysregulation of specific miRNAs may lead to impaired tolerance against self-antigens and to the development of autoimmune diseases. Moreover, dysregulation of miRNAs may lead to sustained inflammation, which is a hallmark of chronic inflammatory diseases [Sonkoly et al., 2009]. RNA toxicity in premutation neurons leads to upregulation of heat shock proteins (e.g. : Hsp70 and αβ-crystallin) [Garcia-Arocena et al., 2010; Wu et al., 2004], which themselves may stimulate immune dysregulation [Georgopoulos et al., 1993]. Hsp 70, whose expression has been found dysregulated in FXTAS [Garcia-Arocena et al., 2010] and in the premutation animal model, is involved in binding antigens and presenting them to the immune system [Nishikawa et al., 2008]. Finally, the sequestration of proteins important for splicing messages including KHDRBS1 and the subsequent mis-splicing of a variety of messages is also known to lead to many forms of autoimmune diseases [Evsyukova et al., 2010].
Our previous findings indicate that there is increased incidence of IMDs in females with the premutation and FXTAS than those female heterozygotes without FXTAS [Winarni et al., 2012]. Here we report four patients, three females with the premutation allele and one woman with a gray zone allele; all demonstrating IMD and subsequent development of FXTAS. We have included a woman with a gray zone allele because FXTAS has been reported in this repeat range [Hall et al., 2012; Liu et al., 2012], suggesting that RNA toxicity can lead to FXTAS also in gray zone heterozygotes.
In these four patients we observed a temporal link between IMD symptoms and onset of FXTAS, where a pattern is emerging with onset of IMD, such as autoimmune thyroid disease, Sjögren’s syndrome, lupus-like symptoms, or related problems such as fibromyalgia, chronic fatigue syndrome or inflammatory bowel disease, followed by development of tremor and/or ataxia. We hypothesize that IMDs or related disorders could accelerate the development of FXTAS by a variety of mechanisms such as enhancing oxidative stress, further dysregulating miRNA levels or enhancing microglia activation [Greco et al., 2008]. Although there is controversy as to whether fibromyalgia is indeed an IMD [Schmidt-Wilcke et al., 2011], fibromyalgia patients demonstrate a decreased cytokine response in stimulated peripheral blood mononuclear cell cultures, thereby implying impaired cell-mediated immunity [Behm et al., 2012]. All of the patients with fibromyalgia reported here also had other IMD and these problems occurred before the onset of FXTAS.
The presence of stressors such as anxiety, depression, divorce, accidents as well as surgery involving general anesthesia are hypothesized to act as triggers for the development of IMD symptoms and/ or the onset of FXTAS [Hagerman et al., 2013]. Emotional responsiveness, anxiety, depression and long-term memory are thought to be influenced by the RNA toxicity manifested by the FXTAS intranuclear inclusions that are abundant in the amygdala and hippocampus [Greco et al., 2002; 2006]. Amygdala dysfunction may underlie the social deficits in some individuals with the premutation [Farzin et al., 2006; Jakala et al., 1997; Hessl et al., 2005, 2007]. Cranial MRI studies have shown that increased levels of anxiety are associated with a decrease in the size of the hippocampus in women with the premutation [Jakala et al., 1997; Adams et al., 2010]. Loss of hippocampal volume may also be associated with mood disorders seen in FXTAS (depression, irritability) [Greco et al., 2002; Bacalman et al., 2006].
There is evidence of hypothalamic-pituitary-adrenal axis dysfunction, with inclusions found in all three locations in humans and the premutation mouse model [Brouwer et al., 2008; Greco et al., 2007; Louis et al., 2006; Hunsaker et al., 2011] leading in turn to enhanced release of the stress hormone cortisol [Brouwer et al., 2008]. Cortisol dysregulation and stress can lead to inflammation and activation of the immune system [Chang et al., 2009]. Perhaps the stress that these women experience can further accelerate the onset of FXTAS.
Adults presenting with immune mediated disorders and symptoms of FXTAS may not know the specifics of their medical histories. They may struggle – as some of our patients did – with an unassembled and incomplete puzzle of various immune mediated disorders and neurological symptoms that may be related to the FMR1 premutation. We recommend that further research in women with the premutation include the variety of medical problems that may be related to the premutation and the temporal sequence of events that may accelerate the onset of FXTAS, particularly immune mediated disorders. Such research may lead to earlier interventions that may decrease the prevalence of FXTAS in this population of women.
ACKNOWLEDGMENTS
The authors thank the families who participated in this study. This work was supported by the National Institute of Health grant HD036071 and HD02274; National Institute on Aging grants AG032119 and AG032115; National Center for Research Resources UL1 RR024146; and Health and Human Services Administration of Developmental Disabilities grant 90DD05969. NR was the recipient of a Beasiswa Unggulan (Excellent scholarship program) from BPKLN, Ministry of National Education and Culture, Government of Indonesia.
Footnotes
CONFLICT OF INTEREST Dr. Hagerman has received funding from Novartis, Roche, Seaside Therapeutics, Forest and Curemark for therapeutic trials in fragile X syndrome and autism. She has also consulted with Novartis and Roche/Genentech regarding treatment of fragile X syndrome. There are no other conflicts of interest from the other authors.
REFERENCES
- Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- Apartis E, Blancher A, Meissner WG, Guyant-Maréchal L, Maltête D, De Broucker T, Legrand AP, Bouzenada H, Thanh HT, Sallansonnet-Froment M, Wang A, Tison F, Roué-Jagot C, Sedel F, Charles P, Whalen S, Héron D, Thobois S, Poisson A, Lesca G, Ouvrard-Hernandez AM, Fraix V, Palfi S, Habert MO, Gaymard B, Dussaule JC, Pollak P, Vidailhet M, Durr A, Barbot JC, Gourlet V, Brice A, Anheim M. FXTAS New insights and the need for revised diagnostic criteria. Neurology. 2012;79:1898–1907. doi: 10.1212/WNL.0b013e318271f7ff. [DOI] [PubMed] [Google Scholar]
- Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Hagerman RJ. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, Gashkoff PA, Gillis BS. Unique immunologic patterns in fibromyalgia. BMC Clinical Pathology. 2012;12:25. doi: 10.1186/1472-6890-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Goetz CG, Leehey MA, Hagerman RJ, Zhang L, Li L, Nguyen D, Hall DA, Tartaglia N, Cogswell J, Tassone F, Hagerman PJ. Neuropathic features in fragile X premutation carriers. Am J Med Genet A. 2007;143:19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Coffey SM, Rivera SM, Hessl D, Gane LW, Tassone F, Greco C, Finucane B, Nelson L, Berry-Kravis E, Grigsby J, Hagerman PJ, Hagerman RJ. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70:852–862. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell JB, Nguyen DV, Hagerman RJ. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2009;72:175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemson R. Altered hypothalamus pituitary adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet Part A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsyukova I, Somarelli JA, Gregory SG, Garcia-Blanco MA. Alternative splicing in multiple sclerosis and other autoimmune diseases. RNA Biol. 2010;7:462–473. doi: 10.4161/rna.7.4.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C, McFarland H. Heat shock proteins in multiple sclerosis and other autoimmune diseases. Immunol Today. 1993;14:373–375. doi: 10.1016/0167-5699(93)90135-8. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007;177:1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- Greco CM, Tassone F, Garcia-Arocena D, Tartaglia N, Coffey SM, Vartanian TK, Brunberg JA, Hagerman PJ, Hagerman RJ. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Arch Neurol. 2008;65:1114–1116. doi: 10.1001/archneur.65.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R, Hagerman PJ. Advances in clinical and molecular understanding of the FMR1premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:8–786. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. 2012;27:296–300. doi: 10.1002/mds.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. 2011;134:863–878. doi: 10.1093/brain/awq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, Hagerman PJ, Hagerman RJ. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Greco CM, Spath MA, Smits AP, Navarro CS, Tassone F, Kros JM, Severijnen LA, Berry-Kravis EM, Berman RF, Hagerman PJ, Willemsen R, Hagerman RJ, Hukema RK. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. 2011. [DOI] [PMC free article] [PubMed]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77:374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakala P, Hanninen T, Ryynanen M, Laakso M, Partanen K, Mannermaa A, Soininen H. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. J Clin Invest. 1997;100:331–338. doi: 10.1172/JCI119538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Goetz CG, Zhang L, Hall DA, Li L, Rice CD, Lara R, Cogswell J, Reynolds A, Gane L, Jacquemont S, Tassone F, Grigsby J, Hagerman RJ, Hagerman PJ. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70:1397–1402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehey MA, Legg W, Tassone F, Hagerman R. Fibromyalgia in fragile X mental retardation 1 gene premutation carriers. Rheumatology (Oxford) 2011;50:2233–2236. doi: 10.1093/rheumatology/ker273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Winarni T, Zhang L, Tassone F, Hagerman R. Fragile X-associated tremor/ataxia syndrome (FXTAS) in grey zone carriers. Clin Genet. 2012;84:74–77. doi: 10.1111/cge.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E, Moskowitz C, Friez M, Amaya M, Vonsattel JP. Parkinsonism, dysautonomia, and intranuclear inclusions in a fragile X carrier: a clinical-pathological study. Mov Disord. 2006;21:420–425. doi: 10.1002/mds.20753. [DOI] [PubMed] [Google Scholar]
- Lu L-F, Liston A. MicroRNA in the immune system, microRNA as an immune system. Immunology. 2009;127:291–298. doi: 10.1111/j.1365-2567.2009.03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E, Ross-Inta C, Wong S, Omanska-Klusek A, Barrow C, Iwahashi C, Garcia-Arocena D, Sakaguchi D, Berry-Kravis E, Hagerman R, Hagerman PJ, Giulivi C. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20:3079–3092. doi: 10.1093/hmg/ddr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Jr., Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Ross-Inta C, Omanska-Klusek A, Wong S, Barrow C, Garcia-Arocena D, Iwahashi C, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Giulivi C. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429:545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: From pathophysiology to therapy. Nat Rev Rheumatol. 2011;7:518–527. doi: 10.1038/nrrheum.2011.98. [DOI] [PubMed] [Google Scholar]
- Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ, Hagerman PJ, Charlet-Berguerand N. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. Embo J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, Alunni V, Moine H, Thibault C, Page A, Tassone F, Willemsen R, Disney MD, Hagerman PJ, Todd PK, Charlet-Berguerand N. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Reports. 2013;3:869–880. doi: 10.1016/j.celrep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seritan AL, Nguyen DV, Farias ST, Hinton L, Grigsby J, Bourgeois JA, Hagerman RJ. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1138–1144. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Pivarcsi Advances in microRNAs: implications for immunity and inflammatory diseases. J Cell Mol Med. 2009;13:24–38. doi: 10.1111/j.1582-4934.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13:555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated Levels of FMR1 mRNA in Carrier Males: A New Mechanism of Involvement in the Fragile-X Syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A Rapid Polymerase Chain Reaction-Based Screening Method for Identification of All Expanded Alleles of the Fragile X (FMR1) Gene in Newborn and High-Risk Populations. The Journal of Molecular Diagnostics. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üçeyler N, Häuser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC musculoskeletal disorders. 2011;12:1–245. doi: 10.1186/1471-2474-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Experimental Biology and Medicine. 2013;238(5):450–460. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SMH, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012;158A:2473–248. doi: 10.1002/ajmg.a.35569. [DOI] [PMC free article] [PubMed] [Google Scholar]