Abstract
Background
Despite more than 60 years of research in the role of serotonin (5-HT) in psychopathology, many questions still remain. From a developmental perspective, studies have provided more insight into how 5-HT dysfunctions acquired in utero or early in life may modulate brain development. This paper discusses the relevance of the developmental role of 5-HT for the understanding of psychopathology. We review developmental milestones of the 5-HT system, how genetic and environmental 5-HT disturbances could affect brain development and the potential role of DNA methylation in 5-HT genes for brain development.
Methods
Studies were identified using common databases (e.g., PubMed, Google Scholar) and reference lists.
Results
Despite the widely supported view that the 5-HT system matures in early life, different 5-HT receptors, proteins and enzymes have different developmental patterns, and development is brain region–specific. A disruption in 5-HT homeostasis during development may lead to structural and functional changes in brain circuits that modulate emotional stress responses, including subcortical limbic and (pre)frontal areas. This may result in a predisposition to psychopathology. DNA methylation might be one of the underlying physiologic mechanisms.
Limitations
There is a need for prospective studies. The impact of stressors during adolescence on the 5-HT system is understudied. Questions regarding efficacy of drugs acting on 5-HT still remain.
Conclusion
A multidisciplinary and longitudinal approach in designing studies on the role of 5-HT in psychopathology might help to bring us closer to the understanding of the role of 5-HT in psychopathology.
Introduction
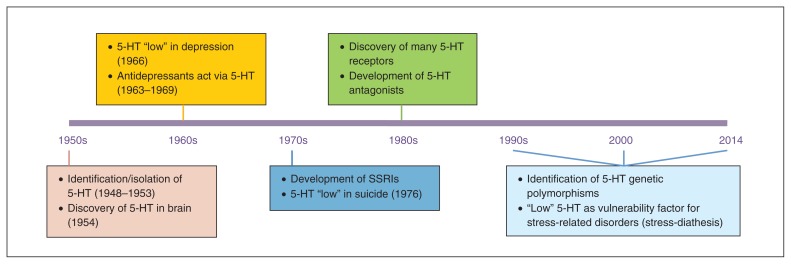
Serotonin (5-HT) has been an active topic of investigation for the past 60–65 years (Fig. 1). While it was originally discovered and isolated as a result of the search for the substance in blood that had a vasoconstrictor action, an important milestone for the role of 5-HT in mental health was the discovery of 5-HT in the brain by 2 independent research groups in the early 1950s.1–3 This led to several other major discoveries in psychiatry and to the publication of several landmark papers, including the first systematic reports of low 5-HT synthesis in depression.4 The observation in mice that tricyclic antidepressants inhibit 5-HT reuptake and that this is involved in their mood-elevating properties5–7 led to the development of selective serotonin reuptake inhibitors (SSRIs) as antidepressants. Furthermore, the observation that low levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid (CSF) is a predictor of suicide8 motivated other researchers to follow up the animal work and show that low 5-HT neurotransmission in humans was implicated in impulsive aggression, using several indices of 5-HT (e.g., 5-HIAA levels in the CSF).9
Fig. 1.
Overview of key findings in serotonin (5-HT) research over the past 65 years. SSRIs = selective serotonin reuptake inhibitors.
In the 1970s and 1980s, the 5-HT hypothesis of depression was a causal model, postulating that a deficit in 5-HT was the primary cause of problems, which could be “reversed” by medications that act on the 5-HT system. However, this so-called “absolute deficit” model had many limitations; not all patients showed alterations in 5-HT, and not all patients seemed to benefit from drugs enhancing 5-HT neurotransmission. Moreover, several drugs devoid of major effects on 5-HT neurotransmission are effective therapeutic agents.10,11
The clearest evidence questioning the absolute deficit model of the consequences of low 5-HT came in the past 25 years from studies conducted in first-degree relatives and recovered patients. Specifically, studies showed that healthy first-degree relatives of patients were more sensitive than healthy controls without patients among their first-degree relatives to 5-HT challenge procedures, such as depletion of the 5-HT precursor tryptophan.12,13 Moreover, altered 5-HT function was often present in recovered patients.14–16 All of these studies favoured a less deterministic model, suggesting that, rather than an absolute deficit, altered/low 5-HT neurotransmission may represent a risk factor for psychopathology, which is more likely to be behaviourally expressed in the presence of environmental stressors (diathesis-stress model). Further evidence for the diathesis-stress model came in the last 20 years when many polymorphisms in genes related to 5-HT receptors and enzymes were identified (see the studies by Lesch and colleagues17 and Walther and colleagues18). Genetic association studies showed a link between variation in certain 5-HT polymorphic variants and (sub)clinical symptoms (e.g., high neuroticism)19 as well as associations between certain 5-HT polymorphisms and several psychiatric diagnoses, especially in the context of early life adversity (see the studies by Caspi and colleagues20, Uher and McGuffin21 and Karg and colleagues22). The 5-HT stress- diathesis model has been the most common 5-HT working model for the understanding of the role of 5-HT in psychiatric disorders for the past 2 decades (Fig. 2).
Fig. 2.
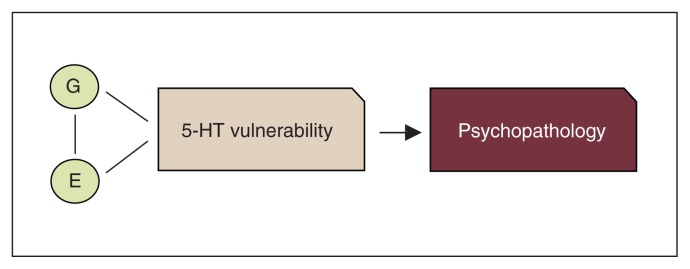
Overview of the common serotonin (5-HT) diathesis-stress model of psychopathology. E = environment; G = gene.
More recently, 5-HT has been reported to be involved in many behavioural, cognitive and (psycho)physiologic functions, including mood regulation, emotional processing, memory, social interaction, cardiac function and hypothalamic–pituitary–adrenal (HPA) axis regulation.23–28 It is possibly the most widely investigated neurotransmitter in mental health research. Postmortem, neuroimaging, genetic and pharmacological challenge studies have shown that alterations in 5-HT function can occur at many different levels, including changes in synthesis, release, receptor function/expression, reuptake and transport. However, despite more than 60 years of research, the underlying physiology of 5-HT neurotransmission in relation to psychopathology is still not known. In fact, 25 years of genetic research has failed to produce even 1 gene of causation despite the sophistication of array technologies. For example, numerous genes, of which a substantial number are involved in 5-HT regulation, have been more or less associated with psychopathology, but all of the identified genes fail to produce consistent replicable results. Second, the 5-HT diathesis-stress model does not account for how the 5-HT risk alleles for low 5-HT neurotransmission–related disorders (e.g., depression) are in fact inconsistently associated with expression levels. For instance, the s allele of the 5-HT transporter (SERT) gene 5-HTTLPR polymorphism has regularly been linked to major depressive disorder. Studies in cell culture showed that expressing the s allele results in decreased expression of the SERT.17,19 By contrast, positron emission tomography (PET) studies in humans, investigating the SERT in vivo, provided evidence that expression of the transporter remained unaffected by this allele.29 Third, although the risk for psychopathology in the presence of low 5-HT is less inevitable according to the low 5-HT diathesis-stress model than the 5-HT deficit model, the 5-HT diathesis-stress model is still somewhat deterministic. For instance, the model does not take into account resilience, nor does it account for non–stress induced relapses, a clinical phenomenon very common in, for example, depressed patients with a history of multiple episodes.30,31 Fourth, the model does not account for findings in animal studies, showing that SSRIs increase anxiety when administered in early life.32 Finally, more recent studies have shown that knockout mice lacking tryptophan hydroxylase-2 (TPH2; required for the production of brain 5-HT)18 were able to survive and showed no overt alterations in brain morphology, psychophysiology and depression- or anxiety-related behaviours,33 thereby further challenging the crucial role of 5-HT in vulnerability to psychopathology. Taken together, these findings support the need for a more refined 5-HT model of psychopathology.
The 5-HT diathesis stress model is gradually being refined, taking into account some of the limitations of the model. An emerging body of experimental evidence showing how dysfunctions in serotonergic tone early in life, whether through genetic ablation or environmental stress, may modulate neuronal growth, brain pathway development, differentiation and maturation, and emotion regulation. In this review, we provide an overview of the role of 5-HT in brain development and discuss whether and how research that focused on the developmental role of 5-HT helped us to better understand the role of 5-HT in psychopathology.
The first part of this paper reviews developmental milestones of the 5-HT system in animals and humans from the time of conception until adulthood. Specific emphasis will be on the timing of first expression of those 5-HT proteins, receptors and enzymes that have commonly been associated with human brain function and psychopathology later in life, such as the SERT, monoamine oxidase-A (MAOA) and TPH. The second part of this paper will review a body of research in animals and humans, demonstrating how interruptions in the 5-HT system at critical windows of development can affect structural and functional brain development. We first describe research targeting specific 5-HT genes directly, involving studies in rodents using knockout models or pharmacological manipulations as well as brain-imaging studies in humans in which brain processes as a function of genotypic or allelic variation in specific 5-HT genes is studied. Next, we describe research on how external adverse environmental factors, such as exposure to psychosocial stress or drugs of abuse at critical time points during development, could affect the 5-HT system and, in turn, could affect brain development. Adversity/environmental stressors have been defined in the literature and assessed in many different ways. In the present review, adversity/environmental stressors are broadly defined and encompass various types of abuse, including emotional and/or physical maternal deprivation, exposure to family dysfunction, neglect and exposure to drugs of abuse and other neurotoxins.
In the third part of this review, we discuss how DNA methylation in 5-HT genes might be an underlying mechanism for physiologic gene–environment interaction in the development of environmentally induced brain alterations.
The overarching hypothesis of this paper is that a disruption in the 5-HT system during development, whether induced by genetics or environmental stressors, could produce subtle changes in brain pathways or structures of brain circuits that are involved in emotion regulation. Alterations in these circuits in turn may increase the risk for a wide variety of psychopathologies.
Developmental milestones of the 5-HT system
Before birth
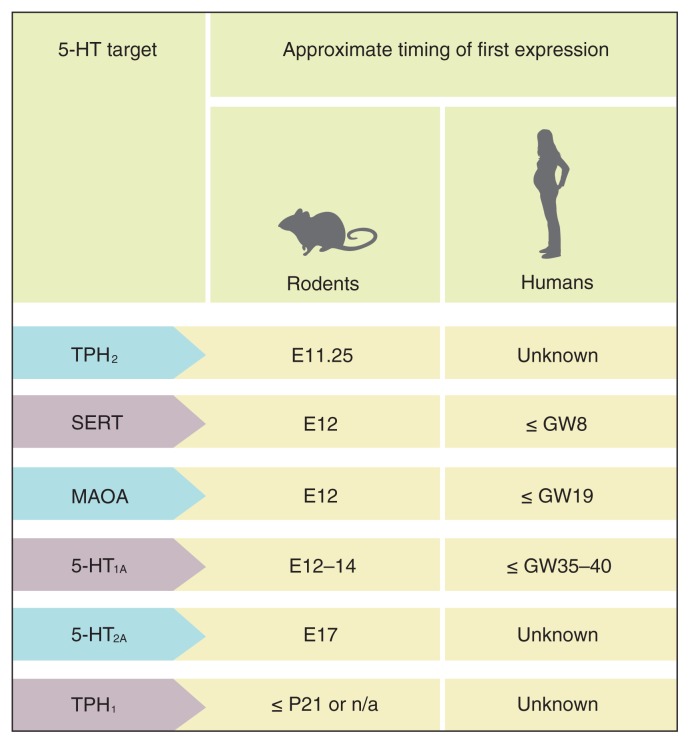
In rodents, the first neurons containing 5-HT appear on days 10–12 of gestation (E10–E12) in the cells of the rostral raphe nuclei. The cells are very small (5–7 μm), but grow long dendrites (up to 135 μm) within 24 hours.34,35 They can synthesize 5-HT about 1 day after neuron formation (E13), and 5-HT is the first monoamine to appear.35 However, the placenta is already able to produce 5-HT as early as E10.5, before actual 5-HT neurons develop, and provides the main source of 5-HT in the forebrain in the earliest stages of development.36 Moreover, before or around the first 5-HT synthesis by neurons, some important 5-HT receptors, proteins and enzymes begin to appear (Fig. 3), some of which increase exponentially after early initial expression (e.g., TPH237). These processes, which happen before or around the time of neuron formation, seem to play a major role in further brain development, including neurogenesis, dendritic maturation, axon connectivity, synaptic plasticity and apoptosis.46
Fig. 3.
Timing of first expression of serotonin (5-HT) proteins, receptors and enzymes in the brain, including tryptophan hydroxylase-2 (TPH2),37,38 5-HT transporter (SERT),39,40 monoamine oxidase-A (MAOA),41,42 5-HT1A receptor,43,44 5-HT2A receptor45 and TPH1.37 E = embryonic day; GW = gestational week; P = postnatal day.
Following initial 5-HT neuron formation around E12, descending 5-HT projections from the caudal part of the raphe to the spinal cord occur by E14, whereas synapses are formed around E17.47 At E15–E16, the number of 5-HT fibres increases substantially in the lateral hypothalamus, and by E17–E19, 5-HT pathways from the rostral part of the raphe (dorsal and medial) to all major brain regions of the frontal cortex have been established.48 Just before birth (E21–E22), 5-HT concentrations in the hindbrain and forebrain have increased by 500%–600% above early embryonic levels.36 These findings seem to parallel the exponential increase in TPH2 expression (enzyme essential for brain 5-HT synthesis) throughout the prenatal period.37
The pattern of 5-HT development in utero in humans appears to be relatively similar overall to the one observed in rodents, with the first 5-HT neurons becoming evident by 5 weeks of gestation.49,50 Prenatal neurodevelopment and the first 10 postnatal days in rats appear to correspond roughly to the first 2 trimesters and the third trimester of pregnancy in humans, respectively.51–54 The number of neurons increases rapidly until the tenth gestational week.55 The SERT-positive fibres from the raphe enter the cortical anlage 8 weeks after gestation, the subplate by 10 weeks of gestation and the plate at gestational week 13.56 In addition, for a short period of time, between 12 and 14 weeks of gestation, SERT is also expressed in non-5-HT neurons, an observation corresponding with what has been observed in rodents, and possibly important for the fine-tuning of the cortex.56 Fifteen weeks after gestation, clustered 5-HT cell bodies can be observed in the raphe nuclei.48 Little is known about specific developmental milestones in the 5-HT system during the second and third trimesters of pregnancy; however, a postmortem study in humans showed peak 5-HT1A receptor densities between 16 and 22 weeks of gestation, with the highest expression levels in the hippocampus and frontal cortex.57
After birth
Research in rodents showed that birth itself causes an overall reduction in extracellular 5-HT, as assessed in the CSF, possibly by increasing 5-HT uptake.58 After birth, 5-HT dendrites quickly grow until the end of the first postnatal week (7 d; P7). While 5-HT fibres reach their target region very early in development (i.e., during the gestation period), the final termination density is not reached in regions such as the hypothalamus and cortex until the end of the second postnatal week.48 Overall, 5-HT neuron distribution and morphology has been matured in the early postnatal period.59 However, many region-specific changes in protein and receptor expression continue to occur after birth, some of them up to adulthood. For instance, SERT density in the forebrain increases up to adulthood, while densities remain steady after P25 in the striatum, midbrain and brain stem.60 Sidor and colleagues61 examined mRNA SERT expression between P14 and P28 in several subregions of the dorsal raphe. Peak SERT mRNA levels were observed at P14 and declined to relatively stable levels between P17 and P28 (adolescence). Several studies investigated the developmental pattern of 5-HT1A receptor expression levels. Overall, the developmental pattern of 5-HT1A expression depended on which specific brain circuit was investigated. For instance, 5-HT1A mRNA is poorly expressed in the cerebral cortex right after birth, but increases rapidly in this region within the first 2 weeks.62 In the visual cortex, expression levels are high between P10 and P30,63,64 but decline markedly in this region during adolescence.63,64 Conversely, expression levels have been found to be relatively stable between P14 and P28 in the amygdala and prefrontal cortex,61 (P28 was the latest age investigated in that study), but increased in that time period by 50% in parts of the raphe and hippocampus.61 Interestingly, a recent study found that 5-HT1A auto-receptors remain uncoupled up to P21.65 Moreover, it was reported, using whole cell recordings in in vitro rat brain slices, that 5-HT1A receptor expression in pyramidal neurons of the postnatal prefrontal cortex increases in adolescence (P7–P16).66 In another study that compared adolescent rats (P35) with adult rats (P70), adolescent rats had 42% more 5-HT1A receptor binding in the amygdala than adult rats, while there were no differences in 5-HT1A mRNA expression.67 Adolescent rats also showed about 40% more 5-HT1A receptor binding and mRNA expression in parts of the hippocampus than adult rats (P70). Taken together, these studies suggest relatively strong fluctuations in 5-HT1A expression in the limbic regions during adolescence.
While TPH2 expression in the raphe exponentially increases prenatally and peaks at birth, it decreases slightly within the first 10 days of life37 and subsequently stabilizes between adolescence and adulthood.37,61,68 Finally, MAOA mRNA expression in the raphe reaches a peak within the first 4 days of life, then decreases and stabilizes at P21.41 Whether these developmental patterns in TPH2 and MAOA expression are brain region–specific is not known.
Studies in human newborns have shown that 5-HIAA levels in the CSF are greatest right after birth and decline sharply 1–2 months later.69,70 Around age 4, 5-HIAA concentrations in the CSF are quite similar to concentrations observed in adults69–73; similar age-matched findings have been observed in rhesus monkeys.74 One study investigated brain 5-HT synthesis in vivo in children.75 This technology is believed to connote aspects of 5-HT synthesis in vivo, hence serving as a proxy for informing on TPH2 functioning. This study showed that 5-HT synthesis rates continue to increase throughout the first 2–5 years of life and then gradually decrease. At age 14 and after, 5-HT synthesis rates are comparable to rates observed in adulthood75 and remain stable throughout adulthood (age 20–80 yr), independent of changes in brain anatomy.76
In humans, MAOA activity in the frontal cortex reaches a peak around birth, decreases rapidly during the first year of life and stabilizes around the age of 2 years,77 a pattern quite consistent with that observed in rodents.
Conclusion
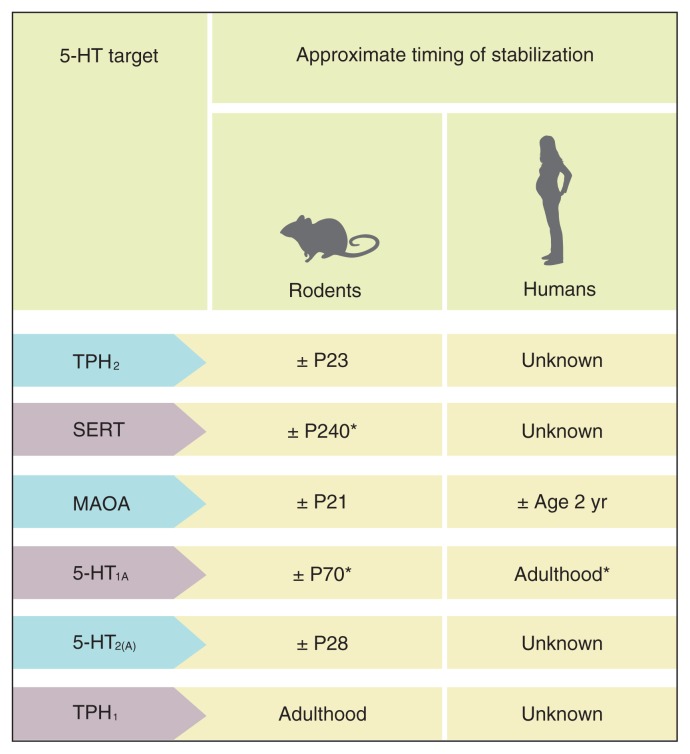
The 5-HT system undergoes major changes in utero and early life. Although the 5-HT system appears to mature early in life in terms of innervation, fibre density and synthesis, each of the 5-HT receptors, enzymes and proteins has a unique developmental pattern, with some of them stabilizing in early life and others not before adulthood, depending on the specific brain region/circuit examined. Some of the main developmental milestones of the 5-HT system in rodents and humans are summarized in Figures 3 and 4.
Fig. 4.
Timing of stabilization of serotonin (5-HT) proteins, receptors and enzymes in the brain, including tryptophan hydroxylase-2 (TPH2),37,38,46 serotonin transporter (SERT),60,78 monoamine oxidase-A (MAOA),41,42 5-HT1A receptor,61,64,79 5-HT2A receptor45,80 and TPH1.68 P = postnatal day. *Published research indicates that the exact timing of stabilization depends on brain region. The number in the table reflects the approximate timing of stabilization across all investigated brain regions.
Disruptions in the 5-HT system and consequences for brain development
Targeting specific 5-HT molecules
Genetic knockout and pharmacological models targeting specific molecules related to 5-HT function have provided increased insight into how a disruption in specific molecules at various time points between conception and adulthood could alter neurodevelopment, with consequences for the behavioural phenotype.46 For instance, prenatal deletion of the SERT in mice leads to increased serotoninergic tone and various subtle neurodevelopmental alterations.27 These alterations can be restored by depleting the excess of neonatal extracellular 5-HT in these mice (caused by a loss of 5-HT clearance) during the first postnatal days, suggesting that excessive 5-HT availability during a critical window of early development underlie these impairments.27 A brief suppression of the SERT in mice during early postnatal development through 1 week of fluoxetine administration resulted in depressive, anxious behaviours and increased stress responses in adulthood.32 These effects were not observed following inhibition of the norepinephrine (NE) transporter, nor when any of the neurotransmitters were inhibited after reaching brain maturity, suggesting that different neurotransmitters have a different role in neurodevelopment and that insult impact on neurotransmitter function is time specific.28
In humans, the role of 5-HT in (altered) brain development has been widely supported by molecular neuroimaging studies, demonstrating how individual differences in 5-HT tone could lead to differences in structure and function of the key brain regions involved in emotion regulation, including the amygdala, anterior cingulate cortex (ACC) and (pre)frontal regions.81 Most studies investigated the effects of polymorphisms of the SERT gene. Consistent with the 5-HT alterations and various subtle neurodevelopmental alterations observed in SERT knockout mice,27,28,32 carriers of the s allele of the SERT 5-HTTLPR polymorphism, a genetic variant associated with reduced expression of the SERT in cell cultures,17 show, upon exposure to emotional stimuli, amygdala hyperreactivity.82 They also show reduced grey matter volume in limbic regions, including the perigenual ACC and the amygdala, as well as reduced functional connectivity between these regions.83 Using a comparable paradigm as that in the studies that focused on the 5-HTTLPR polymorphism,82 amygdala hyperreactivity to emotional stimuli has also been observed in the investigation of polymorphisms of other 5-HT-related genes, including genes encoding TPH2, MAOA activity and 5-HT1A receptor expression.84–86 Hence, many aspects of 5-HT neurotransmission affect the frontal-limbic circuitry.
It is important to note that all of the brain imaging studies discussed involve individuals free of psychopathology. Also, most of these studies in healthy samples show neural differences even though behavioural differences were not reported. Thus, differences in the frontal-limbic circuits, as a function of differences in 5-HT tone, occur even in the absence of obvious behavioural differences. This supports the notion that subtle changes in brain development as a result of 5-HT disruption may pose a risk, but that additional factors may be necessary for an overt behavioural phenotype to be expressed. Counterintuitive as it may be, it should be recognized that until such factors are fully identified, the theoretical possibility that 5-HT neurotransmission is not involved in psychopathology cannot be entirely ruled out.
While genetic knockout mouse models and human molecular imaging studies provided evidence that an excess of 5-HT availability during critical time periods appears to interfere with normal development,87 a rather puzzling but consistent observation is that a partial or complete loss of 5-HT does not appear to lead to any overt brain abnormalities. Although several animal models have been used to study partial or complete 5-HT deficiency,88 of particular interest are mice that lack TPH2, the rate-limiting enzyme in the production of 5-HT. The initial identified TPH isoform, TPH1, is mainly expressed in the periphery and pineal gland, while the second isoform discovered later, TPH2, is expressed exclusively in brain 5-HT neurons and has been shown to have a more prominent role in the brain 5-HT synthesis than TPH1.18,37 TPH2 knockout mice have been shown to have a 98% reduction in brain 5-HT concentrations and 5-HIAA levels in various brain regions, with the greatest reductions in the brain stem.37,89 These studies have shown that 3–4 weeks after birth, TPH2 knockout mice are smaller than wild-type mice and have lower survival rates.33,90 They also showed increased aggression and anxiety, altered physiologic functioning and an inability to provide adequate maternal care to their offspring33 compared with wild-type mice. Notably, however, a consistent finding is that, despite almost complete lack of 5-HT, they show no overt changes in physical morphology, brain structure, neuron formation or fibre distribution.33,89–92
The observations of no overt morphological changes in rodents born with a 5-HT deficiency are at odds with the idea of the crucial role of 5-HT for further brain development. The lack of findings might be attributed to different factors, all of which may interact. First, an almost complete 5-HT deficiency in development might only induce overt changes in brain morphology in the context of severe stress or other biological abnormalities. Second, it could be argued that 5-HT might be specifically implicated in fine-tuning rather than in gross brain development. Hence, morphological methods used in previous studies might not have been sensitive enough to detect subtle alterations. Evidence for the latter comes from a recent study, using mice in which the TPH2 gene was replaced by the enhanced green fluorescent reporter, allowing a more refined examination of 5-HT neurons. Interestingly, it was reported that TPH2 knockout enhanced green fluorescent protein; knock-in mice had severe abnormalities in 5-HT innervation in several parts of the rostral brain.90
In addition, recent studies provided some indications that the impact of 5-HT disruption on brain development may depend on the origins of 5-HT (i.e., is it produced by the embryo’s own neurons, or does it come from the placenta?). First, evidence of sources of 5-HT beyond the embryo’s own neurons dates back to studies showing the presence of endogenous 5-HT93 in vivo in the placenta, before 5-HT neurons appear. More recent studies have supported the role of a maternal 5-HT contribution to the embryo’s brain development. Specifically, it was shown that embryos of mice born to TPH1 knockout mothers had significant brain alterations and were smaller in size, irrespective of their own (or paternal) genotype.94 This study suggests that maternal transmission of 5-HT contributes to the offspring development, irrespective of DNA sequence. Recently, using generated mice models, it was demonstrated that maternal 5-HT in both animals and humans does not cross into the circulation of the embryo, but that the placenta itself is able to produce 5-HT via maternal sources of tryptophan in the very early stages, before 5-HT neurons develop,36 in a time period that corresponds with the late first/early second trimester of human pregnancy. The 5-HT that comes from the placenta is the major source of 5-HT in the forebrain, in the absence of 5-HT neuron formation.36 Taken together, these findings suggest that placental sources of 5-HT might play an important role in the neurodevelopmental origins of altered brain structure or function, possibly resulting in 5-HT-based disease etiologies.
Adversity during gestation and childhood: consequences for brain 5-HT
While the studies discussed show how genetic disruptions in specific 5-HT genes can influence brain development, a number of studies have shown persistent alterations in the 5-HT system, including lower 5-HT innervation, receptor function, protein and enzyme expression, synaptic density and metabolite levels, following in utero or early postnatal exposure to experimentally induced stress95,96 and/or substances of abuse, such as nicotine or alcohol (see the studies by Parker and colleagues,97 Sliwowska and colleagues,98 Slotkin and colleagues99 and Williams and colleagues100). Some of these effects appear to be sex-specific.96,98,99 Studies in mice demonstrate a clear sex effect for the behavioural and physiologic effects of prenatal stress exposure. Male mice exposed to prenatal stress exhibited an increased sensitivity to a low dose of SSRIs.101 Whether such sex-specific effects also occur in humans needs to be further tested.
It is conceivable that some of these environmentally induced changes in the 5-HT system may, in turn, further affect or disrupt brain development. The impact of early environmental changes on human 5-HT neurotransmission has been studied less extensively. Studying this phenomenon in humans poses many obvious methodological challenges. Since it is impossible to randomize or control exposure to early stress or drugs of abuse in vivo in humans, the interpretation of such studies is limited in that it is impossible to tell whether any difference would be due an environmental or genetic effect or to their interaction.
The evidence from experimental animals that is most relevant for the impact of exposure to early stress or drugs of abuse on human 5-HT neurotransmission comes from longitudinal experimental studies of nonhuman primates, in which rhesus monkeys were randomly assigned to different rearing conditions, including maternal deprivation. Maternal deprivation involves separating infants from their mothers at birth, hand-rearing them in a nursery for the first month, and then rearing them with same-age peers until 6 months of age, after which they would be moved into larger groups that also contain mother-reared age-mates and sometimes older adults.102 Both peer-reared and mother-reared monkeys would then continue to live in these mixed social groups at least until puberty. With such monkey separation paradigms, it was shown that, in addition to several behavioural and emotional alterations (e.g., increased anxiety, impulsivity, and aggression103), the monkeys who grew up with their peers had altered levels of the 5-HT metabolite 5-HIAA in the CSF, which remained stable over time, up to adulthood.74 Notably, the impact of maternal deprivation on 5-HT function appears to be more pronounced in those having a short allele of the SERT than in those homozygous for the long allele.104 Using in vivo PET measures of 5-HT, studies involving the maternal separation paradigm have shown that, compared with mother-raised monkeys, juvenile monkeys exposed to early maternal deprivation have decreased SERT and 5-HT1A binding in the raphe and frontal-limbic regions.102,105 Taken together, these findings provide emerging evidence that exposure to stressors in early life could have a long-term effect on the 5-HT system.
Even with its methodological limitations, there is some evidence from human research that exposure occurring during the gestational period, including exposure to antidepressant medications, nicotine, noxious chemicals or malnutrition, could alter 5-HIAA concentrations or tryptophan levels in newborns.106–109
Abuse of toxins, such as alcohol, by pregnant mothers is known to have profound teratogenic effects through the disruption of brain morphology and/or 5-HT function. In a single-photon emission computed tomography (SPECT) study conducted in 5- to 12-year-old children, it was reported that children with fetal alcohol syndrome, a disorder characterized by severe facial dysmorphies, growth deficiencies and central nervous system dysfunction, had reduced medial prefrontal SERT binding relative to control children.110 In addition, there is emerging evidence that prenatal exposure to SSRIs could influence a child’s brain and behavioural development as well as physiologic regulation to stress (for a review, see the study by Oberlander111). One study108 investigated in particular the impact of SSRI exposure during gestation on early measures of 5-HT, physiologic and behavioural phenotypes during the first days of life. It was shown that the babies of depressed mothers who were taking SSRIs during pregnancy had lower blood 5-HT and 5-HIAA concentrations, which were associated with physiologic and motor disturbances at day 4, but no differences at day 14 or 2 months after birth.108 The long-term relevance of these observations, however, is unknown.
While the studies discussed focused mostly on clinical samples, there is also evidence for subtle alterations in the 5-HT system in healthy samples upon exposure to environmental stressors in the perinatal period. We studied the impact of early perinatal stressors on brain 5-HT synthesis capacity using the PET/[11C]Alpha-methyl-tryptophan method in a longitudinal cohort sample of healthy men, followed regularly since childhood. Measures of perinatal and childhood adversity were obtained from various sources, including hospital birth delivery records as well as self and/or family interviews completed at regular follow-ups. Although we did not have any measures of 5-HT synthesis in early life, we reported that variations in perinatal adversity predicted subtle variations in 5-HT synthesis capacity in adulthood in the limbic regions (orbitofrontal cortex, hippocampus), particularly in association with physical distress at birth, lower birth weight and maternal smoking during pregnancy.112 These results are somewhat in line with those of a PET study in rabbits using a similar imaging methodology, suggesting lower 5-HT synthesis in the frontal cortex in animals exposed to endotoxin in utero relative to controls.113 Given that the 5-HT system develops and matures rapidly for a large part, these findings may suggest that the impact of certain stressors on 5-HT synthesis in the limbic pathways may be most pronounced at the time that the 5-HT system undergoes the most developmental changes.
Adversity between childhood and adulthood: consequences for brain 5-HT
The findings that the 5-HT system develops and matures overall relatively soon do not suggest that the system is not sensitive to later stressors through changes in 5-HT neurotransmission. First, as discussed, protein and receptor expression are still fluctuating in some brain regions, some of them up to adulthood. Second, the types of stressors people are exposed to change throughout life. During the periadolescent (8–12 yr) and adolescent periods (12–18 yr), while transitioning from childhood to adulthood, individuals generally spend less time with their families and more time with peers and romantic partners. Hence, the quality of relationships outside the family system might become increasingly influential for further emotional development. Adverse social experiences, such as peer victimization or exposure to dating violence might be of particular relevance in this context, since these adverse experiences are more common in adolescence than in any other phase of life. However, few studies have examined the long-term consequences of such stressors in adolescence on 5-HT despite the widely demonstrated finding that social adverse experiences beyond the family environment, such as bullying at school, predict 5-HT- associated disorders, such as depression and anxiety.114,115 The few existing studies are animal studies using the so-called intruder–resident paradigm (social defeat), in which an adolescent male rodent is put in the cage of another adolescent male rodent. These studies consistently show that chronic exposure to social defeat in adolescence leads to greater levels of anxiety in adulthood.116,117 Yet, effects on 5-HT neurotransmission are often inconclusive. Studies generally focus only on males. For example, a recent study indicated that exposure to fear-inducing stressors in the periadolescent period in male rats led to long-term increases in MAOA in the prefrontal cortex.118 The observation that the 5-HT system is still developing in adolescence, together with the widely demonstrated observation that chronic victimization and aggression exposure severely increase an adolescent’s risk for 5-HT-associated psychopathology, highlights the need for further studies investigating the long-term effects of social stressors in adolescence on 5-HT.
Aside from changes in the type of social stressors in adolescence, people often start their greatest intake of drugs of abuse or alcohol in this phase of life. A number of studies have shown that drugs of abuse could lead to long-term changes in 5-HT neurotransmission.
The effects of the drug 3,4-methylenedioxy-N-methyl-amphetamine (MDMA; ecstasy) have been the most widely studied. That is, extensive studies in adult rodents and primates indicate that MDMA mostly results in axotomy of 5-HT neurons, reduces neuronal signalling and may also lead to other alterations in brain indicators of 5-HT neurotransmission.119,120
Based on the fact that the brain undergoes several critical changes during adolescence, it could be hypothesized that drugs of abuse could have a greater impact on 5-HT neurotransmission during this period. A number of studies in adolescent rodents reported that drugs administered in adolescence induce 5-HT alterations, often in a sex-specific manner.99,121,122 Yet the results obtained from other animal experiments directly comparing the drug effects in adolescent rodents with adult rodents support the opposite view. For instance, cannabinoid administration increases hippocampal 5-HT1A binding and mRNA expression in adult rats, but does not affect 5-HT1A binding in adolescents.67 Anecdotally, MDMA use was reported to reduce SERT binding to a lower extent in the adolescent rat forebrain relative to adult rats.123
Some evidence for greater adverse effects on the 5-HT system on the adult brain, as opposed to the adolescent brain, also exists in humans. Specifically, a younger age of onset of MDMA use is associated with higher SERT binding in the midbrain in adulthood.123 The exact mechanism for these effects is not known, but it might be related to greater plasticity in the adolescent brain than in the adult brain. However, these observations do not suggest that adolescent users are not at increased risk for problems later on. In fact, less adverse effects (or more rewarding effects) may also lead to increased use, which in turn, could lead to increased risk for abuse.123 It would be important to further study the longitudinal consequences for adolescent drug use on the 5-HT system and associated behaviours and how they could interact with the consequences resulting from prenatal and early life environmental “insult”/exposure.
Conclusion
Genetic mouse models and pharmacological studies have shown that interruption of specific 5-HT receptors, proteins or enzymes can affect or interrupt brain development, but the effects appear to be time- and brain region–specific. In some instances, the effects may appear to be subtle; adversity often does not lead to overt brain abnormalities in humans and is not sufficient to cause psychopathology. In addition, a number of studies showed that adversity in utero and in early childhood could affect the 5-HT system, while the impact of adversity in (peri)adolescence on the 5-HT system is not known and is generally believed to be understudied.
In the next part of this review, we describe how epigenetic modifications in the 5-HT system might be one of the molecular mechanisms of how adverse environmental exposure translate into 5-HT alterations.
Disruption in 5-HT homeostasis: role of epigenetic processes
Though the environment may affect brain 5-HT function in many different ways, including direct lesion, via changes in intracellular, transcriptional factors or via interactions with other neurotransmitters, hormones or peptides, one of the underlying mechanisms by which these alterations occur is via environmentally induced stable changes in gene expression.124 These may occur through epigenetic modifications, and do not result in changes of gene sequence or structure.124–127
In contrast to the genome, which is identical across different cell types and through life, the epigenome is dynamic and varies among cell types as well as temporally during life. The epigenome consists of the chromatin and its modifications, a covalent modification of DNA by methylation of cytosine rings found at the dinucleotide sequence CG, and noncoding RNAs.124,128 Though all of these factors are critically involved in gene expression regulation, DNA methylation is part of the covalent structure of the genome and thus, part of the gene sequence itself. DNA methylation is the most widely studied component of the epigenome in relation to mental health. It affects the DNA molecule through enzymatic addition of a methyl group to DNA. It silences expression of the gene, either directly by blocking transcriptional factors from binding to the DNA sequence, or indirectly by recruiting proteins, such as histones, that will precipitate inactive chromatin.129–131
High density of methylation or methylation of critical CpG sites, typically at the level of the gene promoters, could silence gene expression, resulting in a loss of function. This could have a similar consequence to a loss of function by genetic mechanisms, such as mutation. Thus, it could explain reduction in 5-HT function in the absence of a change in genetic sequence.124,125
The DNA methylation pattern is formed primarily during gestation by a series of global methylation and demethylation events, first at the global level, then at the level of specific cell types.132,133 The DNA methylation pattern in this period is highly vulnerable to environmental exposures. Restriction of certain dietary components, such as folic acid and vitamin B12, during gestation is known to affect the DNA methylation pattern in sheep.134 Similarly, a study in humans has shown that individuals exposed to famine in the perinatal period, exhibited, 6 decades later, altered DNA methylation patterns compared with their siblings.135 Though DNA methylation is potentially reversible, it appears to be the most stable epigenetic mark.124,125
Animal studies support the link between early adversity, changes of DNA methylation and subsequent consequences for stress-associated psychophysiology. For instance, in one of the first studies on this topic, low maternal care during the first week of life was associated with alterations in DNA methylation levels, once determined in brain tissue in the regulatory regions of the glucocorticoid receptor (GR) in the hippocampus in the offspring, together with increased neuroendocrine stress responses.136 These changes in methylation were reversible by a postnatal positive environment.136
While DNA methylation processes have been studied for more than 3 decades in relation to cancer, they have been a very active topic of research in mental health for only the past 5 years, and this is equally true with regard to DNA methylation processes in 5-HT genes. After the observation of tissue-specific DNA methylation changes in the hippocampal glucocorticoid receptor gene promoter in relation to childhood abuse in postmortem brains,137 other studies found evidence for DNA methylation changes occurring in peripheral tissues, such as blood or saliva in living humans. Indeed, an increasing number of studies have found associations between early life adversity and methylation in the SERT gene (for a review, see the study by Booij and colleagues125). For instance, rhesus monkeys reared in a nursery, displayed higher SERT methylation and increased separation anxiety during maternal-social separation compared with mother-reared infants.138
In humans, using peripheral cells, several studies indicated that early stress was associated with altered levels of SERT promoter methylation.139–143 In turn, we observed that SERT promoter methylation in the white blood cells of adults was associated with lower in vivo measures of brain 5-HT synthesis in the lateral orbitofrontal cortex and greater childhood aggression.144 It was hypothesized that early stress may have been associated with altered methylation levels of the SERT promoter, with consequences here for brain chemistry and behaviour. This was further supported by a very recent report that greater SERT methylation assessed in whole blood DNA was associated with lower volume of the hippocampus, a brain region with rich 5-HT innervation that is important for stress regulation.145
It is important to remember that, in addition to the restrictions previously mentioned (measurement to peripheral instead of direct in vivo brain measures), the interpretation of the role of DNA methylation for brain development is limited by the fact that almost all DNA methylation research is correlational and thus cannot provide any insight into cause and effect. Little is known about the impact of (peri)adolescent stressors on 5-HT methylation; yet, one study reported that exposure to bullying (peer victimization) in childhood may have an impact on methylation in the SERT transporter gene.146
Notwithstanding this paucity of established knowledge, how does early stress translate into changes in brain development? Exposure to early adversity may result in activation of signalling pathways in different cells, including neurons and hematopoietic stem cells. These in turn may activate and target DNA and chromatin modifying enzymes to a cluster of genes resulting in a long-term response to adversity. This “programming” results in stable changes in gene function and influences the brain, behaviour and psychophysiology. Given the role of 5-HT genes in the development of frontal-limbic brain circuits, it can be speculated that early stressors lead to stable changes in the programming of 5-HT genes, thereby influencing the frontal-limbic circuits and their functional development.
Conclusion
A growing number of studies showed that DNA methylation in 5-HT genes might be an underlying mechanism of how genes and adversity (environmental stressors) physiologically interact, thereby increasing vulnerability for psychopathology. Experimental longitudinal and/or intervention studies are needed to dissect more thoroughly the neurobiology of risk and resilience, as it pertains to modulate the effects of 5-HT genes and generating phenocopies.
Discussion
Integrative summary
Serotonin is perhaps the most widely studied neurotransmitter in relation to mental health. From a developmental perspective, 5-HT has been shown to play a major role in brain development. A number of animal and (more recent) human studies, have shown that various types of insults, whether genetic or environmental, could alter the trophic properties of 5-HT at play during critical periods of brain development. The aim of this review was to highlight some of the research illustrating the relevance of the developmental role of 5-HT to the understanding of its role in human psychopathology. The research described in this review illustrated that, despite the widely supported view that the 5-HT system in both rodents and humans is largely matured and stabilized in early life, expression levels of 5-HT proteins, receptors and enzymes have unique developmental patterns, with some of them stabilizing in early life (e.g., MAOA) and others still fluctuating until adulthood (e.g., SERT) in specific brain regions. The expression of specific receptors, enzymes and generally relevant proteins appears, in turn, to modulate specific brain development processes.46 Although it is clear that no specific 5-HT molecule is unequivocally associated with any specific behaviour, it would be of interest for longitudinal studies to further examine developmental changes in 5-HT expression levels over time and how these changes in 5-HT molecules are associated with developmental changes in specific behaviours.
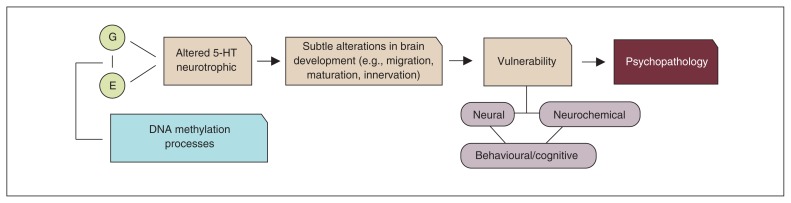
In the second and third part of this review, we discussed research showing that, in addition to genetic factors, environmental stressors could adversely affect the 5-HT system, with possible consequences for brain development. Epigenetic mechanisms might be one of the underlying physiologic mechanisms through which genes and environment interact. However, even when changes in the 5-HT system occur, not all individuals become ill, and there is no unequivocal association with a specific insult or with a single disorder. Rather, the association seems conditioned by multilevel processes: an early disruption of 5-HT homeostasis may lead to structural and functional changes in specific brain circuits that modulate emotional responses to stress, including at the level of subcortical limbic and (pre)frontal areas. A disruption in these circuits may predispose individuals to psychopathology, such as mood and impulse control disorders. Figure 5 illustrates this notion (i.e., combining the neurodevelopmental role of 5-HT with the 5-HT diathesis-stress model of psychopathology).
Fig. 5.
Neurodevelopmental stress-diathesis model of psychopathology. 5-HT = serotonin; E = environment; G = gene.
Role of protective factors and resilience, and other limitations/venues for future research
Though taking into account the neurodevelopmental role of 5-HT in understanding its role in psychopathology may be compelling, little is known about the specific role of protective factors; such factors may include social support and the use of non–maternal care interventions, which all have been shown to mitigate the impact of other adversity factors, like maternal depression and low socioeconomic status on vulnerability for psychopathology later in life.147–150 Protective factors might also mitigate early influences on the neurochemical correlates of risk, including those mediated through 5-HT genes.151
Multiple other biological adaptations that may promote resilience have also been identified. These include, but are not restricted to, high levels of dehydroepiandrosterone (DHEA), which has been shown to counter the effects of the stress hormone cortisol; high levels of testosterone; and high blood neuropeptide Y (NPY).152 All of these biological factors have been shown to interact with the 5-HT system (see the studies by Croonenberghs and colleagues,153 Guy and colleagues154 and Porter and colleagues155).
More generally, even in the absence of protective factors, it is important to mention that the long-term negative consequences of adversity cannot be written in stone. A recent hypothesis in this context is the so-called “mismatch” hypothesis. This implies that not an adverse environment by itself, but a high degree of mismatch between the prenatal or early life and the current environment, would predict maladaptive behaviours. Hence, an individual who has experienced early adversity may be adapted to cope with high levels of stressors later in life.156,157 Taking into account the wide variety of literature showing a robust, frequent, or prolonged activation of the body’s stress-response system following (“toxic”) stressors,158 it is reasonable to assume that the mismatch hypothesis holds for moderate but not severe stressors. Longitudinal studies on the consequences of different types of stressors are needed to further test the mismatch hypothesis.
In addition, it is important to mention that the research on the role of stress on development and the developmental role of 5-HT so far has focused mainly on perinatal and childhood stressors. Serotoninergic changes as a result of adversity in (peri)adolescence have been inconclusive and understudied. Second, DNA methylation research in humans has been mostly correlational, allowing no inferences about cause and effect. For instance, much of the literature implies that DNA methylation induced by stress might increase the risk for psychopathology, but it is reasonable to assume that psychopathology itself via, for example, depression-induced alterations of other biological systems or past or current treatment126,159 could affect the epigenome. Intervention studies or longitudinal studies in at-risk human samples (e.g., first- degree relatives) are needed to disentangle cause and effect. Causal associations between environmental conditions and DNA methylation could also be studied in individuals who have undergone changes in early family environments as part of preventive mental health interventions.160 Such studies could be interpreted as the human equivalent of cross-fostering experiments with rats136 and monkeys.161
Finally, 5-HT has been implicated in a wide variety of psychopathologies. Similar genetic variations and similar adversities have been linked to a wide variety of psychiatric disorders. In particular, when considering specifically early-life abuse, there appears to be no specific association between abuse “mode” and a specific disorder.162
As described in this review, 5-HT neurotransmission and protein expression are also influenced by multiple factors other than the trophic factors considered here, including age, sex, timing of adversity, genetics and the impact of other biological systems. The specific consequences of disrupting 5-HT neurotransmission on behaviour, risk or resilience, if any, ultimately depend on the complex interplay among these various factors.
Conclusion
The 5-HT hypothesis of psychopathology: What has it taught us and where does it lead?
The 5-HT hypothesis of psychopathology has been one, if not the most, dominating hypothesis for psychopathology for almost 50 years. In the 1980s and 1990s the 5-HT hypothesis of psychiatric disease shifted gradually from a simple causal model to a less deterministic model. This shift was largely because of research in the first 30 years after the discovery of 5-HT, considering that not all people respond to drugs that act on the 5-HT system and that healthy, vulnerable individuals believed to be carriers of risk as well as recovered patients still have some 5-HT alterations in the absence of symptoms.
The number of studies on the role of 5-HT in psychopathology has increased steadily over the past 20 years; yet, the specific role(s) of 5-HT in terms of onset, relapse and effective treatment are far from being understood. For instance, 5-HT medications have been on the market for more than 30 years; yet nonresponse rates are still high, and factors associated with adequate or adverse responses are still not known. It still seems impossible to identify people at risk or those who are most vulnerable for relapse after recovery from a disease in which altered 5-HT neurotransmission plays a role.
With the gain in insight resulting from neurodevelopmental studies, we may be closer to dissecting the role(s) of 5-HT in psychiatric disease. On the positive side, much progress has been made in the past 1–2 decades in terms of refinements in techniques to study 5-HT. For instance, transgenic technologies have provided the possibility to generate mice lines with inactive 5-HT receptors or proteins that normally control certain aspects of the 5-HT system. Some of the very recent promising developments involve 5-HT knockout mice models, allowing the investigation of time- and region-specific changes. For instance, a very recent study investigated TPH2 knockout mice in whom the TPH2 gene locus was replaced by the enhanced green fluorescent protein, allowing for a more careful and detailed visualization of the development and the formation of the 5-HT circuitry.90 Moreover, “reversible” knockout models make it possible to study time- and region-specific consequences of developmental interruptions in receptors.163 For instance, it has recently been shown that knockout of the 5-HT1A autoreceptor in 5-HT raphe neurons 14–30 days after birth (corresponding to early childhood) leads to long-lasting increases in anxiety, decreased social behaviours and subtle alterations in neuron physiology.164 In humans, improvements in compounds that permit the visualization of 5-HT target molecules in the human brain have helped us to understand the expression of receptors or proteins in the human brain. Developments in genetic array technologies make it possible to identify new genes and carry out (epi)genetic analyses on a large-scale basis. New developments in imaging techniques provide the possibility to visualize even the smallest brain structures and measure aspects of 5-HT function in vivo. Combining psychopharmacological and genetic techniques with sensitive brain imaging techniques provides a fruitful area of research, allowing us to model specific complex biological processes in the brain relevant for the understanding of treatment and mechanisms of disease, such as 5-HT1A autoreceptor internalization165 or neurochemical 5-HT sensitization processes.166
Research in both mice and humans has demonstrated that it appears to be important to take into account not only the individuals’ early-life history when studying the role of 5-HT in development, but also the developmental events happening between the time of conception and birth as well as the molecular processes in prior generations. Large-scale multidisciplinary studies with longitudinal samples are the most optimal design to help our understanding of the role of 5-HT in development and psychopathology. Such multidisciplinary and longitudinal approaches are crucial to better understand an individual’s vulnerability to psychopathology over the lifespan. Moreover, the designing of experimental interventions that could foster a more optimal brain development, thereby offsetting the deleterious effects of altered 5-HT tone early in life, could prove important in the prevention of psychopathology.
Acknowledgements
L. Booij is supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR). L. Booij thanks the Canadian College of Neuropsychopharmacology (CCNP) for the CCNP 2013 Young Investigator Award, which involved the writing of this invited manuscript. The authors thank J-B. Ng Man Sun, for assistance with the figures.
Footnotes
Competing interests: M. Szyf holds the GlaxoSmithKline–Canadian Institutes of Health Research professorship in pharmacology. No other competing interests declared.
Contributors: L. Booij and C. Benkelfat designed the review. All authors reviewed and interpreted parts or all of the literature. L. Booij wrote the article, which all authors reviewed and approved for publication.
References
- 1.Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;175:157–61. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Sjoerdsma A, Palfreyman MG. History of serotonin and serotinin disorders. Ann N Y Acad Sci. 1990;600:1–7. doi: 10.1111/j.1749-6632.1990.tb16869.x. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 3.Amin AH, Crawford TB, Gaddum JH. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol. 1954;126:596–618. doi: 10.1113/jphysiol.1954.sp005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft GW, Crawford TB, Eccleston D, et al. 5-hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological diseases. Lancet. 1966;2:1049–52. doi: 10.1016/s0140-6736(66)92028-9. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson A. Structural specificity for inhibition of [14C]-5- hydroxytryptamine uptake by cerebral slices. J Pharm Pharmacol. 1970;22:729–32. doi: 10.1111/j.2042-7158.1970.tb08419.x. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson A, Fuxe K, Ungerstedt U. The effect of imipramine of central 5-hydroxytryptamine neurons. J Pharm Pharmacol. 1968;20:150–1. doi: 10.1111/j.2042-7158.1968.tb09706.x. [DOI] [PubMed] [Google Scholar]
- 7.Wong DT, Perry KW, Bymaster FP. Case history: The discovery of fluoxetine hydrochloride (prozac) Nat Rev Drug Discov. 2005;4:764–74. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 8.Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33:1193–7. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 9.Brown GL, Goodwin FK, Ballenger JC, et al. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–9. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 10.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahl SM, Lee-Zimmerman C, Cartwright S, et al. Serotonergic drugs for depression and beyond. Curr Drug Targets. 2013;14:578–85. doi: 10.2174/1389450111314050007. [DOI] [PubMed] [Google Scholar]
- 12.Benkelfat C, Ellenbogen MA, Dean P, et al. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–97. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 13.LeMarquand DG, Benkelfat C, Pihl RO, et al. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. Am J Psychiatry. 1999;156:1771–9. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- 14.Bhagwagar Z, Rabiner EA, Sargent PA, et al. Persistent reduction in brain serotonin1a receptor binding in recovered depressed men measured by positron emission tomography with [11C]way-100635. Mol Psychiatry. 2004;9:386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 15.Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–9. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- 16.Booij L, Van der Does W, Benkelfat C, et al. Predictors of mood response to acute tryptophan depletion. A reanalysis. Neuropsychopharmacology. 2002;27:852–61. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, Balling U, Gross J, et al. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 18.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 19.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 20.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-htt gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 21.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–46. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 22.Karg K, Burmeister M, Shedden K, et al. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 24.Booij L, Swenne CA, Brosschot JF, et al. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol Psychiatry. 2006;60:507–14. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13:268–76. doi: 10.1159/000104854. [DOI] [PubMed] [Google Scholar]
- 26.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations [Review] Mol Psychiatry. 2003;8:951–73. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 27.Esaki T, Cook M, Shimoji K, et al. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–7. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy NV, Selvaraj S, Cowen PJ, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] dasb binding in the living human brain. Neuroimage. 2010;52:50–4. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. Am J Psychiatry. 2002;159:1133–45. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- 32.Ansorge MS, Zhou M, Lira A, et al. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–81. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 33.Alenina N, Kikic D, Todiras M, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–7. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- 35.Olson L, Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1972;137:301–16. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- 36.Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–50. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutknecht L, Kriegebaum C, Waider J, et al. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from tph2 knockout mice. Eur Neuropsychopharmacol. 2009;19:266–82. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Zill P, Buttner A, Eisenmenger W, et al. Analysis of tryptophan hydroxylase 1 and 2 mRNA expression in the human brain: a postmortem study. J Psychiatr Res. 2007;41:168–73. doi: 10.1016/j.jpsychires.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Bruning G, Liangos O, Baumgarten HG. Prenatal development of the serotonin transporter in mouse brain. Cell Tissue Res. 1997;289:211–21. doi: 10.1007/s004410050868. [DOI] [PubMed] [Google Scholar]
- 40.Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Brain Res Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]
- 41.Vitalis T, Fouquet C, Alvarez C, et al. Developmental expression of monoamine oxidases a and b in the central and peripheral nervous systems of the mouse. J Comp Neurol. 2002;442:331–47. doi: 10.1002/cne.10093. [DOI] [PubMed] [Google Scholar]
- 42.Lewinsohn R, Glover V, Sandler M. Development of benzylamine oxidase and monoamine oxidase a and b in man. Biochem Pharmacol. 1980;29:1221–30. doi: 10.1016/0006-2952(80)90278-6. [DOI] [PubMed] [Google Scholar]
- 43.Hillion J, Catelon J, Raid M, et al. Neuronal localization of 5-HT1A receptor mRNA and protein in rat embryonic brain stem cultures. Brain Res Dev Brain Res. 1994;79:195–202. doi: 10.1016/0165-3806(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 44.del Olmo E, Lopez-Gimenez JF, Vilaro MT, et al. Early localization of mrna coding for 5-ht1a receptors in human brain during development. Brain Res Mol Brain Res. 1998;60:123–6. doi: 10.1016/s0169-328x(98)00149-1. [DOI] [PubMed] [Google Scholar]
- 45.Roth BL, Hamblin MW, Ciaranello RD. Developmental regulation of 5-HT2 and 5-HT1C mRNA and receptor levels. Brain Res Dev Brain Res. 1991;58:51–8. doi: 10.1016/0165-3806(91)90236-c. [DOI] [PubMed] [Google Scholar]
- 46.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 47.Wallace JA, Lauder JM. Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain Res Bull. 1983;10:459–79. doi: 10.1016/0361-9230(83)90144-2. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 49.Sundstrom E, Kolare S, Souverbie F, et al. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res. 1993;75:1–12. doi: 10.1016/0165-3806(93)90059-j. [DOI] [PubMed] [Google Scholar]
- 50.Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–85. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 51.Bayer SA, Altman J, Russo RJ, et al. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 52.Clancy B, Finlay BL, Darlington RB, et al. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cronise K, Marino MD, Tran TD, et al. Critical periods for the effects of alcohol exposure on learning in rats. Behav Neurosci. 2001;115:138–45. doi: 10.1037/0735-7044.115.1.138. [DOI] [PubMed] [Google Scholar]
- 54.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitaker-Azmitia PM. Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev. 1991;43:553–61. [PubMed] [Google Scholar]
- 56.Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- 57.Bar-Peled O, Gross-Isseroff R, Ben-Hur H, et al. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neurosci Lett. 1991;127:173–6. doi: 10.1016/0304-3940(91)90787-t. [DOI] [PubMed] [Google Scholar]
- 58.Toda T, Homma D, Tokuoka H, et al. Birth regulates the initiation of sensory map formation through serotonin signaling. Dev Cell. 2013;27:32–46. doi: 10.1016/j.devcel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Lidov HGW, Molliver ME. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- 60.Moll GH, Mehnert C, Wicker M, et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119:251–7. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 61.Sidor MM, Amath A, MacQueen G, et al. A developmental characterization of mesolimbocortical serotonergic gene expression changes following early immune challenge. Neuroscience. 2010;171:734–46. doi: 10.1016/j.neuroscience.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 62.Miquel MC, Kia HK, Boni C, et al. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res Dev Brain Res. 1994;80:149–57. doi: 10.1016/0165-3806(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 63.Dyck RH, Cynader MS. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. J Neurosci. 1993;13:4316–38. doi: 10.1523/JNEUROSCI.13-10-04316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Rood BD, Calizo LH, Piel D, et al. Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J Neurosci. 2014;34:4809–21. doi: 10.1523/JNEUROSCI.1498-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beique JC, Campbell B, Perring P, et al. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–17. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zavitsanou K, Wang H, Dalton VS, et al. Cannabinoid administration increases 5HT1A receptor binding and mrna expression in the hippocampus of adult but not adolescent rats. Neuroscience. 2010;169:315–24. doi: 10.1016/j.neuroscience.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura K, Sugawara Y, Sawabe K, et al. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci. 2006;26:530–4. doi: 10.1523/JNEUROSCI.1835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson GM, Hoder EL, Shaywitz BA, et al. Neurotransmitter precursors and metabolites in csf of human neonates. Dev Med Child Neurol. 1985;27:207–14. doi: 10.1111/j.1469-8749.1985.tb03771.x. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto H. Studies on CSF tryptophan metabolism in infantile spasms. Pediatr Neurol. 1991;7:411–4. doi: 10.1016/0887-8994(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 71.Constantino JN, Murphy DL. Monoamine metabolites in ‘leftover’ newborn human cerebrospinal fluid–a potential resource for biobehavioral research. Psychiatry Res. 1996;65:129–42. doi: 10.1016/s0165-1781(96)02976-9. [DOI] [PubMed] [Google Scholar]
- 72.Hyland K, Surtees RA, Heales SJ, et al. Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population. Pediatr Res. 1993;34:10–4. doi: 10.1203/00006450-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Langlais PJ, Walsh FX, Bird ED, et al. Cerebrospinal fluid neurotransmitter metabolites in neurologically normal infants and children. Pediatrics. 1985;75:580–6. [PubMed] [Google Scholar]
- 74.Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32:127–45. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 75.Chugani DC, Muzik O, Behen M, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–95. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 76.Rosa-Neto P, Benkelfat C, Sakai Y, et al. Brain regional alpha-[11C] methyl-l-tryptophan trapping, used as an index of 5-HT synthesis, in healthy adults: absence of an age effect. Eur J Nucl Med Mol Imaging. 2007;34:1254–64. doi: 10.1007/s00259-007-0365-x. [DOI] [PubMed] [Google Scholar]
- 77.Kornhuber J, Konradi C, Mack-Burkhardt F, et al. Ontogenesis of monoamine oxidase-A and -B in the human brain frontal cortex. Brain Res. 1989;499:81–6. doi: 10.1016/0006-8993(89)91136-0. [DOI] [PubMed] [Google Scholar]
- 78.Galineau L, Kodas E, Guilloteau D, et al. Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study. Neurosci Lett. 2004;363:266–71. doi: 10.1016/j.neulet.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–85. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morilak DA, Ciaranello RD. Ontogeny of 5-hydroxytryptamine2 receptor immunoreactivity in the developing rat brain. Neuroscience. 1993;55:869–80. doi: 10.1016/0306-4522(93)90447-n. [DOI] [PubMed] [Google Scholar]
- 81.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HT-TLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 84.Canli T, Congdon E, Gutknecht L, et al. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J Neural Transm. 2005;112:1479–85. doi: 10.1007/s00702-005-0391-4. [DOI] [PubMed] [Google Scholar]
- 85.Fakra E, Hyde LW, Gorka A, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders–a review. Ups J Med Sci. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernandez SP, Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. 2012;62:144–54. doi: 10.1016/j.neuropharm.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 89.Gutknecht L, Araragi N, Merker S, et al. Impacts of brain serotonin deficiency following TPH2 inactivation on development and raphe neuron serotonergic specification. PLoS ONE. 2012;7:e43157. doi: 10.1371/journal.pone.0043157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Migliarini S, Pacini G, Pelosi B, et al. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18:1106–18. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- 91.Savelieva KV, Zhao S, Pogorelov VM, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kriegebaum C, Song NN, Gutknecht L, et al. Brain-specific conditional and time-specific inducible TPH2 knockout mice possess normal serotonergic gene expression in the absence of serotonin during adult life. Neurochem Int. 2010;57:512–7. doi: 10.1016/j.neuint.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Yavarone MS, Shuey DL, Sadler TW, et al. Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta. 1993;14:149–61. doi: 10.1016/s0143-4004(05)80257-7. [DOI] [PubMed] [Google Scholar]
- 94.Côté F, Fligny C, Bayard E, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104:329–34. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: Specific effects or induction of general susceptibility? Psychol Bull. 2004;130:115–42. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- 96.Van den Hove DL, Leibold NK, Strackx E, et al. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur Neuropsychopharmacol. 2014;24:595–607. doi: 10.1016/j.euroneuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Parker MO, Annan LV, Kanellopoulos AH, et al. The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Prog Neuropsychopharmacol Biol Psychiatry. 2014 Mar. doi: 10.1016/j.pnpbp.2014.03.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sliwowska JH, Song HJ, Bodnar T, et al. Prenatal alcohol exposure results in long-term serotonin neuron deficits in female rats: modulatory role of ovarian steroids. Alcohol Clin Exp Res. 2014;38:152–60. doi: 10.1111/acer.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slotkin TA, MacKillop EA, Rudder CL, et al. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32:1082–97. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- 100.Williams SK, Lauder JM, Johns JM. Prenatal cocaine disrupts serotonin signaling-dependent behaviors: implications for sex differences, early stress and prenatal ssri exposure. Curr Neuropharmacol. 2011;9:478–511. doi: 10.2174/157015911796557957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21:415–20. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ichise M, Vines DC, Gura T, et al. Effects of early life stress on [11C] DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006;26:4638–43. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevens HE, Leckman JF, Coplan JD, et al. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:114–27. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- 104.Bennett AJ, Lesch KP, Heils A, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–22. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 105.Spinelli S, Chefer S, Carson RE, et al. Effects of early-life stress on serotonin(1A) receptors in juvenile rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–53. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berlin I, Heilbronner C, Georgieu S, et al. Reduced monoamine oxidase A activity in pregnant smokers and in their newborns. Biol Psychiatry. 2009;66:728–33. doi: 10.1016/j.biopsych.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 107.Takser L, Campagna D, Blot P, et al. Could monoamine plasma levels and erythrocyte membrane atpase activities at birth be predictive for future hand performance? Pediatr Res. 2003;54:358–63. doi: 10.1203/01.PDR.0000077484.55921.A0. [DOI] [PubMed] [Google Scholar]
- 108.Laine K, Heikkinen T, Ekblad U, et al. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–6. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- 109.Grigoriadis S, Vonderporten EH, Mamisashvili L, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ. 2014;348:f6932. doi: 10.1136/bmj.f6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riikonen RS, Nokelainen P, Valkonen K, et al. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-cit-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol Psychiatry. 2005;57:1565–72. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 111.Oberlander TF. Fetal serotonin signaling: setting pathways for early childhood development and behavior. J Adolesc Health. 2012;51:S9–16. doi: 10.1016/j.jadohealth.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Booij L, Benkelfat C, Leyton M, et al. Perinatal effects on in vivo measures of human brain serotonin synthesis in adulthood: a 27-year longitudinal study. Eur Neuropsychopharmacol. 2012;22:419–23. doi: 10.1016/j.euroneuro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Kannan S, Saadani-Makki F, Balakrishnan B, et al. Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J Cereb Blood Flow Metab. 2011;31:738–49. doi: 10.1038/jcbfm.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: ‘Much ado about nothing’? Psychol Med. 2010;40:717–29. doi: 10.1017/S0033291709991383. [DOI] [PubMed] [Google Scholar]
- 115.Fleisher WP, Schwartz L. Mental health sequelae of bullying: a review and case report. Can Child Adolesc Psychiatr Rev. 2003;12:13–7. [PMC free article] [PubMed] [Google Scholar]
- 116.Watt MJ, Burke AR, Renner KJ, et al. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic mono-amines as adults. Behav Neurosci. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vidal J, Bie J, Granneman RA, et al. Social stress during adolescence in wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–30. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 118.Marquez C, Poirier GL, Cordero MI, et al. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Capela JP, Carmo H, Remiao F, et al. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–71. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- 120.Booij L, Soucy J-P, Young SN, et al. Brain serotonin synthesis in MDMA (ecstasy) polydrug users: an α-[11C]-methyl-l-tryptophan study. J Neurochem. 2014 Jul 18; doi: 10.1111/jnc.12826. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 121.Piper BJ, Fraiman JB, Meyer JS. Repeated mdma (“ecstasy”) exposure in adolescent male rats alters temperature regulation, spontaneous motor activity, attention, and serotonin transporter binding. Dev Psychobiol. 2005;47:145–57. doi: 10.1002/dev.20085. [DOI] [PubMed] [Google Scholar]
- 122.Lopez-Rodriguez AB, Llorente-Berzal A, Garcia-Segura LM, et al. Sex-dependent long-term effects of adolescent exposure to THC and/or mdma on neuroinflammation and serotoninergic and cannabinoid systems in rats. Br J Pharmacol. 2014;171:1435–47. doi: 10.1111/bph.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klomp A, den Hollander B, de Bruin K, et al. The effects of ecstasy (MDMA) on brain serotonin transporters are dependent on age-of-first exposure in recreational users and animals. PLoS ONE. 2012;7:e47524. doi: 10.1371/journal.pone.0047524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009;1790:878–85. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 125.Booij L, Wang D, Levesque ML, et al. Looking beyond the DNA sequence: the relevance of DNA methylation processes for the stress-diathesis model of depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120251. doi: 10.1098/rstb.2012.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nestler EJ. Epigenetic mechanisms of depression. JAMA Psychiatry. 2014;71:454–6. doi: 10.1001/jamapsychiatry.2013.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis. 2010;39:66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 128.Razin A. Cpg methylation, chromatin structure and gene silencing — a three-way connection. EMBO J. 1998;17:4905–8. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and mecp2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–91. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 130.Nan X, Ng HH, Johnson CA, et al. Transcriptional repression by the methyl-cpg-binding protein mecp2 involves a histone deacetylase complex. Nature. 1998;393:386–9. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 131.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 132.Benvenisty N, Mencher D, Meyuhas O, et al. Sequential changes in DNA methylation patterns of the rat phosphoenolpyruvate carboxykinase gene during development. Proc Natl Acad Sci U S A. 1985;82:267–71. doi: 10.1073/pnas.82.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Razin A, Cedar H. DNA methylation and embryogenesis. EXS. 1993;64:343–57. doi: 10.1007/978-3-0348-9118-9_15. [DOI] [PubMed] [Google Scholar]
- 134.Sinclair KD, Allegrucci C, Singh R, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104:19351–6. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–49. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: Let’s call the whole thing off. Epigenetics. 2007;2:22–8. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- 137.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kinnally EL, Capitanio JP, Leibel R, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010;9:575–82. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beach SRH, Brody GH, Todorov AA, et al. Methylation at SLC6A4 is linked to family history of child abuse: an examination of the iowa adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:710–3. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beach SRH, Brody GH, Todorov AA, et al. Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the iowa adoptee sample. Psychosom Med. 2011;73:83–7. doi: 10.1097/PSY.0b013e3181fdd074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Devlin AM, Brain U, Austin J, et al. Prenatal exposure to maternal depressed mood and the mthfr c677t variant affect SLC6A4 methylation in infants at birth. PLoS ONE. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang HJ, Kim JM, Stewart R, et al. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:23–8. doi: 10.1016/j.pnpbp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 143.van IJzendoorn MH, Caspers K, Bakermans-Kranenburg MJ, et al. Methylation matters: Interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biol Psychiatry. 2010;68:405–7. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang D, Szyf M, Benkelfat C, et al. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE. 2012;7:e39501. doi: 10.1371/journal.pone.0039501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Booij L, Szyf M, Carballedo A, et al. The role of SLC6A4 DNA methylation in stress-related changes in hippocampal volume: a study in depressed patients and healthy controls [lecture]. 29th CINP World Congress of Neuropsychopharmacology meeting; 2014 June 22–26; Vancouver. [Google Scholar]
- 146.Ouellet-Morin I, Wong CC, Danese A, et al. Increased serotonin transporter gene (sert) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: a longitudinal study of discordant monozygotic twins. Psychol Med. 2013;43:1813–23. doi: 10.1017/S0033291712002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Côté SM, Boivin M, Nagin DS, et al. The role of maternal education and nonmaternal care services in the prevention of children’s physical aggression problems. Arch Gen Psychiatry. 2007;64:1305–12. doi: 10.1001/archpsyc.64.11.1305. [DOI] [PubMed] [Google Scholar]
- 148.Herba CM, Tremblay RE, Boivin M, et al. Maternal depressive symptoms and children’s emotional problems: Can early child care help children of depressed mothers? JAMA Psychiatry. 2013;70:830–8. doi: 10.1001/jamapsychiatry.2013.1361. [DOI] [PubMed] [Google Scholar]
- 149.Giles L, Davies M, Whitrow M, et al. Structured regression analyses of life course processes: an example exploring how maternal depression in early childhood affects children’s subsequent internalizing behavior. Ann Epidemiol. 2011;21:654–9. doi: 10.1016/j.annepidem.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 150.Lee LC, Halpern CT, Hertz-Picciotto I, et al. Child care and social support modify the association between maternal depressive symptoms and early childhood behaviour problems: a US national study. J Epidemiol Community Health. 2006;60:305–10. doi: 10.1136/jech.2005.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Booij L, Tremblay RE, Leyton M, et al. Brain serotonin synthesis in adult males characterized by physical aggression during childhood: a 21-year longitudinal study. PLoS ONE. 2010;5:e11255. doi: 10.1371/journal.pone.0011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Russo SJ, Murrough JW, Han MH, et al. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Croonenberghs J, Spaas K, Wauters A, et al. Faulty serotonin–DHEA interactions in autism: results of the 5-hydroxytryptophan challenge test. Neuroendocrinol Lett. 2008;29:385–90. [PubMed] [Google Scholar]
- 154.Guy J, Pelletier G, Bosler O. Serotonin innervation of neuropeptide y-containing neurons in the rat arcuate nucleus. Neurosci Lett. 1988;85:9–13. doi: 10.1016/0304-3940(88)90419-3. [DOI] [PubMed] [Google Scholar]
- 155.Porter RJ, Gallagher P, Watson S, et al. Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology (Berl) 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- 156.Champagne DL, Bagot RC, van Hasselt F, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–45. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Daskalakis NP, Claessens SE, Laboyrie JJ, et al. The newborn rat’s stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm Behav. 2011;60:165–76. doi: 10.1016/j.yhbeh.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 158.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–9. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 159.Melas PA, Rogdaki M, Lennartsson A, et al. Antidepressant treatment is associated with epigenetic alterations in the promoter of p11 in a genetic model of depression. Int J Neuropsychopharmacol. 2012;15:669–79. doi: 10.1017/S1461145711000940. [DOI] [PubMed] [Google Scholar]
- 160.Tremblay RE. Developmental origins of disruptive behaviour problems: the ‘original sin’ hypothesis, epigenetics and their consequences for prevention. J Child Psychol Psychiatry. 2010;51:341–67. doi: 10.1111/j.1469-7610.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 161.Provençal N, Suderman MJ, Guillemin C, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and t cells. J Neurosci. 2012;32:15626–42. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bulik CM, Prescott CA, Kendler KS. Features of childhood sexual abuse and the development of psychiatric and substance use disorders. Br J Psychiatry. 2001;179:444–9. doi: 10.1192/bjp.179.5.444. [DOI] [PubMed] [Google Scholar]
- 163.Tanaka KF, Ahmari SE, Leonardo ED, et al. Flexible accelerated stop tetracycline operator-knockin (fast): a versatile and efficient new gene modulating system. Biol Psychiatry. 2010;67:770–3. doi: 10.1016/j.biopsych.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Donaldson ZR, Piel DA, Santos TL, et al. Developmental effects of serotonin 1a autoreceptors on anxiety and social behavior. Neuropsychopharmacology. 2014;39:291–302. doi: 10.1038/npp.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sibon I, Benkelfat C, Gravel P, et al. Decreased [18F]MPPF binding potential in the dorsal raphe nucleus after a single oral dose of fluoxetine: a positron-emission tomography study in healthy volunteers. Biol Psychiatry. 2008;63:1135–40. doi: 10.1016/j.biopsych.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 166.Slotkin TA, Seidler FJ. Nicotine exposure in adolescence alters the response of serotonin systems to nicotine administered subsequently in adulthood. Dev Neurosci. 2009;31:58–70. doi: 10.1159/000207494. [DOI] [PubMed] [Google Scholar]