Abstract
Background
Oxytocin (OXT) plays a prominent role in social cognition and may have clinical applications for disorders such as autism, schizophrenia and social anxiety. The neural basis of its mechanism of action remains unclear.
Methods
We conducted a systematic literature review of placebo-controlled imaging studies using OXT as a pharmacological manipulator of brain activity.
Results
We identified a total of 21 studies for inclusion in our review, and after applying additional selection criteria, 11 of them were included in our fMRI voxel-based meta-analysis. The results demonstrate consistent alterations in activation of brain regions, including the temporal lobes and insula, during the processing of social stimuli, with some variation dependent on sex and task. The meta-analysis revealed significant left insular hyperactivation after OXT administration, suggesting a potential modulation of neural circuits underlying emotional processing.
Limitations
This quantitative review included only a limited number of studies, thus the conclusions of our analysis should be interpreted cautiously. This limited sample size precluded a more detailed exploration of potential confounding factors, such as sex or other demographic factors, that may have affected our meta-analysis.
Conclusion
Oxytocin has a wide range of effects over neural activity in response to social and emotional processing, which is further modulated by sex and task specificity. The magnitude of this neural activation is largest in the temporal lobes, and a meta-analysis across all tasks and both sexes showed that the left insula demonstrated the most robust activation to OXT administration.
Introduction
The role of oxytocin (OXT) in influencing social behaviour has been well established in animal research,1,2 but over the past decade it has been increasingly shown to be relevant in humans.3 Early research focused on its core role in parturition, milk ejection, sexual function and parenting, but recent studies have focused on its effects on social behaviour.1 The use of evolving technologies in animal models, such as OXT receptor agonists and antagonists, receptor knockouts and autoradiography, have all helped to identify analogous areas of interest for examination in human research2,4 and have contributed the basis for further investigation of the effects of OXT on human behaviour and social cognition.5
In behavioural studies OXT has been linked to social behaviours, such as trust and parental bonding. It has also been shown to increase the ability to identify emotions, increase empathy toward others and attenuate aversion to angry faces.2,6 Furthermore, its role in facilitating social interactions by ameliorating social bias and response to emotional faces has led it to be considered a possible drug to use in patients who have disorders with severe social deficits, such as autism and schizophrenia.7,8
The neurophysiological effects of OXT were initially investigated in animal models before testing in humans. Although OXT is produced and secreted in the same areas in both humans and animals, the receptor distribution can vary in a species-specific manner.5,9 Much of this variation may be due to species-specific differences in the way social stimuli are perceived and processed — with subsequent reorganization of neural connections in favour of more developed areas in each species. For example, in rats, the primary source of social input is through odour, whereas in humans it is based on visual and auditory cues and relies heavily on facial cues.2 Oxytocin is synthesized in the hypothalamic paraventricular parvocellular neurons (PVN) and the supraoptic nuclei (SON) and is then secreted by the posterior pituitary. Neurons from the PVN project to various areas in the limbic system (hippocampus, amygdala, striatum, hypothalamus and nucleus accumbens), which are involved in social cognition.5 With this in mind, recent neuroimaging techniques have allowed scientists to investigate in vivo the neurophysiological correlates of the effect of OXT. By examining fluctuations in the hemodynamic activity in the brain using fMRI after the administration of OXT compared with the administration of a placebo, the network and regions that contribute to OXT’s influence over brain activity have become clearer.
The present systematic review and meta-analysis aims to provide an integrative and comprehensive review of placebo-controlled neuroimaging studies of OXT. We report the magnitude of change in activation of various brain regions as a result of OXT administration versus a placebo during tasks related to social behaviour by computing its effect sizes in the limbic system, including the amygdala; the reward system; the frontal lobe; and the temporal lobe. Finally, we performed a voxel-based meta-analysis of the effect of OXT administration on the human brain, as addressed by various fMRI studies. We also performed an examination of animal studies, which is presented in the Appendix, available at jpn.ca.
Methods
Search strategies
We used a systematic search strategy following the PRISMA guidelines for systematic reviews10 to identify relevant studies for inclusion in our review. Initially, we searched Embase, Medline and PsychInfo to identify relevant studies (published from 1806 up to Feb. 7, 2013). To be included, each study had to be an original study using OXT as a pharmacological manipulator of brain activity. Since our review was intended to cover all types of brain imaging, search parameters were expanded to include different brain imaging techniques. Search terms included “oxytocin,” “magnetic resonance imaging,” “MRI,” “fMRI,” “magnetoencephalography,” “MEG,” “electroencephalography,” “EEG,” “positron emission tomography” and “PET.”
The bibliographies in the retrieved articles were also manually searched to uncover relevant papers that may have been missed. Although no limits were placed on the language for an article, all articles found that fit our selection criteria were published in English. No articles were found for magnetoencephalography (MEG) or positron emission tomography (PET) research using OXT as a pharmacological probe. Studies were included only if they were an original publication in a peer-reviewed journal.
Selection criteria
We included only the studies that explored the effects of intranasal administration of OXT in a placebo-controlled double-blind design and used functional neuroimaging or electrophysiological techniques. Studies that used a patient population but also reported findings for healthy controls were included if they satisfied all other criteria. Exclusion criteria were postmortem studies, structural imaging techniques, studies looking at only endogenous OXT and studies on vasopressin or OXT antagonists, as these studies did not examine how OXT administration affected brain activity. Only intranasal administration in humans was explored, as this is the most common method of administering OXT in current imaging studies and there is a body of research supporting its influence over brain regions by potentially increasing its concentration in the cerebrospinal fluid (CSF).11,12
For the whole brain fMRI voxel-based meta-analysis, further criteria were applied. Only studies using emotional stimuli in healthy controls and reporting the peak coordinates of homogeneously thresholded OXT effects at the whole-brain level were included, as there were not enough studies using social interactions alone to allow for an additional comparison.
Systematic review
For each retrieved study we recorded the following variables: number of participants, study design, sex, dosage of OXT, the tasks implemented, major findings, areas attenuated or activated by OXT administration and their corresponding coordinates, contrasts and statistical values.
Results are comprehensively reported in tables. In particular, we discuss the core findings with respect to different brain areas of interest — the basal ganglia, insula, thalamus, temporal lobes, prefrontal cortex (PFC) and amygdala — as these are the areas most commonly reported in studies using OXT.
Effect sizes
We analyzed the magnitude of the OXT effects (effect sizes) on the regions of interest (ROIs). Where sufficient information was provided in a study to assess the significance of the results (e.g., the presence of t values, p values, F values, or means and standard deviations), we calculated the effect size.
Studies that fit the criteria for effect size analysis accounted for 8 of the 15 studies reporting modulation from OXT aside from connectivity measures and 12 of the 15 studies when looking at the amygdala alone. The effect size is a dimensionless number that facilitates the integration of findings across studies that used different types of measurements. The choice of effect size estimator is a much debated and still unresolved issue13 and is related to the choice of whether or not greater reliance should be laid on studies carried out on larger samples when the effect size is to be computed. We chose to use an effect size estimator corrected for the number of participants included in each study using the Cohen d statistic14 generated by the SPSS program. We calculated these values because most studies had fairly small samples. When the power of a study is insufficient to show statistically significant differences between or within groups, even when such is the case in the population, type II errors occur. By examining effect sizes rather than statistical significance, we can better understand what differences exist in the general population and whether these differences might merit further study. Effect size (d) is taken to mean “the degree to which a phenomenon is present in the population”; we indexed d according to Cohen’s scheme.14 Cohen placed the value of d for small effects at 0.2, for medium effects at 0.5 and for large effects at 0.8.
Voxel-based meta-analysis
Meta-analysis of the OXT effects at the whole brain level was conducted using effect size signed differential mapping (ES-SDM, www.sdmproject.com/),15,16 a technique that has already been applied to the study of both healthy controls17 and to many individuals with neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder (ADHD),18 anxiety,19 autism20 and schizophrenia.21,22 Briefly, we used the information reported in the papers about the brain peaks of maximum OXT effects to recreate a statistical parametric map for each study. We then conducted a standard meta-analysis to obtain a meta-analytic brain map of the effects of OXT. First, peak coordinates were converted to Talairach space, and their t values were converted into effect sizes and their standard errors (i.e., accounting for study precision and sample size). It must be noted that SDM software was modified to account for the fact that some of the studies had conducted 1-sample tests, whereas others had conducted 2-sample tests. Second, each peak was used to recreate a cluster of voxels with significant OXT effects by assigning an effect size to the voxels close to the peak, taking the distance of each voxel to the different close peaks into account. Finally, the different recreated maps were voxel-wise combined by fitting random-effects models, and p values were derived from a permutation test. Results were thresholded with a voxel p ≤ 0.005, peak z ≥ 2 and cluster extent ≥ 10 voxels. For further details, see the study by Radua and Mataix-Cols.16
This analysis was complemented with 2 others to assess the robustness of the meta-analytic findings. First, we conducted a jack-knife test, which consisted of repeating the meta-analysis many times, each time including all studies but 1, to infer the replicability of the findings. Second, funnel plots were drawn from the meta-analytic peaks to discard publication bias and gross abnormalities. Potential publication biases were further assessed with Egger tests.
Results
Database
Our literature search uncovered 21 papers that met our inclusion criteria. Details of the retrieved studies are given in Table 1.
Table 1.
Overview of included studies
| Technique, study | Sample size* | Within/between | Sex | OXT dose, IU | Task used | Brain areas analyzed | Effects of OXT |
|---|---|---|---|---|---|---|---|
| fMRI | |||||||
| Baumgartner et al.23 | 49 (23 PBO, 26 OXT) | Between | M | 24 | Trust game | Amygdala, brainstem, caudate, putamen, insula, thalamus | Increased trust in partner after trust had been violated |
| Domes et al.24 | 13 | Within | M | 24 | Implicit facial emotion processing by identifying sex of fearful, angry and happy faces | Amygdala, temporal pole, TPJ, thalamus, PFC | Increased ability to identify emotions |
| Domes et al.25 | 16 | Within | F | 24 | Explicit facial emotion processing by rating arousal of fearful, angry and happy faces | Amygdala, brainstem, temporal pole, STG, fusiform gyrus, insula, PFC, thalamus | Greater arousal in rating facial emotions |
| Gamer et al.26 | 46 (23 PBO, 23 OXT) | Between | M | 24 | Explicit facial emotion processing by classifying emotion of fearful and happy faces | Amygdala, superior colliculus | Increased ability to identify emotions; gaze starts at mouth and is redirected to eyes for longer period |
| Kirsch et al.27 | 15 | Within | M | 27 | Implicit facial emotion processing by matching fearful and angry faces | Amygdala, brainstem | Increased ability to identify emotion |
| Labuschagne et al.28†‡ | 18 | Within | M | 24 | Implicit facial processing by matching angry, fearful, happy and neutral faces | Amygdala | Insignificant decreases in amygdala activity |
| Labuschagne et al.29† | 18 | Within | M | 24 | Explicit facial processing using emotional classification of sad, happy and neutral faces | mPFC, ACC, thalamus, STG | General attenuation in areas associated with processing social stimuli, cognitive control and emotion regulation |
| Lischke et al.30 | 14 | Within | F | 2 | Explicit processing by rating emotional arousal of negative, positive and neutral scenes | Amygdala, temporal pole, fusiform gyrus | Increased threat sensitivity in the amygdala to scenes depicting social and nonsocial threat; no changes in eye tracking |
| Petrovic et al.31 | 27 (12 PBO, 15 OXT) | Between | M | 32 | Aversely conditioned face processing by rating likeability of faces that were previously paired with a negative experience | Amygdala, ACC, vlPFC, fusiform area | Increased likeability of faces even after being linked to aversive stimuli |
| Pincus et al.32 | 9 | Within | 1M/8F | 40 | Explicit emotional processing using Reading of the Mind in the Eyes test by emotional classification | Caudate, amygdala, ACC, STG, GP | OXT increased activity in areas associated with reward and processing of social stimuli |
| Riem et al.33§ | 42 (21 PBO, 21 OXT) | Between | F | 24 | Auditory exposure to babies crying | Amygdala, insula | Decrease in amygdala to sound clips of crying babies |
| Riem et al.34 | 42 (20 PBO, 22 OXT) | Between | F | 16¶ | Auditory exposure to infant laughter | Connectivity between the amygdala and the OFC, ACC, hippocampus, precuneus, supramarginal gyri and MTG | Increased functional connectivity between the amygdala and other areas involved in regulating emotion and decrease in amygdala when listening to laughter over control noise |
| Rilling et al.35 | 60 (34 PBO, 26 OXT) | Between | M | 24 | Altruistic interaction | Amygdala, caudate, ventral PFC, insula, putamen | Differences in activity in reciprocated and unreciprocated cooperativism; increase in amygdala only in reciprocated interaction |
| Singer et al.36 | 20 | Between | M | 32 | Empathy for pain and experience of pain | PFC, OFC, amygdala | No significant observations in response to viewing their partner in pain; reduced amygdala activation when receiving painful stimulation |
| Sripada et al.37 | 15 | Within | M | 24 | Resting state connectivity | Amygdala to ACC/rmFC | Increased connectivity during resting state |
| Striepens et al.38 | 70 (35 PBO, 35 OXT) | Between | M | 24 | Implicit processing of social scenes using a memory task with negative and neutral pictures paired with nouns | Insula, amygdala | Increased memory for negative pictures at the expense of neutral stimuli; accompanied by an increase in the left insula and changes in connectivity between the insula and amygdala |
| Wittfoth-Schardt et al.39 | 21 | Within | M | 24 | Implicit facial processing of familiar and unfamiliar faces | GP, MTG, caudate | Reduced activity and functional connectivity to the GP from reward-related regions to pictures of own child and unknown child |
| EEG | |||||||
| Born et al.40 | 17 | Within | M | 40 | Auditory mismatch paradigm | N2 and P3 | No effect of OXT |
| Fehm-Wolfsdorf et al.41 | 30 | Between | M | 20 | Memory recall of 25 nouns, tone counting | Auditory evoked potentials | No effect of OXT on memory or auditory stimuli |
| Huffmeijer et al.42 | 47 | Between | F | 15.6 | Altruistic interaction | Frontal asymmetry | Increased donations from OXT corresponded with decrease in left/increase in right frontal activity |
| Huffmeijer et al.43 | 48 | Within | F | 16 | Combination of feedback processing and facial processing | VPP and LPP | More positive VPP and LPP after OXT, which was heightened more for those experiencing less love withdrawal |
| Perry et al.44 | 24 | Within | M | 24 | Point light experiment to mimic biological and nonbiological motion | μ rhythms | Better able to identify biological movement, which corresponded to μ suppression across the whole scalp |
ACC = anterior cingulate cortex; EEG = electroencephalography; F = female; fMRI = functional magnetic resonance imaging; GP = globus pallidum; LPP = late positive potential; M = male; mPFC = medial prefrontal cortex; MTG = middle temporal gyrus; OXT = oxytocin; PBO = placebo; PFC = prefrontal cortex; rmFC = rostromedial frontal cortex; STG = superior temporal gyrus; TPJ = temporoparietal junction; vlPFC = ventrolateral prefrontal cortex; VPP = vertex positive potential; VTA = ventral tegmental area.
Sample size incudes only the participants in the OXT and PBO samples. Other participants may have been included in other arms of the study.
Also included 18 patients with generalized anxiety disorder, but only results from healthy controls are reported.
Excluded from analysis other than that of the amygdala because no direction of effects were reported.
Also included patients with depression, but only results from healthy controls are reported.
Originally reported as 20 IU, but a subsequent erratum confirmed it was 16 IU.
To make the anatomic labelling of the relevant areas reported more consistent, all coordinates from each study were converted to Talairach coordinates using icbm2tal for the appropriate template (i.e., SPM, FSL).45 These coordinates were then entered into Talairach client46 to create a standardized list of areas reported by each study. When a study reported a different area, this was noted as shown in Table 2 and is referenced throughout the systematic review.
Table 2.
Oxytocin-related results from fMRI studies in the systematic review
| Study | Sample size | Sex | OXT dose, IU | Task, contrast | Effect of OXT* | WB/ROI |
|---|---|---|---|---|---|---|
| Baumgartner et al.23 | 49 | M | 24 | Trust interaction as trustee with human and risk interaction with a computer | ||
| Trust feedback | ↓ L/R amygdala | WB | ||||
| ↓ L brainstem | WB | |||||
| ↓ L/R caudate | WB | |||||
| ↓ L postcentral gyrus (superior parietal lobe) | WB | |||||
| ↓ L putamen/insula | WB | |||||
| Trust prefeedback | ↑ R thalamus/pulvinar | WB | ||||
| ↓ R ACC | WB | |||||
| Domes et al.24 | 13 | M | 24 | Implicit facial processing by indicating sex of happy, angry, fearful and neutral faces | ||
| Implicit facial processing (fearful > neutral) | ↓ L paracentral gyrus | WB | ||||
| ↓ L frontal medial lobe (MFG) | WB | |||||
| ↓ L medulla | WB | |||||
| ↓ anterior cerebellum/culmen | WB | |||||
| ↓ L inferior temporal lobe (MTG) | WB | |||||
| ↓ L MTG | WB | |||||
| Implicit facial processing (angry > neutral) | ↓ L thalamus/pulvinar | WB | ||||
| ↓ L postcentral gyrus (paracentral lobe) | WB | |||||
| ↓ R precentral gyrus (postcentral gyrus) | WB | |||||
| Implicit facial processing (happy > neutral) | ↓ L/R inferior temporal lobe (STG) | WB | ||||
| ↓ L paracentral gyrus | WB | |||||
| ↓ L MTG | WB | |||||
| Domes et al.25 | 16 | F | 24 | Explicit facial processing by rating emotional arousal of fearful, angry, happy and neutral faces | ||
| Explicit facial processing (fearful > neutral) | ↑ L MTG (temporal pole) | WB | ||||
| ↑ L amygdala (subcallosal gyrus) | WB | |||||
| ↑ L/R fusiform gyrus (L cerebullum/R parahippocampal gyrus) | WB | |||||
| ↑ L STG | WB | |||||
| ↑ L insula | WB | |||||
| ↑ R cerebellum | WB | |||||
| ↑ R brainstem (SN) | WB | |||||
| ↓ R dlPFC (SFG) | WB | |||||
| Explicit facial processing (angry > neutral) | ↑ L rolandic operculum (insula) | WB | ||||
| ↑ R rolandic operculum (thalamus) | WB | |||||
| ↑ R dlPFC (MFG) | WB | |||||
| ↑ L/R vlPFC (MFG) | WB | |||||
| Explicit facial processing (happy > neutral) | ↑ L MTG (temporal pole) | WB | ||||
| ↑ R hippocampus (amygdala) | WB | |||||
| ↑ L STG (transverse temporal gyrus) | WB | |||||
| ↑ L fusiform gyrus (cerebellum) | WB | |||||
| ↑ L insula (postcentral gyrus) | WB | |||||
| ↑ R rolandic operculum (precentral gyrus) | WB | |||||
| ↑ R cerebellum | WB | |||||
| ↓ R dlPFC (SFG) | WB | |||||
| Gamer et al.26 | 46 | M | 24 | Explicit facial processing by emotional classification of fearful, happy and neutral faces | ||
| Explicit facial processing (fearful > neutral) | ↓ L/R amygdala | ROI | ||||
| Gazing toward eyes | ↑ R posterior amygdala | ROI | ||||
| Gazing toward mouth | ↑ L/R superior colliculus (cerebellum) | WB | ||||
| Kirsch et al.27 | 14 | M | 27 | Implicit emotional processing by matching fearful/threatening scenes and angry/fearful faces or shapes | ||
| All social stimuli, but greater for faces | ↓ L amygdala | ROI | ||||
| Connectivity measures | ↓ L amygdala, L/R brainstem | ROI/ROI | ||||
| Labuschagne et al.29† | 18 | M | 24 | Explicit facial processing using emotional classification of sad, happy and neutral faces | ||
| Explicit facial processing (sad > neutral) | ↓ R ACC | WB | ||||
| ↓ R MFG | WB | |||||
| ↓ R supplementary motor cortex (MFG) | WB | |||||
| ↓ L superior parietal cortex (precuneus) | WB | |||||
| ↓ L thalamus | WB | |||||
| Explicit facial processing (happy > neutral) | ↓ L cerebellum | WB | ||||
| ↓ L cerebellum/fusiform | WB | |||||
| ↓ L calcarine fissure/cerebellum | WB | |||||
| ↓ R mPFC (MFG) | WB | |||||
| ↓ L MFG | WB | |||||
| ↓ L precuneus (cuneus) | WB | |||||
| ↑ R STG (IFG) | WB | |||||
| Lischke et al.30 | 14 | F | 24 | Explicit processing by rating emotional arousal of negative, positive and neutral social scenes | ||
| Explicit social processing (negative > neutral scenes) | ↑ L/R amygdala (L/R putamen) | ROI | ||||
| ↑ L MTG | WB | |||||
| ↑ L ITG (fusiform gyrus) | WB | |||||
| ↑ L temporal pole, STG | WB | |||||
| ↑ L postcentral gyrus | WB | |||||
| Explicit social processing (positive > neutral scenes) | ↓ R supplementary motor cortex (SFG) | WB | ||||
| Petrovic et al.31 | 27 | M | 32 | Conditioned neutral faces with direct and indirect gaze to aversive stimuli (implicit processing) | ||
| Fear conditioning (main effect) | ↓ R anterior MTL (MFG) | ROI | ||||
| ↓ R vmPFC (ACC) | WB | |||||
| ↓ L lateral OFC (IFG) | WB | |||||
| ↓ R ACC | WB | |||||
| ↓ R rostral ACC (MFG) | WB | |||||
| ↓ L vlPFC (IFG) | WB | |||||
| Fear conditioning for direct gaze | ↓ R amygdala (subcallosal gyrus) | ROI | ||||
| ↓ L/R caudal ACC | WB | |||||
| ↓ R subgenual ACC | WB | |||||
| ↑ L vlPFC (MFG) | WB | |||||
| ↓ R vlPFC (MFG) | WB | |||||
| ↓ R fusiform face area | WB | |||||
| Pincus et al.32‡ | 9 | M/F | 40 | Explicit facial processing using Reading of the Mind in the Eyes test and emotional classification | ||
| (1M/8F) | All stimuli | ↑ L caudate | WB | |||
| ↑ R parahippocampal gyrus | WB | |||||
| ↑ R amygdala | WB | |||||
| ↑ R IFG (MFG) | WB | |||||
| ↑ L lentiform nucleus (GP) | WB | |||||
| ↑ L ACC | WB | |||||
| ↑ R parahippocampal gyrus | WB | |||||
| ↑ L caudate (parahippocampal gyrus) | WB | |||||
| ↑ L STG (MTG) | WB | |||||
| ↑ L STG (MTG) | WB | |||||
| ↑ L ACC (subcallosal gyrus) | WB | |||||
| ↑ L ACC (MFG) | WB | |||||
| Riem et al.33 | 42 | F | 24 | Auditory exposure to babies crying | ||
| Implicit emotional processing (infant’s cry > control noise) | ↓ R amygdala (GP) | ROI | ||||
| ↑ L planum polare (STG) | WB | |||||
| ↑ R IFG (Insula) | WB | |||||
| Riem et al.34 | 42 | F | 16 | Auditory exposure to infant laughter | ||
| Implicit emotional processing (infant’s laughter > control noise) | ↓ L/R amygdala | ROI | ||||
| Connectivity (laughter > control noise) | ↑ R amygdala, L hippocampus | ROI/WB | ||||
| ↑ R amygdala, L precuneus | ROI/WB | |||||
| ↑ R amygdala, R MTG | ROI/WB | |||||
| ↑ R amygdala, R parahippocampal gyrus | ROI/WB | |||||
| ↑ R amygdala, L OFC | ROI/WB | |||||
| ↑ R amygdala, L angular gyrus | ROI/WB | |||||
| ↑ R amygdala, L ACC | ROI/WB | |||||
| ↓ R amygdala, R lateral occipital cortex | ROI/WB | |||||
| ↑ L amygdala, ACC | ROI/WB | |||||
| ↑ L amygdala, R lateral occipital cortex | ROI/WB | |||||
| Rilling et al.35 | 60 | M | 24 | Altruistic interaction using the prisoner’s dilemma | ||
| Reciprocated cooperation | ↑ L caudate (parahippocampal gyrus) | WB | ||||
| ↑ L amygdala (parahippocampal gyrus) | WB | |||||
| ↑ R putamen/insula | WB | |||||
| Unreciprocated cooperation | ↑ L vlPFC (MFG) | WB | ||||
| ↑ L vmPFC (MFG) | WB | |||||
| Connectivity | ↑ R amygdala, R MTG/STG | ROI/WB | ||||
| ↑ R amygdala, R IFG | ROI/WB | |||||
| ↑ R amygdala, L MFG/postcentral gyrus | ROI/WB | |||||
| ↑ R amygdala, L anterior insula/IFG | ROI/WB | |||||
| ↑ R amygdala, L planum polare/STG | ROI/WB | |||||
| ↑ R amygdala, L MTG | ROI/WB | |||||
| ↓ R amygdala, L/R cerebellum | ROI/WB | |||||
| ↓ R amygdala, R superior parietal lobe | ROI/WB | |||||
| ↓ R amygdala, L STG/MTG | ROI/WB | |||||
| ↓ R amygdala, R Cuneus | ROI/WB | |||||
| ↓ R amygdala, L MFG | ROI/WB | |||||
| Singer et al.36 | 20 | M | 32 | Empathetic responses to pain inflicted in self and others | ||
| Pain inflicted in self | ↑ R mOFC (IFG) | WB | ||||
| ↑ R lOFC (MFG) | WB | |||||
| ↓ R amygdala/striatum (GP) | ROI | |||||
| Pain inflicted in self (prosocial > selfish) | ↓ L frontoinsular PFC (MFG) | WB | ||||
| ↓ L mPFC (MFG) | WB | |||||
| Pain inflicted in self (selfish > prosocial) | ↓ L amygdala | WB | ||||
| ↓ R amygdala (parahippocampal gyrus) | ROI | |||||
| Pain inflicted in other | ↓ R OFC (IFG) | WB | ||||
| Sripada et al.37 | 15 | M | 24 | Resting state connectivity | ↑ R amygdala, ACC/rmFC | ROI/WB |
| Striepens et al.38 | 70 | M | 24 | Implicit processing of social scenes using a memory task with negative and neutral pictures paired with nouns | ||
| Later remembered negative > later forgotten neutral | ↑ L anterior insula (IFG) | WB | ||||
| Negative and neutral > baseline | ↑ R amygdala | ROI | ||||
| Negative > baseline | ↑ R amygdala | ROI | ||||
| Neutral > baseline | ↑ R amygdala | ROI | ||||
| Connectivity (all stimuli) | ↓ L amygdala, L ACC | ROI/WB | ||||
| Connectivity (negative > neutral stimuli) | ↓ R amygdala, L insula | ROI/WB | ||||
| ↑ L anterior insula, L IFG | ROI/WB | |||||
| ↑ L anterior insula, L basolateral amygdala | ROI/WB | |||||
| Wittfoth-Schardt et al.39 | 21 | M | 24 | Implicit facial processing where fathers viewed pictures of their own children, a familiar and unfamiliar child | ||
| Implicit facial processing (own child > familiar child) | ↓ L GP | ROI | ||||
| Implicit facial processing (unfamiliar child > familiar child) | ↓ L GP (putamen) | ROI | ||||
| ↓ L precentral gyrus | WB | |||||
| ↓ L hippocampus (amygdala) | ROI | |||||
| ↓ L/R MTG (L STG/R ITG) | WB | |||||
| ↓ L STG | WB | |||||
| ↓ L supramarginal gyrus (inferior parietal lobe) | WB | |||||
| Implicit facial processing (own child > unfamiliar child) | ↑ L caudate | WB | ||||
| Connectivity (own child > familiar child) | ↓ L GP, R GP | ROI/WB | ||||
| ↓ L GP, L MFG | ROI/WB | |||||
| ↓ L GP, L hippocampus | ROI/WB | |||||
| ↓ L GP, R superior parietal lobe | ROI/WB |
ACC = anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; F = female; GP = globus pallidum; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; lOFC = lateral orbitofrontal cortex; M = male; MFG = medial frontal gyrus; mOFC = medial orbitofrontal cortex; mPFC = medial prefrontal cortex; MTG = middle temporal gyrus; OFC = orbitofrontal cortex; OXT = oxytocin; PFC = prefrontal cortex; rmPFC = rostromedial prefrontal cortex; ROI = region of interest; SN = substantia nigra; STG = superior temporal gyrus; vlPFC = ventrolateral prefrontal cortex; WB = whole brain analysis.
Areas in italics represent areas that were different when the coordinates were entered into Talairach client; all other areas are those reported by the studies listed.
Also included patients with social anxiety, but only results for healthy controls are reported.
Also included patients with depression, but only results for healthy controls are reported.
Sixteen of the studies used fMRI and 5 used electroencephalography (EEG) to explore the effects of OXT. The voxel-based meta-analysis in fMRI studies included a total of 11 studies. Details of the literature search are described in the PRISMA flowchart in Figure 1.
Fig. 1.
Study selection process. OXT = oxytocin.
Systematic review
The present systematic review aims to elucidate the effect of OXT in various regions of the brain and their corresponding networks. The specific brain areas modulated by OXT in each study are detailed in Table 2, and further details on the differences between OXT receptors and its administration between humans and animals can be found in the Appendix. We also assessed the magnitude of OXT effects by providing the Cohen d for specific brain areas (Table 3).
Table 3.
Effect of OXT on parts of the human brain*
| Part of brain; study | OXT dose, IU | Sex | Task | Contrast | Left | Cohen d | Right | Cohen d | Area (Talairach) or ROI/WB |
|---|---|---|---|---|---|---|---|---|---|
| Insula | |||||||||
| Baumgartner et al.23 | 24 | M | Trust interaction | Trust game postfeedback | ↓ | 0.92 | ← → | — | |
| Domes et al.25 | 24 | F | Explicit facial processing | Angry > neutral† | ↑ | ← → | — | ||
| Fearful > neutral | ↑ | ← → | — | ||||||
| Happy > neutral‡ | ↑ | ← → | — | ||||||
| Riem et al.33 | 24 | F | Listening to infant crying | Infant cry > control noise† | ← → | — | ↑ | ||
| Rilling et al.35 | 24 | M | Trust interaction | Reciprocated cooperation | ← → | — | ↑ | 0.91 | |
| Striepens et al.38 | 24 | M | Implicit processing of social scenes | Later remembered negative > later forgotten neutral‡ | ↓ | 1.47 | ← → | — | |
| Thalamus | |||||||||
| Baumgartner et al.23 | 24 | M | Trust interaction | Trust game prefeedback | ← → | — | ↓ | 1.03 | |
| Domes et al.24 | 24 | M | Implicit facial processing | Fearful > neutral | ↓ | 1.69 | ← → | — | |
| Domes et al.25 | 24 | F | Explicit facial processing | Angry > neutral† | ↑ | ← → | — | ||
| Labuschagne et al.29 | 24 | M | Explicit facial processing | Sad > neutral | ↑ | 1.27 | ← → | — | |
| Basal ganglia | |||||||||
| Baumgartner et al.23 | 24 | M | Trust interaction | Trust game postfeedback | ↓ | 0.81 | ↓ | 0.90 | Caudate |
| ← → | — | ↓ | 0.84 | Caudate | |||||
| ← → | — | ↓ | 0.84 | Caudate | |||||
| ↓ | 0.92 | ← → | — | Putamen | |||||
| Domes et al.25 | 24 | F | Explicit facial processing | Fearful > neutral† | ← → | — | ↑ | Brainstem (SN) | |
| Lischke et al.30 | 24 | M | Emotion arousal rating task | Negative > neutral scenes‡ | ← → | — | ↑ | Putamen (ROI) | |
| Pincus et al.32 | 40 | M/F | Explicit facial processing | Across all facial valances Across all facial valances‡ | ↓ | 1.97 | ← → | — | Caudate |
| ↓ | 2.12 | ← → | — | Caudate | |||||
| ↓ | 1.86 | ← → | — | GP | |||||
| Rilling et al.35 | 24 | M | Trust interaction | Reciprocated cooperation | ↓ | 1.03 | ← → | — | Caudate |
| ← → | — | ↑ | 0.91 | Putamen | |||||
| Wittfoth-Schardt et al.39 | 24 | M | Viewing familiar or unfamiliar child | Own child > unfamiliar child | ↓ | ← → | — | Caudate | |
| Own child > familiar child | ↓ | ← → | — | GP | |||||
| Unfamiliar child > familiar child‡ | ↓ | ← → | — | GP (Putamen) | |||||
| Temporal lobes | |||||||||
| Domes et al.24 | 24 | M | Implicit facial processing | Fearful > neutral | ↓ | 1.44 | ← → | — | Inferior temporal lobe |
| ↓ | 1.38 | ← → | — | MTG | |||||
| Happy > neutral | ↓ | 1.45 | ↓ | 1.44 | STG | ||||
| ↓ | 1.38 | ← → | — | MTG | |||||
| Domes et al.25 | 24 | F | Explicit facial processing | Fearful > neutral | ↑ | ← → | — | MTG (temporal pole) | |
| ← → | — | STG fusiform gyrus | |||||||
| Happy > neutral | ↑ | ← → | — | MTG (temporal pole) | |||||
| ↑ | ← → | — | STG | ||||||
| ↑ | ← → | — | Fusiform gyrus | ||||||
| Labuschagne et al.29 | 24 | M | Explicit facial processing | Happy > neutral | ← → | — | ↑ | 1.67 | STG |
| Lischke et al.30 | 24 | F | Emotional arousal rating task | Negative > neutral scenes | ↓ | ← → | — | STG (temporal pole) | |
| ↑ | ← → | — | MTG | ||||||
| ↓ | ← → | — | ITG (fusiform gyrus) | ||||||
| Petrovic et al.31 | 32 | M | Fear conditioning to faces | Main effect | ↓ | 1.49 | ← → | — | MTG |
| Fear conditioning with direct gaze | ← → | — | ↓ | 1.49 | Fusiform gyrus | ||||
| Pincus et al.32 | 40 | M/F | Explicit facial processing | ↓ | 1.97 | ← → | — | STG (MTG) | |
| ↑ | 1.85 | ← → | — | STG (MTG) | |||||
| Riem et al.33 | 24 | F | Listening to infant crying | Infant cry > control noise | ↓ | ← → | — | Planum polare (STG) | |
| Wittfoth-Schardt et al.39 | 24 | M | Viewing familiar or unfamiliar child (implicit facial processing) | Unfamiliar child > familiar child | ↓ | ↓ | MTG | ||
| ↓ | ← → | — | STG | ||||||
| PFC | |||||||||
| Domes et al.24 | 24 | M | Implicit facial processing | Fearful > neutral | ↓ | 1.38 | ← → | — | MFG (dlPFC) |
| Domes et al.25 | 24 | F | Explicit facial processing | Fearful > neutral | ← → | — | ↓ | dlPFC | |
| Angry > neutral | ↑ | ↓ | vlPFC | ||||||
| ← → | — | ↑ | dlPFC | ||||||
| Happy > neutral | ← → | — | ↓ | dlPFC | |||||
| Labuschagne et al.29 | 24 | M | Explicit facial processing | Sad > neutral | ← → | — | ↓ | 1.55 | MFG |
| Happy > neutral | ↓ | 1.39 | ← → | — | MFG | ||||
| ← → | — | ↓ | 1.29 | mPFC | |||||
| ← → | — | ↓ | 1.21 | mPFC | |||||
| Petrovic et al.31 | 32 | M | Fear conditioning | Main effect | ← → | — | ↓ | 1.66 | vlPFC |
| Direct gaze | ↓ | 1.90 | ↓ | 2.02 | vlPFC | ||||
| Pincus et al.32 | 40 | M/F | Explicit facial processing | ← → | — | ↓ | 1.87 | IFG | |
| Rilling et al.35 | 24 | M | Trust interaction | Unreciprocated cooperation | ↓ | 0.92 | ← → | — | vlPFC |
| ↓ | 0.92 | ← → | — | vmPFC | |||||
| Singer et al.36 | 32 | M | Observing pain inflicted in self and others | Pain in self | ← → | — | ↓ | mOFC | |
| ← → | — | ↓ | lOFC | ||||||
| Pain inflicted in self (prosocial > selfish) | ↓ | ← → | — | Frontoinsular PFC | |||||
| ↓ | ← → | — | mPFC | ||||||
| Pain inflicted in other | ← → | — | ↓ | OFC | |||||
| Amygdala | |||||||||
| Baumgartner et al.23 | 24 | M | Trust interaction | Trust game postfeedback | ↓ | — | ↓ | — | WB |
| Domes et al.24 | 24 | M | Implicit facial processing | Fearful > neutral | ↓ | — | ↓ | 0.91 | ROI |
| Angry > neutral | ↓ | — | ↓ | 1.05 | ROI | ||||
| Happy > neutral | ↓ | — | ↓ | 0.91 | ROI | ||||
| Domes et al.25 | 24 | F | Explicit facial processing | Fearful > neutral | ↑ | 0.79 | ← → | — | WB |
| Happy > neutral† | ↓ | — | ↑ | 0.39 | WB | ||||
| Gamer et al.26 | 24 | M | Explicit facial processing | PBO × OXT × facial valence interaction | ↓ | 0.92 | ↓ | — | ROI |
| ↓ | — | ← → | — | ROI | |||||
| Gaze at faces | Gaze preference to eyes | ↓ | 0.99 | ← → | — | ROI | |||
| Kirsch et al.27 | 27 | M | Implicit facial and scene processing | Main effect for faces and scenes | ↓ | 1.02 | ← → | — | ROI |
| Main effect for faces | ↓ | 0.83 | ← → | — | ROI | ||||
| Main effect for scenes | ↓ | 0.74 | ← → | — | ROI | ||||
| Labuschagne et al.28 | 24 | M | Implicit facial processing | Angry > neutral | ↓ | — | ↓ | — | ROI |
| Fearful > neutral | ↓ | — | ↓ | — | ROI | ||||
| Happy > neutral | ↓ | — | ↓ | — | ROI | ||||
| Petrovic et al.31 | 32 | M | Fear conditioning (direct gaze) | Gaze to negative stimulus > neutral | ← → | — | ↓ | 0.96 | ROI |
| Main effect | ← → | — | ↓ | — | ROI | ||||
| Pincus et al.32 | 40 | M/F | Explicit facial processing | Reading of the Mind in the Eyes test | ← → | — | ↑ | 1.33 | WB |
| Riem et al.33 | 24 | F | Listening to crying babies | Cry > control noise | ← → | — | ↓ | 0.43 | ROI |
| Riem et al.34 | 16 | F | Listening to laughing babies | Laughter > control noise | ↓ | ↓ | ROI | ||
| Rilling et al.35 | 24 | M | Trust interaction | Reciprocated cooperation | ↓ | 0.47 | ← → | — | WB |
| [(reciprocated cooperation human > computer) OXT > (reciprocated cooperation human > computer) PBO] | ↓ | 1.03 | ← → | — | WB | ||||
| Singer et al.36 | 32 | M | Empathy for pain | Pain in self | ← → | — | ↓ | 0.64 | ROI |
| Self PBO > OXT, selfish > prosocial | ↓ | 0.64 | ← → | WB | |||||
| ← → | ↓ | 0.75 | ROI | ||||||
| Striepens et al.38 | 24 | M | Implicit scene processing and subsequent memory test | Negative and neutral > baseline | ← → | — | ↓ | ROI | |
| Negative > baseline | ← → | — | ↓ | ROI | |||||
| Neutral > baseline | ← → | — | ↑ | ROI | |||||
| Wittfoth-Schardt et al.39 | 24 | M | Viewing familiar or unfamiliar child | Viewing own child, familiar child, unknown child | ← → | — | ← → | — | ROI |
dlPFC = dorsolateral prefrontal cortex; F = female; GP = globus pallidum; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; lOFC = lateral orbitofrontal cortex; M = male; MFG = medial frontal gyrus; mOFC = medial orbitofrontal cortex; mPFC = medial prefrontal cortex; MTG = middle temporal gyrus; OFC = orbitofrontal cortex; OXT = oxytocin; PBO = placebo; PFC = prefrontal cortex; ROI = region of interest; SN = substantia nigra; STG = superior temporal gyrus; WB = whole brain; vlPFC = ventrolateral prefrontal cortex.
The arrows indicate the direction of differences in blood oxygen level–dependent response during the selected contrast; studies that did not provide enough information to calculate effect size were left blank.
Region reported as different area in study, but was found to be in the area indicated when converting to Talairach coordinates; see Table 1.
Region reported in the study, but was found to be in a different area when converting to Talairach coordinates; see Table 1.
fMRI studies in humans
Reward system
Social learning is largely influenced by how reward is processed in the reward system and can be modulated by OXT manipulation. The reward system influences social behaviour by driving the evaluation and interpretation of choices and their associated values.47 It also houses a large proportion of the OXT receptors in the human brain, the highest concentration of which is located in the substantia nigra (SN),9,48 an area heavily implicated in reward processing, prediction error and dopamine (DA) signalling.5,9,48 However, this area is very small, and its between-subjects variability makes it difficult to show robust activation in human imaging studies. Thus, its relative omission from the current human imaging studies of OXT is not surprising. However, the SN still plays a role in reward-mediated social behaviours owing to its influence over other areas of the basal ganglia, such as the caudate and putamen, the limbic system and other areas of the brain that have shown consistent changes in brain activity after OXT administration. After correcting for location by standardizing to Talaraich coordinates, 1 study that had originally reported activation in the brainstem after OXT administration showed that OXT elicited increased activation in the right SN while explicitly processing fearful faces over neutral faces.25
We examined 6 studies addressing the reward system after OXT administration.23,25,30,32,35,39 We found that OXT administration has an effect over reward-related learning in that it seems to increase activation associated with reward-related learning in the caudate while decreasing learning effects. It has a similar influence on activation in the putamen, but is specific to learning during social interactions. How OXT affects neural activity during social tasks in the globus pallidum (GP) is less clear, but it appears to play a role in attachment. We review the available evidence in each of these areas in the sections that follow.
Basal ganglia
We identified 6 studies addressing OXT effects on the activation within the basal ganglia (5 using whole brain analysis; Table 3).23,25,30,32,35,39 The basal ganglia are important areas for reward processing in the brain and comprise the GP,32,39 SN,25 putamen,23,30,35 caudate23,32,35,39 and ventral striatum. Some of these areas, such as the ventral striatum, show strong connections to the amygdala47 and play an important role in reward-based learning by interpreting actions and their corresponding outcomes to make future decisions.47
Caudate
The caudate plays a role in processing feedback when there exists a perception of contingency between the action and outcome, especially when the task incorporates an element of trust;49–55 therefore, the caudate is an important region to address when exploring the effects of OXT administration, as it may help modulate how decisions are made based on preconceptions of trust. Four studies have addressed the role of the caudate after OXT administration.23,32,35,39 The correlation between action and outcome in terms of action contingency appears to be activated exclusively within the left caudate, and activation correlates with prediction error during instrumental conditioning.52,53 On the other hand, bilateral activation of the caudate is observed in games involving trust where increased activity was related to an “intention to trust.”49,50 Furthermore, increased activation within the caudate has been shown to correlate with more benevolent partners49,54 by reinforcing actions that could potentially lead to further rewards.52 In a task using a simple trust interaction involving money between 2 participants, Baumgartner and colleagues23 found that activation in the bilateral caudate was attenuated by OXT administration in participants after receiving feedback about the trustworthiness of the partners with whom they had just interacted. In contrast, using a standard prisoner’s dilemma paradigm to explore trust, Rilling and colleagues35 found that the left caudate was significantly activated after OXT administration in response to trustworthiness in the form of reciprocated cooperation in human opponents, but there was no change in activation in the caudate after unreciprocated cooperation, thus reinforcing the role of the caudate in action contingency. It is important to note that the differences between these findings may be attributed to the fact that Baumgartner and colleagues23 did not distinguish between trust affirming and trust negating trials or reciprocated and unreciprocated trust; as such, the difference in caudate activity may be attributable to differences in trial types between the studies. Another explanation for the diminished activity may be that caudate activation diminished as learning progressed or that participants believed their partners to be morally “good.”49,54,55 Furthermore, the increased activation in the caudate after OXT administration may have predicted their belief in the likelihood of future cooperation.49
The caudate is also of interest in other studies incorporating the processing of social stimuli, such as novel and familiar faces. A study by Wittfoth-Schardt and colleagues39 involved a father viewing pictures of his own child, a familiar child and an unfamiliar child after being administered OXT. They reported that OXT administration increased activation in the left caudate when the men viewed their own children versus an unfamiliar child. This may reflect the enhanced rewarding effects of parental attachment with one’s own child, which is augmented by OXT administration. A study by Pincus and colleagues32 also showed an increase in neural activation in the left caudate after OXT administration while assessing social stimuli during a Reading the Mind in the Eyes test. Furthermore, Labuschagne and colleagues28 showed a main effect of OXT in the activation within the left caudate while explicitly processing emotional faces by matching angry, happy and fearful faces. These interactions may potentially reflect the neural effects of learning, attachment and evaluating novelty in terms of learning reward contingencies when combined with OXT administration, showing that OXT administration increases reward-related responses while decreasing learning-related activation.
Putamen
The putamen is adjacent to the caudate and plays a role in associative learning.56 It has been demonstrated to show greater activation than the caudate during performance of actions associated with reward, but shows similar levels of activation as the caudate when still learning the optimal action associated with a reward.57 Three studies have addressed the changes in the activation of the putamen after being administered OXT.23,30,35 Two studies included in the systematic review used trust interactions between partners to demonstrate greater activation in the putamen extending into the insula after receiving OXT and feedback about the partner.23,35 One study by Rilling and colleagues35 used a prisoner’s dilemma game to demonstrate greater activation in the right putamen as a response to reciprocated cooperation after OXT was administered. Another study used a trust game49 to demonstrate an attenuation in the activation within the left putamen after OXT administration following feedback on a partner’s trustworthiness.23 A study in women conducted by Lischke and colleagues30 showed that the right putamen was more active after receiving OXT when rating negative scenes over neutral scenes. However, the activation observed in the putamen was part of an ROI analysis centred around the amygdala, and activation in these regions was not evident during whole brain analysis. Overall, the putamen showed reliable changes in activation only after OXT administration during games involving trust manipulations and when there was concurrent activation within the caudate. Therefore, the putamen, along with the caudate, may play a role during the processing of rewards related to interactions with other people.
Globus pallidus
Another area of the reward system that has been implicated in OXT studies, albeit to a lesser extent, is the GP, which has been associated with general reward signal processing58 as well as maternal attachment.59,60 Although we recognize that the external (GPe) and internal (GPi) GP have different functions, restrictions in the number of studies involving the GP made it difficult to draw further conclusions on subsections. Two studies have addressed the effect of OXT administration on activation within the GP.32,39 A study by Wittfoth-Schardt and colleagues39 showed that OXT administration attenuated activity in the GP when participants viewed pictures of their own children versus viewing a familiar child and when viewing pictures of an unfamiliar child versus a familiar child. This suggests a role for OXT administration in attenuating activation in the GP related to reward and novelty and also coincides with areas associated with maternal attachment.59,60 An earlier study by Pincus and colleagues32 also showed an effect of OXT administration in the activation within the left GP during a Reading the Mind in the Eyes test. However, these findings in the GP after OXT administration have not been robustly replicated.
Temporal lobes
Although primarily known for their role in auditory processing61 and vision, the temporal lobes also play an important role in memory and the interpretation of social cues through visual processing and spatial recognition to help determine socially relevant information.62,63 The temporal lobes are also known to be a target of output from the basal ganglia,64 which may show how OXT administration influences neural processes in this area through learning and reward processing of social stimuli. Of the areas in the temporal lobe, the middle (MTG) and superior temporal gyrus (STG) have been shown to be consistently activated in studies involving human emotional face processing65 and during mentalizing/theory of mind.66–68 Eight of the 12 studies reporting whole brain findings found differential activation in the temporal lobe after OXT administration, most of which used faces as social stimuli24,25,29–33,39 (Table 3). In addition, another study found that connectivity to the temporal lobe from the amygdala was increased after OXT administration.35 This consistency of activation made this the most robust region showing activation after OXT administration.
Domes and colleagues24 used implicit facial processing in men to demonstrate decreases in the activation within the left MTG and bilateral STG after OXT administration in response to happy facial stimuli as well as increased activation within the left inferior temporal lobe and MTG in response to fearful facial stimuli. On the other hand, in a different study Domes and colleagues25 used an explicit facial processing task in women to show increased activation in the left MTG and STG after OXT administration in response to happy and fearful facial stimuli. Labuschagne and colleagues28 used explicit and implicit processing tasks in men to show a main effect of OXT administration on the activation within the left MTG and bilateral STG when implicitly processing a range of emotional faces, although the direction of this effect was not reported. In a follow-up report,29 they showed increased activation within the right STG after OXT administration while explicitly processing happy faces. Wittfoth-Schardt and colleagues39 also showed that in men activation is attenuated in the bilateral MTG and left STG after OXT administration when implicitly processing the faces of an unfamiliar child versus a familiar child. This effect of OXT administration on neural attenuation associated with facial valence is not only limited to the processing of faces, but also extends to influencing activity where negative stimuli have been paired with faces. In a task pairing a shock with different faces to condition for fear, Petrovic and colleagues31 showed attenuation in the activation within the right anterior MTG after OXT administration across all fear conditioning trials. However, increased activity when viewing faces appears to be mainly evident in women; Lischke and colleagues30 asked women to rate emotional arousal from a series of faces and demonstrated greater activation in the STG and inferior temporal gyrus (ITG) after OXT administration. Furthermore, a study by Pincus and colleagues32 involving mainly women (8 of 9 participants) showed greater activation in the STG during the Reading of the Mind in the Eyes test after OXT administration. A similar increase in activation within the STG was reported in women administered OXT before auditory stimuli of crying infants in a study by Riem and colleagues.33 It is important to note that all of these studies in women (except the study by Riem and colleagues33) used explicit emotional processing, whereas the studies in men used tasks with implicit facial processing (except the study by Labuschagne and colleagues,29 who also showed increased activation in the temporal lobes after OXT administration, contrary to other studies in men). Thus, task type and sex may play a large role in how OXT administration affects neural activity in the temporal lobes.
Of the areas in the temporal lobes associated with social processing, the fusiform gyrus has been the area most consistently activated in response to tasks incorporating facial stimuli69 and is known to be modulated by facial valence.70 Neural activity in this area has also been shown to be modulated by OXT administration. In women, Domes and colleagues25 showed an increase in activation across various regions of the temporal lobes when viewing fearful and happy faces but not angry faces after OXT administration. However, in men, Petrovic and colleagues31 showed a decrease in activation in the left MTG and right fusiform gyrus following OXT administration after fear conditioning using faces with direct gaze. Overall, OXT administration appears to play a role in the increase and decrease of activity in the temporal lobes with sex-specific effects; OXT administration increases activation in the temporal lobes in women and generally attenuates activation in the temporal lobes in men. However, this difference in activation within the temporal lobes between women and men may also be attributable to the variability in task types; OXT administration may attenuate activation in the temporal lobes during implicit facial processing but may increase activation in the temporal lobes during explicit facial processing.
Insula
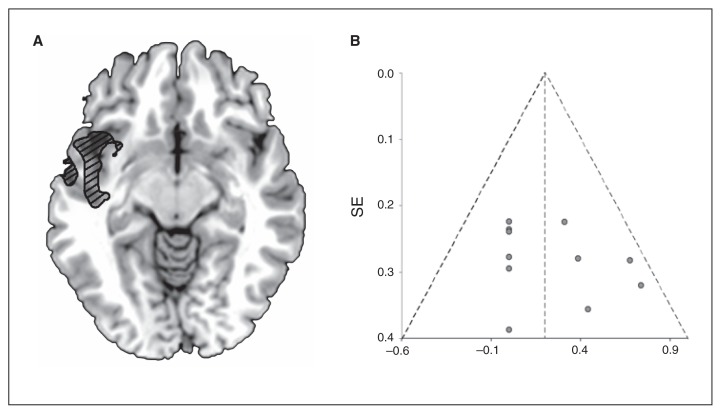
The activation of the insula after OXT administration may reflect neural processing to minimize risk prediction errors71 and reward anticipation in a social context.72,73 Activity in the insula may also be associated with emotion regulation74 and facilitating a sense of emotional involvement when interacting with others in a trust game.75 Its activation during trust games implicates it in making decisions about the risk of trusting another person during social interactions. Five studies in our review have investigated neural activity in this area.23,25,33,35,38 Overall, activity in the insula is generally augmented after OXT administration, suggesting a heightened role in modulating risk prediction and reward anticipation in a social context. There does not appear to be a significant effect of sex in this area. Furthermore, our meta-analysis of all whole brain imaging data found the left insula to be the most robustly activated area among all the studies included in the meta-analysis (Fig. 2).
Fig. 2.
Voxel-based signed differential mapping meta-analysis of placebo-controlled fMRI studies using acute oxytocin (OXT) challenge in humans. (A) This cluster reflects increased regional activation after OXT administration in humans consistent with the left insula. Results were thresholded with voxel p ≤ 0.005, uncorrected, peak z ≥ 2, and cluster extent ≥ 10 voxels. (B) The Egger test for funnel plot asymmetry was not significant (t = 1.1, p = 0.30), and no gross abnormalities could be observed. SE = standard error.
In a trust game, Baumgartner and colleagues23 showed attenuation of activation within the left putamen and insula after OXT administration in response to all feedback about their partner’s trustworthiness, whereas Rilling and colleagues35 demonstrated an increased activation in the right putamen and insula after OXT administration following reciprocated cooperation but not unreciprocated cooperation. This may show a lateralization of the effect of OXT on the processing of trust-related stimuli in the putamen and insula or may reflect a difference caused by cooperation bias that was not explicitly established by Baumgartner and colleagues,23 as they did not differentiate between cooperative and uncooperative trials.
The insula is also important in evaluating tasks with an element of emotional processing. Domes and colleagues25 found that, in women only, OXT administration intensified activation in the insula in response to fearful and happy emotional faces compared with neutral faces when asked to identify sex. Another study in women conducted by Riem and colleagues33 also demonstrated a greater activation within the insula after being administered OXT and listening to a crying infant. This effect may reflect an implicit empathizing that is intensified by OXT administration in women but not men. However, Striepens and colleagues38 also showed an increase in activation within the left anterior insula in men after OXT administration when viewing negative social scenes that were later remembered over neutral social scenes that were later forgotten. This may reflect the role of the insula in the recall of emotional information. They went on to show that the connections between the insula and other regions were also important in determining which negative social scenes were remembered over neutral social scenes after OXT administration. Furthermore, functional connectivity increased between the left anterior insula and the left inferior frontal gyrus (IFG) as well as the left basolateral amygdala after OXT administration. However, connectivity between the right amygdala and left insula was attenuated after OXT administration. Of these areas, the basolateral amygdala was shown to be the major projection area of OXT-related sharing of information between the left insula and the left amygdala, suggesting that the insula may have been using some of the modulatory functions of the amygdala to bias emotional processing.38
Limbic and paralimbic system
The limbic system is important in the regulation of autonomic and endocrine functioning as well as the processing of and response to emotional stimuli (for a review see the study by Phan and colleagues74).
All of the fMRI studies presented in this review have investigated some aspect of the limbic system.23–33,35–39 Its role has been one of the focuses of neuroendocrine research, most notably research on OXT, and has mainly revolved around the role of the amygdala, which is explored in greater detail below. This systematic review explores the role of other areas in the limbic system in addition to the amygdala relative to OXT.
Amygdala
In all but one39 of the fMRI studies involving OXT administration retrieved in the present systematic review, the amygdala was one of the main ROI (Table 3). Its involvement in numerous animal studies has implicated it as an important area for OXT binding and release.5,76 In humans, the role of the amygdala in social cognition77 and, notably, facial processing65 has established it as a very important region in OXT research. Although no OXT receptors have been detected in the amygdala in any human studies5,9,48 its size in humans has been shown to vary in relation to the OXT gene78,79 and it is consistently implicated in studies involving OXT administration (Table 3).
Studies using implicit processing of social stimuli consistently found attenuated activation within the amygdala after OXT administration.24,27,31,33 However, pairing implicit facial processing with a negative stimulus to condition for fear showed nonsignificant attenuation in the amygdala.31 In tasks using explicit processing of social stimuli, there were more mixed results for the direction of activation in the amygdala resulting from OXT administration. However, when separating samples by sex, studies involving all or mainly women reported that OXT administration increased activation in the amygdala,25,32 but in studies involving men OXT administration attenuated activation in the amygdala.26 In a study involving a trust interaction, OXT administration increased activation in the amygdala when cooperation was reciprocated, and this increase in activation was shown to be specific to a participant being told they were playing another human versus a computer. These studies show that the type of study and participant sex play a large role in how OXT influences activity in the amygdala. It is also important to note that only 3 of these studies reported that activation in the amygdala was significant at the whole brain level;25,35,36 the majority of these studies used ROI analysis or small volume correction (SVC) to extract significant activation within the amygdala. Two other studies used ROI analysis and reported insignificant findings bilaterally.23,28 Furthermore, our meta-analysis of all studies reporting whole brain fMRI findings did not show any hypo- or hyperactivation of the amygdala in response to OXT administration during tasks using emotional stimuli (Fig. 2, Table 4).
Table 4.
Results for voxel-based SDM meta-analysis of placebo controlled fMRI studies employing acute OXT challenge in humans*
| Group | Peak Talairach x, y, z | Z score | p value | Cluster voxels | Breakdown |
|---|---|---|---|---|---|
| OXT > PBO | |||||
| Left insula (extending to superior temporal gyrus and precentral frontal gyrus) | −46,−6,−6 | 2.451 | < 0.001 | 1182 | Left BA 13 (222) |
| Left BA 22 (219) | |||||
| Left BA 38 (174) | |||||
| Left BA 6 (165) | |||||
| Left BA 21 (120) | |||||
| Left BA 43 (70) | |||||
| Left BA 44 (39) | |||||
| Left BA 4 (37) | |||||
| Left BA 47 (31) | |||||
| Left BA 42 (26) | |||||
| Left BA 9 (19) | |||||
| Left BA 20 (12) | |||||
| OXT < PBO | — | — | — | — | — |
BA = Brodmann area; OXT = oxytocin; PBO = placebo; SDM = signed differential mapping.
Results were thresholded with voxel p ≤ 0.005, uncorrected; peak z ≥ 2; and cluster extent ≥ 10 voxels. Breakdown regions with an extent < 10 voxels extent are not reported.
Thalamus
The thalamus has been implicated in various studies involving OXT administration. Its involvement may be due to its connections with other regions of the brain that help mediate and control social interactions and behaviour. In addition to serving as the brain’s relay board, the thalamus has been associated with selective attention and levels of arousal23,80 as well as consciousness81 and may be used to relay and modulate important emotional and cognitive information.82 During a trust game, OXT administration increased the amount of activity in the right thalamus before participants received feedback on the outcome of the game.23 However, in an implicit emotion processing task where participants viewed faces of varying valence at varying intensities, OXT administration attenuated activation in the left thalamus in response to angry faces.24 In other studies using explicit emotional processing where participants had to match faces with the same facial valence, there was a main effect of OXT administration in the left thalamus for sad28 and angry25 facial valence. Together, these findings may reflect differential mediation and relaying of signals from emotional stimuli to corresponding areas connected to the thalamus, which varies with task design.
Prefrontal and anterior cingulate cortex
The prefrontal cortex (PFC) and anterior cingulate cortex (ACC) are associated with the limbic system. Most of the imaging studies involving men have shown that OXT administration attenuates activation in both of these regions during the processing of social stimuli.23,29,31,36 Overall, the studies show that, at least in men, the involvement of the orbitofrontal cortex (OFC) and ACC are minimized after OXT administration. Furthermore, attenuated activity in the dorsolateral (dlPFC) and ventrolateral PFC (vlPFC) after OXT administration may indicate an attenuated need for emotion regulation and cognitive control.
The PFC is important for social interactions owing to its role in action selection and evaluation83 as well as facial processing65 and mentalizing or interpreting the actions of others.66 Specifically, the roles of the ACC and OFC are important in helping to compute the value of various choices.83 More specifically, the OFC is important for evaluating current choices, whereas the ACC encodes choice predictions.83 Most of the imaging studies involving men have shown that OXT administration attenuates both of these regions during the processing of social stimuli.23,29,31,36 However, 1 study involving mainly women showed an increase in activation within the ACC after receiving OXT and performing a Reading the Mind in the Eyes test.32 This may reflect a sex difference in how social stimuli are evaluated after OXT administration, but there are not enough studies to reach a strong conclusion. Overall, the studies show that, at least in men, the involvement of the OFC and ACC are minimized after OXT administration and may indicate a greater efficiency in evaluating social stimuli.
Other areas of the PFC, such as the dlPFC, which is involved in cognitive control,84,85 and the vlPFC which is implicated in emotion regulation,86 play a role in encoding and processing value and activation in response to explicit and implicit facial processing and have also been shown to be attenuated by OXT administration.24,25,31,35 This effect was stable across both sexes and across different task types except in part of 1 study25 in which women explicitly processing an angry face over the neutral face showed increased activation in the bilateral vlPFC and right dlPFC after OXT administration. However, in this same study, the authors also found that activation in the dlPFC was attenuated for fearful and happy faces after OXT administration. There is evidence that women process angry faces differently than men in the PFC.65,87 Women show greater activation in the PFC while explicitly processing angry faces as opposed to neutral faces with no difference in happy or sad faces,87 which may explain why women exhibit this difference in activation with regard to facial valance after OXT administration. Thus, attenuated activation in these areas after OXT administration may indicate an attenuated need for emotion regulation and cognitive control.
EEG studies in humans
Studies using EEG have demonstrated how OXT administration can attenuate cortical activity and its association with social tasks.41,42,44 To date, only 5 studies have used EEG and OXT in humans and only 2 have explored the social implications of altered cortical activity due to OXT administration while performing a task with social stimuli40–44 (Table 4).
A few studies have been done using EEG and OXT administration without incorporating any social elements. Unsurprisingly, these studies did not find any effect of OXT administration. A study by Fehm-Wolfsdorf and colleagues41 explored the potential for OXT administration to facilitate nonsocial learning and long-term recall of 25 unrelated nouns and its effect on auditory evoked potentials using a series of tone pips. Another study by Born and colleagues40 examined OXT administration in participants during an auditory mismatch task; no difference was found in cortical activity after OXT administration. Both of these experiments highlight that OXT administration did not appear to have any systematic effect on brain activity in tasks lacking social elements.
To demonstrate that OXT administration can have an effect over cortical activity during social tasks, Perry and colleagues44 used EEG and OXT administration along with stimuli that demonstrated biological motion. Participants were presented with a series of point lights that reflected either biological or nonbiological movements. After receiving OXT, participants demonstrated improved performance between trials associated with biological movement and those with random movement, and participants who received OXT elicited widespread μ and α suppression compared with those who received a placebo. This change in μ/α activity may indicate potential processing of higher social information as well as activation of the mirror neuron system.88 Another study with social stimuli explored the cognitive effects of charitable donations and their modulations by OXT administration. Huffmeijer and colleagues42 measured frontal α asymmetry and parental love withdrawal (i.e., how often parents would withhold love and affection to discipline their children for misbehaving or failing to attend to an instruction) after OXT and placebo administration and then gave participants a chance to donate to a charitable organization. They found that in participants with lower degree of love withdrawal and relative lower right to left frontal α activity OXT administration increased charitable donations. In addition, participants who showed higher relative left frontal activity gave larger donations than those with higher relative right frontal activity. No differences in frontal α asymmetry were found after OXT administration; however, EEGs were recorded only at rest and with no social or cognitive probes, which may be why no changes were observed. Huffmeijer and colleagues43 went on to use faces as a social probe in women. They used the Eriksen flanker task89 in which each trial was followed by either a happy or disgusted face and an indicator as to whether or not they were correct. The authors found that OXT administration increased the amplitudes of the vertex positive potential (VPP) and late positive potential (LPP), which indicated greater attention to the feedback stimuli (from the LPP) and an increased ability to process faces (from the VPP) regardless of the facial valence or feedback. This finding supports fMRI data showing that OXT administration has an effect over facial valence perception and that perception of facial valence is altered across both early (VPP) and later (LPP) stages of processing. Overall, these studies show how using EEG can increase our understanding of how OXT administration exerts its influence over cortical activity in addition to hemodynamic findings and how these changes in neural activity may influence social cognition.
These studies show altered EEG activity after OXT administration corresponding with changes in performance on social tasks. No effects were found in experiments without a social aspect, highlighting that OXT does not appear to have any systematic effect on brain activity during tasks lacking social elements. The study by Perry and colleagues44 is particularly important, as it highlights the specificity of cortical activity elicited by OXT administration in a social context. Their study also highlights the importance of OXT administration in manipulating widespread cortical activity and provides a possible translation to the importance of mirror neurons and their association with OXT administration. Collectively, these studies show how using EEG can increase our understanding of how OXT administration exerts its influence over cortical activity in addition to hemodynamic findings and give a more complete picture of how OXT administration may influence social cognition.
Effect sizes
The magnitude of the effect of OXT administration is provided in Table 3 for each ROI. For the human fMRI studies, we observed a Cohen d ranging from 0.39 to 2.12 for the emotional tasks and 0.47 to 1.03 for the social cognition tasks involving trust.
The largest effect size was observed in the temporal lobes (1.56 ± 0.21). This reflects the large number of studies that showed activity in the temporal lobes, mainly during tasks incorporating facial processing.
The smallest effect size was observed in the amygdala (0.82 ± 0.24). This smaller effect may be due to the large number of studies that used an ROI approach to show that the amygdala was significant in their study.
Voxel based meta-analysis
We performed a meta-analysis using all studies that reported whole brain findings using emotional stimuli. Eleven studies fit all the criteria and were included in the meta-analysis (Fig. 1).5,24,25,29–33,36,38,39 Taking into account 1- and 2-sample studies, we found that the left insula was the only area that reliably showed greater activation after OXT administration across all studies (Fig. 2, Table 4). These findings were found across all task types and across both sexes, and thus may have important implications for the role of OXT research by showing that OXT administration augments neural responses to social tasks in the left insula regardless of task type and sex (Table 1, Fig. 2).
Discussion
In the present study, we examined the acute effect of OXT administration on neural activity within the brain. First, we conducted a systematic review of the neurophysiological and electrophysiological placebo-controlled studies administering OXT. Second, we measured the magnitude of change in activation of various brain regions as a result of OXT administration versus placebo in specific key areas relevant to the behavioural effects of OXT. Finally, to our knowledge, we performed the first fMRI voxel-based meta-analysis of the effects of OXT administration. We found that sex and task type seem to significantly impact the neurophysiological effects of OXT administration. The biggest effect sizes from OXT administration were observed in the temporal lobes, and the smallest were observed in the amygdala. The fMRI voxel-based meta-analysis revealed significant insular hyperactivation during the OXT administration versus placebo contrasts.
Our systematic review indicates that OXT administration appears to influence different brain regions in varying ways. In the reward system, OXT administration increases activity related to social reward, but decreases learning social contingencies; this is the most evident during the evaluation of tasks involving trust and reciprocity.23,35 In the temporal lobes, the modulation of neural activity after OXT administration varies according to task type and sex. Functional MRI studies in women have shown that OXT exerts an almost opposite effect to that seen in men. However, most of the tasks in women used explicit emotional processing, whereas most of those in men used implicit emotional processing (for a meta-analysis of brain activation during explicit v. implicit emotional processing see the study by Fusar-Poli and colleagues65). Explicit emotional processing tasks in women tended to augment activity in the temporal lobes after OXT administration,25,30,32 whereas implicit emotional processing in men tended to attenuate activity after OXT administration.24,31,39
This may show that the manner in which each sex attends to social information varies, but it may also reflect the differences in how OXT administration modulates activity based on task type. This shows the importance of the temporal lobes in processing social stimuli and shows how OXT administration influences how social stimuli are perceived and how we attend to socially salient information.
There is a consistent association between OXT administration and amygdala function in human OXT studies with widespread ROIs and small volume correction around the amygdala. Our systematic review supports this view by showing consistent attenuation of amygdala activity in implicit emotion judgement in men. However, the negative results in our voxel-wise meta-analysis uncovers relevant heterogeneity among the surveyed studies, most notably between men and women and between implicit and explicit tasks. It is still unclear whether or not the amygdala has any OXT receptors. So far, only 1 group has unsuccessfully attempted to localize OXT receptors in the amygdala, using a relatively nonspecific approach (autoradiography).9,48 More studies are necessary to clearly show or exclude OXT receptor presence and abundance in the human brain. However, magnocellular OXT neurons project to areas such as the central amygdala,90 which may indicate 1 way in which OXT influences the amygdala. Recent PET imaging in rats has identified a potential ligand to look at OXT receptor density,91 which may give better insight into the receptor densities in humans, although recent research by the same group has also shown that this particular radiotracer may have limited brain penetration in primates.92
There is evidence linking midbrain and mesolimbic DA with the effects of OXT on the processing of social stimuli. Some evidence suggests that DA is involved in mediating some of the same maternal behaviours that are exhibited under the influence of OXT93,94 as well as pair bonding.95–98 Furthermore, OXT has been implicated in helping to mediate addiction and withdrawal in the mesolimbic dopaminergic pathways,99 and differences in peripheral and central levels of OXT have been found in patients with disorders with potential DA abnormalities, such as autism,100–103 schizophrenia104–108 and social anxiety disorder.109–112 In addition, preclinical studies have shown that dopaminergic fibres may regulate OXT release113,114 and that the receptor binding sites and neuronal fibres of both of these neurochemicals reside in the same regions of the central nervous system and are often next to each other.92,99,115 Further evidence suggests that, at least in the ventral and dorsal striatum, DA D2 receptors and OXT are heteromers with facilitatory receptor-to-receptor interactions.116 Although most studies reporting links between DA and OXT have been performed in a preclinical population and most evidence for an association between DA and OXT in humans is speculative, a few recent studies in humans have also shown these 2 systems to be more directly associated. Sauer and colleagues117 found an association between OXT genes, DA and OXT administration during responses to social stimuli. They postulated that the availability of DA may regulate OXT secretion within the amygdala. Furthermore, Love and colleagues118 found that dopaminergic responses to stress could be explained by variability within the OXT gene. Evidence from these studies lead to the proposal that DA and OXT may work together to regulate behavioural responses to social stimuli, such that DA affects the assignment of emotional salience,108,119 whereas OXT modulates amygdala tone.120 However, further review of the association between DA and OXT is outside the scope of this paper, and we would encourage those interested to look at reviews already available on this subject.99,120–123
Limitations
Our fMRI voxel-based meta-analysis clearly showed that the insula is consistently modulated by OXT administration across all task types and both sexes. The insula is known for its role in emotion regulation by aiding in the recall and self-induction of emotion74 as well as facilitating a sense of empathy under uncertainty.124 In the context of processing emotional stimuli after OXT administration, the role of the insula may be one of increased recall of emotional information by self-induction of these emotions. Connectivity analyses have shown that, after OXT administration, connectivity between the insula and other areas important to social cognition, such as the amygdala, increases.35,38 Despite this intriguing result, there are many potential confounding factors that were hard to account for and may have biased the voxel-based analysis. It is important to note that this meta-analysis included relatively few studies (n = 11); therefore, it was difficult to conduct moderator analysis to take into account different study design characteristics that may have affected effect sizes. Thus our work represents a more preliminary analysis of the general effects of OXT administration without taking into account factors such as sex, past experiences and in-group versus out-group comparisons. For example, in some areas of the brain, such as the temporal lobes, there is a strong indication that women and men have opposite patterns of activity as a result of OXT administration. This may have caused some areas to appear as null, which in a sex-specific analysis may have been significant. However, we did not have enough studies of each sex to test this hypothesis. In addition, the type of task varied among studies, but no specific type of task had enough power to create its own meta-analysis, so results may have been biased in this regard too. As discussed previously, it appears that implicit processing of social stimuli decreases activity, whereas processing explicit stimuli increases activity, for example, in the amygdala. The significant activation of the insula in our meta-analysis highlights it as an important area to include in future studies for further exploration.
Conclusion
Oxytocin is a complex molecule with many facets that have yet to be understood. Although it is clear that OXT administration has an effect on neural activity of cortical and subcortical areas over a range of social tasks, it is not clear yet precisely how OXT administration influences neural activity across these domains. We have shown evidence that OXT may play differential roles across the sexes. The neurophysiological effects of OXT on subcortical areas, such as the amygdala, could also be influenced by task type. Our systematic review indicates small effect sizes in the amygdala, whereas our voxel-based meta-analysis indicates no effect in this region. Conversely, we found a significant effect of OXT administration in the left insula, which survived across all task types and both sexes. The temporal lobes were also shown to be influenced by OXT administration by our systematic review as these demonstrate the largest effect sizes across sexes and task type.
Further research into OXT and the areas it modulates are necessary to elucidate more on this neuropeptide. More studies examining social tasks using OXT administration that incorporate differing neural domains will help clarify why OXT administration appears to attenuate activity in some tasks or in 1 sex and augment it in another task or the other sex. Studying tasks that implicate the reward system may also help elucidate the role of DA and its ties to OXT. The current links between OXT and DA and the pronounced effects over tasks in the social domain mean that OXT is of particular interest to clinical groups.125 Preliminary studies have shown its potential efficacy in patients with schizophrenia126–128 and autism129 and have shown behavioural effects in patients with schizophrenia,130,131 depression,32 generalized anxiety disorder28,29 and autism.132 Importantly, the prosocial effects of OXT may offer potential for synergistic pharmaco-therapeutic and psychotherapeutic treatments of these severe disorders.133 One study demonstrating such potential benefits is a recent meta-analysis done in behavioural studies of clinical populations.134 One advantage of OXT is that it is an endogenous hormone that has been used therapeutically in other areas of medicine, so its properties as a drug are fairly well known. In patients with disorders such as psychosis, there is an increasing recognition that nondopaminergic drugs are needed to advance care, and there exists great interest in alternative therapeutic targets to DA. There are currently no drug treatments available for social dysfunction, so the concept of a treatment for social ailments is novel. Further studies are warranted to examine how OXT administration instantiates these behavioural changes in these groups and whether or not OXT administration produces the same pronounced effects over time.
Acknowledgements
P. Fusar-Poli was supported in part by a 2014 NARSAD Young Investigator Award.
Footnotes
Competing interests: None declared.
Contributors: R. Wigton, J. Radua, S. Shergill and P. Fusar-Poli designed the study. R. Wigton and P. Fusar-Poli acquired the data, which all authors analyzed. R. Wigton, J. Radua, P. Allen and P. Fusar-Poli wrote the article, which all authors reviewed and approved for publication.
References
- 1.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 2.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–8. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Lindenberg A, Domes G, Kirsch P, et al. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 4.Neumann ID, Landgraf R. Advances in vasopressin and oxytocin–from genes to behaviour to disease. Preface. Prog Brain Res. 2008;170:xi–xiii. doi: 10.1016/S0079-6123(08)00448-2. [DOI] [PubMed] [Google Scholar]
- 5.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 6.Bartz JA, Zaki J, Bolger N, et al. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–8. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Evans S, Shergill SS, Averbeck BB. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacology. 2010;35:2502–9. doi: 10.1038/npp.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loup F, Tribollet E, Dubois-Dauphin M, et al. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–32. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Ijzendoorn MH, Bhandari R, van der Veen R, et al. Elevated salivary levels of oxytocin persist more than 7 h after intranasal administration. Front Neurosci. 2012;6:174. doi: 10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Born J, Lange T, Kern W, et al. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 13.Hunter J, Schmidt F. Methods of meta-analysis. Newbury Park (CA): Sage; 1990. [Google Scholar]
- 14.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–11. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Radua J, Mataix-Cols D. Meta-analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord. 2012;2:6. doi: 10.1186/2045-5380-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–17. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 19.Radua J, van den Heuvel OA, Surguladze S, et al. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–11. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 20.Radua J, Via E, Catani M, et al. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–50. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- 21.Fusar-Poli P, Radua J, McGuire P, et al. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–33. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner T, Heinrichs M, Vonlanthen A, et al. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Domes G, Heinrichs M, Glascher J, et al. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–90. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci U S A. 2010;107:9400–5. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labuschagne I, Phan KL, Wood A, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–13. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labuschagne I, Phan KL, Wood A, et al. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol. 2011 Oct. doi: 10.1017/S1461145711001489. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Lischke A, Gamer M, Berger C, et al. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–8. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Petrovic P, Kalisch R, Singer T, et al. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pincus D, Kose S, Arana A, et al. Inverse effects of oxytocin on attributing mental activity to others in depressed and healthy subjects: a double-blind placebo controlled FMRI study. Front Psychiatry. 2010;1:134. doi: 10.3389/fpsyt.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riem MM, Bakermans-Kranenburg MJ, Pieper S, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry. 2011;70:291–7. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Riem MM, van I Jzendoorn MH, Tops M, et al. No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology. 2012;37:2174. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rilling JK, DeMarco AC, Hackett PD, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–61. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer T, Snozzi R, Bird G, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8:781–91. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sripada CS, Phan KL, Labuschagne I, et al. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol. 2013;16:255–60. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- 38.Striepens N, Scheele D, Kendrick KM, et al. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci U S A. 2012;109:18144–9. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittfoth-Schardt D, Grunding J, Wittfoth M, et al. Oxytocin modulates neural reactivity to children’s faces as a function of social salience. Neuropsychopharmacology. 2012;37:1799–807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Born JBR, Pietrowsky R, Fehm-Wolfsdorf G, et al. Influences of vasopressin and oxytocin on human event-related brain potentials in an attention task. J Psychophysiol. 1987;4:351–60. [Google Scholar]
- 41.Fehm-Wolfsdorf G, Bachholz G, Born J, et al. Vasopressin but not oxytocin enhances cortical arousal: an integrative hypothesis on behavioral effects of neurohypophyseal hormones. Psychopharmacology (Berl) 1988;94:496–500. doi: 10.1007/BF00212844. [DOI] [PubMed] [Google Scholar]
- 42.Huffmeijer R, Alink LR, Tops M, van IMH, et al. Asymmetric frontal brain activity and parental rejection predict altruistic behavior: moderation of oxytocin effects. Cogn Affect Behav Neurosci. 2012;12:382–92. doi: 10.3758/s13415-011-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huffmeijer R, Alink LR, Tops M, et al. The impact of oxytocin administration and maternal love withdrawal on event-related potential (ERP) responses to emotional faces with performance feedback. Horm Behav. 2013;63:399–410. doi: 10.1016/j.yhbeh.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Perry A, Bentin S, Shalev I, et al. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–53. doi: 10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 48.Loup F, Tribollet E, Dubois-Dauphin M, et al. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res. 1989;500:223–30. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- 49.King-Casas B, Tomlin D, Anen C, et al. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 50.Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Annu Rev Neurosci. 2006;29:417–48. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- 51.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 53.Gromann PM, Heslenfeld DJ, Fett AK, et al. Trust versus paranoia: abnormal response to social reward in psychotic illness. Brain. 2013;136:1968–75. doi: 10.1093/brain/awt076. [DOI] [PubMed] [Google Scholar]
- 54.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005;8:1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 55.Delgado MR, Miller MM, Inati S, et al. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–73. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–59. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 57.Hikosaka O, Nakamura K, Sakai K, et al. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–22. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 58.Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–9. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Leibenluft E, Gobbini MI, Harrison T, et al. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Simons CJ, Tracy DK, Sanghera KK, et al. Functional magnetic resonance imaging of inner speech in schizophrenia. Biol Psychiatry. 2010;67:232–7. doi: 10.1016/j.biopsych.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 63.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–9. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 64.Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci U S A. 1996;93:8683–7. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- 66.Frith C, Frith U. Theory of mind. Curr Biol. 2005;15:R644–6. doi: 10.1016/j.cub.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 67.Hampton AN, Bossaerts P, O’Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci U S A. 2008;105:6741–6. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fett AK, Gromann PM, Giampietro V, et al. Default distrust? An fMRI investigation of the neural development of trust and cooperation. Soc Cogn Affect Neurosci. 2014;9:395–402. doi: 10.1093/scan/nss144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarthy G, Luby M, Gore J, et al. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–4. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- 70.Pujol J, Harrison BJ, Ortiz H, et al. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychol Med. 2009;39:1177–87. doi: 10.1017/S003329170800500X. [DOI] [PubMed] [Google Scholar]
- 71.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Villafuerte S, Heitzeg MM, Foley S, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2012;17:511–9. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wittmann M, Lovero KL, Lane SD, et al. Now or later? Striatum and insula activation to immediate versus delayed rewards. J Neurosci Psychol Econ. 2010;3:15–26. doi: 10.1037/a0017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phan KL, Wager T, Taylor SF, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 75.Singer T, Kiebel SJ, Winston JS, et al. Brain responses to the acquired moral status of faces. Neuron. 2004;41:653–62. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- 76.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 77.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 78.Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36:891–7. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inoue H, Yamasue H, Tochigi M, et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry. 2010;68:1066–72. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Portas CM, Rees G, Howseman AM, et al. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Posner MI. Attention: the mechanisms of consciousness. Proc Natl Acad Sci U S A. 1994;91:7398–403. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shane MS, Stevens M, Harenski CL, et al. Neural correlates of the processing of another’s mistakes: a possible underpinning for social and observational learning. Neuroimage. 2008;42:450–9. doi: 10.1016/j.neuroimage.2007.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kennerley SW, Behrens TE, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14:1581–9. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annu Rev Psychol. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- 85.Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–47. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- 86.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 87.McClure EB, Monk CS, Nelson EE, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–55. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: a link between action observation and social skills. Soc Cogn Affect Neurosci. 2007;2:62–6. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of target letters in a nonsearch task. Percept Psychophys. 1974;16:143–9. [Google Scholar]
- 90.Sofroniew MV, Weindl A, Schrell U, et al. Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta Histochem Suppl. 1981;24:79–95. [PubMed] [Google Scholar]
- 91.Smith AL, Freeman SM, Voll RJ, et al. Carbon-11 N-methyl alkylation of L-368,899 and in vivo PET imaging investigations for neural oxytocin receptors. Bioorg Med Chem Lett. 2013;23:902–6. doi: 10.1016/j.bmcl.2012.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith AL, Freeman SM, Voll RJ, et al. Investigation of an F-18 oxytocin receptor selective ligand via PET imaging. Bioorg Med Chem Lett. 2013;23:5415–20. doi: 10.1016/j.bmcl.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shahrokh DK, Zhang TY, Diorio J, et al. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–86. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Douglas AJ. Baby love? Oxytocin-dopamine interactions in mother-infant bonding. Endocrinology. 2010;151:1978–80. doi: 10.1210/en.2010-0259. [DOI] [PubMed] [Google Scholar]
- 95.Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–28. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 96.Smeltzer MD, Curtis JT, Aragona BJ, et al. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–51. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 97.Strathearn L. Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. J Neuroendocrinol. 2011;23:1054–65. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smeltzer MD, Curtis JT, Aragona BJ, et al. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–51. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 99.Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al-Ayadhi LY. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences. 2005;10:47–50. [PubMed] [Google Scholar]
- 101.Anderson BM, Schnetz-Boutaud N, Bartlett J, et al. Examination of association to autism of common genetic variationin genes related to dopamine. Autism Res. 2008;1:364–9. doi: 10.1002/aur.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamilton PJ, Campbell NG, Sharma S, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18:1315–23. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Previc FH. Prenatal influences on brain dopamine and their relevance to the rising incidence of autism. Med Hypotheses. 2007;68:46–60. doi: 10.1016/j.mehy.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 104.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laruelle M, Abi-Dargham P. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–71. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 107.Sasayama D, Hattori K, Teraishi T, et al. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophr Res. 2012;139:201–6. doi: 10.1016/j.schres.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 108.Winton-Brown TT, Fusar-Poli P, Ungless MA, et al. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37:85–94. doi: 10.1016/j.tins.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Bell C, Bhikha S, Colhoun H, et al. The response to sulpiride in social anxiety disorder: D2 receptor function. J Psychopharmacol. 2013;27:146–51. doi: 10.1177/0269881112450778. [DOI] [PubMed] [Google Scholar]
- 110.Fink M, Akimova E, Spindelegger C, et al. Social anxiety disorder: epidemiology, biology and treatment. Psychiatr Danub. 2009;21:533–42. [PubMed] [Google Scholar]
- 111.Schneier FR, Abi-Dargham A, Martinez D, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety. 2009;26:411–8. doi: 10.1002/da.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoge EA, Pollack MH, Kaufman RE, et al. Oxytocin levels in social anxiety disorder. CNS Neurosci Ther. 2008;14:165–70. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindvall O, Bjorklund A, Skagerberg G. Selective histochemical demonstration of dopamine terminal systems in rat di- and telen-cephalon: new evidence for dopaminergic innervation of hypothalamic neurosecretory nuclei. Brain Res. 1984;306:19–30. doi: 10.1016/0006-8993(84)90352-4. [DOI] [PubMed] [Google Scholar]
- 114.Melis MR, Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci Biobehav Rev. 2011;35:939–55. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz JC, Diaz J, Griffon N, et al. Multiple dopamine receptors: the D3 receptor and actions of substances of abuse. EXS. 1994;71:81–92. doi: 10.1007/978-3-0348-7330-7_9. [DOI] [PubMed] [Google Scholar]
- 116.Romero-Fernandez W, Borroto-Escuela DO, Agnati LF, et al. Evidence for the existence of dopamine d2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol Psychiatry. 2013;18:849–50. doi: 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- 117.Sauer C, Montag C, Reuter M, et al. Imaging oxytocin x dopamine interactions: an epistasis effect of CD38 and COMT gene variants influences the impact of oxytocin on amygdala activation to social stimuli. Front Neurosci. 2013;7:45. doi: 10.3389/fnins.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Love TM, Enoch MA, Hodgkinson CA, et al. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol Psychiatry. 2012;72:198–206. doi: 10.1016/j.biopsych.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fett AK, Shergill SS, Joyce DW, et al. To trust or not to trust: the dynamics of social interaction in psychosis. Brain. 2012;135:976–84. doi: 10.1093/brain/awr359. [DOI] [PubMed] [Google Scholar]
- 120.Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophr Bull. 2011;37:1077–87. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014;119:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abi-Dargham A. Is it in our genes: oxytocin, dopamine, stress, and sex. Biol Psychiatry. 2012;72:171–2. doi: 10.1016/j.biopsych.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 124.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 125.Ferris CF. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. Prog Brain Res. 2008;170:305–20. doi: 10.1016/S0079-6123(08)00425-1. [DOI] [PubMed] [Google Scholar]
- 126.Feifel D, Macdonald K, Cobb P, et al. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res. 2012;139:207–10. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 127.Feifel D, Macdonald K, Nguyen A, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–80. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 128.Pedersen CA, Gibson CM, Rau SW, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132:50–3. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 129.Hollander E, Novotny S, Hanratty M, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–8. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- 130.Evans S, Shergill SS, Chouhan V, et al. Patients with schizophrenia show increased aversion to angry faces in an associative learning task. Psychol Med. 2011;41:1471–9. doi: 10.1017/S0033291710001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Averbeck BB, Bobin T, Evans S, et al. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42:259–66. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 133.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–8. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 134.Bakermans-Kranenburg MJ, van I Jzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]