Abstract
The study of immunity has become an important area of investigation for researchers in a wide range of areas outside the traditional discipline of immunology. For the last several decades, psychoneuroimmunology (PNI) has strived to identify key interactions among the nervous, endocrine and immune systems and behavior. More recently, the field of ecological immunology (ecoimmunology) has been established within the perspectives of ecology and evolutionary biology, sharing with PNI an appreciation of the environmental influences on immune function. The primary goal of ecoimmunology is to understand immune function within a broadly integrative, organismal context, typically from an ultimate, evolutionary perspective. To accomplish this ecoimmunology, like PNI, has become a broadly integrative field of investigation, combining diverse approaches from evolution and ecology to endocrinology and neurobiology. The disciplines of PNI and ecoimmunology, with their unique yet complementary perspectives and methodologies, have much to offer one another. Researchers in both fields, however, remain largely unaware of each other's findings despite attempts at integration. The goal of this review is to share with psychoneuroimmunologists and other mechanistically-oriented researchers some of the core concepts and principles, as well as relevant recent findings, within ecoimmunology with the hope that this information will prove relevant to their own research programs. More broadly, our goal is to attempt to integrate both the proximate and ultimate perspectives offered by PNI and ecoimmunology respectively into a common theoretical framework for understanding neuro-endocrine-immune interactions and behavior in a larger ecological, evolutionary context.
Keywords: cytokines, sickness behavior, coinfection, energetics of immunity, wisdom of the body
1. What is Ecoimmunology?
Immunology is clearly not just for immunologists anymore. The immune system, once studied almost exclusively by “card-carrying” immunologists, has become an important area of investigation for a considerably larger sphere of scientists, among them animal physiologists, ecologists, and behavioral neuroscientists. These researchers now employ a wealth of knowledge about the immune system as a means for appreciating how environmental factors drive changes in disease susceptibility, as well as understanding the neural and neuroendocrine mechanisms that mediate these changes. As readers of this journal are well aware, seminal studies in the 1980's demonstrating the conditioning of immune responses, as well as the identification of key interactions among psychological processes and the nervous, endocrine and immune systems, gave birth to the field of psychoneuroimmunology (PNI) (Ader, 1981). More recently, a similar appreciation of the environmental influences (both biotic and abiotic) on immune system function has occurred within the fields of ecology and evolutionary biology, leading to the development of the field of ecological immunology (ecoimmunology) (Demas and Nelson, 2012; Folstad and Karter, 1992; Sheldon and Verhulst, 1996). As with PNI, the goal of ecoimmunology is to understand immune function within a broadly integrative, organismal context, albeit from a more ultimate, evolutionary perspective. Much of ecoimmunology focuses on interactions and tradeoffs between immunity and other life-history traits, both within and across individuals, populations, and species. Thus, a major goal of the field is to understand the extrinsic and intrinsic factors leading to changes in immune system function and how these changes contribute to disease susceptibility across a wide range of animal species (often, but not exclusively, non-model systems). To accomplish this, ecoimmunology, like its older sibling PNI, has become a broadly integrative field of investigation, combining diverse approaches from evolution, ecology and life history theory on the one hand, to endocrinology, neuroscience, molecular biology and behavior on the other.
Despite these two important fields of investigation, the majority of research within each is still predominantly focused on only a single level of analysis; whereas ecoimmunology traditionally focuses on ultimate questions (e.g., evolution and ecology of parasites and their effects on life histories), PNI more often emphasizes proximate questions (e.g., neural and endocrine factors that regulate stress, learning, immunity and disease). While there has been some degree of overlap, as well as attempts at continuing synthesis, in recent years (e.g., “Psychoneuroimmunology meets Integrative Biology” symposium at the 2009 meeting of the Society for Integrative and Comparative Biology), we have yet to bridge the gap. In truth, many ecoimmunologists and psychoneuroimmunologists still remain all too ignorant of each other's unique research perspectives. Ecological and evolutionary forces, however, clearly drive the evolution, development, and production of immune responses, and in turn, physiological mechanisms serve as the critical mediators of these responses. We cannot afford to ignore either aspect when attempting to develop a synthetic approach to the study of environmental influences on immune function and disease. Thus, while the evolutionary biologist Theodore Dobzhansky was most certainly correct when he stated that “nothing makes sense except in the light of evolution” (Dobzhansky, 1973), an equally strong case can be made that “nothing makes sense in biology without an understanding of the underlying mechanisms” as well (Demas et al., 2012). Quite simply, the time is ripe for us to bridge the gap between psychoneuroimmunology and ecoimmunology.
The overarching goal of this minireview is to share with psychoneuroimmunologists, as well as other, more mechanistically-oriented researchers, recent findings and concepts within the discipline of ecoimmunology that they may find interesting, and perhaps even relevant, to their own research programs. More broadly, our goal is to attempt to integrate both the proximate and ultimate perspectives into a common theoretical framework. We do not pretend, however, to be able to accomplish this in a single review; continued progress towards a unified framework is surely needed to guide future research endeavors towards a more integrative, synthetic approach to the study of “Evo-Eco-Psycho-Neuro-Immunology.”
2. Why should Psychoneuroimmunologists care about Ecoimmunology?
In general, ecologists are quite fond of exploring life-history trade-offs; not surprisingly then, ecoimmunologists like exploring how life-history traits (e.g., reproduction, growth, thermoregulation) specifically trade off with immunity. In fact, one of the main targets for research in ecoimmunology has been to understand the costs of immunity. “Costs”, of course, can mean different things to different people and unfortunately ecoimmunologists are not always in agreement on what constitutes a cost of immunity. One simple yet elegant categorization divides costs into “evolutionary costs”, variation in the expression of a component of the immune system that may simultaneously affect another fitness-related trait (e.g. growth, reproduction), and “use costs”, the costs of maintenance and deployment of immune defenses, including energetic costs (Schmid-Hempel, 2003). These latter costs may contribute to energetic allocations (or reallocations) to potentially competing life history traits (Demas et al., 2012).
In this line of research, ecoimmunologists have learned that analyzing data with respect to environmental context is critical for interpreting the results of specific manipulations or treatments on immune function. Below we highlight some of these critical themes and findings within the field of ecoimmunology that have shaped experimental approaches, interpretation of results, and appreciation of environmental context within the field and will hopefully transform our understanding of the immune system across fields.
2.1. Studying Animals under Natural Field Conditions
Ecoimmunologists generally study immunity within both laboratory and field settings; however, it is the comparisons across those environments that have highlighted the importance of taking environmental context under consideration when interpreting results, as the same experimental protocol can lead to different conclusions in controlled versus natural environments (French and Moore, 2008; French et al., 2009). For example, when healing rates of experimentally-induced wounds are examined in reproductive and non-reproductive ornate tree lizards (Urosaurus ornatus) under both field and laboratory conditions, paradoxical findings are the result. In the field, wound healing responses are suppressed during vitellogenesis, an energetically costly reproductive stage in female lizards during which vitellogenin is produced and deposited in follicles, supporting the idea of a trade-off between reproduction and immune function (French and Moore, 2008). When this same relationship is explored in the laboratory, however, no such difference is detectable. To test the idea that resource availability serves as a key mediator between these potential trade-offs, controlled laboratory experiments were conducted in which food resources were manipulated. The results demonstrate that restricting resources in vitellogenic females suppresses wound healing, mimicking what was previously observed under natural field conditions (French et al., 2007). No such suppression, however, is present in either ad lib-fed vitellogenic animals or food-restricted non-reproductive females (French et al., 2007). Therefore, the suppression is only detectable during energetically costly reproductive stages, when resources are limiting, as is often the case within an organism's natural environment. In short, the trade-off is context-dependent. Similar findings can be found in invertebrates as well (e.g., Moret and Schmid-Hempel, 2000) suggesting context-depend trade-offs exist throughout the animal kingdom.
Furthermore, there is evidence from ecoimmunological studies that immunity and the magnitude and type of trade-offs with immunity can differ with season (Buehler et al., 2008; Nelson, 2004), life stage (Palacios et al., 2011), diet quality and availability (Bourgeon et al., 2010; Lochmiller and Deerenberg, 2000; Ruiz et al., 2010), social environment (Archie et al., 2012; Hawley et al., 2006), and through interactions between or among these and other factors (Hegemann et al., 2012; Love et al., 2008). Additionally, these immune costs and environmental contributions are not limited to only non-human animals, as humans are subject to similar influences on our immune systems (Muehlenbein, 2010). This research, focused through an environmental lens, has opened ecoimmunologists' eyes to the context-dependent nature of immunity and has highlighted the necessity for considering the results of immunological studies with regard to the influence of environmental factors.
2.2. Using Natural Variation among Individuals
As opposed to more traditional, biomedically based immunological research, ecoimmunologists often search out patterns of natural variation in immunity. Just as variation in the environment can influence immunity, so too can variation in the genetics of individual organisms lead to differences in immunity. Ecoimmunologists are generally interested in natural variation in constitutive and induced immune responses because this variation may have consequences for the survival and reproductive success of individuals (Pedersen and Babayan, 2011). For example, ecoimmunologists have discovered that antibody concentrations predict overwinter survival in some populations (Graham et al., 2010; Nussey et al., 2014; Raberg and Stjernman, 2003), highlighting the influence of immune variation in population dynamics. Although it may be easier to weed out or ignore natural variation in immunity when addressing neuro-endocrine-immune interactions, it is important to keep in mind that variation in immunity is the natural state. Ignoring sources of natural variation is, to an ecoimmunologist's way of thinking, akin to “throwing the baby out with the bath water.” For example, in a comparison of immune profiles of wild caught mice and inbred (C57BL/6) mice, the wild caught mice had greater inter-individual variability in many of the immune indices as compared to the lab strain (Abolins et al., 2011). Sampling wild populations has not only revealed great variation in immunity, but this sampling has also revealed substantial individual variation in endocrine responses and profiles (Williams, 2008). This individual variation in immunity (and endocrine and neuroendocrine responses) will likely have profound impacts on an animal's response to a pathogen and its ability to survive an infection. Furthermore, human populations are genetically diverse and variable, so examining natural variation in immune and neuroendocrine responses will undoubtedly provide us with a greater understanding of factors influencing human health and disease spread.
Along with this awareness of genetic diversity, ecoimmunologists also appreciate the role that behavioral variation can have in influencing immunity and disease transmission. For example, within populations, animals can vary greatly in their social rank and dominance status, and these variations in rank and status can influence resource access, contact with conspecifics, potential for injury, and hormone profiles. These effects of rank and status can then result in immune system modulation and changes in pathogen exposure (Fairbanks and Hawley, 2012). Furthermore, behavior itself can be one of the more important immune strategies an animal can use, as simply avoiding pathogens allows an animal to beat an infection before it can even begin. House finches (Carpodacus mexicanus) show individual variation in the whether they avoid or do not avoid a conspecific that is displaying experimentally-induced sickness behaviors, and this avoidance or lack of avoidance is related to their acute phase responses. Specifically, avoiders show lower levels of a major acute-phase protein than non-avoiders, suggesting that avoiders may not have to invest as much in immune defenses because they invest in their behavioral defenses (Zylberberg et al., 2013). In contrast, male house finches actually preferentially feed by conspecifics infected with Mycoplasma gallisepticum, whereas females show no preference between infected and uninfected individuals (Bouwman and Hawley, 2010). These results suggest that not only does avoidance behavior differ among individuals but that these behaviors can be pathogen-dependent as well. Understanding the relationships between behavioral variation and immunity is an exciting ecoimmunological direction, and this research direction will undoubtedly add an important component of context to our understanding of environmental influences on the immune system.
2.3. Employing Non-Model Organisms to Assess Patterns across Phylogenies
In general, most ecoimmunologists study non-model organisms (e.g., not inbred, commercially available, or domesticated species) in which congeneric antibodies and reagents do not exist or for which tests that can measure specific immune cell types, proteins, or signaling molecules are not readily available. The lack of species-specific tools has required ecoimmunologists to be resourceful in developing their own assay “tool-kits” which can be shared among laboratories and generalized across species. It has also garnered reasonable criticism, however, in that these tool-kits are often restricted in scope and limited to simply what works. Some of the more commonly used assays in the ecoimmunological field include ex vivo bacterial killing, delayed-type hypersensitivity (e.g., phytohemagglutinin [PHA]), wound healing, and measures of sickness in response to bacterial and viral mimetics (Demas et al., 2011). Many of these measures assess multiple aspects of the immune system simultaneously. By utilizing what ecoimmunologists call “integrative” measures of immunity (and what immunologists may call “messy”), researchers are able to explicitly test inter-specific hypotheses within a study (Lee, 2006; Martin et al., 2007; Tieleman et al., 2005) and perform cross-taxa meta-analyses to address long-standing evolutionary, behavioral, and physiological questions within the field (Boonekamp et al., 2008; Hasselquist and Nilsson, 2012; Nunn et al., 2009; Roberts et al., 2004). These comparative approaches have been valuable in the field of ecoimmunology because they have brought into question long-standing assumptions (e.g., testosterone is immunosuppressive; Roberts et al., 2004) and have allowed researchers to re-evaluate these hypotheses that may have important implications for our understandings of how the immune system functions. One key limitation, however, especially compared to more traditional techniques employed within PNI, is that this same integrative aspect of these measures precludes the identification of the specific, individual immune parameters that contribute to the findings reported in these studies. Despite these limitations, the current ecoimmunological tool-kit is beginning to expand at an unprecedented rate, yielding new challenges to choosing techniques and standardizing protocols across studies (Boughton et al., 2011; Demas et al., 2011; Downs et al., 2014). The availability of powerful new laboratory and field technologies have increased dramatically while, in turn, the costs have declined substantially in the past decade alone, opening the potential for ecoimmunologists and organismal biologists to explore physiological mechanisms driving immune variation in their study systems (Downs et al., 2014). Further, the development of multiplex technologies to quantify gene expression has begun to enable quantification of a wide range (and large number) of cytokines, chemokines, and immunologically-relevant markers in very small quantities of a range of biological fluids across any species with a sequenced genome. It will be no surprise then, that as these technologies continue to develop at an accelerated pace, the range of questions ecoimmunologists hope to answer will expand concomitantly in new and exciting directions (Downs et al., 2014).
2.4. Linking Immunity to Disease
Although many ecoimmunologists appreciate the underlying immunological mechanisms that contribute to an immune response, in general, ecoimmunologists are most concerned with understanding “optimal immunity.” Ultimately, as ecoimmunologists (and psychoneuroimmunologists), we expect our research to translate to understanding of and applications to disease states; however, the results of our immune assays often do not directly correlate with disease resistance (Adamo, 2004). Many ecoimmunologists have begun to integrate objectives from the field of disease ecology into their own research programs in order to understand how environmental influences on immunity relate to natural host-pathogen systems (Brock et al., 2014; Hawley and Altizer, 2011). Continued pursuit of this integration will allow for a more comprehensive understanding of the factors that contribute to disease susceptibility and prevalence.
One exciting avenue of research in ecoimmunology that has emerged from its integration with disease ecology has been to understand how coinfection influences immune responses. Just as there is considerable natural variation in immunity among organisms, there is also great variation in what an individual is infected with at any given time. It is clear that an animal's immune system is not always engaged in just one “battle” with a pathogen or parasite, and these coinfection scenarios can have strong impacts on how the host's immune system reacts (Budischak et al., 2012; Ezenwa et al., 2010; Graham, 2008; Telfer et al., 2010). Furthermore, these effects of coinfection with multiple pathogens on the immune system may be different depending on environmental characteristics at the time of sampling (Beechler et al., 2012). In human populations, coinfection is generally more common than uncommon (Petney and Andrews, 1998) and is often associated with a reduction in host health and increased pathogen abundance within individuals (Griffiths et al., 2011). Thus, coinfection may be the default, rather than the exception and is a ripe area for research across all immune-related fields. Psychoneuroimmunologists have shown us that infection and immune activation influence the neuro-endocrine system and vice-versa. However, if coinfection is standard, then we have a lot more to learn about how these two systems interact and influence the dynamics of multiple infections in natural systems (O'Neal, 2013). Undoubtedly, gaining an increased understanding of the environmental and neuroendocrine factors that influence the immune dynamics of coinfection could have great impacts on our understanding of human and wildlife disease dynamics.
3. What can Ecoimmunologists learn from PNI?
While the majority of the readers of Brain, Behavior and Immunity focus primarily on mechanistic approaches in their research, it is only fair to acknowledge the need for ecoimmunologists to learn from psychoneuroimmunologists as well. Integration, after all, is a two-way street. While ecoimmunologists have typically done an admirable job nesting the study of immune function and disease ecology in an environmental, ecological context (Brock et al., 2014; French et al., 2011; Hawley and Altizer, 2011), there remains a need for the field to look within the organism and more carefully consider the role that physiological mechanisms play in mediating environmental influences on immunity. All too often the brain (and other relevant organs and tissues) is a “missing link” in ecoimmunology. Incorporating mechanistic approaches will allow for a richer analysis in ecoimmunology (Figure 1).
Figure 1.
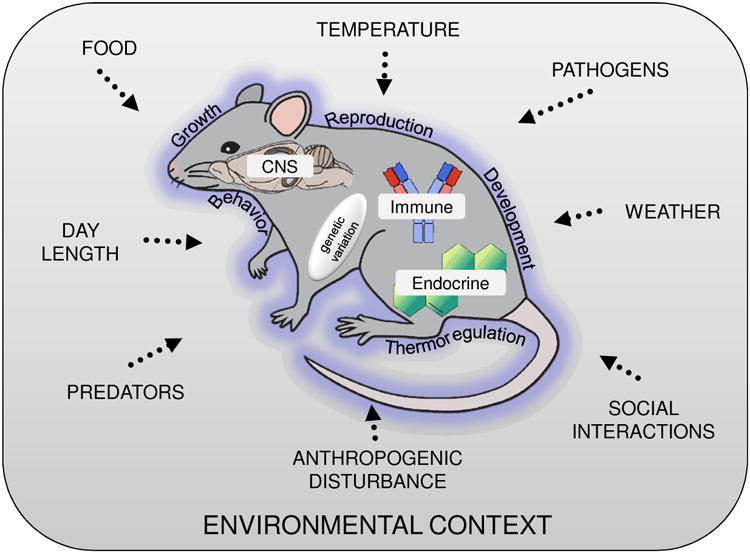
Graphic model displaying the respective research emphases within the fields of ecoimmunology and psychoneuroimmunology. PNI largely focuses on the interactions of internal physiological systems, represented in the diagram by the traditional laboratory mouse. These systems include, but are not limited to, interactions among the nervous, endocrine and immune systems and their concomitant signaling molecules (e.g., neurotransmitters, neurotropic factors, hormones and cytokines). Also nested with the organism is genetic variability across individuals, which will vary considerably depending on whether the study system is a wild animal or an inbred model organism. In either case, this variability (or lack thereof), has the potential to modify the interactions among the physiological systems. Morphological, physiological, and behavioral outputs from such interactions are represented by the outer “skin” of the mouse, and include what are traditionally considered by ecoimmunologists as phenotypic traits (e.g., reproduction, growth and development, thermoregulation, and behavior). Our hypothetical mouse is nested within a larger environmental context (represented by the surrounding box). Within this context is included select examples from a nearly infinite suite of environmental variables that can range from biotic and abiotic factors, behavioral interactions with conspecifics, and the pathogen environment present in the animal's environmental niche. These environmental factors often vary across time (e.g., seasons, time of day) and space (geographic or population differences) and play an important role as “environmental input signals” to the organism. Lastly, the areas of shading represent the areas of overlap, or lack of overlap, between PNI and ecoimmunology, and serve to highlight key areas where greater integration and synthesis across the fields are needed.
3.1. Contributions of PNI to Ecoimmunology and Disease Ecology
As PNI has demonstrated, a deep knowledge of the mechanistic underpinnings of the immune system is critical to understanding the more large-scale patterns of disease, something that has only recently begun to be appreciated within ecoimmunology. Thus, disease susceptibility is driven as much by host resistance and tolerance (Raberg et al., 2007) (which in turn are based on host physiology), as it is on pathogen prevalence across environmental contexts. Complex interactions between several physiological systems can result in changes in disease transmission. One of the key strengths of PNI is its focus on proximate control underlying neuroendocrine and immunological interactions, providing a reasonably comprehensive understanding of these complex mechanisms. It is often difficult, however, to apply such findings to natural populations, where environmental conditions, including energy availability, stressors, and pathogen abundance, are not static across time or space. For example, we have demonstrated energetic trade-offs between immune function and other energetically costly physiological and behavioral responses (Demas et al., 2012). Manipulations that reduce total energy stores, such as photoperiod-induced reductions in body mass in seasonally breeding rodents (Drazen et al., 2001) or surgical removal of adipose tissues (Demas et al., 2003), suppress specific immune responses. These immune deficits are often transient, however, as immune function will return to normal if optimal energy balance is restored (Demas et al., 2003). Interestingly, it is not necessarily total energy availability per se, but signals of current energy budget that appear to be the critical factor in mediating energetic trade-offs with immunity (Demas, 2004). The adipose tissue hormone leptin appears to be one such signal that regulates immune responses. Experimental provisioning of exogenous leptin, which provides a “false signal” of increased energy availability, will restore immune function in animals with suppressed immunity due to reduced energy stores (Demas and Sakaria, 2005). Leptin is only one among many potential signaling molecules that provide a biochemical signal of available energy resources (e.g., Garcia et al., 2010), and therefore provides critical information for animals to properly allocate resources among systems, especially in the face of changing physiological states and external environmental conditions.
Another well-studied class of hormones involved in energy regulation, and thus energetic trade-offs, are glucocorticoids (i.e., cortisol, corticosterone) which serve as a means for mobilizing glucose during times of increased energetic demands. Elevations in these hormones are also highly correlated with environmental stressors and are often used as a proxy for determining stress in ecoimmunological studies. All too often, however, they are used as the singular measure of stress in ecoimmunology. As psychoneuroimmunologists generally appreciate, the stress response represents a coordinated set of biological processes involving multiple physiological systems. While glucocorticoids are clearly involved, they are rarely synonymous with stress. Many other factors, including adrenal catecholamines, endorphins, neuropeptides, and the sympathetic nervous system innervation of peripheral tissues, are likely equally critical in regulating stress effects on immunity. While the precise role of these signaling molecules in mediating immune trade-offs may be easier to determine under constant lab conditions where energy budgets are generally limitless, few researchers actually bring such physiological analyses into the field.
More work needs to be centered on understanding how various alterations in physiological states affect immune function and thus alter disease susceptibility in natural populations in the field. For example, much effort has been placed on understanding temporal fluctuations in disease transmission as a product of pathogen abundance; however, environmentally-induced fluctuations in immune system maintenance and response can often be overlooked when trying to assess the causes of changes in disease dynamics. Because disease transmission is very much a product of both pathogen abundance and host resistance and physiology, if we want to fully understand patterns of disease, we cannot ignore the contributions of neuroendocrine-immune interactions to disease susceptibility. Only by applying the lessons learned from PNI can a comprehensive understanding of both the regulatory biology underlying neuroendocrine-immune interactions and how these relate to the prevalence and transmission of infectious diseases within ecological context be fully achieved.
3.2. Contributions of PNI to Life History Theory
Connections between the neuroendocrine and immune systems require a large number of specializations (e.g., signaling molecules with cognate receptors in requisite tissues and cells, mechanisms to transmit peripheral signals to the brain and across blood-brain barriers) (Dantzer, 2004). It is unlikely that such an array of molecules and physiological mechanisms would evolve in animals across phyla unless bi-directional communication between neuroendocrine and immune systems serves important adaptive functions. Thus, selection likely acts on the evolution of specific physiological processes, which in turn, drive integrative immune responses within individuals. Understanding these connections from both physiological and evolutionary and comparative perspectives is necessary to make sense of the complexity of their interactions. One of these complex interactions is that of stress and immunity; stress can have both enhancing and suppressive effects on immune responses that range across the scale from adaptive, neutral, to maladaptive (Dhabhar, 2009). As ecoimmunologists gain a better understanding of the roles that these neuro-endocrine factors involved in the stress response (e.g., glucocorticoids, catecholamines) play in immune modulation, we may be able to better elucidate the consequences and evolutionary significance of these relationships to maximizing survival in a stressful and pathogen-rich world. Crickets have emerged as one “model system” in which to address some of these relationships. Research has already shown, through experimentally-induced stress scenarios and manipulations of the invertebrate stress-response neurohormone octopamine, that stress effects on immunity are not only mechanisms to shift resources toward or away from the immune system (Adamo, 2014). Rather, octopamine and other stress hormones released during these stressful events can act to reconfigure the immune system. This allows for optimal immune functioning despite molecular resources being shifted toward other physiological processes and reduces the effects of immune-related oxidative damage on the host in these already oxidative stress-filled stressful scenarios (Adamo, 2014). Thus, integrating a psychoneuroimmunological perspective into our studies of life history trade-offs and immunity will hopefully allow us to look beyond the standard assumptions of our field (e.g., resource-based trade-offs cause immune suppression) and appreciate other adaptive causes and mechanisms of immune modulation in our future studies.
4. A Return to Wisdom
In the early 1930's Walter Cannon, one of the most influential American physiologists of modern times, developed the concept of homeostasis (based on Claude Bernard's early work on the “milieu interior”). In Cannon's own words “the organism which with the aid of increased adrenal secretion can best muster its energies, call forth sugar to supply the labouring muscles, lessen fatigue, and send blood to the parts essential in the run or the fight for life.” Cannon referred to the idea that the body is exquisitely adapted to respond to subtle, or often not-so-subtle, changes in the environment in order to maintain homeostasis as the “wisdom of the body.” A concept similar to Canon's “canon,” that of allostasis and allostatic load, has been developed more recently to capture the dynamic nature of homeostatic mechanisms (McEwen and Wingfield, 2003). This idea of “constancy through change” was, and continues to be, extremely important, and one with relevance for both ecoimmunology and psychoneuroimmunology. For example, in the 1960s and 1970s Eugene Weinberg demonstrated the concept of iron withholding, whereby sick organisms avoid ingesting foods rich in iron (Weinberg, 1974). Iron is an essential nutrient for many bacteria and parasites to grow and survive, as such, avoiding iron is an adaptive response by an organism to prevent “feeding the pathogen.” Further, a series of elegant and groundbreaking studies by Matthew Kluger and colleagues demonstrated the adaptive value of a fever response (Kluger et al., 1975; Vaughn et al., 1974). His lab demonstrated that ectothermic lizards that are unable to mount a physiological fever response, will voluntarily leave a previously thermoneutral position and move to a warmer portion of a thermocline when injected with bacteria (or the bacterial mimetic lipopolysaccharide [LPS]), thus inducing “behavioral fever.” Sick lizards return to the original cooler location once sickness had passed. Further, bacterially-treated lizards prevented from inducing behavioral fever results in near complete mortality in these individuals. Over twenty-five years ago, based on the findings from these and other studies like them, Benjamin Hart published a now classic paper (Hart, 1988) re-couching the idea of sickness behavior from that of a collection of non-specific, maladaptive byproducts of disease to one of a coordinated set of adaptive responses that help an organism effectively fight off infection.
Sickness responses are characterized by fever (or hypothermia), anorexia, cachexia, and reductions in social, pleasurable, and sexual behaviors. These responses can be displayed at varying intensities within and among individuals, and the adaptive nature of these sickness responses is made clear by the context-dependent nature of their magnitude. For instance, several seasonally breeding animals (e.g., Siberian hamsters, song sparrows, white-crowned sparrows) show variation in the intensity of their responses to sickness-inducing LPS across seasons (Bilbo et al., 2002; Owen-Ashley et al., 2008; Owen-Ashley et al., 2006; Owen-Ashley and Wingfield, 2006). In order to understand how and why these animals may be modulating their sickness responses across seasons, we can compare across studies and find that there is no consistent season in which animals display a weak or strong sickness response (suggesting that seasonal photoperiodic cues or reproductive status may not be driving this variation). Rather, only one pattern persists across all studies—the expression of sickness responses is attenuated in the season in which these organisms have the lowest energy reserves. In addition, individual variation in body mass is a strong predictor of individual variation in sickness response in white-crowned sparrows and Siberian hamsters. In both of these species, there is a negative correlation between initial body mass and percent change in body mass in response to LPS across all seasons, such that animals with greater initial body mass exhibit greater mass loss in response to LPS-induced sickness (Owen-Ashley et al., 2006; Carlton and Demas, unpublished data). These results are not particularly surprising when one considers the substantial energetic costs that come from mounting a fever or actively avoiding eating. These patterns suggest that animals may modulate sickness based upon their energy limitations, such that the magnitude of a sickness display is constrained by a minimum body mass that an animal can reach before it risks not being able to recover and survive (Ashley and Wingfield, 2012).
The beauty of adopting a comparative approach, while also examining individual variation, to determine a potential cause of variation in sickness responses is that we can then use our hypotheses to generate experiments to determine the neuro-endocrine mechanisms that might mediate this diversity in responses (Carlton et al., 2012). The value of this ecoimmunological approach to psychoneuroimmunological studies (and vice-versa) becomes apparent when we consider that these same patterns emerge in biomedical models. For instance, diet induced obese (DIO) Wistar rats show more intense LPS-induced sickness responses as compared to lean controls (Pohl et al., 2014 ; Pohl et al., 2009). This result on its own suggests that obesity exacerbates sickness response symptoms, which can be interpreted as a potential obesity-induced dysregulation of the inflammatory response. When looking across individuals ranging in all weight indices from lean to obese, however, there are strong positive correlations between body weight and both the length of time it takes for an animal to recover from LPS-induced sickness and the total number of sickness symptoms an animal displays (Pohl et al., 2014). In contrast to the previous results, these correlations suggest that the sickness responses of these lean and obese rats may be functioning in the same manner and utilizing the same mechanisms as the birds and hamsters mentioned earlier. Mounting an appropriate sickness response will always be a balance of killing the pathogen while not killing oneself by losing too much energy in the process. There is no reason to think that, even in our more biomedical systems, these adaptive immunological “strategies” no longer function or exist.
In addition to the role of energetics in immunity, investigations into the effects of social interactions on immunity are another research aim that spans across the psychoneuroimmunological and ecoimmunological disciplines. For example, psychoneuroimmunologists often turn to a well-established laboratory based social defeat paradigm (i.e., a staged “resident-intruder” interaction where a conflict occurs between a generally smaller “intruder” animal and a larger “resident animal; the intruder is placed into and remains in the home cage of the resident until the intruder submits in defeat; this defeat is generally accompanied by increased hypothalamic-pituitary-adrenocorticol (HPA) axis activation (Huhman et al., 1990)) in order to determine the effects of this social stressor on immune function (Bartolomucci, 2007). Ecoimmunologists may investigate the effects of resource-driven aggression and subsequent social dominance shifts within natural or experimentally-formed groups in order to address this same aim (Fairbanks and Hawley, 2012). Although we may use different names in reference to our behavioral paradigms, we are all ultimately examining the same stressor that is ubiquitous across much of the animal kingdom.
Research into how and why social interactions influence immunity may be one aim in which we can start to integrate across these two disciplines. There is considerable evidence that social defeat often suppresses humoral immunity (Bartolomucci, 2007), but integrating an ecoimmunological approach to this paradigm may increase our understanding of both the proximate mechanisms underlying this immune modulation and the functional consequences of this suppression to disease resistance. For example, the effects of social defeat on immunity may vary across individuals and be highly context-dependent. As such, house finches that are allowed to form dominance hierarchies in flocks and then experimentally separated into two flocks—one that contains all the dominant individuals from previous flocks, one that contains all the subordinate individuals from previous flocks—exhibit different effects of social status change on immunity (Hawley, 2006). Only the previously dominant individuals showed corresponding reductions in humoral immunity as their social status decreased in their new flock, while there was no relation between humoral immunity and change in social status in the subordinate flock (Hawley, 2006). Examining the hormonal and immunological characteristics of the birds throughout these shifts in social status may allow us to detect individual behavioral predictors and physiological correlates of these changes in immunity. These findings could shed light on neuroendocrine and immune factors that contribute to this commonly observed phenomena of social defeat-induced suppression of humoral immunity.
We can also gain insights into how social defeat may be modulating immunity by embracing a “whole organism approach” and determining whether the effects of social defeat on immunity may be adaptive based upon the changes that are observed in other socially-sensitive aspects of phenotype after the defeat. For example, in Syrian hamsters (Mesocricetus auratus), both a single social defeat, as well as repeated exposure, suppresses humoral immunity (Jasnow et al., 2001), but repeated social defeat also results in increased food intake, body mass, and adiposity in this species (Foster et al., 2006). Although these two phenomena have not been examined together in this species, an ecoimmunological approach may allow this kind of integration. Before concluding that both of these effects are maladaptive for the animal (i.e., immunosuppression and obesity), we could take an ecoimmunological approach to determine if suppressed immunity and increased food intake are beneficial to the animal's survival or potential for future reproductive success. For example, if social defeat reflects a loss of access to food resources in these animals in their natural setting, then it would be very practical for a defeated animal to eat any food that is readily available and to slow down its metabolism to store energy. Additionally, if survival is at stake, an animal may not want to allocate resources to maintaining humoral immunity but may want to shift allocation to its immediate immune defenses. For example, Siberian hamsters (Phodopus sungorus) that are socially defeated show reduced humoral immunity but actually show increased innate immunity (Chester et al., 2010). This result not only highlights how measuring multiple arms of the immune system can change the interpretation of a study, but it also truly illustrates the “wisdom of the body.” The immediate risks of a social defeat are likely to come from exposed wounds or bites, while pathogen exposure may be a later consequence. Thus, in this context, allocation to innate immunity may be incredibly important, while humoral immunity may not be a priority. Furthermore, an organism is not just an immune system and social defeat “stress” may influence the redistribution of resources to other more immediately important physiological and behavioral processes than humoral immunity (Segerstrom, 2010). From this standpoint, decreases in humoral immunity are no longer a negative response to social defeat but a wise strategy for extending survival after this defeat.
4.1 Cytokines: They're Not Just for Sickness Anymore
More recent evidence within PNI has suggested an important adaptive role for pro-inflammatory cytokines in mediating normal species-typical behavior. While increases in cytokine production are most often associated with adverse conditions such as neuroinflammation (e.g., “cytokine storm”), recent findings require us to revise this view (Williamson et al., 2011). Specifically, neonatally-infected and control rats were treated in adulthood with LPS or saline (control) 24h prior to a learning task (fear conditioning involving an initial period of context exploration followed by foot shock) or a control task involving foot shock only without context exploration (Williamson et al., 2011). In adult rats from the neonatal control condition, interleukin (IL)-1β protein expression is modestly (but significantly) elevated, but only in the learning condition, while context exploration has no effect on cytokine expression. Interestingly, neonatally-infected rats challenged with LPS in adulthood display exaggerated IL-1β levels in response to learning. In a follow-up study, rats displaying exaggerated cytokine responses showed reduced learning and memory on a fear conditioning test. Taken together, these findings suggest that a modest increase in cytokine production, rather than being maladaptive, is critical to learning; exaggerated cytokine responses, in contrast, can inhibit learning and memory, suggesting there exists an “optimal” level of cytokine production (Bilbo and Schwarz, 2012). In our opinion, this conclusion would not have been reached purely within the context of disease; rather, these findings represent an adaptive response to a novel environmental context.
Unfortunately, in the modern era dominated by translational science, Cannon's Canon has been largely replaced by a “disease dictum” in which we focus our research efforts on studying how and why the body fails during disease states. In this light, metabolism has been replaced with metabolic syndrome, sickness behaviors with neuroinflammation, and normal healthy genomes have been co-opted by “the gene(s) for diseases x, y and z.” Canon's “wisdom of the body”, the idea that the normal day-to-day responses of our bodies to environmental challenges are healthy, adaptive responses that only occasionally go awry (leading to disease), has been largely supplanted by a biology of disease. Thus, perhaps one of the most important lessons from ecoimmunology is the need for a “return to wisdom.” That is, by understanding the basic biology of naturally occurring fluctuations in immunity in relatively healthy animals (under either field or laboratory conditions) we have the potential to make real and substantial contributions to our understanding of the role of environmental influences on the immune system. In doing so, we will be able to view fluctuations in immune status as adaptive responses rather than deleterious consequences (although not denying the potential for disease). This basic approach to studying the immune system has been, and will continue to be, an extremely productive strategy with which to complement more applied, biomedical approaches using disease models. Careful consideration of context, from both proximate and ultimate perspectives, will allow us to develop a common theoretical framework for understanding neuro-endocrine-immune interactions and behavior within a larger ecological and evolutionary context.
Acknowledgments
The authors thank Dr. Staci Bilbo for inviting us to submit this review, and for suggesting the first part of the title. We also wish to thank the Indiana University “Mechanisms of Behavior (MoBsters)” research group for providing feedback on several of the ideas presented within, and Sarah Keesom for kindly providing the mouse graphic used in Figure 1. The writing of this manuscript, and some of the data discussed in the mini-review, were supported by NSF IOS 0543798, an NSF Graduate Research Fellowship, a National Institutes of Health T32 training grant HD049336, and generous funds provided by Indiana University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abolins SR, Pocock MJO, Hafalla JCR, Riley EM, Viney ME. Measures of immune function of wild mice, Mus musculus. Molecular Ecology. 2011;20:881–892. doi: 10.1111/j.1365-294X.2010.04910.x. [DOI] [PubMed] [Google Scholar]
- Adamo SA. How should behavioural ecologists interpret measurements of immunity? Anim Behav. 2004;68:1443–1449. [Google Scholar]
- Adamo SA. The effects of stress hormones on immune function may be vital for the adaptive reconfiguration of the immune system during fight-or-flight behavior. Integr Comp Biol. 2014 doi: 10.1093/icb/icu005. [DOI] [PubMed] [Google Scholar]
- Ader R. Psychoneuroimmunology. Academic Press; New York: 1981. [Google Scholar]
- Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proc Natl Acad Sci U S A. 2012;109:9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley NT, Wingfield JC. Sickness Behavior in Vertebrates: Allostasis, Life-History Modulation, and Hormonal Regulation. In: Demas GE, Nelson RJ, editors. Ecoimmunology. Oxford University Press; New York: 2012. pp. 45–91. [Google Scholar]
- Bartolomucci A. Social stress, immune functions and disease in rodents. Front Neuroendocrinol. 2007;28:28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Beechler BR, Broughton H, Bell A, Ezenwa VO, Jolles AE. Innate immunity in free-ranging African buffalo (Syncerus caffer): associations with parasite infection and white blood cell counts. Physiol Biochem Zool. 2012;85:255–264. doi: 10.1086/665276. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proceedings Biological sciences / The Royal Society. 2002;269:447–454. doi: 10.1098/rspb.2001.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonekamp JJ, Ros AHF, Verhulst S. Immune activation suppresses plasma testosterone level: a meta-analysis. Biology Letters. 2008;4:741–744. doi: 10.1098/rsbl.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton RK, Joop G, Armitage SAO. Outdoor immunology: methodological considerations for ecologists. Funct Ecol. 2011;25:81–100. [Google Scholar]
- Bourgeon S, Kauffmann M, Geiger S, Raclot T, Robin JP. Relationships between metabolic status, corticosterone secretion and maintenance of innate and adaptive humoral immunities in fasted re-fed mallards. Journal of Experimental Biology. 2010;213:3810–3818. doi: 10.1242/jeb.045484. [DOI] [PubMed] [Google Scholar]
- Bouwman KM, Hawley DM. Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol Lett. 2010;6:462–465. doi: 10.1098/rsbl.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock PM, Murdock CC, Martin LB. The History of Ecoimmunology and Its Integration with Disease Ecology. Integr Comp Biol. 2014 doi: 10.1093/icb/icu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budischak SA, Jolles AE, Ezenwa VO. Direct and indirect costs of co-infection in the wild: Linking gastrointestinal parasite communities, host hematology, and immune function. International journal for parasitology Parasites and wildlife. 2012;1:2–12. doi: 10.1016/j.ijppaw.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler DM, Piersma T, Matson KD, Tieleman BI. Seasonal Redistribution of Immune Function in a Migrant Shorebird: Annual-Cycle Effects Override Adjustments to Thermal Regime. Am Nat. 2008;172:783–796. doi: 10.1086/592865. [DOI] [PubMed] [Google Scholar]
- Carlton ED, Demas GE, French SS. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm Behav. 2012;62:272–279. doi: 10.1016/j.yhbeh.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Chester EM, Bonu T, Demas GE. Social defeat differentially affects immune responses in Siberian hamsters (Phodopus sungorus) Physiology & behavior. 2010;101:53–58. doi: 10.1016/j.physbeh.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. European journal of pharmacology. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Demas GE. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proceedings Biological sciences / The Royal Society. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Greives TJ, Chester EM, French SS. The Energetics of Immunity: Mechanisms Mediating Trade-offs in Ecoimmunology. In: Demas GE, Nelson RJ, editors. Ecoimmunology. Oxford University Press; New York: 2012. pp. 259–296. [Google Scholar]
- Demas GE, Nelson RJ, editors. Ecoimmunology. Oxford University Press; New York: 2012. [Google Scholar]
- Demas GE, Sakaria S. Leptin regulates energetic tradeoffs between body fat and humoural immunity. P Roy Soc B-Biol Sci. 2005;272:1845–1850. doi: 10.1098/rspb.2005.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS. Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol. 2011;80:710–730. doi: 10.1111/j.1365-2656.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 1973;35:125–129. [Google Scholar]
- Downs CJ, Adelman JS, Demas GE. Mechanisms and Methods in Ecoimmunology: Integrating Within-Organism and Between-Organism Processes. Integr Comp Biol. 2014 doi: 10.1093/icb/icu082. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Demas GE, Nelson RJ. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus) Endocrinology. 2001;142:2768–2775. doi: 10.1210/endo.142.7.8271. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A, Jolles AE. Hidden Consequences of Living in a Wormy World: Nematode-Induced Immune Suppression Facilitates Tuberculosis Invasion in African Buffalo. Am Nat. 2010;176:613–624. doi: 10.1086/656496. [DOI] [PubMed] [Google Scholar]
- Fairbanks B, Hawley DM. Interactions between Host Social Behavior, Physiology, and Disease Susceptibility: The Role of Dominance Status and Social Context. In: Demas GE, Nelson RJ, editors. Ecoimmunology. Oxford University Press; New York: 2012. pp. 440–467. [Google Scholar]
- Folstad I, Karter AJ. Parasites, Bright Males, and the Immunocompetence Handicap. The American Naturalist. 1992;139:603–603. [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. American journal of physiology Regulatory, integrative and comparative physiology. 2006;290:R1284–1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- French SS, Dearing MD, Demas GE. Leptin as a Physiological Mediator of Energetic Trade-Offs in Ecoimmunology: Implications for Disease. Integr Comp Biol. 2011;51:505–513. doi: 10.1093/icb/icr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SS, DeNardo DF, Moore MC. Trade-offs between the reproductive and immune systems: Facultative responses to resources or obligate responses to reproduction? Am Nat. 2007;170:79–89. doi: 10.1086/518569. [DOI] [PubMed] [Google Scholar]
- French SS, Moore MC. Immune function varies with reproductive stage and context in female and male tree lizards, Urosaurus ornatus. Gen Comp Endocr. 2008;155:148–156. doi: 10.1016/j.ygcen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- French SS, Moore MC, Demas GE. Ecological immunology: The organism in context. Integr Comp Biol. 2009;49:246–253. doi: 10.1093/icb/icp032. [DOI] [PubMed] [Google Scholar]
- Garcia NW, Greives TJ, Zysling DA, French SS, Chester EM, Demas GE. Exogenous insulin enhances humoural immune responses in short-day, but not long-day, Siberian hamsters (Phodopus sungorus) Proceedings Biological sciences / The Royal Society. 2010;277:2211–2218. doi: 10.1098/rspb.2009.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL. Ecological rules governing helminth-microparasite coinfection. Proc Natl Acad Sci U S A. 2008;105:566–570. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–665. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- Griffiths EC, Pedersen AB, Fenton A, Petchey OL. The nature and consequences of coinfection in humans. J Infection. 2011;63:200–206. doi: 10.1016/j.jinf.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and biobehavioral reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Nilsson JA. Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim Behav. 2012;83:1303–1312. [Google Scholar]
- Hawley DM. Asymmetric effects of experimental manipulations of social status on individual immune response. Anim Behav. 2006;71:1431–1438. [Google Scholar]
- Hawley DM, Altizer SM. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol. 2011;25:48–60. [Google Scholar]
- Hawley DM, Lindstrom K, Wikelski M. Experimentally increased social competition compromises humoral immune responses in house finches. Horm Behav. 2006;49:417–424. doi: 10.1016/j.yhbeh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hegemann A, Matson KD, Both C, Tieleman BI. Immune function in a free-living bird varies over the annual cycle, but seasonal patterns differ between years. Oecologia. 2012;170:605–618. doi: 10.1007/s00442-012-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Bunnell BN, Mougey EH, Meyerhoff JL. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiology & behavior. 1990;47:949–956. doi: 10.1016/0031-9384(90)90023-w. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Drazen DL, Huhman KL, Nelson RJ, Demas GE. Acute and chronic social defeat suppresses humoral immunity of male Syrian hamsters (Mesocricetus auratus) Horm Behav. 2001;40:428–433. doi: 10.1006/hbeh.2001.1708. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Love OP, Salvante KG, Dale J, Williams TD. Sex-specific variability in the immune system across life-history stages. Am Nat. 2008;172:E99–112. doi: 10.1086/589521. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP. Evolutionary Medicine, Immunity, and Infectious Disease. In: Muehlenbein MP, editor. Human Evolutionary Biology. Cambridge University Press; New York: 2010. pp. 459–490. [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends in immunology. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nunn CL, Lindenfors P, Pursall ER, Rolff J. On sexual dimorphism in immune function. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009;364:61–69. doi: 10.1098/rstb.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey DH, Watt KA, Clark A, Pilkington JG, Pemberton JM, Graham AL, McNeilly TN. Multivariate immune defences and fitness in the wild: complex but ecologically important associations among plasma antibodies, health and survival. Proceedings Biological sciences / The Royal Society. 2014;281:20132931. doi: 10.1098/rspb.2013.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal DM. Eco-endo-immunology across avian life history stages. Gen Comp Endocrinol. 2013;190:105–111. doi: 10.1016/j.ygcen.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Hasselquist D, Raberg L, Wingfield JC. Latitudinal variation of immune defense and sickness behavior in the white-crowned sparrow (Zonotrichia leucophrys) Brain, behavior, and immunity. 2008;22:614–625. doi: 10.1016/j.bbi.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii) Horm Behav. 2006;49:15–29. doi: 10.1016/j.yhbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Wingfield JC. Seasonal modulation of sickness behavior in free-living northwestern song sparrows (Melospiza melodia morphna) J Exp Biol. 2006;209:3062–3070. doi: 10.1242/jeb.02371. [DOI] [PubMed] [Google Scholar]
- Palacios MG, Winkler DW, Klasing KC, Hasselquist D, Vleck CM. Consequences of immune system aging in nature: a study of immunosenescence costs in free-living Tree Swallows. Ecology. 2011;92:952–966. doi: 10.1890/10-0662.1. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Babayan SA. Wild immunology. Mol Ecol. 2011;20:872–880. doi: 10.1111/j.1365-294X.2010.04938.x. [DOI] [PubMed] [Google Scholar]
- Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. International Journal for Parasitology. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- Pohl J, Sheppard M, Luheshi GN, Woodside B. Diet-induced weight gain produces a graded increase in behavioral responses to an acute immune challenge. Brain, behavior, and immunity. 2014;35:43–50. doi: 10.1016/j.bbi.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Pohl J, Woodside B, Luheshi GN. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology. 2009;150:4901–4910. doi: 10.1210/en.2009-0526. [DOI] [PubMed] [Google Scholar]
- Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- Raberg L, Stjernman M. Natural selection on immune responsiveness in blue tits Parus caeruleus. Evolution. 2003;57:1670–1678. doi: 10.1554/02-417. [DOI] [PubMed] [Google Scholar]
- Roberts MLL, Buchanan KLL, Evans MRR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav. 2004;68:227–239. [Google Scholar]
- Ruiz M, French SS, Demas GE, Martins EP. Food supplementation and testosterone interact to influence reproductive behavior and immune function in Sceloporus graciosus. Horm Behav. 2010;57:134–139. doi: 10.1016/j.yhbeh.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. P Roy Soc B-Biol Sci. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC. Resources, stress, and immunity: an ecological perspective on human psychoneuroimmunology. Ann Behav Med. 2010;40:114–125. doi: 10.1007/s12160-010-9195-3. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and tradeoffs in evolutionary ecology. Trends in ecology & evolution. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman BI, Williams JB, Ricklefs RE, Klasing KC. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. P Roy Soc B-Biol Sci. 2005;272:1715–1720. doi: 10.1098/rspb.2005.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn LK, Bernheim HA, Kluger MJ. Fever in the lizard Dipsosaurus dorsalis. Nature. 1974;252:473–474. doi: 10.1038/252473a0. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron and susceptibility to infectious disease. Science. 1974;184:952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Williams TD. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:1687–1698. doi: 10.1098/rstb.2007.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberberg M, Klasing KC, Hahn TP. House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. Biol Lett. 2013;9:20120856. doi: 10.1098/rsbl.2012.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]


