Abstract
Objective
To evaluate the labor curves of patients undergoing preterm induction of labor (IOL) and assess possible predictors of vaginal delivery (VD).
Study Design
Data from the NICHD Consortium on Safe Labor were analyzed. A total of 6,555 women undergoing medically-indicated IOL before 37 weeks gestational age (GA) were included in this analysis. Patients were divided into four groups based on gestational age: A: 24-27+6, B: 28-30+6, C: 31-33+6, and D: 34-36+6 weeks. Pregnant women with a contraindication to VD, IOL at or after 37 weeks and those without data from cervical exam on admission were excluded. ANOVA was used to assess differences between GA groups. Multiple logistic regression was used to assess predictors of VD. A repeated measures analysis was used to determine average labor curves.
Results
Rates of vaginal live births increased with GA, from 35% (Group A) to 76% (Group D). Parous women [odds ratio (OR)=6.78, 95% confidence interval (CI) 6.38-7.21] and those with a favorable cervix at the start of IOL (OR=2.35, 95% CI 2.23-2.48) were more likely to deliver vaginally. Analysis of labor curves in nulliparous women showed shorter duration of labor with increasing GA; the active phase of labor was, however, similar across all GA.
Conclusion
The majority of women undergoing medically-indicated preterm IOL between 24 and 36+6 weeks’ GA deliver vaginally. The strongest predictor of VD was parity. Preterm IOL had a limited influence on estimated labor curves across gestational age.
Keywords: preterm, induction of labor, labor curve
Introduction
Approximately 12% of all deliveries in the United States occur before 37 weeks gestational age (GA).1 Preterm birth is the leading cause of neonatal mortality and morbidity, contributing to over 35% of total infant health care spending, well over 5 billion dollars per year.2, 3 Spontaneous labor precedes approximately 50% of preterm deliveries, the remainder are guided by medical necessity due to either maternal or fetal indications.4 Cervical favorability, as assessed by Bishop scoring, cervical length, and maternal parity predicted vaginal delivery following IOL at term.5, 6 However, data regarding predictors of vaginal delivery and labor curves in pregnancies undergoing preterm IOL are limited. Using an interval censored analysis, Zhang and colleagues revisited the median progression of labor at term.7, 8 Active labor occurred most commonly after 6 cm of dilation, and cervical dilation progressed more slowly than previously thought, especially between 4 and 6 cm. These results represent a departure from the Friedman curve9 and now inform our clinical knowledge of median labor progression in modern obstetric practice. Additionally, inherent differences in the progress of labor have been attributed to specific patient characteristics or clinical conditions. Maternal obesity,10, 11 gestational age beyond 37 weeks12 and even fetal sex13 have been shown to influence labor progression. In our study, we examined a large, contemporary U.S. labor database to identify labor curves and predictors of vaginal delivery in pregnant women undergoing medically indicated preterm IOL. We hypothesized that gestational age would influence labor curves in women undergoing preterm IOL.
Materials and Methods
This was a retrospective analysis of de-identified data from the Consortium on Safe Labor (CSL). The CSL is a multicenter, retrospective, observational study with detailed labor and delivery information from electronic medical records at 12 clinical centers (which included a total of 19 U.S. hospitals) from 2002 to 2008, with 87% of the deliveries occurring between 2005 and 2007. Data collected from electronic medical records included demographics, past medical history, labor and delivery information as well as obstetrical, post partum and neonatal outcomes. Patient data were supplemented with maternal discharge ICD-9 codes for each delivery. Each site transferred data in electronic format to the data coordinating center where data were mapped to common categories for each pre- defined variable. Validation studies indicated that the electronic medical record data represented the medical charts accurately.14 This analysis was approved by the Institutional Review Board of MedStar Health Research Institute.
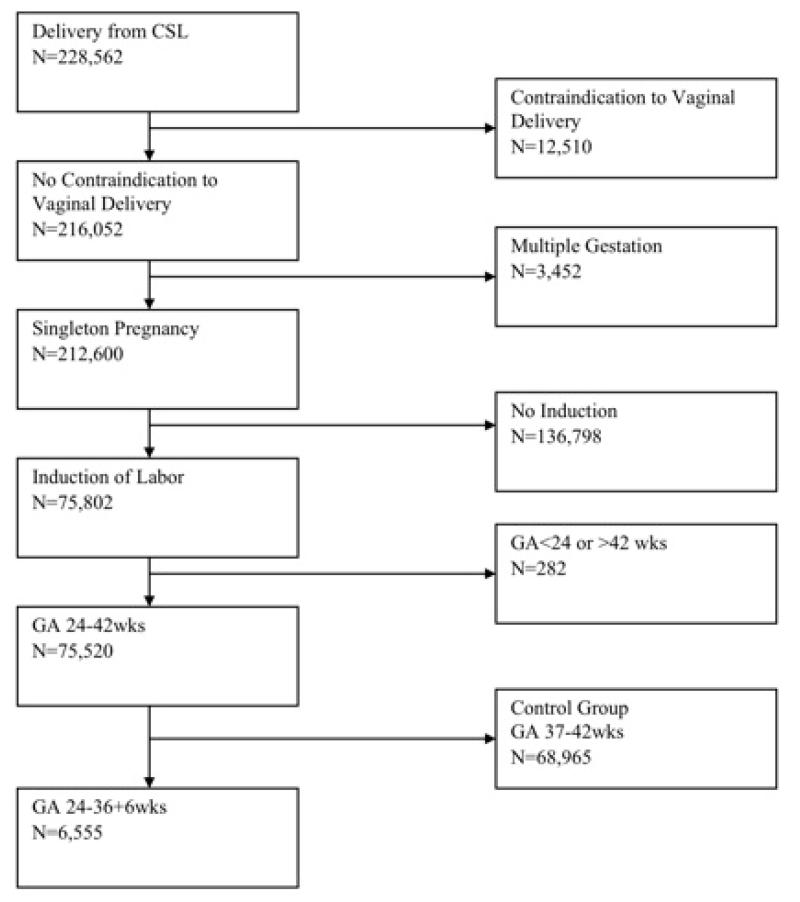
The CSL cohort includes information on 233,844 births from 228,562 pregnancies. Induction of labor was a predefined variable when either the patient’s electronic medical record indicated that there was an induction or a start time was recorded in the patient’s chart. The database included a distinct variable for labor augmentation. The indication for induction was used to identify the precursors of delivery and classified using a previously described hierarchy.15 One site did not provide indications for induction and was not included in the precursor analysis. Four hospitals did not report methods of induction, and 2 hospitals did not report cervical dilation at admission, leaving cases from 13 hospitals available for analysis (Figure 1). Fewer than 20% of the remaining cases had an original Bishop score with all 5 components reported. Therefore, we used the previously described simplified Bishop score comprised of dilation, effacement, and station.16 We defined an unfavorable cervix as a simplified Bishop score ≤4 because of similar sensitivity and specificity to the original Bishop score ≤6.17
Figure 1.
Patient selection diagram Flow diagram for study cohort
After excluding women with any contraindication to vaginal delivery (i.e., vasa previa, complete placenta previa, breech presentation, previous myomectomy or classical cesarean delivery), multifetal gestation, those with spontaneous labor, and GA <24 weeks, our cohort included 6,555 gravidas undergoing attempted IOL at 24+0 to 36+6 completed weeks (Fig. 1). Outcomes were grouped and analyzed by GA, determined from the labor and delivery admission records: 24+0 to 27+6 weeks (Group A), 28+0 to 30+6 weeks (Group B), 31+0 to 33+6 weeks (Group C) and 34+0 to 36+6 weeks (Group D), with further comparison to a control group of women undergoing IOL at 37+0 to 41+6 weeks (Group E). ANOVA and pairwise comparisons were used to assess differences between GA groups in demographic characteristics and rates of vaginal delivery. Multiple logistic regression analysis, controlling for maternal age, parity, body mass index (BMI), cervical effacement, cervical dilation, and fetal station was used to determine which clinical characteristics, available at the time of admission, were most associated with subsequent vaginal delivery following preterm IOL. A repeated measures analysis with an eight-degree polynomial model 7 was used to determine average labor curves for live births in each GA group. This method takes into account both the interval-censored and repeated-measure nature of cervical dilation data. Stillbirth cases were excluded from the labor curve analysis, due to expected variation in clinical management of these cases, especially at an early gestational age. Since we only sought to describe labor patterns by GA, we did not perform any statistical comparisons of the labor curves among various groups Significance was considered at p<0.05.
Results
Age, pre-pregnancy BMI and current BMI were overall similar across GA groups. The earliest GA group (24-27+6 weeks) had a higher proportion of African American subjects, parous women, cases with a history of cesarean delivery, and those with a prior preterm delivery. Within each group, more than half of subjects delivered vaginally following IOL. Vaginal delivery rates differed among most GA groups. Rates of vaginal live births were similar in Groups A and B, then increased gradually and significantly with GA, from 57% (Group A) to 80% (Group E) (Table 1).
Table 1.
Study Population Demographics Grouped according to Gestational Age
| Gestational Age (weeks) | ||||||
|---|---|---|---|---|---|---|
| A: 24-27+6 (n= 258) |
B: 28-30+6 (n= 339) |
C:31-33+6 (n =902) |
D: 34-36+6 (n=5,056) |
E: 37-42 (n= 68,965) |
P-value a (Pairwise comparisons) |
|
| Age (years) | 28.3±6.8 | 26.9±6.7 | 26.6±6.8 | 27.4±6.7 | 27.7±6.1 | <0.001 (1,2,3,7,8,9,10) |
| African American |
51.7 | 48.9 | 43.8 | 36.4 | 22.0 | <0.001 (2,3,4,6,7,8,9,10) |
| Pre-pregnancy BMI (kg/m2) |
26.9±7.4 | 26.6±7.3 | 27.1±7.9 | 26.6±7.3 | 25.6±6.2 | <0.001 (4,9,10) |
| Current BMI (kg/m2) |
30.5±7.7 | 30.8±7.2 | 31.8±7.9 | 32.0±7.6 | 31.2±6.3 | <0.001 (2,3,6,10) |
| Nulliparous | 44.2 | 58.1 | 53.0 | 50.8 | 46.8 | <0.001 (1,2,3,6,7,9,10) |
| Previous cesarean delivery |
7.4 | 3.5 | 4.6 | 3.7 | 3.2 | <0.001 (1,3,4,9,10) |
| Prior preterm delivery |
16.7 | 13.0 | 15.4 | 13.0 | 4.5 | <0.001 (4,7,9,10) |
| Vaginal delivery rate (live and stillbirths) (%) |
70.5 | 64.0 | 69.1 | 77.4 | 80.3 | <0.001 (3,4,6,7,8,9,1 0) |
| Vaginal delivery rate (live births only) |
56.9 | 54.2 | 66.7 | 77.1 | 80.2 | <0.001 (2,3,4,5,6,7,8,9,10) |
Data presented as % unless stated otherwise.
Overall difference across all GA groups assessed by ANOVA. Additional result of pairwise comparisons is listed in the parentheses and denoted by the following numbers: 1: A≠B, 2: A≠C, 3: A≠D, 4: A≠E, 5: B≠C, 6: B≠D, 7: B≠E, 8: C≠D, 9: C≠E, 10: D≠E
Hypertensive disease was the precursor indication for preterm IOL in 35 % of cases in Group A, 51% in Group B, 53% in Group C and 41% in Group D (Table 2). Within this category, the most common underlying pathophysiology was preeclampsia, followed by chronic hypertension. Fetal anomalies (25-33%) and antepartum stillbirth accounted for up to one third of preterm inductions at less than 31 weeks. By comparison, hypertensive disease, followed by fetal (25%) and maternal (24%) conditions were the most common indications for delivery in GA groups at or beyond 31 weeks. Premature rupture of membranes preceded 20% to 25% of preterm labor inductions. Chorioamnionitis was noted in up to 15% of cases before 31 weeks, but occurred less often beyond 34 weeks (3%). Rates of gestational and pre-existing diabetes were similar across GA groups. Unspecified fetal and maternal reasons were the most common precursors to induction in the term IOL control group.
Table 2. Clinical Precursors Preceding Induction of Labor by Gestational Age.
| Gestational Age (weeks) | ||||||
|---|---|---|---|---|---|---|
| Precursora | Group A: 24-27+6 (n= 258) |
Group B: 28-30+6 (n= 339) |
Group C: 31-33+6 (n =902) |
Group D: 34-36+6 (n=5,056) |
Group E: 37-42 (n= 68,965) |
P-valueb |
| Premature rupture of membranes |
19.8 | 19.2 | 25.3 | 19.6 | 5.5 | <0.001 |
| Chorioamnionitis | 15.1 | 10.9 | 5.8 | 2.9 | 3.3 | <0.001 |
| Decidual hemorrhage/abruption |
8.9 | 12.1 | 7.3 | 3.3 | 0.8 | <0.001 |
| Hypertensive disease (overall category) |
35.3 | 51.0 | 52.7 | 40.9 | 14.2 | <0.001 |
| Gestational Hypertension |
8.1 | 10.6 | 12.5 | 13.2 | 6.1 | <0.001 |
| Preeclampsia | 20.5 | 36.9 | 39.8 | 26.7 | 6.5 | <0.001 |
| Superimposed preeclampsia |
12.4 | 17.7 | 16.6 | 8.8 | 1.4 | <0.001 |
| Eclampsia | 2.3 | 2.1 | 1.4 | 0.7 | 0.1 | <0.001 |
| Chronic hypertension | 16.3 | 20.9 | 21.1 | 13.6 | 3.98 | <0.001 |
| Unspecified hypertensive disease |
2.3 | 3.8 | 2.3 | 3.0 | 1.3 | <0.001 |
| Maternal medical conditionc |
17.8 | 20.7 | 23.7 | 24.0 | 13.8 | <0.001 |
| Maternal Pregestational Diabetes |
7.4 | 5.0 | 8.0 | 7.4 | 2.4 | <0.001 |
| Maternal Gestational Diabetes |
5.0 | 5.3 | 8.4 | 9.3 | 6.1 | <0.001 |
| Fetal anomaly | 32.6 | 24.8 | 16.1 | 9.7 | 5.7 | <0.001 |
| Antepartum stillbirth (IUFD) |
31.4 | 15.9 | 7.7 | 1.9 | 0.4 | <0.001 |
| Fetal conditiond | 28.7 | 28.6 | 25.4 | 24.7 | 15.4 | <0.001 |
| Maternal fever on admission |
14.0 | 10.0 | 6.8 | 5.4 | 6.1 | <0.001 |
| Admission for fetal reason, not otherwise specifiede |
0.4 | 0.3 | 0.1 | 0.3 | 0.5 | 0.136 |
| Admission for maternal reason, not otherwise specifiede |
1.6 | 1.2 | 1.8 | 4.9 | 18.2 | <0.001 |
| History of maternal/obstetric conditionf |
0.4 | 0 | 0.2 | 0.2 | 0.3 | 0.870 |
| History of fetal condition |
3.9 | 2.7 | 2.0 | 3.4 | 2.9 | 0.125 |
| Prior uterine scar | 7.8 | 3.8 | 4.6 | 3.8 | 3.2 | <0.001 |
Data presented as %
Sum of precursors can exceed 100% because women could have more than one precursor.
From analysis of variance assessing overall relationship between variable and gestational age category.
Maternal medical conditions are maternal medical problems. The percent of women with diabetes is listed.
Fetal conditions included conditions such as intrauterine growth restriction and abnormal antenatal testing.
Admission for fetal or maternal reasons were included only if there was no other pregnancy condition
History of fetal or maternal/obstetrical conditions included pregnancy complications in a prior pregnancy (e.g. history of fetal demise, or traumatic first delivery, respectively).
e and f are the only two indicated categories that are exclusive of other indications.
One site did not provide indications for inductions and was excluded.
From 28+0 to 36+6 weeks GA, nulliparous and parous women who delivered vaginally following IOL had a higher median simplified Bishop score when compared with those who subsequently required cesarean delivery (Table 3). Intravenous oxytocin infusion was the most common method of induction, regardless of gestational age, parity or cervical favorability (data not shown). Overall, misoprostol and prostaglandin E2 were used more commonly than mechanical methods to ripen an unfavorable cervix, whereas use of mechanical ripening was similar in nulliparas, both at term and preterm (data not shown).
Table 3. Simplified Bishop Score on Admission by Gestational Age.
| A. Nulliparous† | |||||
|---|---|---|---|---|---|
| Gestational Age (weeks) | |||||
| Group A: 24-27+6 (n= 60) |
Group B: 28-30+6 (n= 112) |
Group C: 31-33+6 (n =309) |
Group D: 34-36+6 (n=1,668) |
Group E: 37-42 (n= 23,215) |
|
| (VD, C/S)§ | (VD, C/S) | (VD, C/S) | (VD, C/S) | (VD, C/S) | |
| Median Simplified Bishop Score |
(3, 0.5)* | (3, 1.5)* | (3, 2)* | (4, 2)* | (5, 3)* |
| Simplified Bishop Score > 4 (%) |
(21.1, 9.1) | (35.3, 20.5) | (35.1, 6.7)* | (39.5, 13.0)* | (53.5, 31.5)* |
| B. Parous‡ | |||||
|---|---|---|---|---|---|
| Gestational Age (weeks) | |||||
| Group A: 24-27+6 (n= 67) |
Group B: 28-30+6 (n= 80) |
Group C: 31-33+6 (n =258) |
Group D: 34-36+6 (n=1,633) |
Group E: 37-42 (n= 26,641) |
|
| (VD, C/S) | (VD, C/S) | (VD, C/S) | (VD, C/S) | (VD, C/S) | |
| Median Simplified Bishop Score |
(2, 1) | (3, 2)* | (3, 2)* | (4, 3)* | (5, 3)* |
| Simplified Bishop Score > 4 (%) |
(26.5, 5.6) | (25.4, 0)* | (32.1, 15.2)* | (37.1, 15.7)* | (53.9, 29.7)* |
Only 71% (25364/35615) of records of nulliparous women had a complete simplified bishop score.
Only 72% (28679/39905) of records of parous women had a complete simplified bishop score.
VD: Vaginal delivery, C/S: Cesarean delivery
p <0.05 (comparison made within same gestational age group)
The odds ratios for vaginal delivery as a function of GA group compared with women undergoing induction at term were: Group A 0.49 (95% CI 0.30-0.79), Group B 0.45 (95% CI 0.31-0.65), Group C 0.69 (95% CI 0.55-0.87), and Group D 1.07 (95% CI 0.96-1.19).Not surprisingly, parity was the strongest predictor of vaginal delivery (OR =6.78, 95% CI 6.38-7.21), followed by the presence of a favorable cervix (OR =2.35, 95% CI 2.23-2.48). In contrast, older maternal age and current BMI were significant but minor negative predictors of vaginal delivery. There was no statistically significant difference in the odds of vaginal delivery between Groups A, B and C. Similarly, there was no statistically significant difference in the odds for vaginal delivery between Groups D and E. However, after controlling for the other variables, women with a gestational age <34 weeks were less likely to deliver vaginally compared to those with a gestational age at 34 weeks or above.
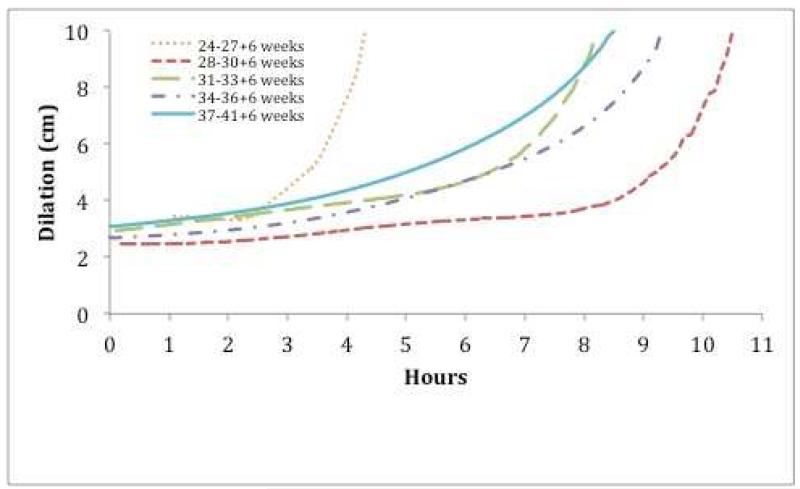
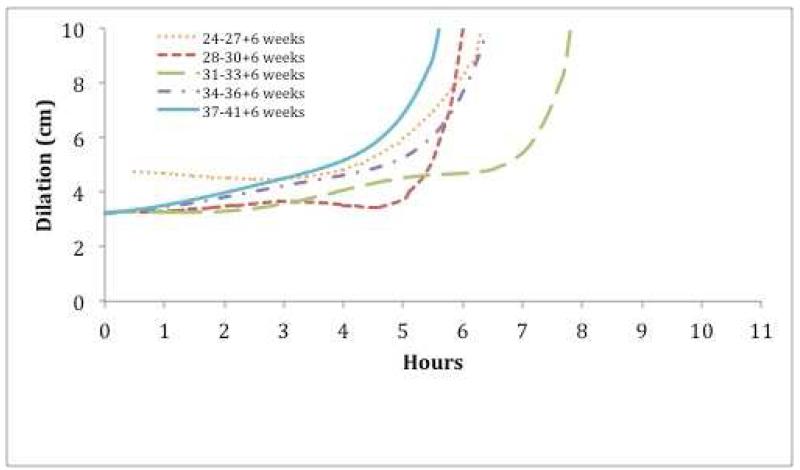
In nulliparous women, labor appeared to accelerate with increasing GA, ultimately approaching the rate observed after 37 weeks, except for those under 27 weeks GA (Figure 2A). In parous women, labor curves were comparable across all GA groups. Overall, latent labor was longer for nulliparous compared to parous women (Figure 2B). For all subjects, labor accelerated after 4 to 6 cm of cervical dilation. Regardless of parity, the curve for the active phase labor, represented by the period of increasing slope, was similar across gestational age groups.
Figure 2.
Average Labor Curves for Live Births Following Preterm Induction of Labor
A. Nulliparous Women
B. Parous Women
Cervical dilation over time for (A) nulliparous women and (B) parous women.
Comment
This study describes maternal and obstetric characteristics of preterm induction of labor in a large, modern cohort of pregnant women across the United States. Vaginal delivery rates increased with gestational age. Hypertensive disease, maternal conditions including diabetes mellitus, and fetal conditions were the most common indications for induction. Parity significantly increased the likelihood of a successful vaginal delivery. While indications for induction differed by gestational age, labor curves were similar overall, with minimal differences comparing the active phases across gestational ages.
In our study, vaginal delivery occurred in 57% to 80% of women undergoing preterm IOL, increasing with gestational age. The increase in vaginal delivery rates with increasing gestational age was comparable to, but greater than, that of Nassar and colleagues,18 who reported a success rate of 31.6% at ≤28 weeks, and 62.5% at >32 weeks in their series of 145 patients undergoing IOL for severe preeclampsia remote from term. The higher vaginal delivery rates we observed may be related to heterogeneity in the indications for IOL in our cohort, including conditions with less threatening maternal and fetal risks than severe preeclampsia.
Published strategies to predict the likelihood of vaginal delivery at the time of induction have had low predictive value.5, 6, 19 Our analysis confirmed a highly significant effect of parity in predicting vaginal delivery in women undergoing preterm IOL. In seeking to assess cervix favorability and the likelihood of a successful vaginal delivery, limitations in our database required us to rely on a simplified Bishop score, evaluating cervical dilatation, station, and effacement. However, this simplified Bishop score has been shown previously to predict vaginal delivery comparably to the original Bishop score.16 Our study demonstrated that, as in term pregnancies, cervical favorability predicted a higher likelihood of vaginal delivery. By contrast, obesity was a negative predictor of vaginal delivery. This observation is in accord with a significant association between BMI the risk for cesarean delivery in laboring women at term.10 Furthermore, obesity has been linked to a slower labor progression, mainly due to a prolonged latent phase.11
In nulliparas, preterm labor following induction accelerates with increasing GA, eventually approximating the labor curve of patients undergoing induction at term. Qualitative comparison of labor curves revealed that nulliparas have a longer duration of labor compared to parous women, mainly due to a longer length of the latent phase. The active phase of labor was similar across the range of gestational ages, regardless of parity. We only analyzed the labor curves from live births, since management of stillbirth can differ, especially at early gestational age. Nulliparous women undergoing preterm IOL before 28 weeks appear to have an accelerated labor with a short latent phase. This may be due to a small number of subjects, limited cervical examinations and poor documentation of the start of the induction, limiting conclusions based on this finding. Overall, our findings are comparable to recent data on labor progression. In a previous report from the CSL, Zhang and colleagues reported that spontaneous labor in singleton gestations at term progressed rapidly following 6 cm dilation,14 differing from Friedman’s classic observation that the active phase starts at 4 cm dilation.9, 20 A recent analysis of IOL cases in the CSL cohort noted that most cesarean deliveries occurred during the latent phase at <6 cm dilation for fetal indications or in the settings of preeclampsia or diabetes mellitus.15 Nassar et al18 reported that 88.2% of cesarean delivery due to “failure to progress,” occurred in the latent phase in women with a diagnosis of severe preeclampsia remote from term. Labor progress to complete cervical dilation was slower in women undergoing induction compared to those laboring spontaneously, primarily due to a longer latent phase.21 Taken together, these data suggest that both maternal and obstetric characteristics should be considered in clinical assessment of labor progression.
Our study was limited by some missing data on the clinical indications for induction. We inferred missing clinical indications by using patient-level information available on other medical, obstetrical or fetal conditions. However, it is possible that those precursor conditions may not have been the actual clinical indications for delivery and that the true incidence of indicated precursors was less than we reported. The majority of cases with unknown indications for IOL occurred in patients at term, most likely reflecting elective inductions. By contrast, some of the preterm cases with no recorded indication may be due to underreporting of maternal or fetal conditions. Incomplete information about methods of induction limited our ability to assess any potential association between a specific method and successful vaginal delivery. It seems likely that documentation of fewer than all three components of the simplified Bishop score, was based on clinicians’ preferences, rather than inherent differences among women undergoing induction. The retrospective nature of our study limited our ability to control for variation in clinical practice with regards to decision for induction, choice of induction methods, intervals for cervical examination and decision for cesarean delivery. While our results may not be applicable to all populations, the major strengths of the study are the large sample size of this modern obstetrical cohort across the United States and the use of electronic medical records with validation of data collection, rather than administrative or birth certificate database.
In summary, our findings suggest that vaginal delivery is likely following preterm IOL irrespective of gestational age. Parity and cervical favorability are the first and second strongest predictors of a successful vaginal delivery, respectively. Labor curves accelerate with each category of GA, ultimately approximating those observed following term IOL. In the absence of fetal or maternal contraindications, IOL should be considered in women requiring preterm delivery and considerations should be made for gestational age in assessing labor progress.
Condensation: Vaginal delivery occurs in most women undergoing preterm induction of labor, more frequently in parous women with cervical favorability. Gestational age has a limited effect on labor curves.
Acknowledgment
The Consortium on Safe Labor was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Contract No. HHSN267200603425C. Institutions involved in the Consortium include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas. The named authors alone are responsible for the views expressed in this manuscript, which does not necessarily represent the decisions or the stated policy of the NICHD. This analysis was supported, in part, by a grant from National Center for Advancing Translational Sciences, UL1TR000101, to the Georgetown-Howard Universities Center for Clinical and Translational Science.
The Consortium on Safe Labor was supported by National Institute of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development Contract No. HHSN267200603425C. This analysis was supported, in part, by a grant from National Center for Advancing Translational Sciences, UL1TR000101, to the Georgetown-Howard Universities Center for Clinical and Translational Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
References
- 1.Hamilton BE Mj, Ventura SJ. National vital statistics reports web release. 2011. Births: Preliminary Data for 2010; p. 60. [PubMed] [Google Scholar]
- 2.Lewit EM, Baker LS, Corman H, Shiono PH. The direct cost of low birth weight. Future Child. 1995;5:35–56. [PubMed] [Google Scholar]
- 3.Russell RB, Green NS, Steiner CA, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists ACOG Practice Bulletin. Assessment of risk factors for preterm birth. Clinical management guidelines for obstetrician-gynecologists. Number 31, October 2001. (Replaces Technical Bulletin number 206, June 1995; Committee Opinion number 172, May 1996; Committee Opinion number 187, September 1997; Committee Opinion number 198, February 1998; and Committee Opinion number 251, January 2001) Obstet Gynecol. 2001;98:709–16. [PubMed] [Google Scholar]
- 5.Friedman EA, Niswander KR, Bayonet-Rivera NP, Sachtleben MR. Relation of prelabor evaluation to inducibility and the course of labor. Obstet Gynecol. 1966;28:495–501. [PubMed] [Google Scholar]
- 6.Hughey MJ, Mcelin TW, Bird CC. An evaluation of preinduction scoring systems. Obstet Gynecol. 1976;48:635–41. [PubMed] [Google Scholar]
- 7.Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281–7. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Troendle J, Mikolajczyk R, Sundaram R, Beaver J, Fraser W. The natural history of the normal first stage of labor. Obstet Gynecol. 2010;115:705–10. doi: 10.1097/AOG.0b013e3181d55925. [DOI] [PubMed] [Google Scholar]
- 9.Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567–89. doi: 10.1016/s0029-7844(02)02398-0. [DOI] [PubMed] [Google Scholar]
- 10.Kominiarek MA, Vanveldhuisen P, Hibbard J, et al. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264 e1–7. doi: 10.1016/j.ajog.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kominiarek MA, Zhang J, Vanveldhuisen P, Troendle J, Beaver J, Hibbard JU. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244 e1–8. doi: 10.1016/j.ajog.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey AB, Nicholson JM, Cheng YW, Lyell DJ, Washington AE. Induction of labor and cesarean delivery by gestational age. Am J Obstet Gynecol. 2006;195:700–5. doi: 10.1016/j.ajog.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Cahill AG, Roehl KA, Odibo AO, Zhao Q, Macones GA. Impact of fetal gender on the labor curve. Am J Obstet Gynecol. 2012;206:335 e1–5. doi: 10.1016/j.ajog.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326 e1–26 e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. 2012;206:486 e1–9. doi: 10.1016/j.ajog.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laughon SK, Zhang J, Reddy UM. Using a simplified bishop score to predict vaginal delivery. Obstet Gynecol. 2011;118:360. doi: 10.1097/AOG.0b013e3182114ad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OBSTETRICS ACOPB-- ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114:386–97. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 18.Nassar AH, Adra AM, Chakhtoura N, Gomez-Marin O, Beydoun S. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am J Obstet Gynecol. 1998;179:1210–3. doi: 10.1016/s0002-9378(98)70133-4. [DOI] [PubMed] [Google Scholar]
- 19.Dhall K, Mittal SC, Kumar A. Evaluation of preinduction scoring systems. Aust N Z J Obstet Gynaecol. 1987;27:309–11. doi: 10.1111/j.1479-828x.1987.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedman EA. Labor in multiparas; a graphicostatistical analysis. Obstet Gynecol. 1956;8:691–703. [PubMed] [Google Scholar]
- 21.Harper LM, Caughey AB, Odibo AO, Roehl KA, Zhao Q, Cahill AG. Normal progress of induced labor. Obstet Gynecol. 2012;119:1113–8. doi: 10.1097/AOG.0b013e318253d7aa. [DOI] [PubMed] [Google Scholar]