Abstract
We have recently shown that several classes of glucuronide metabolites, including the morphine metabolite morphine-3-glucuronide and the ethanol metabolite ethyl glucuronide, cause toll like receptor 4 (TLR4)-dependent signalling in vitro and enhanced pain in vivo. Steroid hormones, including estrogens and corticosterone, are also metabolized through glucuronidation. Here we demonstrate that in silico docking predicts that corticosterone, corticosterone-21-glucuronide, estradiol, estradiol-3-glucuronide and estradiol-17-glucuronide all dock with the MD-2 component of the TLR4 receptor complex. In addition to each docking with MD-2, the docking of each was altered by pre-docking with (+)-naloxone, a TLR4 signaling inhibitor. As agonist versus antagonist activity cannot be determined from these in silico interactions, an in vitro study was undertaken to clarify which of these compounds can act in an agonist fashion. Studies using a cell line transfected with TLR4, necessary co-signaling molecules, and a reporter gene revealed that only estradiol-3-glucuronide and estradiol-17-glucuronide increased reporter gene product, indicative of TLR4 agonism. Finally, in in vivo studies, each of the 5 drugs was injected intrathecally at equimolar doses. In keeping with the in vitro results, only estradiol-3-glucuronide and estradiol-17-glucuronide caused enhanced pain. For both compounds, pain enhancement was blocked by the TLR4 antagonist lipopolysaccharide from Rhodobacter sphaeroides, evidence for the involvement in TLR4 in the resultant pain enhancement. These findings have implications for several chronic pain conditions, including migraine and tempromandibular joint disorder, in which pain episodes are more likely in cycling females when estradiol is decreasing and estradiol metabolites are at their highest.
Keywords: corticosterone, corticosterone-21-glucuronide, estradiol, estradiol-17-glucuronide, estradiol-3-glucuronide, TLR4, (+)-naloxone
1. Introduction
Recent evidence suggests that certain glucuronide metabolites have neuroinflammatory actions. Specifically, morphine-3-glucuronide, ethyl-β-D-glucuronide and glucuronic acid have been shown to activate the innate immune toll-like receptor 4 (TLR4) and cause TLR4-dependent enhanced pain when administered intrathecally (Lewis et al., 2010; Lewis et al., 2013) or systemically (Due et al., 2012). Another major class of molecules with glucuronidated metabolites is the steroid hormones. Androgens (Belanger et al., 1994), estrogens (Guillemette et al., 2004), and corticosteroids (Ikegawa et al., 2009) are all glucuronidated as part of metabolism. However, the potential for TLR4 activation and/or enhanced nociception by these glucuronidated metabolites has not been studied.
Several of the steroid hormones also have associations with enhanced pain, including corticosterone and estradiol. Acute stress and acute stress-associated corticosterone release in rats has, by and large, been shown to inhibit nociception, while chronic stress has been linked to hyperalgesia (Imbe et al., 2006), although analgesic effects have also been repeatedly reported (reviewed in (Miguez et al., 2014). Clinically, chronic stress has also been associated with chronic pain conditions such as fibromyalgia, chronic headache, inflammatory bowel disease and tempromandibular joint pain (McEwen and Kalia, 2010). Corticosterone, intriguingly, has both anti-inflammatory and pro-inflammatory effects on inflammation caused by the classic TLR4 ligand lipopolysaccharide (LPS). Corticosterone inhibits proinflammation by LPS when LPS precedes corticosterone, whereas corticosterone potentiates proinflammation by LPS when LPS is given 2 or 24 hours following corticosterone (Frank et al., 2010). One potential explanation for this finding is that the anti-inflammatory effect of corticosterone was modulated by a proinflammatory effect of its glucuronidated metabolite. Corticosterone is metabolized by extensive degradation or conjugation with a glucuronide group to form corticosterone-21-glucuronide (CortG). CortG has been found in mouse brain homogenates, indicating that it has the potential to directly affect the central nervous system (Kallonen et al., 2009; Maeda et al., 2013). In humans, the major stress hormone cortisol and several of its metabolites are also glucuronidated, with over 90% of cortisol metabolites in the urine being glucuronide conjugated (Kornel and Saito, 1975).
Large epidemiology studies, in different countries and cultures, show women are more likely to report chronic pain (Blyth et al., 2001; Johannes et al., 2010) and chronic pain that limits daily activities (Blyth et al., 2001). Estrogens, including estradiol, are the primary female sex hormones and have reports of both pro-inflammatory and pro-nociceptive activity as well as antinociceptive effects (for review, Craft, 2007). Notably, the drop from peak estradiol levels during pre-menses in women is associated with increased incidence of migraine (Martin and Behbehani, 2006; Somerville, 1972) and temporomandibular joint pain (LeResche et al., 2003). This period of declining estradiol coincides with peak to consistent levels of glucuronidated estradiol metabolites (Stanczyk et al., 1980), as glucuronidated metabolites typically have longer half-lives than parent molecules. The declining estradiol and steady glucuronidated metabolites in this time period increases the relative effect of the glucuronidated metabolites. Estradiol may also affect the LPS response. Chronic estradiol administration in ovariectomized females and male rats increased the LPS–stimulated inflammatory response in hippocampal microglia ex vivo and increased proinflammatory cytokine transcription in vivo (Loram et al., 2011a). Estradiol is metabolized into estradiol-3-glucuronide (E2-3-G) and estradiol-17-glucuronide (E2-17-G), and both of these metabolites have also been found in brain tissue homogenates, indicating that they have access to the central nervous system (Kallonen et al., 2009). Neither metabolite is believed to have activity at the estrogen receptors (Guillemette et al., 2004).
The first step to determine if glucuronidated corticosterone and estradiol metabolites could contribute to the pain-enhancing effects of the parent hormones is to determine if the metabolites have the ability to potentiate pain. Given the TLR4-dependent allodynia caused by other glucuronidated metabolites, we hypothesize that CortG, E2-3-G and E2-17-G will cause an increase in TLR4 signaling as well as TLR4-dependent enhanced pain.
2. Materials and methods
2.1. Drugs
Corticosterone, estradiol, E2-3-G, and E2-17-G were purchased from Sigma (St. Louis, MO). CortG was synthesized by the authors (MMF, TS) from D-(+)-glucurono-6,3-lactone. The lactone was converted to the protected trichloroacetimidate by the procedure of Nakajima et al. (2005), coupled with corticosterone, then deprotected according to the procedure of Ciuffredaa et al. (2009). The identity of the product was confirmed by comparison of 1H and 13C NMR data with that reported by Ciuffredaa et al. (2009). The competitive TLR4 antagonist lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS) was purchased from Invivogen (Thousand Oaks, CA) and (+)-naloxone was obtained from the National Institute on Drug Abuse, synthesized by an author (KR). CortG, E2-3-G, LPS-RS and (+)-naloxone were dissolved in endotoxin-free sterile water (Hospira, Lake Forest, IL) for in vitro studies (Experiment 2) and endotoxin-free, sterile 0.9% saline (Hospira, Lake Forest, IL) for in vivo studies (Experiment 3). E2-17-G was dissolved in 10% DMSO (Sigma, St. Louis, MO) and sterile water for in vitro studies (Experiment 2) and 1% DMSO and sterile saline for in vivo studies (Experiment 3). Corticosterone and estradiol were dissolved in 100% DMSO for both in vitro and in vivo studies. Corticosterone, CortG, estradiol, E2-3-G, and E2-17-G, (+)-naloxone, saline and water were all confirmed to be endotoxin-free by the limulus amebocyte lysate (LAL) assay (Lonza, Walkersville, MD). Where appropriate, doses are reported as a free base concentration.
2.2. In silico docking simulations
In silico docking simulation methods were similar to those previously described in detail (Hutchinson et al., 2012; Hutchinson et al., 2010b). These were employed to examine the docking of corticosterone, CortG, estradiol, E2-3-G, and E2-17-G to the TLR4/MD-2 complex. The in silico docking analyses were conducted in Experiment 1 using the recently published high-resolution crystalline structure of the dimer of human TLR4 and MD-2 (Park et al., 2009) and the in silico docking software vina, PyRx and the AutoDockTools package. Briefly, the complexed human TLR4 and MD-2 pdb file was obtained from RCSB Protein Data Bank database (PDBID: 3fxi). Docking was conducted using Vina (version 1.1.2; (Trott and Olson, 2010) within PyRx (version 0.8; (Wolf, 2009)). An exhaustiveness factor of 8 was used for all simulations, with the Vina search space dimensions and center defined using the auto-maximize function. Structures were gathered using PubChem isomeric SMILES then converted to .pdb using a structure file generator (http://cactus.nci.nih.gov/services/translate/).
2.3. In vitro assay for TLR4 signaling
A human embryonic kidney-293 (HEK 293) cell line was used in Experiment 2. This cell line was stably transfected by Invivogen (San Deigo, CA) to over-express human TLR4 and co-receptor molecules (MD-2, CD14) (293-htlr4a-md2cd14; referred to here as HEK-TLR4). In addition, these cells stably express an optimized alkaline phosphatase reporter gene under the control of a promoter inducible by transcription factors, such as NFκB and AP-1, activated as part of the TLR4 signaling cascade. Secreted alkaline phosphatase (SEAP) protein is produced as a consequence of TLR4 activation.
HEK-TLR4 cells were grown at 37°C (5% CO2; VWR incubator model 2300) in 10 cm dishes (Greiner Bio-One, CellStar 632171; Monroe, NC, USA) in normal supplement selection media (DMEM media [Invitrogen, Carlsbad, CA, USA] supplemented with 10% fetal bovine serum [Hyclone; Logan, UT, USA], HEK-TLR4 selection [Invivogen]; Penicillin 10,000 U/ml [Invitrogen]; streptomycin 10 mg/ml [Invitrogen], Normocine [Invivogen], and 200 nM L-Glutamine [Invitrogen]). The cells were then plated for 48 h in 96 well plates (Microtest 96 well plate, flat bottom, Becton Dickinson; 5 × 103 cells/well) with the same media. After 48 h, supernatants were removed and replaced with fresh media. Drugs tested were added in concentrations indicated and incubated for 24 h. Supernatants (15 μl) were then collected from each well for immediate assay.
SEAP levels in the supernatants were assayed using the Phospha-Light System (Applied Biosystems) according to the manufacturer’s instructions. This chemiluminescent assay incorporates Tropix CSPD chemiluminescent substrate. The 15 μl test samples were diluted in 45μl of 1x dilution buffer, transferred to 96-well plates (Thermo, Walthma, MA, USA), and heated at 65°C in a water bath (Model 210, Fisher Scientific, Pittsburgh, PA, USA) for 30 min, then cooled on ice to room temperature. Assay buffer (50 μl/well) was added and, 5 min later, reaction buffer (50 μl/well) is added and allowed to incubate for 20 min at room temperature. The light output is then measured in a microplate luminometer (Dynex Technologies, #IL213.1191, Chantilly, VA, USA).
2.4. In vivo behavioral assessments
2.4.1. Animals
Adult, male, pathogen-free Sprague-Dawley rats (Harlan Labs, Madison, WI) were used for all in vivo experiments. Rats (325–375 g at time of experiments) were housed in temperature (23±3 °C) and light (12 h: 12 h light:dark) controlled rooms with water and food given ad libitum. All habituation and behavioral testing procedures were performed during the light phase of the daily cycle. All procedures were approved by the University of Colorado-Boulder Institutional Animal Care and Use Committee and in compliance with the International Association for the Study on Pain guidelines for the ethical use of animals. Each experimental group contains 6–8 rats.
2.4.2. Intrathecal catheter implantation and injections
Acute intrathecal injections were used to administer drugs in Experiment 3. Catheter placements via the L5/L6 intervertebral approach and drug microinjections were performed based on Milligan et al. (1999). Rats were briefly anaesthetized under isoflurane anesthesia. An 18-gauge needle was placed between L5 and L6 into the intrathecal space and served as a guide. Polyethylene-10 tubing was threaded rostrally through the guide, terminating over the lumbosacral enlargement. The catheters were pre-loaded with drugs at the intrathecal end and the remainder filled with sterile saline. Upon insertion, the drug was injected with an 8.5 μl flush to ensure complete drug delivery, and the needle and catheter then removed. The rats were anaesthetized for, on average, 5 min and had no skin incision or implantations upon awakening and hence required no additional analgesics.
All drugs administered in vivo in Experiment 3 were given intrathecally over lumbosacral spinal cord. All doses were equimolar with the doses of glucuronide metabolites (morphine-3-glucuronide, ethyl glucuronide and glucuronic acid) that were sufficient to cause allodynia in prior studies (Lewis et al., 2010; Lewis et al., 2013). Corticosterone was given at a dose of 0.56 μg and CortG was given at a dose of 0.85 μg. Estradiol was given at a dose of 0.44 μg, E2-3-G and E2-17-G were both given at doses of 0.76 μg. All doses were equimolar to each other. LPS-RS was given at an intrathecal dose of 40 μg, consistent with previous literature showing this dose was capable of reversing neuropathic pain (Hutchinson et al., 2008).
2.4.3. von Frey test for tactile allodynia
All testing was conducted by an experimenter blind to group assignment. Rats received at least four 1 h habituations to the appropriate testing apparatus and environment prior to baseline testing, as in our previous studies (Lewis et al., 2010).
The response threshold to light touch on both plantar hind paws was measured using calibrated microfilaments (von Frey hairs; Stoelting, Wood Dale, IL, USA), as described in Milligan et al. (2001). Briefly, a logarithmic range of hairs from 0.406-15.136 g force were used, allowing both analgesia and allodynia to be measured, following the standard up-down procedures previously described (Chaplan et al., 1994). Responses were fitted to a Gaussian integral psychometric function using a maximum-likelihood fitting method as described in Milligan et al. (2001).
2.5. Statistics
GraphPad Prism (version 5 for Windows, San Diego, CA) software was used for all statistical analyses. One-way ANOVAs with appropriate Bonferroni post hocs were used to compare experimental groups on in vitro experiments and to confirm that there were no baseline differences on behavioral measures. Two-way repeated-measures ANOVAs with Bonferroni post-hoc tests when appropriate were used to determine statistical significance between behavioral measures. For all analyses, p<0.05 was considered significant.
2.6. Experimental Procedures
2.6.1. Experiment 1A: Does in silico docking predict that corticosterone, CortG, estradiol, E2-3-G, and/or E2-17-G will bind to TLR4/MD-2?
Initially, the in silico docking of corticosterone, CortG, estradiol, E2-3-G, and E2-17-G to the entire TLR4 MD-2 dimer complex was conducted. All the ligands were docked to MD-2 alone with greater resolution. The lowest energy and highest interaction docking conformation was visualized and the residues on MD-2 that interacted with that conformation determined for each of the experimental drugs.
2.6.2. Experiment 1B: For drugs predicted to dock to the TLR4/MD-2 complex in Experiment 1A, would previously docked (+)-naloxone reduce the likelihood of their in silico docking?
The (+)-naloxone conformation from a previous study (Lewis et al., 2010) was saved as a combined MD-2/(+)-naloxone pdb file and the corticosterone, CortG, estradiol, E2-3-G, and/or E2-17-G in silico docking was repeated on the combined MD-2/(+)-naloxone complex to determine the change in docking due to the presence of already docked (+)-naloxone.
2.6.3. Experiment 2A: Does in vitro corticosterone, CortG, estradiol, E2-3-G, and/or E2-17-G cause an increase in TLR4-dependent SEAP expression in HEK-TLR4 cells?
The experimental drugs, corticosterone, CortG, estradiol, E2-3-G, and/or E2-17-G were each applied to HEK-TLR4 cells at concentrations of 0, 1, 10 and 100 μM as described above. Cells were incubated for 24 h, then supernatants removed and assessed for SEAP activity, indicative of TLR4-induced NFκB activity.
2.6.4. Experiment 2B: Is the in vitro increase in TLR4-dependent SEAP expression seen in Experiment 2A blocked by the TLR4 inhibitors LPS-RS and/or (+)-naloxone?
For drugs which increase HEK-TLR4 SEAP production in Experiment 2A, the most effective dose (100 μM for each) was applied to HEK-TLR4 cells as described above. Additionally, one of two TLR4 antagonists was coadministered. One, lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS, Invivogen, San Diego, CA), lacks functional TLR4 activity and acts as a competitive TLR4 antagonist. LPS-RS was applied to the HEK-TLR4 cells at the same time as the estradiol, E2-3-G, or E2-17-G at concentrations of 0, 0.1, 1 or 10 ng/ml. The other, (+)-naloxone, is the opioid-inactive isomer of naloxone and has been shown to antagonize LPS-induced increases in HEK-TLR4 signaling (Hutchinson et al., 2010b). (+)-Naloxone was applied to the HEK-TLR4 cells at the same time as the estradiol, E2-3-G, and/or E2-17-G at concentrations of 0, 1, 10 or 100 μM. For all assays, cells were incubated for 24 h, then supernatants removed and assessed for SEAP activity, indicative of TLR4 activity.
2.6.5. Experiment 3: Does intrathecal corticosterone, CortG, estradiol, E2-3-G, and/or E2-17-G cause tactile allodynia? Is that allodynia TLR4 dependent?
Rats received either corticosterone (0.56 μg), CortG (0.85 μg), estradiol (0.44 μg), E2-3-G (0.76 μg), and/or E2-17-G (0.76 μg) or equivolume (1 μl) vehicle via an acute intrathecal injection, as described above. Both injections had a saline flush for a total injection volume of 9.5 μl. Rats were tested for tactile allodynia 1 d prior to injection (baseline; BL) and again 1 and 3 h post-injection.
The drugs that produced significant allodynia (in this case, E2-3-G and E2-17-G) were coadministered with the TLR4 antagonist LPS-RS. Rats received 0.76 μg of either E2-3-G, E2-17-G or equivolume (1 μl) saline with 40 μg LPS-RS via an acute intrathecal injection. LPS-RS was selected over (+)-naloxone due to its longer half-life which permitted coadministration and assessment of pain behaviors 3 hours later.
3. Results
3.1. Experiment 1A: In silico modeling predicts that corticosterone, CortG, estradiol, E2-3-G, and E2-17-G will bind to MD-2
An in silico docking study was done to determine if corticosterone, CortG, estradiol, E2-3-G, and/or E2-17-G would be predicted to dock to the TLR4/MD-2 complex. This in silico technique has been successfully used previously to model the docking of morphine-3-glucuronide, other opioid drugs, tricyclic antidepressants, ethanol glucuronide, glucuronic acid and other compounds (Hutchinson et al., 2010a; Hutchinson et al., 2010b; Lewis et al., 2010; Lewis et al., 2013).
The in silico docking of corticosterone, CortG, estradiol, E2-3-G, and E2-17-G to the entire TLR4 MD-2 dimer complex was initially conducted (AutoGrid center set 3.438, -7.805, 2.034; 126 grid points expanding in all directions; genetic algorithm running number of 100, Max Evals 5 × 106 and 1.0 Å spacing). These data demonstrated that the ligands each docked with human MD-2 independent of human TLR4 interactions. Therefore, the ligands were then docked to MD-2 alone with greater resolution. The lowest energy and highest interaction docking conformation was visualized for each drug and the residues on MD-2 that each drug interacted with are listed in Table 1. Additionally, corticosterone, CortG, estradiol, E2-3-G, and E2-17-G all had a preferred conformation with a negative predicted docking energy, suggesting that they would be likely to dock to MD-2.
Table 1.
In silico docking studies predict that corticosterone (Cort), CortG, estradiol (E2), E2-3-G, and E2-17-G will dock to the MD-2 portion of the TLR4-MD-2 complex. Residues that interacted with lipid A chains in the crystal structure determined by Park et al., (2009) are included for reference. The residues on MD-2 at which these compounds interact with MD-2 are denoted with an X. A B signifies a residue identified to be critical for TLR4 MD-2 interactions. When the docking simulations were repeated with (+)-naloxone ((+)-Nal) already bound to the MD-2 molecule, the residues at which each drug interacted were altered. The residues that lost interaction with MD2 when (+)-naloxone was already docked are denoted with a -- in the appropriate column. Residues that gained interaction with MD-2 when (+)-naloxone was already docked are denoted with a G. Note that these docking results have been obtained using Vina and supersede previous autodock 4 simulations.
| residue | lipid A | (+)-Nal | Cort alone (+)-Nal | Cort G alone (+)-Nal | Estradiol alone (+)-Nal | E2-3-G alone (+)-Nal | E2-17-G alone (+)-Nal | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | X | |||||||||||
| 32 | X | X | X | -- | X | -- | ||||||
| 46 | X | X | X | -- | X | -- | X | -- | ||||
| 48 | X | X | X | -- | X | -- | ||||||
| 52 | X | X | X | -- | X | -- | X | -- | ||||
| 54 | X | G | ||||||||||
| 61 | X | X | -- | X | X | G | ||||||
| 63 | X | X | -- | X | -- | X | X | G | ||||
| 65 | X | -- | X | X | ||||||||
| 71 | X | X | -- | X | -- | |||||||
| 74 | X | X | -- | -- | G | G | ||||||
| 76 | X | X | -- | X | X | G | ||||||
| 78 | X | G | X | X | G | |||||||
| 80 | X | G | G | |||||||||
| 87 | X | G | ||||||||||
| 90 | B | G | ||||||||||
| 92 | G | |||||||||||
| 94 | X | G | G | |||||||||
| 104 | X | X | -- | X | X | G | ||||||
| 117 | X | G | ||||||||||
| 119 | X | X | -- | G | ||||||||
| 121 | X | |||||||||||
| 126 | X | G | G | |||||||||
| 131 | X | G | ||||||||||
| 133 | G | G | G | |||||||||
| 135 | X | X | X | -- | ||||||||
| 147 | X | X | -- | X | X | |||||||
| 151 | X | X | X | -- | X | -- | X | -- | X | -- | ||
| 153 | X | X | -- | X | X | X | -- | |||||
3.2. Experiment 1B: (+)-Naloxone alters the predicted MD-2 docking conformations of corticosterone, CortG, estradiol, E2-3-G, and E2-17-G in silico
Given prior evidence that (+)-naloxone altered the predicted in silico docking of other glucuronide molecules and successfully blocked HEK-TLR4 signaling of these same molecules in vitro (Hutchinson et al., 2010b; Lewis et al., 2010; Lewis et al., 2013), we hypothesized that CortG, E2-3-G, and E2-17-G would dock on MD-2 such that when (+)-naloxone was already docked to MD-2, the drugs of interest would be less likely to dock. Similarly, in Experiment 1A, corticosterone and estradiol were also predicted to dock to MD-2 at similar residues to the glucuronide metabolites, and we hypothesize that this docking would also be altered by the presence of (+)-naloxone.
The in silico docking analysis performed in Experiment 1A was repeated utilizing a saved combined MD-2/(+)-naloxone pdb file to determine the change in docking due to the presence of (+)-naloxone already docked to the MD-2 molecule. Additionally, for all the molecules, the residues at which the lowest binding energy conformation in Experiment 1A interacted were altered by the presence of (+)-naloxone (Table 1). However, the functional effects of any of these compounds docking to MD-2 are beyond the capability of this in silico study.
3.3. Experiment 2A: In vitro TLR4-dependent SEAP expression in HEK-TLR4 cells is increased by E2-3-G and E2-17-G and decreased by estradiol and corticosterone
Experiment 1 provided initial evidence suggesting that corticosterone, CortG, estradiol, E2-3-G and E2-17-G may have effects on TLR4. In order to determine if these effects were present in a cellular system, HEK-TLR4 cells were utilized. As detailed in the methods, HEK-TLR4 cells have been transfected by Invivogen for overexpression of TLR4 and its co-receptors and an NFκB-dependent SEAP reporter gene. Thus, an increase in TLR4 signaling will lead to an increase in NFκB transcription and subsequent increase in SEAP levels measured. Previous studies have confirmed that HEK cells with the SEAP reporter gene but without TLR4 overexpression did not increase SEAP expression to the classic TLR4 agonist LPS (Hutchinson et al., 2010b). The dose-response functions for corticosterone, CortG, estradiol, E2-3-G and E2-17-G on HEK-TLR4 SEAP expression were calculated.
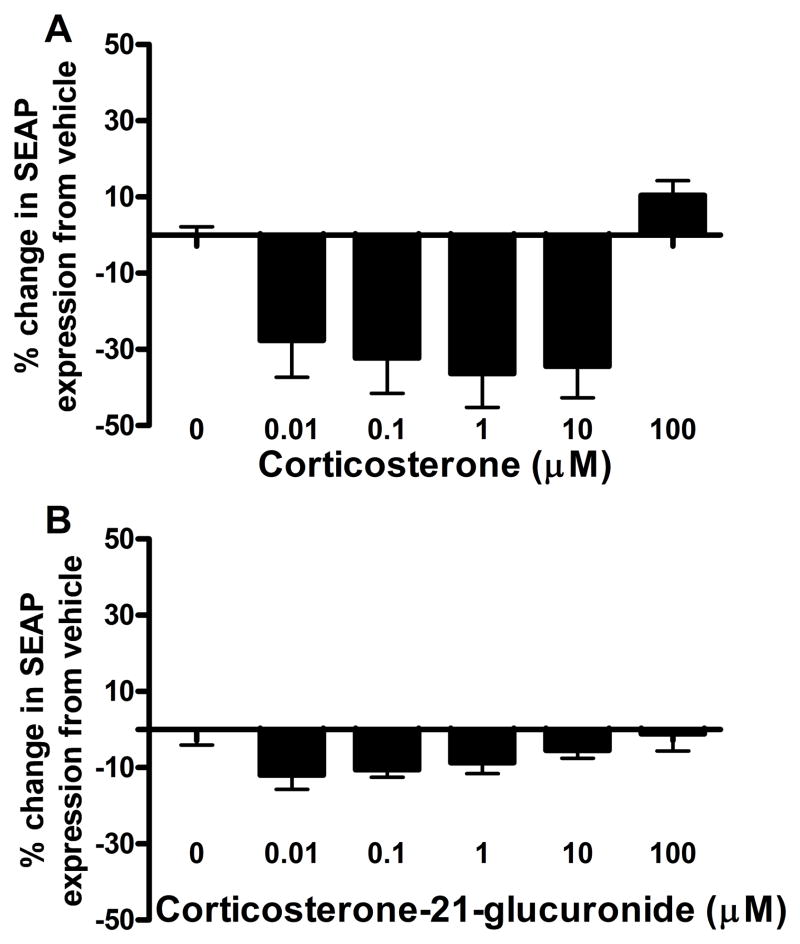
Corticosterone caused a significant decrease in SEAP production in the HEK-TLR4 cells relative to its 1% DMSO vehicle (F(5, 35)=9.05, p<0.05). Post-hoc tests showed that corticosterone decreased SEAP expression relative to vehicle at 0.1, 1 and 10 μM concentrations, but was not significantly different from vehicle at the 100 or 0.01 μM concentrations (Figure 1A). CortG, however, caused no significant alterations from its saline vehicle (F(5,23)=1.72, p>0.05, Figure 1B).
Figure 1.
Corticosterone caused a significant decrease in HEK-TLR4 cell SEAP reporter expression (F(5,25)=9.05, p<0.05, A). Bonferroni post-hoc tests showed a significant decreased between vehicle and 10 (t=1.15, p<0.05), 1 (t=1.84, p<0.05), and 0.1 (t=1.84, p<0.05) μM corticosterone concentrations. CortG did not cause any changes in HEK-TLR4 SEAP expression compared to vehicle (F(5,23)=1.72, p>0.05, B).
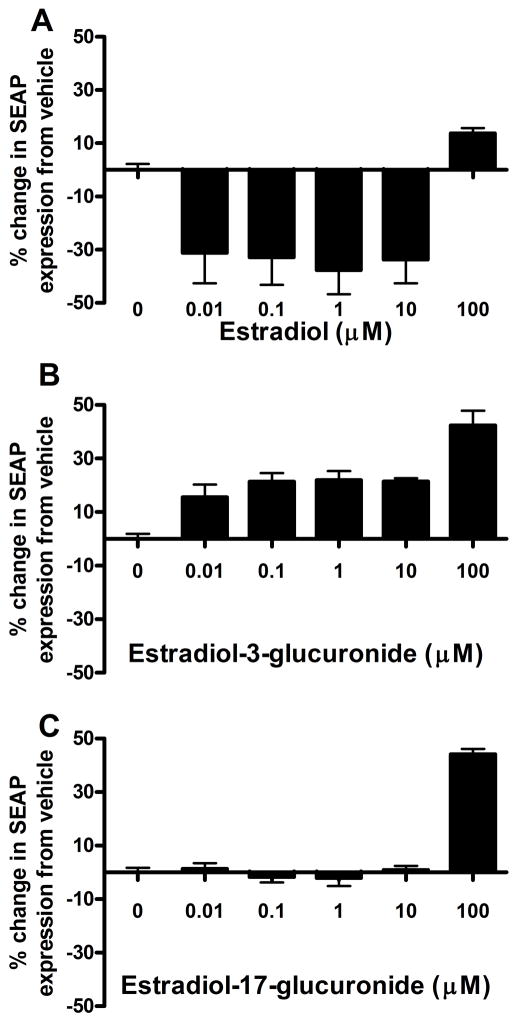
Estradiol caused a significant decrease in SEAP expression in the HEK-TLR4 cells (F(5, 30)=9.60, p<0.05). Post-hoc tests showed that estradiol decreased SEAP expression relative to vehicle at the 0.01, 0.1, 1 and 10 μM concentrations, but caused no change at the 100 μM concentration (Figure 2A). E2-3-G, on the other hand, caused an increase in SEAP production in the HEK-TLR4 cells (F(5, 21)=19.2, p<0.05). Post-hoc tests confirmed that all five concentrations of E2-3-G tested (0.01. 0.1, 1, 10 and 100 μM) were significantly increased relative to vehicle (Figure 2B). Finally, E2-17-G also caused a significant increase in HEK-TLR4 SEAP expression (F(5, 18)=72.81, p<0.05), but post-hoc tests showed this difference only at the 100 μM concentration (Figure 2C). The potential inhibitory effects of corticosterone and estradiol could be due to an effect on TLR4 or on other effects leading to decreased NFkB activation. As the focus of this paper is the glucuronide metabolite activation of TLR4, these effects remain for further investigation.
Figure 2.
Estradiol caused a significant decrease in HEK-TLR4 cell SEAP reporter gene expression relative to 1% DMSO vehicle (F(5, 30)=9.60, p<0.05, A). Bonferroni post-hoc tests showed a significant decrease from vehicle at 10 (t=3.696, p<0.05), 1 (t=4.14, p<0.05), 0.1 (t=3.61, p<0.05) and 0.01 (t=3.426, p<0.05) μM estradiol concentrations. E2-3-G caused a significant increase in HEK-TLR4 cell NFκB-dependent SEAP expression relative to water (F=19.2, p<0.05, B). A Bonferroni post-hoc test showed E2-3-G significantly increased SEAP expression at 100 (t=42.35, p<0.05), 10 (t=4.84, p<0.05), 1 (t=4.95, p<0.05), 0.1 (t=4.82, p<0.05) and 0.01 (t=3.505, p<0.05) μM E2-3-G concentrations. E2-17-G also caused a significant increase in HEK-TLR4 cell SEAP expression relative to 0.1% DMSO vehicle at the 100 μM concentration (F(5,18)=72.81, p<0.05, Bonferroni post-hoc t=14.63, p<0.05, C).
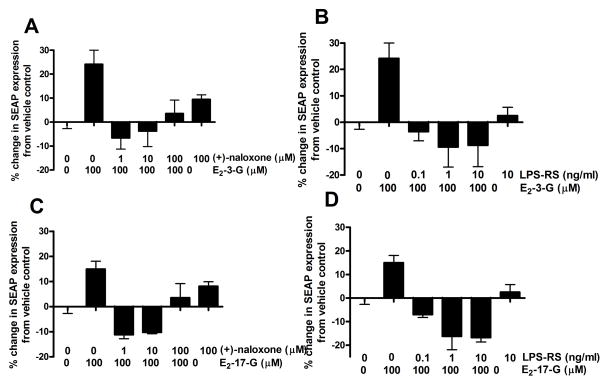
3.4. Experiment 2B: In vitro E2-3-G and E2-17-G induced increases in SEAP expression are blocked by the TLR4 inhibitors LPS-RS and (+)-naloxone
Both E2-3-G and E2-17-G caused a robust increase in SEAP expression in the HEK-TLR4 cells in Experiment 2A. To test whether this effect was via actions at TLR4, two TLR4 inhibitors, LPS-RS and (+)-naloxone, were separately coincubated with E2-3-G and E2-17-G to determine if the TLR4 inhibitors each blocked the SEAP increases seen with the estrogen degradation products alone. LPS-RS acts as a competitive antagonist of LPS binding to the TLR4 complex, as it is structurally similar to LPS but fails to produce a TLR4-dependent signaling-induced proinflammatory response. The second inhibitor, (+)-naloxone, has also been shown to antagonize the LPS response in vitro (Hutchinson et al., 2008) and in vivo (Hutchinson et al., 2009) and has been documented by a broad receptor, enzyme, and second messenger screen to have no identified off-target effects (Hutchinson et al., 2012).
(+)-Naloxone effectively blocked the increase in HEK-TLR4 cell SEAP expression caused by E2-3-G (F(5, 17)=6.89, p<0.05, Figure 3A) and E2-17-G (F(5,14)=9.17, p<0.05, Figure 3C). Posthoc tests showed that when 100 μM E2-3-G was incubated with 1 or 10 μM (+)-naloxone, SEAP expression was significantly decreased compared to 100 μM E2-3-G alone. Similarly, when 100 μM E2-17-G was incubated with 10 or 1 μM (+)-naloxone, SEAP expression was also significantly decreased compared to 100 μM E2-17-G alone.
Figure 3.
The TLR4 inhibitor (+)-naloxone significantly attenuated the HEK-TLR4 cell SEAP increases caused by 100 μM E2-3-G (F(5,17)=6.89, p<0.05, A), or 100 μM E2-17-G (F(5,14)= 9.18, p<0.05, C) at the 1 and 10 μM concentrations of (+)-naloxone. Bonferroni post-hoc tests showed a significant decrease in SEAP expression when 100 μM E2-3-G was incubated with 10 (t=4.49, p<0.05) or 1 μM (t=4.96, p<0.05) (+)-naloxone compared to 100 μM E2-3-G alone. Similarly, Bonferroni post-hoc tests showed a significant decrease in SEAP expression when 100 μM E2-17-G was incubated with 10 (t=5.06, p<0.05) or 1 μM (t=5.25, p<0.05) (+)-naloxone compared to 100 μM E2-3-G alone. The TLR4 antagonist LPS-RS significantly attenuated the HEK-TLR4 cell SEAP increases caused by 100 μM E2-3-G (F(5,19)=5.22, p<0.05, B) or 100 μM E2-17-G (F(5,16)=7.81, p<0.05, D) at all three concentrations tested (0.1, 1 and 10 ng/ml). Bonferroni post-hoc tests showed a significant decrease in SEAP expression when 100 μM E2-3-G was coincubated with 10 (t=4.29, p<0.05), 1 (t=4.38, p<0.05), or 0.1 (t=3.62, p<0.05) ng/ml LPS-RS. Similarly, Bonferroni post-hoc tests showed a significant decrease in SEAP expression when 100 μM E2-17-G was coincubated with 10 (t=4.29, p<0.05), 1 (t=4.38, p<0.05), or 0.1 (t=3.62, p<0.05) ng/ml LPS-RS.
LPS-RS also blocked the increase in HEK-TLR4 cell SEAP expression caused by E2-3-G (F(5,19)=5.22, p<0.05, Figure 3B) and E2-17-G (F(5, 16)=7.81, p<0.05, Figure 3D). Posthoc tests showed that when 100 μM E2-3-G was incubated with 0.1, 1 or 10 ng/ml LPS-RS, SEAP expression was significantly decreased compared to 100 μM E2-3-G alone. Similarly, when 100 μM E2-17-G was incubated with 0.1, 1 or 10 ng/ml LPS-RS, SEAP expression was also significantly decreased compared to 100 μM E2-17-G alone.
3.5. Experiment 3: E2-3-G and E2-17-G cause tactile allodynia that is blocked by the TLR4 antagonist LPS-RS
The in silico and in vitro studies above strongly suggest that E2-3-G and E2-17-G, but not corticosterone, CortG or estradiol, are capable of activating TLR4. Previous studies have shown that other glucuronide metabolites that can act as TLR4 agonists are able to produce pain in vivo (Lewis et al., 2010). To determine if the drugs investigated here caused increased tactile sensitivity, equimolar corticosterone, CortG, estradiol, E2-3-G and E2-17-G were injected via an acute intrathecal injection over the lumbar enlargement and allodynia measured 1 and 3 hours following injection. Experiments 1 and 2 predict that E2-3-G and E2-17-G will enhance TLR4 signaling. Previous studies suggest that TLR4 activity can cause enhanced pain in vivo (Hutchinson et al., 2008; Lewis et al., 2010). To determine if TLR4 activity was necessary for drugs that caused allodynia in this study, a TLR4 antagonist, LPS-RS was coadministered intrathecally and allodynia again assessed 1 and 3 hours post-injection. LPS-RS was chosen to test as it successfully blocked E2-3-G and E2-17-G induced SEAP expression in HEK-TLR4 cells in vitro and has previously been used to block allodynia induced by intrathecal glucuronic acid or ethyl glucuronide administration in vivo (Lewis et al., 2010).
No differences between right and left hind paw responsiveness were detected. Thus, results are reported as the average of the response thresholds recorded for both paws. There were also no baseline differences between any of the behavior groups (F(9, 52)=1.86, p>0.05).
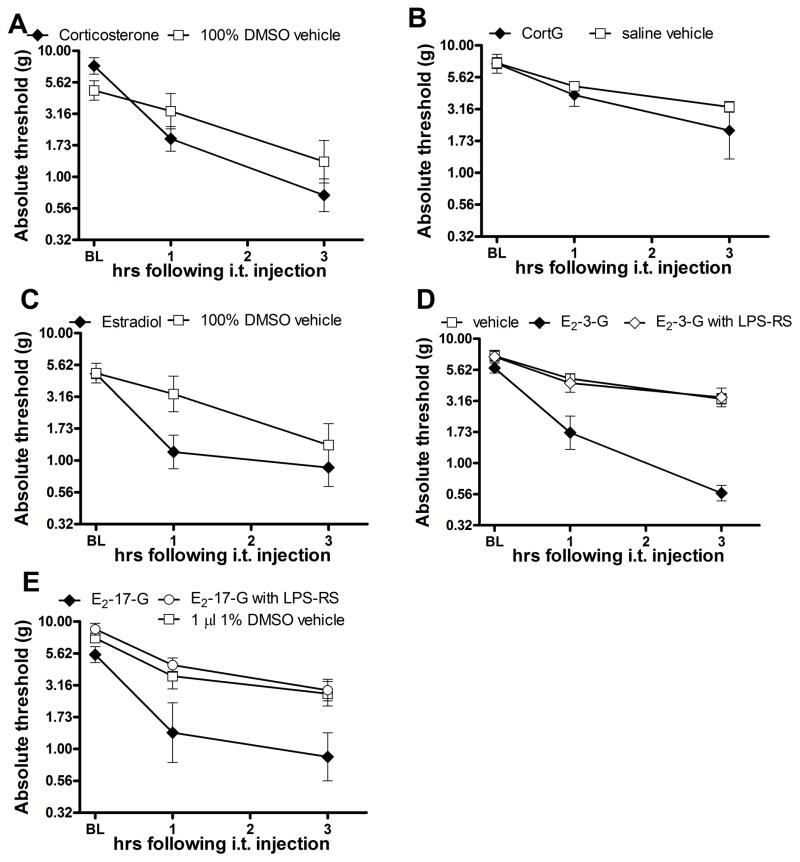
Intrathecal corticosterone (0.56 μg) and its equivolume (1 μl) 1% DMSO vehicle each caused an increase in tactile sensitivity relative to baseline, and did not differ from each other on post-hoc tests (F(2, 22), interaction=3.59, p<0.05; F(2,22), main effect time=35.33, p<0.05; F(1, 22) main effect treatment = 0.53, p>0.05, Figure 4A). Hence intrathecal corticosterone failed to induce allodynia relative to its vehicle control. However, due to the induction of allodynia by the vehicle, it is difficult to ascertain the nociceptive effect of corticosterone, if any, independent of its vehicle. Intrathecal CortG (0.85 μg) also failed to produce allodynia to develop relative to its saline vehicle (F(2, 20) interaction=0.93, p>0.05; F(2,20) main effect time=21.11, p<0.05; F(1,20) main effect treatment=0.87, p>0.05 Figure 4B).
Figure 4.
Intrathecal injection of corticosterone (0.56 μg, F(2,22)=3.59, p<0.05, A), CortG (0.85 μg, F(2,22)=0.93, p>0.05, B) or E2 (0.44 μg, F(2,22)=2.82, p>0.05, C) failed to cause significant allodynia relative to vehicle controls. Intrathecal injection of E2-3-G (0.76 μg, F(4, 40)=11.98, p<0.05, D) and E2-17-G (0.76 μg, F(4,34), p<0.05, E) caused significant allodynia to develop, which was blocked by LPS-RS (40 μg intrathecal) coadministration. Bonferroni post-hoc tests showed a significant increase in sensitivity was present at 1 (t=4.61, p<0.05) and 3 (t=8.04, p<0.05) hours following E2-3-G administration. The allodynia caused by E2-3-G injection was blocked at 1 (t=4.10, p<0.05) and 3 (t=7.89, p<0.05) hours by coadministration of 40 μg LPS- RS. Similarly, Bonferroni post-hoc tests showed a significant increase in sensitivity following E2-17-G administration at 3 hours (t=3.99, p<0.05) which was blocked by LPS-RS (40 μg intrathecal; t=5.02, p<0.05) coadministration.
Similar to corticosterone, intrathecal estradiol (0.44 μg) and its equivolume (1 μl) 1% DMSO vehicle both caused an increase in sensitivity relative to baseline but did not differ from each other (F(2, 22) interaction=2.82, p>0.05; F(2, 22) main effect time = 23.46, p<0.05; F(1,22) main effect treatment = 2.17, p>0.05, Figure 4C). Hence, intrathecal estradiol failed to induce allodynia relative to its vehicle control although the nociception caused by the vehicle makes it difficult to interpret estradiol nociceptive effects independent of vehicle effects. Intrathecal E2-3-G (0.76 μg), however, caused a robust tactile allodynia 1 and 3 hours post injection, which was blocked by 40 μg LPS-RS (F(4,40) interaction=11.98, p<0.05; F(2,40) main effect time= 73.28, p<0.05; F(2, 40) main effect treatment=24.53, p<0.05, Figure 4D). Bonferroni post-hoc tests showed that animals administered E2-3-G had significant more tactile allodynia at 1 and 3 hours post-injection than either saline or E2-3-G with LPS-RS treated animals. E2-17-G (0.76 μg) also caused significant tactile allodynia to develop 3 hours post injection that was blocked by 40 μg LPS-RS (F(4,34)interaction=3.585, p<0.05; F(4,34) main effect time = 43.42, p<0.05; F(2,34) main effect treatment=9.303, p<0.05) Figure 4E). Bonferroni post-hoc tests showed that E2-17-G treated animals had significantly lower thresholds at 1 and 3 hours than either saline or E2-17-G with LPS-RS treated animals.
4. Discussion
We present the first evidence that the naturally occurring glucuronide metabolites E2-3-G and E2-17-G cause TLR4 activation in vitro and a potent TLR4-dependent pain response in vivo. Additionally, each molecule tested, including corticosterone, CortG, and estradiol as well as E2-3-G and E2-17-G, was predicted to dock to MD-2 in an in silico model utilizing a recently published TLR4-MD-2 crystal structure (Park et al., 2009). Further, the docking of each was altered when in silico modeling was repeated using an MD-2 structure with the TLR4 inhibitor (+)-naloxone already docked, indicating a (+)-naloxone sensitive docking. However, whether that interaction is as an antagonist or agonist to TLR4 signaling is unknown from the in silico docking alone. An in vitro study using HEK cells transfected with TLR4 and necessary co-signaling molecules showed that E2-3-G and E2-17-G caused a dose-dependent increase in NFκB-dependent SEAP reporter gene protein expression, indicating agonism at TLR4. In contrast, antagonism of TLR4 signaling was observed with corticosterone and estradiol, as each significantly reduced reporter gene protein expression relative to their 1% DMSO vehicle. CortG exhibited no apparent TLR4 activity, as it produced no significant changes on TLR4 reporter protein expression. When administered intrathecally, E2-3-G and E2-17-G induced mechanical allodynia that was blocked by the TLR4 antagonist LPS-RS, hence supporting and extending the in vitro and in silico results. As predicted from in vitro results, corticosterone, CortG and estradiol did not alter pain responsiveness relative to their vehicles.
One inherent issue with corticosterone and estradiol results is that, in addition to the potential for TLR4 activity, they also have potent effects on the glucocorticoid and estrogen receptors, respectively. The larger, polar glucuronide metabolite of corticosterone, CortG, cannot cross the cell membrane to interact with the cytosolic glucocorticoid receptors. E2-3-G and E2-17-G are not believed to have effects on estrogen receptors (Guillemette et al., 2004). Another concern with these metabolites is that the vehicle (1% DMSO) also appeared to cause TLR4 activity. The purpose of the present studies was to determine the potential of these steroid metabolites to cause TLR4 activity, resulting in increased pain in vivo. Future studies might more directly assess the potential for TLR4 activity independent of glucocorticoid or estrogen receptor function by coadministering glucocorticoid or estrogen receptor antagonists, or to assess the potential of these drugs as TLR4 antagonists. The present studies have not investigated potential, but unlikely, dual roles for these compounds, in which the hormone receptor and TLR4 might cause opposing effects.
CortG is the first of six glucuronide metabolites tested in this methodology that failed to either increase HEK-TLR4 cell reporter protein in vitro or cause potentiated pain responses in vivo (Lewis et al., 2010; Lewis et al., 2013). Glucocorticoids, including corticosterone, are known to inhibit many inflammatory functions (Barnes, 2010), although they may also “prime” an enhanced LPS-induced inflammation after a delay (Frank et al., 2010). The anti-inflammatory actions of glucocorticoid agonists may mask a potential pro-inflammatory TLR4 agonist effect in vivo when both were present. Another possible explanation is that the structure of CortG is such that it lacks TLR4 agonism despite docking predicted in silico. Additionally, the delayed proinflammatory effects of corticosterone have been shown to be blocked by a glucocorticoid receptor antagonist, evidence that the glucocorticoid receptor itself modulates the inflammatory effects of corticosterone (Frank et al., 2012). Elucidating which explanation is correct will aid in the understanding of the nature of the TLR4 agonism in glucuronidated molecules. Further, the effects of human cortisol and its glucuronidated metabolites remain to be investigated.
The finding that estrogen metabolites can, by themselves, have TLR4-dependent, pain-producing effects has implications for several chronic pain disorders. For instance, among women who suffer from migraines, about 60% are more likely to have a migraine during perimenstrual stage of their menstrual cycle, between ovulation and menses (Kornstein and Parker, 1997). During this stage, there is a precipitous drop in circulating levels of the parent estrogen molecule with maintenance of the longer lived glucuronated metabolite. Notably, very high levels of estrogens, as in the third trimester of pregnancy, as well as ongoing very low levels of estrogens and their metabolites, as in post-menopause, both sharply reduce the risk of migraines (Craft, 2007). The risk conferred during the perimenstrual stage, then, is not due exclusively to the level of estrogens, but rather due to the abrupt decline in circulating levels of the parent estrogen molecule during this stage. A similar link between the perimenstrual stage and pain episodes was found in women suffering from tempromandibular joint disorder (LeResche et al., 2003). As noted, the perimenstrual stage of estrogen withdrawal is also the period during which estradiol-glucuoronide concentrations will be maximal (LeResche et al., 2003; Stanczyk et al., 1980). The studies here suggest that the TLR4 activation and enhanced pain caused by E2-3-G and E2-17-G may potentially contribute to the increase risk, in women affected by the disorder, of menstrual migraine or tempromandibular joint pain in the perimenstrual period. Were this link substantiated in future studies, it would create a novel treatment target in these challenging disorders.
The disparity between the length of time to develop a SEAP signal in vitro and the length of time for onset of pain behaviors in vivo is not surprising given the very different environments and end points. There are several potential explanations for this disparity. First, the threshold for physiologic effect may be quite different than the threshold for detection in the SEAP assay. Second, numerous studies have shown rapid release of cytokines from cells in the CNS (Loram et al., 2011b; Milligan et al., 2001), suggesting that these molecules are not synthesized de novo following stimulation in vivo, while the HEK-TLR4 SEAP assay requires gene expression. Third, the HEK-TLR4 system is designed to be a reporter system, not to mimic physiological effects, and only measures one potential downstream effect of TLR4 activation (i.e. increased NFkB transcription). Finally, the cellular environment and cell type (HEK cell vs. CNS cells), including costimulatory molecule expression, cell surface molecules and second signals, are remarkably different in vivo than in vitro, and thus the response of the cells to TLR4 signaling is likely to also be quite different.
These studies have shown that naturally produced steroid hormone metabolites, E2-3-G and E2-17-G are predicted to bind to MD-2 in silico, increase HEK-TLR4 cell reporter gene protein levels and cause TLR4-dependent pain. The finding that endogenously produced, naturally circulating molecules can have TLR4 effects is somewhat counterintuitive. TLR4s are generally thought to detect danger signals, such as lipopolysaccharide from gram negative bacterial cell walls, indicative of bacterial invasion, and degraded membrane components that are not typically found extracellularly, indicative of tissue damage (Osterloh and Breloer, 2008). While glucuronic acid, another endogenous molecule, has also been shown to activate TLR4 (Lewis et al., 2013), it is typically found conjugated to form uridine diphosphoglucuronic acid or polymerized to form hyaluronan, rather than freely available. We present the first evidence that an endogenously produced molecule can activate TLR4 and produce increased pain sensation.
There are several possible explanations for why TLR4 detects numerous compounds with a glucuronide component. First, several classes of bacteria, such as streptococci, have a hyaluronan capsule surrounding their cell walls (Maclennan, 1956). Several studies have shown that TLR4 detects low molecular weight hyaluronan as the polymers degrade (Leu et al., 2011; Li et al., 2010). It is possible that the destruction of bacterial capsules and presence of low molecular weight hyaluronan became a danger signal of bacterial invasion, and one which was detected by TLR4. Another potential explanation is that, while the chemical structure of the exogenously made estradiol glucuronide metabolites is identical to that produced endogenously, that other differences between endogenously and exogenously produced metabolites exist that result in the effects observed. All drugs tested free of endotoxin contamination on the LAL assay, one obvious concern, but there could be more subtle differences in the metabolism of these steroid hormones and the molecular environment in which they are encountered by TLR4 receptors. Finally, TLR4 is a pattern recognition receptor that responds to a range of molecular structures. While it evolved to detect danger signals, it is possible that it also detects similar molecules due to similarities in epitope patterning. That is, molecules that are structurally similar to the danger signals detected by TLR4 also act as agonists without being danger signals themselves.
The studies here present evidence that the estradiol metabolites, E2-3-G and E2-17-G, are predicted to bind to MD-2 in silico, increase the production of a HEK-TLR4 reporter protein in vitro and cause potent allodynia when administered intrathecally. Additionally, we determined that the parent molecules, corticosterone and estradiol as well as the glucuronide metabolite CortG, are not TLR4 agonists in sum with other potential physiological effects, and when administered acutely at low doses. These findings may potentially have broad implications for migraine and other disorders, such as fibromyalgia and tempromandibular join disorder (Craft, 2007), in which female hormone levels have been linked to painful episodes.
Acknowledgments
This work was supported in part by NIH Grants DA023132, DA024044, and DE017782. This work was also supported in part by the NIH Intramural Research Programs of NIDA and NIAAA and the Colorado Bioscience Discovery Evaluation Grant Program. Mark R. Hutchinson is a NHMRC CJ Martin Fellow (ID 465423; 2007–2010) and an Australian Research Council Research Fellow (DP110100297). We thank Dr. Kirk Johnson from Avigen for the gift of the HEK-TLR4 cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Ciuffreda P, Casati S, De Mieri M, Ferraboschi P. Corticosteroids 21-glucuronides: synthesis and complete characterization by (1)H and (13)C NMR. Steroids. 2009;74:870–875. doi: 10.1016/j.steroids.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation. 2012;9:200. doi: 10.1186/1742-2094-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette C, Belanger A, Lepine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6:246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Loram LC, Zhang Y, Shridhar M, Rezvani N, Berkelhammer D, Phipps S, Foster PS, Landgraf K, Falke JJ, Rice KC, Maier SF, Yin H, Watkins LR. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience. 2010a;168:551–563. doi: 10.1016/j.neuroscience.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Ramos KM, Loram LC, Wieseler J, Sholar PW, Kearney JJ, Lewis MT, Crysdale NY, Zhang Y, Harrison JA, Maier SF, Rice KC, Watkins LR. Evidence for a role of heat shock protein-90 in toll like receptor 4 mediated pain enhancement in rats. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010b;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegawa S, Hasegawa M, Okihara R, Shimidzu C, Chiba H, Iida T, Mitamura K. Simultaneous determination of twelve tetrahydrocorticosteroid glucuronides in human urine by liquid chromatography/electrospray ionization-linear ion trap mass spectrometry. Anal Chem. 2009;81:10124–10135. doi: 10.1021/ac9018632. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kallonen SE, Tammimaki A, Piepponen P, Raattamaa H, Ketola RA, Kostiainen R. Discovery of neurosteroid glucuronides in mouse brain. Anal Chim Acta. 2009;651:69–74. doi: 10.1016/j.aca.2009.07.059. [DOI] [PubMed] [Google Scholar]
- Kornel L, Saito Z. Studies on steroid conjugates-VIII. Isolation and characterization of glucuronide-conjugated metabolites of cortisol in human urine. J Steroid Biochem. 1975;6:1267–1284. doi: 10.1016/0022-4731(75)90118-1. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Parker AJ. Menstrual migraines: etiology, treatment, and relationship to premenstrual syndrome. Curr Opin Obstet Gynecol. 1997;9:154–159. [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Leu SW, Shi L, Xu C, Zhao Y, Liu B, Li Y, Shiedlin A, Xiang C, Shen H, Quinn DA, Hales CA, Zhao H. TLR4 through IFN-beta promotes low molecular mass hyaluronan-induced neutrophil apoptosis. J Immunol. 2011;186:556–562. doi: 10.4049/jimmunol.1001630. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Zhang Y, Hund DK, Maier SF, Rice KC, Watkins LR. Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause Toll-like receptor 4 (TLR4) activation and enhanced pain. Brain Behav Immun. 2013;30:24–32. doi: 10.1016/j.bbi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Potts EN, Piantadosi CA, Foster WM, Hollingsworth JW. Hyaluronan fragments contribute to the ozone-primed immune response to lipopolysaccharide. J Immunol. 2010;185:6891–6898. doi: 10.4049/jimmunol.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Loram LC, Taylor FR, Sholar P, Konrad B, Zhang Y, Hutchinson MR, Berkelhammer D, Babb JA, Day HEW, Maier SF, Rice KC, Watkins LR. Chronic estradiol potentiates microglial pro-inflammatory response induced by lipopolysaccharide and morphine in ovariectomized female rats. 2011a. In Preparation. [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun. 2011b;25:1408–1415. doi: 10.1016/j.bbi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan AP. The production of capsules, hyaluronic acid and hyaluronidase by 25 strains of group C streptococci. J Gen Microbiol. 1956;15:485–491. doi: 10.1099/00221287-15-3-485. [DOI] [PubMed] [Google Scholar]
- Maeda N, Tanaka E, Suzuki T, Okumura K, Nomura S, Miyasho T, Haeno S, Yokota H. Accurate determination of tissue steroid hormones, precursors and conjugates in adult male rat. J Biochem. 2013;153:63–71. doi: 10.1093/jb/mvs121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis--part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(Suppl 1):S9–15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: Conditioned analgesia and hyperalgesia. Acta Psychol (Amst) 2014;145:10–20. doi: 10.1016/j.actpsy.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Ono M, Aiso S, Akita H. Synthesis of methyl 1-O-(4-hydroxymethamphetaminyl)-alpha-D-glucopyranouronate. Chem Pharm Bull (Tokyo) 2005;53:684–687. doi: 10.1248/cpb.53.684. [DOI] [PubMed] [Google Scholar]
- Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol. 2008;197:1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Miyakawa I, Goebelsmann U. Direct radioimmunoassay of urinary estrogen and pregnanediol glucuronides during the menstrual cycle. Am J Obstet Gynecol. 1980;137:443–450. doi: 10.1016/0002-9378(80)91125-4. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf L. New software and Websites for the Chemical Enterprise. Chem Eng News. 2009:87. [Google Scholar]