Abstract
Objective
To evaluate the risk of reclassification on serial biopsy for Caucasian and African American (AA) men with very low risk PCa enrolled in a large prospective AS registry.
Methods
The Johns Hopkins AS registry is a prospective observational study that has enrolled 982 men since 1994. Including only men who met all National Comprehensive Cancer Network VLR criteria (clinical stage ≤T1, Gleason ≤6, PSA <10 ng/ml, PSA density <0.15 ng/ml/cc, positive cores <3, percent cancer per core ≤50), we analyzed a cohort of 654 men (615 Caucasian, 39 AA). The association of race with reclassification on serial biopsy was assessed with competing risks regressions.
Results
AA on AS were more likely than Caucasians to experience upgrading on serial biopsy (36% vs 16%, adjusted p<0.001). Adjusting for PSA, prostate size, volume of cancer on biopsy, treatment year, and BMI, AA race was an independent predictor of biopsy reclassification (subdistribution hazard ratio [sHR] 1.8, p=0.003). Examining specific modes of reclassification, AA race was independently associated with reclassification by grade (sHR 3.0, p=0.002) but not by volume.
Conclusions
AA with VLR PCa followed on AS are at significantly higher risk of grade reclassification as compared to Caucasians. Therefore, if the goal of AS is to selectively monitor men with low grade disease, AA men may require alternate selection criteria.
Keywords: active surveillance, prostate cancer, black, racial disparities, progression
Introduction
Active surveillance (AS) is a preferred management option for men with very low risk prostate cancer (PCa), particularly those with life expectancy <20 years (guidelines from the National Comprehensive Cancer Network (NCCN) and European Association of Urology).1,2 Oncologic outcomes of men enrolled in large AS cohorts are excellent, with near zero prostate cancer specific mortality over up to 7 years of follow-up.3,4 However, these cohorts under-represent minorities, with only 6–10% of AS cohorts comprised of African Americans (AA).3,5 Thus the oncologic outcomes of AA in AS are largely unknown, which is worrisome because AA are known to have markedly worse PCa outcomes in general.6–11
Recent studies have reported that AA men with very low risk PCa face elevated oncologic risks. We recently reported on a surgical cohort of men who met all AS criteria, in which AA were more likely to have adverse pathologic features at surgery such as upgrading (33% vs 13%).12 Additionally, recent work exploring outcomes of AA in AS have demonstrated similar increases in oncologic risk. Iremashvili et al. analyzed a cohort of 24 AA in the University of Miami AS cohort who had Gleason ≤6, clinical stage ≤T2a, positive cores <3, and percent cancer per core ≤20: here AA had an adjusted hazard ratio of 3.8 for reclassification on serial biopsy.5 Abern et al. analyzed a cohort of 32 AA in the Duke University AS cohort who had Gleason ≤6, clinical stage <T3, proportion of positive cores <33%, and prostate specific antigen (PSA) <10 ng/ml: here AA had an adjusted hazard ratio of 3.1 for treatment due to disease reclassification.13 These findings are concerning but merit independent confirmation.
Therefore, we studied the Johns Hopkins prospective AS registry of men with NCCN very low risk PCa to assess comparative risks of reclassification on serial biopsy between Caucasians and AA. The primary outcomes were overall pathologic reclassification, reclassification by grade≥7, and reclassification by volume (cores ≥3 or percent per core >50).
Materials and Methods
Study cohort
The AS program at Johns Hopkins is an institutional review board approved registry that prospectively follows men by annual transrectal ultrasound-guided biopsy and semi-annual digital rectal exam and serum PSA. Men with NCCN very low risk features, and particularly those with life expectancy <10 years, are counseled that AS is a viable management option. NCCN very low risk PCa is defined by Gleason ≤6, PSA <10 ng/ml, PSA density (PSAD) <0.15 ng/ml/cc, clinical stage <T2, positive cores <3, and percent cancer involvement per core ≤50.1 Due to either personal preference or co-morbidities, some men who do not meet all NCCN very low risk criteria are also followed on AS.3
We studied our institutional AS registry, which contains 982 men, diagnosed between 1994 and 2012. 691 (70.4%) met all NCCN very low risk criteria and 62 (6.3%) were AA. Men who do not meet all very low risk criteria were excluded, as were 26 (2.6%) who were non-Caucasian and non-AA, and 11 (1.1%) who had incomplete follow-up information. The final analysis cohort was comprised of 654 men (615 Caucasian, 39 AA).
Study Outcomes
Characteristics at program entry and time of censor (most recent biopsy or treatment) were calculated by race. The primary outcomes were reclassification by grade ≥7, reclassification by volume (≥3 positive cores or >50% cancer involvement per core), and overall reclassification by grade or volume. All tissue cores were analyzed centrally at our center by dedicated genitourinary pathologists.
Statistical Analysis
Means were compared with t-tests, medians of non-normally distributed variables were compared by Wilcoxon-Mann-Whitney tests, and proportions were compared with chi-squared tests. Competing risks regression analyses accounting for progression to treatment were performed to analyze race-based differences in reclassification. Kaplan-Meier survival analysis was performed in order to analyze differences in treatment-free survival between groups. Univariate and multivariable subdistribution hazard ratios (sHR) were computed to analyze the associations of AA race (compared to Caucasians) with reclassification on serial biopsy. Covariates in multivariable models were PSA, prostate volume, number of positive biopsy cores, maximum percent core involvement, body mass index (BMI), year of entry into AS, and center where the patient underwent prostate biopsy (Johns Hopkins versus elsewhere).
All statistical tests were two-sided, with significance pre-defined at the p≤0.05 level. Analyses were computed with Stata 11.0 (StataCorp, College Station, Texas).
Results
At entry into AS, baseline characteristics between AA (n=39) and Caucasians (n=615) were similar, though AA had slightly higher median BMI (27.1 vs 26.3 kg/m2, p=0.01) (Table 1). The number of total cores obtained at the first surveillance biopsy was known for 206 men, and did not differ between race groups (Table 1). Overall 91.3% of men were sampled with ≥12 cores. Median follow-up was 32.0 months (IQR 14.9–59.9 months) for the entire cohort, and was similar between race groups (Table 1).
Table 1.
Baseline characteristics and biopsy outcomes, JHH Prostate Cancer Active Surveillance cohort, 1994–2012
| Caucasian | African-American | p | |
|---|---|---|---|
|
| |||
| N | 615 | 39 | - |
|
| |||
| Median age (years) (IQR) | 65.6 (61.9, 68.8) | 65.8 (62.1, 71.1) | 0.346† |
|
| |||
| Median BMI (kg/m2) (IQR) | 26.3 (24.4, 28.7) | 27.1 (25.7, 30.4) | 0.013† |
|
| |||
| Median PSA at diagnosis (ng/ml) (IQR) | 4.4 (3.1, 5.6) | 4.2 (2.2, 5.4) | 0.255† |
|
| |||
| Median PSA density at diagnosis (ng/ml/cc) (IQR) | 0.09 (0.06, 0.11) | 0.07 (0.06, 0.09) | 0.058† |
|
| |||
| Gleason at diagnosis ≤6 |
615 (100%) | 39 (100%) | - |
|
| |||
| Positive cores at diagnosis | 0.099 | ||
| 1 | 450 (75.9%) | 25(64.1%) | |
| 2 | 143 (24.1%) | 14 (35.9%) | |
|
| |||
| Total cores sampled at first AS biopsy (mean, median, (IQR)) | 12.9, 12.0 (12.0, 12.0) | 12.4, 12.0 (12.0, 12.5) | 0.948† |
|
| |||
| Maximum percent cancer per core at diagnosis (median, IQR) | 2.0 (1.0, 10.0) | 5.0 (1.0, 10.0) | 0.641† |
|
| |||
| Median PSA velocity (ng/ml/year) (IQR) | 0.13 (−0.75, 1.15) | 0.08 (−0.58, 1.45) | 0.557† |
|
| |||
| Median PSA doubling time (months) (IQR) | 100.0 (38.9, 100.0) | 100.0 (48.6, 100.0) | 0.409† |
|
| |||
| Location of first AS biopsy | <0.001 | ||
| Johns Hopkins | 100 (16.3%) | 17 (43.6%) | |
| Outside center | 515 (83.7%) | 22 (56.4%) | |
|
| |||
| Reclassification on biopsy | |||
| Grade | 99 (16.1%) | 14 (35.9%) | <0.001‡ |
| Volume | 322 (52.4%) | 19 (48.7%) | 0.903‡ |
| Either | 366 (59.5%) | 27 (69.2%) | 0.019‡ |
| Both | 181 (29.4%) | 15 (38.5%) | 0.156‡ |
|
| |||
| Reclassification to treatment | 213 (34.6%) | 17 (43.6%) | 0.304§ |
|
| |||
| Treatment subtypes | 0.142 | ||
| Radical prostatectomy | 108/213 (50.9%) | 5/17 (29.4%) | |
| EBRT* +/− ADT** | 93/213 (43.7%) | 9/17 (52.9%) | |
| Brachytherapy | 9/213 (4.2%) | 2/17 (11.8%) | |
| Other | 3/213 (1.4%) | 1/17 (5.9%) | |
|
| |||
| Median follow-up (months) IQR |
36.3 (18.4, 61.3) | 30.5 (16.4, 59.1) | 0.536† |
Wilcoxon-Mann-Whitney rank-sum p-value
Competing risks regression p-value (unadjusted)
Log-rank p-value
External beam radiotherapy
Androgen deprivation therapy
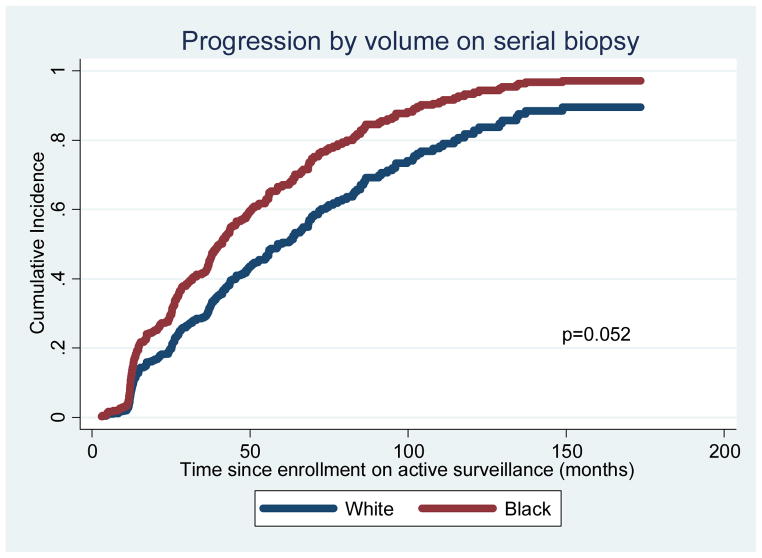
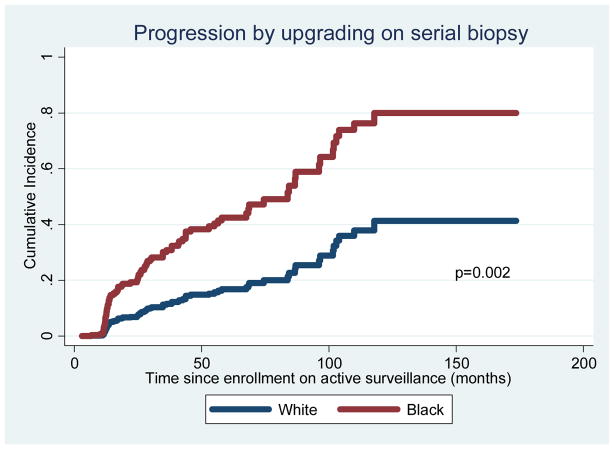
AA men were more likely to progress on serial biopsy by either grade or volume (69% vs 60%, adjusted p=0.003) (Tables 1, 2, and Figure S1). AA were also more likely to experience instances involving progression by both grade and volume (39% vs 29%, adjusted p=0.001) (Tables 1, 2, and Figure S2). When analyzing reclassification by volume (subsequent biopsies with ≥3 positive cores or >50% cancer involvement per core), AA were not more likely to progress (Table 1, Figure 1). However, the difference in overall reclassification between race groups was driven by reclassification by grade (subsequent biopsies with Gleason grade ≥7): 36% among AA vs 16% among Caucasians (p=0.002) (Tables 1, 2, Figure 2).
Table 2.
Predictors of reclassification on serial biopsy: Competing risks regression models
| RECLASSIFICATION OVERALL (GRADE OR VOLUME) | ||||||
|---|---|---|---|---|---|---|
| UNIVARIATE | MULTIVARIABLE | |||||
| sHR | 95% C.I. | p | sHR | 95% C.I. | p | |
| AA race | 1.46 | 1.06, 2.00 | 0.020 | 1.80 | 1.23, 2.65 | 0.003 |
| PSA | 1.12 | 1.07, 1.18 | <0.001 | 1.11 | 1.04, 1.20 | 0.003 |
| Gland vol | 1.01 | 1.00, 1.01 | 0.001 | 1.01 | 1.00, 1.01 | 0.005 |
| 2 pos cores | 1.60 | 1.27, 2.01 | <0.001 | 1.51 | 1.15, 1.98 | 0.003 |
| % pos/core | 1.02 | 1.01, 1.03 | <0.001 | 1.02 | 1.00, 1.03 | 0.004 |
| BMI ≥25 | 1.23 | 0.97, 1.56 | 0.091 | 1.18 | 0.91, 1.53 | 0.207 |
| Year | 1.13 | 1.09, 1.17 | <0.001 | 1.25 | 1.20, 1.31 | <0.001 |
| Bx at JHH | 1.00 | 0.78, 1.27 | 0.973 | 0.96 | 0.72, 1.29 | 0.803 |
| RECLASSIFICATION BY GRADE AND VOLUME | ||||||
|---|---|---|---|---|---|---|
| UNIVARIATE | MULTIVARIABLE | |||||
| sHR | 95% C.I. | p | sHR | 95% C.I. | p | |
| AA race | 1.46 | 0.87, 2.45 | 0.156 | 2.44 | 1.44, 4.14 | 0.001 |
| PSA | 1.02 | 0.97, 1.08 | 0.427 | 1.05 | 0.95, 1.16 | 0.308 |
| Gland vol | 1.00 | 0.99, 1.00 | 0.404 | 1.00 | 0.99, 1.01 | 0.908 |
| 2 pos cores | 1.90 | 1.40, 2.59 | <0.001 | 1.73 | 1.19, 2.49 | 0.004 |
| % pos/core | 1.02 | 1.01, 1.03 | 0.001 | 1.02 | 1.00, 1.03 | 0.022 |
| BMI ≥25 | 1.20 | 0.85, 1.70 | 0.297 | 1.09 | 0.75, 1.58 | 0.646 |
| Year | 1.05 | 1.00, 1.10 | 0.041 | 1.17 | 1.11, 1.23 | <0.001 |
| Bx at JHH | 0.79 | 0.53, 1.17 | 0.239 | 0.68 | 0.41, 1.13 | 0.137 |
| RECLASSIFICATION BY GRADE | ||||||
|---|---|---|---|---|---|---|
| UNIVARIATE | MULTIVARIABLE | |||||
| sHR | 95% C.I. | p | sHR | 95% C.I. | p | |
| AA race | 2.67 | 1.55, 4.58 | <0.001 | 3.02 | 1.48, 6.18 | 0.002 |
| PSA | 1.14 | 1.05, 1.24 | 0.003 | 1.29 | 1.13, 1.49 | <0.001 |
| Gland vol | 1.00 | 0.99, 1.01 | 0.812 | 0.99 | 0.98, 1.00 | 0.129 |
| 2 pos cores | 2.29 | 1.56, 3.37 | <0.001 | 1.72 | 1.12, 2.64 | 0.012 |
| % pos/core | 1.02 | 1.01, 1.04 | 0.004 | 1.02 | 1.00, 1.04 | 0.024 |
| BMI ≥25 | 1.04 | 0.67, 1.61 | 0.865 | 0.97 | 0.62, 1.53 | 0.911 |
| Year | 1.09 | 1.03, 1.16 | 0.002 | 1.17 | 1.01, 1.25 | <0.001 |
| Bx at JHH | 0.72 | 0.42, 1.23 | 0.228 | 0.58 | 0.32, 1.05 | 0.071 |
Figure 1.
Freedom from reclassification by volume (adjusted for covariates)
Figure 2.
Freedom from reclassification by grade (adjusted for covariates)
Ultimately, 44% of AA and 35% of Caucasian men underwent definitive PCa treatment. The most common treatment modality among Caucasian men was radical prostatectomy (51%) and among AA, external beam radiotherapy with or without concurrent androgen deprivation (53%) (Table 1). There were no significant differences in treatment-free survival (p=0.304) (Table 1, Figure S3). In sub-analyses of men who were treated at the time of pathologic reclassification and of men who were treated in the absence of reclassification (due to 'anxiety' or physician recommendation), there were still no differences in reclassification to treatment between race groups (data not shown).
Because AA experienced significantly more frequent overall reclassification and reclassification by grade, these outcomes were modeled using competing risks regressions. Adjusting for PSA and prostate volume at entry, number of positive cores, volume of cancer per core, BMI, year of diagnosis, and biopsy location, AA race was an independent risk factor for overall biopsy reclassification (sHR 1.80, p =0.003, Table 2). Higher PSA and higher volume of cancer on biopsy were also significant predictors of overall reclassification (Table 2). In a similar model, AA race was an independent risk factor for reclassification by upgrading to Gleason ≥7 (sHR 3.02, p=0.002, Table 2). Having two (versus one) positive cores at diagnostic biopsy was also an independent predictor of overall progression, progression by grade and volume, and progression by grade only (sHR range 1.51–1.73, Table 2).
In an alternative competing risks regression adjusting for PSAD as a substitute for PSA and gland volume, PSAD was also associated with upgrading (adjusted p=0.002 when computing as a continuous variable; adjusted p≤0.03 when computing as a binary variable with cutoff points 0.05–0.13 ng/ml/cc; adjusted p-value non-significant at other PSAD cut-off values). In sub-analyses of men whose total biopsy core information was unknown, AA remained a significant risk factor in a univariate competing risk regression (sHR 2.48, p=0.018), and approached statistical significance in the multivariable model (sHR 2.73, p=0.065). We repeated competing risks regression models of progression by grade including only men who were still on surveillance 18 months after enrollment (572 Caucasians and 33 AA). Here, AA race remained predictive of upgrading on surveillance though lost statistical significant on multivariable analysis (univariate sHR 2.68, p=0.007, adjusted sHR 2.36, p=0.087).
Discussion
Here we demonstrate that in a cohort of men with very low risk PCa undergoing AS, AA have a significantly higher risk of pathologic reclassification on serial biopsies; and the specific driving force behind this finding is that AA men have a significantly higher risk of reclassification by grade. This study serves to validate recent reports of AA in AS facing higher risks of biopsy reclassification and reclassification to treatment.5,13 The present study is also unique, in several aspects. First, though the sample size of AA men is modest (n=39), it is to our knowledge the largest reported. By comparison, the sample sizes in prior AS cohorts analyzing race ranged from only 19–24 (AA men on AS with ≥2 biopsies).5,13 Importantly, the overall paucity of AA men in the current report and prior studies underscores the reality that minorities are under-represented in AS programs and thus previously reported AS outcomes14 may not be applicable to them.
Second, men who are encouraged to join the Johns Hopkins AS registry, and thus the men we analyzed, met all NCCN very low risk criteria. Accordingly, unlike the prior studies that analyzed men with low grade, low volume, and low stage disease, the current study is distinguished by using PSAD <0.15 ng/ml as an inclusion criterion. PSAD has been shown to be an independent risk factor for reclassification in AS, as reported here and previously, at levels as low as 0.08–0.09 ng/ml/cc.15
Third, the specific outcomes in the current study are unique. Men in the University of Miami AS cohort were found to be at increased risk of reclassification on serial biopsy5,16; here we studied overall biopsy reclassification but also separated the two types of reclassification (grade vs volume) and found that upgrading was the major risk that AA face. Patients studied in the Duke University AS cohort were found to be at increased risk of reclassification to treatment for any reason and in the setting of pathologic reclassification; here we show, in contrast, that AA men had a higher hazard of pathologic reclassification though they did not have worse treatment-free survival. Though the theme of the present study and prior reports are similar, certain key differences may thus be related to limited sample size, the decision to use PSAD <0.15 ng/ml as an inclusion criterion, or both.
The finding in our prospective AS registry that AA men have a PCa disparity mainly due to reclassification by grade (36% vs 16%) echoes the main finding of a retrospective surgical series from our institution.12 In study of 256 AA men who qualified for AS (based on NCCN very low risk criteria), but underwent immediate radical prostatectomy, AA had adverse pathologic outcomes compared to Caucasians, most notably increased upgrading to Gleason ≥7 at surgery (33% vs 13%).12 Taken together these studies of distinct patient cohorts suggest that AA with very low risk disease face a distinctly elevated profile of oncologic risk, so current risk stratification and AS inclusion criteria may not be applicable to AA men.
In a detailed pathological examination of the surgical specimens of very low risk men who underwent radical prostatectomy, we recently found that AA were more likely to harbor dominant tumor nodules in the anterior aspect of the prostate (51% vs 29%), and the discrepancy was even greater when examining high-grade dominant nodules.17 If it is also true that AA in AS have a higher prevalence of high-grade index lesions in the anterior prostate (which are potentially missed by transrectal biopsies directed from a posterior approach), and if this is the reason upgrading is more common among AA in AS; then current biopsy and staging conventions may not be sufficient in AA men. In particular, AA may benefit from alternate biopsy templates or adjunctive prostate imaging tests to detect high-grade tumors that are potentially missed with standard transrectal biopsies. To shed light on this matter, a study characterizing differences in tumor nodule distribution among men in AS who underwent surgery due to pathologic reclassification is currently ongoing. Our sub-analysis of progression by upgrading including only men who were progression free 18 months into surveillance (thus excluding men who progressed at or around their first annual surveillance biopsy) showed that AA race was still a risk factor for progression though the association was weaker. This serves to demonstrate that AA men are at highest risk for upgrading at earlier time points during their time on surveillance. Consistent with the subanalysis results, the 'progression by grade' adjusted cumulative incidence curves continue to diverge over time (Figure 2), suggesting that the conditional probability of upgrading for AA men on surveillance decreases over time but remains higher than for Caucasians.
Alternatively, it is possible that the AA men in our AS registry were properly staged and that their elevated risk of pathologic reclassification reflects an increased de novo risk to develop high grade and/or high volume tumors over short time periods despite starting with very low risk disease. This may reflect race-based biological differences in carcinogenesis and PCa reclassification. The biological mechanisms underlying PCa disparities in AA are largely unknown, though some investigators have begun to shed light on certain molecular differences.18–21 Studies analyzing the molecular basis of tumorigenesis in the anterior aspect of the prostate may reveal insights into racial disparities in PCa, which are also underway.
There are several limitations to this study, one of which is limited follow-up. Though many men have been followed for many years, the accrual of new patients to the registry has partly contributed to an overall median follow-up of 36 months. This is not a unique issue, however--in the only other study of racial disparities in AS that reported follow-up duration, median follow-up was 23 months.13 It is unknown if the disparities in reclassification we report here may increase or diminish with longer follow-up. Second, while over 90% of men with known total biopsy cores had ≥12 samples obtained, this information was available on approximately one third of the analyzed cohort. Therefore, while we hold reasonable confidence in the point estimate that at least 90% of men were sampled with contemporary extended sampling techniques, it is a possibility that men with unknown total cores may have had less extensive biopsies. Third, AA were more likely to undergo surveillance biopsy within our center (as opposed to undergoing biopsies at referring clinics and have the slides interpreted by Hopkins pathologists). The significance of this is unknown but may relate to referral patterns to our center. If biopsy techniques systematically differed between other centers and ours, it raises the question of whether AA men were found to have higher reclassification despite (or because of) a greater likelihood of undergoing biopsy at Johns Hopkins. Fourth, the number of biopsies undergone by patients prior to entry into AS was unknown. Increased prior negative biopsies in the Caucasian cohort is a scenario, for example, that might have lead to improved selection and less progression. Finally, the sample size of AA men was relatively small, thus limiting the power of the study to detect race-based differences in this surveillance cohort. These results should be considered tentative until validated with a larger cohort.
Conclusions
In conclusion, this study demonstrates a significant racial disparity among men with very low risk PCa managed by AS. In particular AA men are at significantly higher risk (approximately three to four-fold) of reclassification on serial biopsy, and in particular, of reclassification by upgrading to Gleason ≥7. The reason for this difference in surveillance outcomes is unknown and may be an interesting focus for future study. Therefore, if the goal of AS is to selectively monitor men with low grade disease, alternate selection criteria may be indicated for AA men.
Supplementary Material
Figure S1. Freedom from reclassification on serial biopsy (reclassification defined as Gleason grade ≥7 or number of positive cores ≥2 or percent cancer involvement per core ≥50) (adjusted for covariates)
Figure S2. Freedom from reclassification on serial biopsy by grade and volume (adjusted for covariates)
Figure S3. Freedom from treatment
Acknowledgments
Research supported by NIH NIDDK training grant T32DK007552 (DS), Johns Hopkins Physician Scientist Award (AER), Prostate Cancer Foundation (HBC), AUA/Astellas Rising Star Award (EMS), HHMI Early Career Physician Scientist Award (EMS)
Footnotes
Disclosures
All authors: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NCCN. Clinical practice guidelines in oncology: prostate cancer. Natl Compr cancer Netw. 2012 Version 3. Available at: NCCN.org.
- 2.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 4.Dall’era MA, Albertsen PC, Bangma C, et al. Active Surveillance for Prostate Cancer: A Systematic Review of the Literature. Eur Urol. 2012:1–8. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 5.Iremashvili V, Soloway MS, Rosenberg DL, et al. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187(5):1594–9. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 7.Moul JW, Sesterhenn IA, Connelly RR, et al. Prostate-Specific Antigen Values at the Time of Prostate Cancer Diagnosis in African-American Men. JAMA. 1995;274(16):1277–1281. [PubMed] [Google Scholar]
- 8.Sanchez-Ortiz RF, Troncoso P, Babaian RJ, et al. African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer. 2006;107(1):75–82. doi: 10.1002/cncr.21954. [DOI] [PubMed] [Google Scholar]
- 9.Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183(5):1792–6. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chornokur G, Dalton K, Borysova ME, et al. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71(9):985–97. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis SD, Blackard B, Carpenter WR, et al. Receipt of National Comprehensive Cancer Network guideline-concordant prostate cancer care among African American and Caucasian American men in North Carolina. Cancer. 2013;119(12):2282–90. doi: 10.1002/cncr.28004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundi D, Ross AE, Humphreys EB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31(24):2991–7. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abern MR, Bassett MR, Tsivian M, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2013;16(1):85–90. doi: 10.1038/pcan.2012.38. [DOI] [PubMed] [Google Scholar]
- 14.Conti SL, Dall’era M, Fradet V, et al. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181(4):1628–33. doi: 10.1016/j.juro.2008.11.107. discussion 1633–4. [DOI] [PubMed] [Google Scholar]
- 15.Tseng KS, Landis P, Epstein JI, et al. Risk stratification of men choosing surveillance for low risk prostate cancer. J Urol. 2010;183(5):1779–85. doi: 10.1016/j.juro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iremashvili V, Burdick-Will J, Soloway MS. Improving risk stratification in patients with prostate cancer managed by active surveillance : a nomogram predicting the risk of biopsy progression. BJU Int. 2013;112(1):39–44. doi: 10.1111/bju.12112. [DOI] [PubMed] [Google Scholar]
- 17.Sundi D, Kryvenko ON, Carter HB, et al. Pathological Examination of Radical Prostatectomies in Men with Very Low Risk Disease at Biopsy Reveals Distinct Zonal Distribution of Cancer in Black American Men. J Urol. 2013;191:60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reams RR, Kalari KR, Wang H, et al. Detecting gene-gene interactions in prostate disease in African American men. Infect Agent Cancer. 2011;6 (Suppl 2):S1. doi: 10.1186/1750-9378-6-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magi-Galluzzi C, Tsusuki T, Elson P, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71(5):489–97. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 20.Powell IJ, Dyson G, Land S, et al. Genes associated with prostate cancer are differentially expressed in african american and European american men. Cancer Epidemiol Biomarkers Prev. 2013;22(5):891–7. doi: 10.1158/1055-9965.EPI-12-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinseth MA, Jia Z, Rahmatpanah F, et al. Expression between African American and Caucasian Prostate Cancer Tissue Reveals that Stroma is the Site of Aggressive Changes. Int J Cancer. 2013:1–31. doi: 10.1002/ijc.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Freedom from reclassification on serial biopsy (reclassification defined as Gleason grade ≥7 or number of positive cores ≥2 or percent cancer involvement per core ≥50) (adjusted for covariates)
Figure S2. Freedom from reclassification on serial biopsy by grade and volume (adjusted for covariates)
Figure S3. Freedom from treatment