Abstract
The serotonin 5-HT2C receptor has shown promise in vivo as a pharmacotherapeutic target for alcoholism. For example, recently, a novel 4-phenyl-2-N,N-dimethylaminotetralin (PAT) drug candidate, that demonstrates 5-HT2C receptor agonist activity together with 5-HT2A/2B receptor inverse agonist activity, was shown to reduce operant responding for ethanol after peripheral administration to rats. Previous studies have shown that the 5-HT2C receptor is found throughout the mesoaccumbens pathway and that 5-HT2C receptor agonism causes activation of ventral tegmental area (VTA) GABA neurons. It is unknown what effect 5-HT2C receptor modulation has on GABA release in the nucleus accumbens core (NAcc). To this end, microdialysis coupled to capillary electrophoresis with laser-induced fluorescence was used to quantify extracellular neurotransmitter concentrations in the NAcc under basal and after potassium stimulation conditions, in response to PAT analogs and other 5-HT2C receptor modulators administered by reverse dialysis to rats. 5-HT2C receptor agonists specifically attenuated stimulated GABA release in the NAcc while 5-HT2C antagonists or inverse agonists had no effect. Agents with activity at 5-HT2A receptors had no effect on GABA release. Thus, in contrast to results reported for the VTA, current results suggest 5-HT2C receptor agonists decrease stimulated GABA release in the NAcc, and provide a possible mechanism of action for 5HT2C-mediated negative modulation of ethanol self-administration.
Keywords: 5-HT2C receptor, GABA, microdialysis, capillary electrophoresis, nucleus accumbens
INTRODUCTION
Current pharmacotherapy approved for the treatment of alcoholism is only moderately effective (Edwards et al., 2011). One promising method to alter ethanol intake is modulating the serotonin (5-HT) 5-HT2C G protein-coupled receptor (GPCR) (Tomkins et al., 2002; Buck et al., 2004; Yoshimoto et al., 2012; Kasper et al., 2013). The 5-HT2C GPCR is expressed throughout the mesoaccumbens pathway (Pompeiano et al., 1994; Abramowski et al., 1995; Bubar et al., 2011), a neural pathway associated with ethanol reward (for review, see Soderpalm and Ericson, 2013). Recently, a novel 4-phenyl-2-N,N-dimethylaminotetralin (PAT) analog was reported to be a high affinity 5-HT2C receptor-specific agonist (Booth et al., 2009), and, in preclinical behavioral studies, (−)-trans-PAT was shown to decrease voluntary ethanol consumption in rats when administered peripherally (Kasper et al., 2013).
The relevant brain region(s) and underlying neurocircuitry by which PAT may decrease ethanol consumption includes the mesoaccumbens pathway, where dopamine neurons project from the ventral tegmental area (VTA) to the nucleus accumbens and GABA neurons project from the nucleus accumbens to regions including the VTA (Noori et al., 2012). 5-HT2C receptor agonists increase GABA release in the VTA (Theile et al., 2009), but it is unknown if 5-HT2C receptors modulate GABA release in the nucleus accumbens core (NAcc).
A common difficulty in studying 5-HT2C receptor agonists is that most bind to multiple receptor families and subtypes (Bubar and Cunningham, 2006). In this regard, (−)-trans-PAT is no exception, binding at the histamine H1 GPCR (Choksi et al., 2000) as well as at the three (2A, 2B, 2C) 5-HT2 GPCR subtypes (Booth et al., 2009), all of which signal primarily via Gαq-mediated activation of phospholipase C (PLC; Canal et al., 2013a). Functionally, (−)-trans-PAT specifically activates only 5-HT2C PLC signaling, and is an inverse agonist and competitive antagonist at the other sites (Canal et al., 2013a). Nevertheless, the 5-HT2A, 5-HT2C, as well as, H1 GPCRs are widely expressed in brain, confounding determination of the receptor(s) mechanisms responsible for mediating the effect of (−)-trans-PAT to reduce voluntary ethanol intake (Kasper et al., 2013).
The present study confirms the direct involvement of the 5-HT2C receptor in regulation of the mesoaccumbens reward pathway by co-administering antagonists along with the PAT analogs directly into NAcc of awake freely-moving rats and measuring the effects on GABA release using microdialysis. Measuring GABA by microdialysis is difficult because GABA has both neuronal as well as non-neuronal sources (e.g. glia) which can obfuscate interpretation of results (van der Zeyden et al., 2008). The non-neuronal sources can be minimized by measuring stimulation-evoked neurotransmitter release. Potassium stimulation results in calcium dependent synaptic release of neurotransmitters (Sellstrom and Hamberger, 1977). Specifically, we hypothesized that 5-HT2C receptor activation in the NAcc increases accumbal GABA release, similar to previous studies in the VTA (Theile et al., 2009). Contrary to our hypothesis, we found evidence that 5-HT2C receptor agonists (three novel PAT analogs and Ro60-0175) attenuate stimulated GABA release. These unexpected results provide a possible site of action and mechanism by which 5-HT2C receptor agonists alter neurotransmission in a reward associated brain region.
MATERIALS AND METHODS
Animals and housing
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225 to 250 g, were singly housed in a temperature- and humidity-controlled environment with a 12 hour normal phase light/dark cycle (06:00–18:00). All tests were conducted during the light phase. Rats were acclimated to housing facilities and handled daily for at least 1 week prior to experimentation. The subjects had ad libitum access to food and water throughout the experiment. Rat use was approved by the IACUC and was consistent with the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
Artificial cerebrospinal fluid (aCSF; 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2, 0.45 mM monobasic phosphate, 1.55 mM dibasic phosphate, pH 7.4) and high potassium aCSF (95 mM NaCl, 50 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2, 0.45 mM monobasic phosphate, 1.55 mM dibasic phosphate, pH 7.4) was prepared weekly with components from Fisher Scientific (Pittsburgh, PA). Ro60-0175 and ketanserin were purchased from Tocris Bioscience (Bristol UK). The novel compounds, (−)-trans-4-phenyl-2-N,N-dimethylaminotetralin ((−)-trans- PAT), (−)-trans-4-(4′ [para]-chlorophenyl)-2-N,N-dimethylaminotetralin ((−)-trans-p-Cl-PAT), (−)- trans-4-cylcohexyl-2-N,N-dimethylaminotetralin ((−)-trans-CAT), and (−)-trans-4-(3′ [meta]-chlorphenyl)-6-methoxy-N,N-dimethyl-1,2,3,4-tetrahydronaphthalene-2-amine (m-Cl-6-OMe- PAT) were synthesized in the University of Florida Department of Medicinal Chemistry laboratories with details reported elsewhere (Booth et al., 2009; Canal et al., 2013b; Morgan et al., 2013; Vincek and Booth, 2009). All drugs were prepared in aCSF.
Surgeries and microdialysis
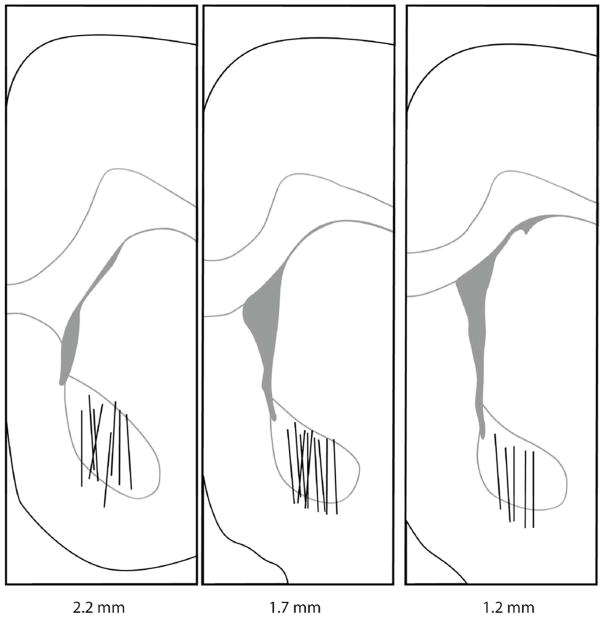
Each rat was anesthetized with isoflurane (induced with 5% isoflurane in an anesthesia chamber) and placed in a stereotaxic instrument for implantation of a guide cannula. Anesthesia was maintained by 2% isoflurane via constant stream through a nosecone. The guide cannula was anchored with two stainless steel screws and dental cement. The following coordinates from bregma were used for implantation into the NAcc: +1.8 mm anteroposterior, +1.3 mm lateral, and −6.2 mm dorsoventral. After this surgery, rats were never exposed to anesthesia again. After cannula implantation, animals were given at least 2 days to recover before microdialysis testing. Microdialysis probes with 2 mm active length and 13,000 molecular weight cut off were constructed as previously described (Peris et al., 2006). After calibration (see below), probes were inserted in the guide cannula (see figure 1 for placement), connected to a dual channel swivel, and perfused with aCSF at 1 μL/min. The swivel was mounted atop a modified home cage lid so that animals were tested in their home cage with free access to food and water throughout the experiment.
Figure 1.
Coronal sections showing microdialysis probe placement within the NAcc. Lines indicate the active dialysis regions. Numbers below the figure represent the position of the slice relative to bregma. Figure was adapted from Paxinos and Watson, 2005.
Capillary electrophoresis with laser-induced fluorescence
Data was gathered using microdialysis coupled with capillary electrophoresis with laser116 induced fluorescence detection which has been described previously (Bowser and Kennedy, 2001; Li et al., 2010). On the experiment day, a standard curve (7 concentrations of glutamate, aspartate, ornithine, GABA, taurine, glutamine, serine, and glycine ranging from 0 to 20 μM) was generated using a microdialysis probe. The validation and characterization of these neurotransmitters by elution time was described by Bowser and Kennedy (2001). After calibration, the probe was implanted in a non-anesthetized and freely moving rat via the guide cannula. The experiment began 2 hours after implantation.
Experimental procedures
In the potassium stimulation experiment, aCSF first was perfused into the NAcc for 5 minutes, followed by high-potassium aCSF for 10 minutes. The dialysate was then switched back to plain aCSF for 40 minutes (wash procedure). The perfusion and wash procedure was repeated two more times, with the second stimulation paired with drug perfusion (see Figure 2). In antagonism experiments to determine receptor site of action for the PATs, potassium stimulation experiments were repeated including constant perfusion of 50 μM ketanserin or mepyramine throughout the experiment, along with drug perfusion paired to the second stimulation as described above. Rat brains were taken immediately after the experiment and frozen. Separate rats were used for each experimental treatment.
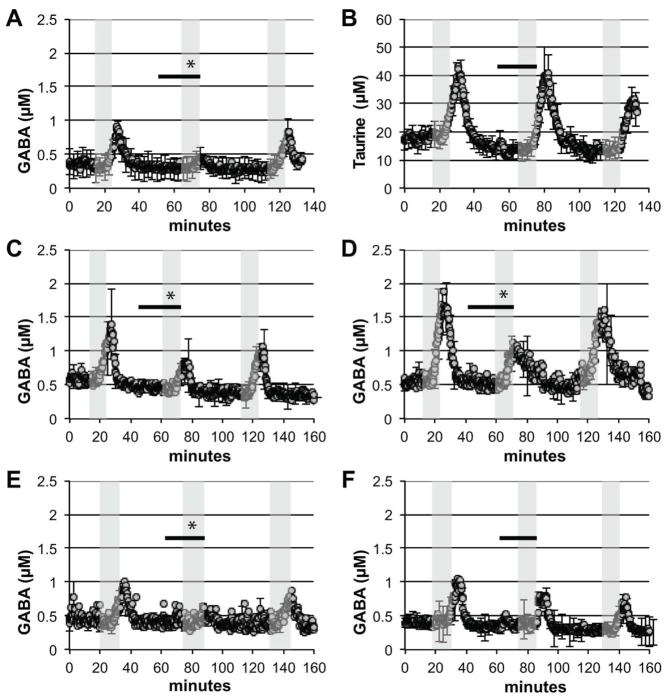
Figure 2.
Agonists for the 5-HT2C receptor decreased potassium-stimulated GABA release in the NAcc. The concentration of neurotransmitter in the dialysate is displayed over time. Grey shaded areas represent perfusion with 50 mM potassium containing aCSF. Black bar indicates when 50 μM 5-HT2C receptor agonist was added to the aCSF. (A, B) (−)-trans-PAT attenuated potassium stimulated GABA release but did not affect taurine release. Potassium stimulated GABA release was also attenuated by the 5-HT2C agonists (C) (−)-trans-p-Cl-PAT, (D) m-Cl-6-OMe-PAT, and (E) Ro60-0175. (F) The 5-HT2 receptor inverse agonist (−)- trans-CAT had no significant effect on potassium stimulated GABA release. The data shown are mean values ± SEMs for n = 3 in each panel. * indicates p < 0.05 by AUC comparison.
Data analysis and histology
Analysis of the concentration of analytes in dialysate was performed using Lab View software to determine the peak height for each measured neurotransmitter in each electropherogram which resulted in one data point every 15 seconds during the experiment as described previously (Li et al., 2008). The peak heights for each analyte were batch analyzed (Shackman et al., 2004), changed to μM concentration based on the above described standard curve, and then graphed over time. Data points from specific time periods, such as during potassium stimulations, were examined using area under the curve (AUC). Basal concentrations of neurotransmitters were determined by finding the average concentration for the 5 minutes before the time period of interest. AUC was determined by subtracting the basal value from the data points during the 10 minute time period of interest resulting in the AUC value. This AUC value was compared to other AUC values in the same experiment using one-way ANOVA to determine if the AUC values were different from each other. Additionally, the three AUC values were then converted to a percent of the first (control) AUC. One-way ANOVA with Tukey post147 hoc tests with significance level p < 0.05 were performed using SPSS on the AUC values to determine significant changes.
A Hacker Instruments cryostat was used to section the frozen brains after the experiment. Coronal sections 30 μm thick were removed until the probe tract was observed. Probe placement was determined by tract location in relation to brain architecture described with a brain atlas (Paxinos and Watson, 2005). Only animals with the majority (over 80%) of the 2 mm long probe in the NAcc were included in the study (Figure 1).
RESULTS
Rats included in the study (n = 33) had over 80% of the active probe length located inside the NAcc (Figure 1). Results from rats with misplaced or malfunctioned probes (n = 14) were not included in the study. Malfunction by membrane leakage (defined as less than 95% of fluid return) is a known problem for probes under high fluid pressure such as those used here.
Potassium stimulation caused an increase in GABA (Figure 2A) and taurine (Figure 2B) concentrations in the NAcc which was reliably expressed after each of three successive potassium stimulations. Taurine stimulation was right shifted compared to GABA and full visualization of the last stimulation was not possible. (−)-trans-PAT reduced stimulated GABA release by approximately 60% (F (2,8) = 180, p<0.0001) (Figure 2A) but had no effect on stimulated taurine release (Figure 2B). (−)-trans-PAT did not alter basal concentrations of GABA (GABA basal was 0.48±0.13 μM and during (−)-trans-PAT was 0.44±0.12 μM representing the average ± SEM, n = 3).
Additional 5-HT2C receptor agonists were then tested for the ability to reduce stimulated GABA release. These included (−)-trans-p-Cl-PAT, m-Cl-6-OMe-PAT, and Ro60-0175. All three compounds were able to reduce stimulated GABA release as shown in Figure 2C (F (2,8) = 220, p<0.0001), Figure 2D (F (2,8) = 39, p<0.01), and Figure 2E (F (2,8) = 75, p<0.001), respectively. To explore the pharmacology of PATs’ inhibition of GABA release, the 5-HT2 receptor inverse agonist (−)-trans-CAT was tested (Figure 2F). However, (−)-trans-CAT caused no change in stimulated GABA release (F (2,8) = 0.55, p>0.05).
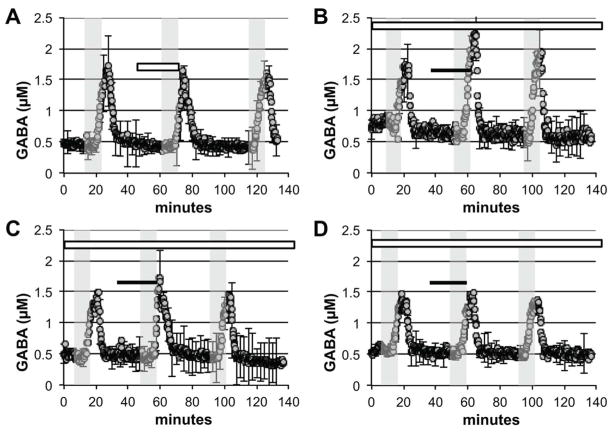
Antagonism experiments were performed to determine which receptor mediated the effects of PAT noted above. In separate experiments, ketanserin and mepyramine were included in the aCSF throughout the experiment to block 5-HT2A/2C and H1 receptors, respectively. Ketanserin (Figure 3A) had no effect on stimulated GABA release when tested alone (F (2,8) = 0.60, p>0.05). When ketanserin was perfused throughout the experiment (Figure 3B), however, it blocked (−)-trans-PAT ability to decrease stimulated GABA release (F (2,8) = 1.6, p>0.05). Ketanserin also blocked the effects of the 5-HT2C receptor agonists (−)-trans182 p-Cl-PAT (Figure 3C) and m-Cl-6-OMe-PAT (Figure 3D) to decrease stimulated GABA release (F (2,8) = 0.72, p>0.05, and F (2,8) = 2.2, p>0.05). The H1 receptor antagonist mepyramine had no effect on stimulated GABA release when tested alone (Figure 4A) (F (2,8) = 3.7, p>0.05) and did not block (−)-trans-PAT from decreasing stimulated GABA release (Figure 4B) (F (2,8) = 180, p<0.0001).
Figure 3.
Ketanserin blocks 5-HT2C agonist induced attenuation of potassium stimulated GABA release. The concentration of GABA in the dialysate is displayed over time. Grey shaded areas represent perfusion with 50 mM potassium containing aCSF. White bar signifies when 50 μM ketanserin was present. Black bar indicates when 50 μM 5-HT2C receptor agonist was present. (A) Ketanserin alone has no effect on potassium stimulated GABA release. However, when constantly perfused throughout the experiment, ketanserin blocks (B) (−)-trans-PAT, (C) (−)-trans-p-Cl-PAT, and (D) m-Cl-6-OMe-PAT from attenuating potassium stimulated GABA release. The data shown are mean values ± SEMs for n = 3 in each panel. * indicates p < 0.05 by AUC comparison.
Figure 4.
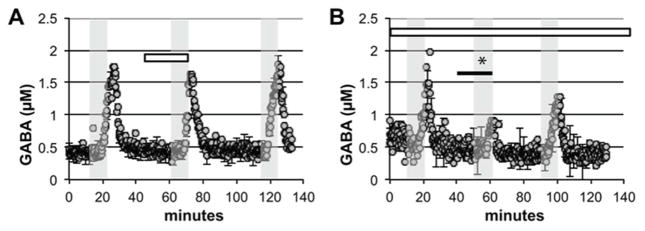
Mepyramine, an H1 antagonist, fails to block 5-HT2C agonist induced attenuation of potassium stimulated GABA release. Grey shaded areas represent perfusion with 50 mM potassium containing aCSF. White bar signifies when 50 μM mepyramine was present. Black bar indicates when 50 μM (−)-trans-PAT was present. (A) Mepyramine alone has no effect on potassium stimulated GABA release. (B) When constantly perfused throughout the experiment, mepyramine fails to block (−)-trans-PAT from attenuating potassium stimulated GABA release. The data shown are mean values ± SEMs for n = 3 in each panel. *indicates p < 0.05 by AUC comparison.
DISCUSSION
This study advanced previous work using microdialysis to measure potassium stimulated neurotransmitter release (Sellstrom and Hamberger, 1977) by testing the effects of local drug perfusion on both basal and stimulated neurotransmitter levels. Using a series of three potassium stimulations, there was a reliable increase in both GABA and taurine release in the NAcc while other neurotransmitters (serine, glycine, glutamine, glutamate and aspartate) remain unchanged. The inability of potassium stimulation to result in increases in all neurotransmitters (e.g. glutamate increase demonstrated in the striatum by Yamamoto and Davy, 1992) has been reported previously with microdialysis in the accumbens (Wydra et al., 2013). Combined with our optimization for GABA detection, it would be difficult to visualize the relatively small increase in glutamate or other putative neurotransmitters without substantially increasing the duration of the potassium stimulation. The advantage of the three potassium stimulation experiments in the present study is reproducibility, which indicates that such repetitive stimulation does not deplete neuronal vesicular neurotransmitter stores.
The 5-HT2C receptor agonists Ro60-0175 and novel PAT analogs (Table 1) reduced stimulated GABA release in NAcc. Taurine concentrations in the potassium stimulation experiments were unaffected by the presence of any of the test compounds, suggesting that 5- HT2C receptor agonists selectively alter GABA in the NAcc. The PAT analog (−)-trans-CAT, a 5- HT2C receptor inverse agonist and the 5-HT2A/2C antagonist ketanserin, had no effect on extracellular neurotransmitter concentrations. To the best of our knowledge, these data represent the first report that 5-HT2C agonists can modulate GABA release in the NAcc. Further experiments comparing full dose-response curves for each compound and ensuring that affinity of each compound for the 5-HT2C receptor predicts potency for attenuating GABA release will strengthen our hypothesis.
Table 1.
Compound 5-HT2 affinity and functional activitya.
| 5-HT2A | 5-HT2C | |
|---|---|---|
| Ro60-0175 | 32 nM agonist | 1 nM agonist |
|
|
||
| Ketanserin | 4 nM antagonist | 120 nM antagonist |
|
|
||
| (−)-trans-PAT | 80 nM inverse agonist | 20 nM agonist |
|
|
||
| (−)-trans-p-Cl-PAT | 240 nM inverse agonist | 130 nM agonist |
|
|
||
| m-Cl-6-OMe-PAT | 35 nM inverse agonist | 17 nM agonist |
|
|
||
| (−)-trans-CAT | 1.6 nM inverse agonist | 14 nM inverse agonist |
|
|
||
Booth et al., 2009; Choksi et al., 2000; Fiorella et al., 1995; Martin et al., 2004; Vincek and Booth, 2009.
Although we were able to reliably measure inhibition of potassium-stimulated GABA release, detection of putative reductions in basal GABA release might have been limited by detection sensitivity and/or confounding non-neuronal GABA sources. For example, non215 neuronal GABA sources (e.g. glial) are well documented to confound interpretation of microdialysis results (Sellstrom and Hamberger, 1977; van der Zeyden et al., 2008). Meanwhile, the limit of detection for GABA using capillary electrophoresis with laser induced fluorescence is about 0.14 μM, with basal GABA concentrations in this study being around 0.5 μM. This may have hampered our ability to reliably measure a decrease in basal GABA levels caused by our compounds.
The test agents (−)-trans-CAT and ketanserin, that are 5-HT2C receptor inverse agonists/antagonists, did not alter stimulated GABA or taurine release in the NAcc. Moreover, ketanserin blocked the reduction of potassium-stimulated GABA release by the 5-HT2C agonist (−)-trans-PAT. These results provide convincing evidence that 5-HT2C receptor activation is responsible for negatively modulating potassium-evoked GABA release. Ketanserin is also an antagonist at 5-HT2A and H1 receptors (Baxter et al., 1995; Ghoneim et al., 2006), as are (−)- trans-PAT, m-Cl-6-OMe-PAT, and (−)-trans-p-Cl-PAT, however, only the test agents with 5-HT2C agonist activity (i.e., the PAT analogs, not ketanserin) attenuate potassium-induced GABA release. Likewise, mepyramine, a high affinity inverse agonist that is specific for the H1 receptor (Fitzsimons et al., 2004) had no effect on basal or stimulated GABA release when directly perfused into the NAcc. This supports the hypothesis that the PAT analogs’ ability to decrease stimulated GABA release is mediated by activation of 5-HT2C receptors and not through their action on H1 receptors. Mepyramine also failed to prevent (−)-trans-PAT from decreasing stimulated GABA release, demonstrating that H1 receptors are not involved.
One weakness in this study is the use of a single drug dose to determine the effect on potassium stimulated GABA release. The perfusion dose of 50 μM was employed based on our pharmacokinetic understanding of the PAT compounds and the ethanol-specific behaviorally active peripheral doses employed previously (Kasper et al., 2013). Thus, we favored a design employing compounds at one behaviorally relevant dose with broad affinity and functional activity (Table 1). Obviously, full dose-response curves are warranted in future studies, especially if the ability of these agents to modulate GABA release is suspected of changing with repeated ethanol exposure.
Previous electrophysiological studies have shown that 5-HT2C receptor agonism directly causes an increase in GABA release onto dopaminergic neurons in the VTA (Theile et al., 2009) which appear to conflict with the results of the present study wherein we observed 5-HT2C receptor agonists decreased stimulated GABA release in the NAcc. This apparent disagreement is rectified in light of other studies (Navailles et al., 2008) wherein 5-HT2C receptors have been shown to differentially regulate cocaine-induced effects on dopamine concentration depending on brain region (VTA vs NAc), suggesting that the effect of 5-HT2C receptor activation on neurotransmission in the mesoaccumbens pathway is region dependent. Taken together, these findings indicate that 5-HT2C receptor agonists modulate GABAergic drive in the mesoaccumbens pathway differently in the NAcc than in the VTA. Indeed, this work suggests an indirect way for 5-HT2C receptor agonists to increase VTA GABAergic drive, i.e., 5-HT2C receptor activation disinhibits accumbal GABAergic projections to the VTA. Measuring GABA release in the VTA after administering a 5-HT2C receptor agonist to the NAcc is the next step in confirming this mechanism.
Determining 5-HT2C receptor agonist mechanisms of action related to treating substance abuse is a subject of much interest (for review, see Filip et al., 2012). Previously, some of these compounds with 5-HT2C receptor agonist properties were shown to be effective in decreasing ethanol consumption, particularly when it was elevated due to a period of ethanol deprivation of the animal (Kasper et al., 2013). The present results, showing 5-HT2C receptor activation negatively modulates stimulated GABA release in NAcc, suggest a mechanistic overlap with the reduction of nucleus accumbens shell GABA concentration seen after administration of carbamathione (Faiman et al., 2013), a metabolite of the drug disulfiram that is used to treat alcoholism. It is possible that attenuating GABA release in the accumbens modulates reinforcement and motivation for alcohol. Thus, the current results suggest that 5-HT2C-specific agonists may have pharmacotherapeutic relevance in the treatment of alcoholism.
Acknowledgments
We would like to thank Sashi Sivendren, Zhuming Sun, Rajeev Sakhuja, and Adam Vincek for providing test compounds. These studies were funded in part by grants from the U.S. National Institutes of Health (DA023928, DA030989, MH081193).
Footnotes
Conflict of interest statement
All authors declare that there are no financial, professional, or personal relationships with people or organizations that can inappropriately influence our work.
Authors contribution
JK, RB, and JP designed the research. JK performed the research. RB designed and provided PAT analogues. All authors wrote the paper, critically reviewed the content and approved the final submission for publication.
References
- Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M. Localization of the 5- hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology. 1995;34(12):1635–45. doi: 10.1016/0028-3908(95)00138-7. [DOI] [PubMed] [Google Scholar]
- Baxter G, Kennett G, Blaney F, Blackburn T. 5-HT2 receptor subtypes: a family re-united? Trends Pharmacol Sci. 1995;(3):105–10. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- Booth RG, Fang L, Huang Y, Wilczynski A, Sivendran S. (1R, 3S)-(−)-trans-PAT: a novel full289 efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity. Eur J Pharmacol. 2009;615(1–3):1–9. doi: 10.1016/j.ejphar.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser MT, Kennedy RT. In vivo monitoring of amine neurotransmitters using microdialysis with on-line capillary electrophoresis. Electrophoresis. 2001;22(17):3668–76. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6(18):1971–85. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Stutz SJ, Cunningham KA. 5-HT2C receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One. 2011;6(6):e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KJ, Reilly MT, Rogers LM, Szeliga K, Grant K, Brodie MS. Serotonin 5-HT2 receptors and alcohol: reward, withdrawal and discrimination. Alcohol Clin Exp Res. 2004;28(2):211–6. doi: 10.1097/01.alc.0000113423.40075.a3. [DOI] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4- iodoamphetamine elicited head-twitch response model. Neuropharmacology. 2013a;70:112–21. doi: 10.1016/j.neuropharm.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Morgan D, Felsing D, Kondabolu K, Rowland NE, Robertson KL, Sakhuja R, Booth RG. A novel aminotetralin-type serotonin (5-HT) 2C receptor-specific agonist and 5-HT2A competitive antagonist/5-HT2B inverse agonist with preclinical efficacy for psychoses. J Pharmacol Exp Ther. 2013b;349(2):310–8. doi: 10.1124/jpet.113.212373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi NY, Nix WB, Wyrick SD, Booth RG. A novel phenylaminotetralin (PAT) recognizes histamine H1 receptors and stimulates dopamine synthesis in vivo in rat brain. Brain Res. 2000;852(1):151–60. doi: 10.1016/s0006-8993(99)02228-3. [DOI] [PubMed] [Google Scholar]
- Edwards SM, Kenna GA, Swift RM, Leggio L. Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Curr Pharm Des. 2011;17(14):1323–32. doi: 10.2174/138161211796150765. [DOI] [PubMed] [Google Scholar]
- Faiman MD, Kaul S, Latif SA, Williams TD, Lunte CE. S-(N, N-diethylcarbamoyl)glutathione (carbamathione), a disulfiram metabolite and its effect on nucleus accumbens and prefrontal cortex dopamine, GABA, and glutamate: a microdialysis study. Neuropharmacology. 2013;75:95–105. doi: 10.1016/j.neuropharm.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Spampinato U, McCreary AC, Przegaliński E. Pharmacological and genetic interventions in serotonin (5-HT)(2C) receptors to alter drug abuse and dependence processes. Brain Res. 2012;1476:132–53. doi: 10.1016/j.brainres.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl) 1995;121(3):347–56. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fitzsimons CP, Monczor F, Fernandez N, Shayo C, Davio C. Mepyramine, a histamine H1 receptor inverse agonist, binds preferentially to a G protein-coupled form of the receptor and sequesters G protein. J Biol Chem. 2004;279(33):34431–9. doi: 10.1074/jbc.M400738200. [DOI] [PubMed] [Google Scholar]
- Ghoneim OM, Legere JA, Golbraikh A, Tropsha A, Booth RG. Novel ligands for the human histamine H1 receptor: synthesis, pharmacology, and comparative molecular field analysis studies of 2-dimethylamino-5-(6)-phenyl-1,2,3,4-tetrahydronaphthalenes. Bioorg Med Chem. 2006;14(19):6640–58. doi: 10.1016/j.bmc.2006.05.077. [DOI] [PubMed] [Google Scholar]
- Kasper J, Tikamdas R, Kim MS, Macfadyen K, Aramini R, Ladd J, Bisceglia S, Booth R, Peris J. The serotonin-2 receptor modulator, (−)-trans-PAT, decreases voluntary ethanol consumption in rats. Eur J Pharmacol. 2013;718(1–3):98–104. doi: 10.1016/j.ejphar.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zharikova A, Bastian J, Esperon L, Hebert N, Mathes C, Rowland NE, Peris J. High temporal resolution of amino acid levels in rat nucleus accumbens during operant ethanol self333administration: involvement of elevated glycine in anticipation. J Neurochem. 2008;106(1):170–81. doi: 10.1111/j.1471-4159.2008.05346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zharikova A, Vaughan CH, Bastian J, Zandy S, Esperon L, Axman E, Rowland NE, Peris J. Intermittent high-dose ethanol exposures increase motivation for operant ethanol self336 administration: possible neurochemical mechanism. Brain Res. 2010;1310:142–53. doi: 10.1016/j.brainres.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Bös M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Köhler C, Delft AM. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286(2):913–24. [PubMed] [Google Scholar]
- Morgan D, Kondabolu K, Kuipers A, Sakhuja R, Robertson KL, Rowland NE, Booth RG. Molecular and behavioral pharmacology of two novel orally-active 5HT2 modulators: potential utility as antipsychotic medications. Neuropharmacology. 2013;72:274–81. doi: 10.1016/j.neuropharm.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by the serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: An in vivo microdialysis study with cocaine. Neuropsycopharmacology. 2008;33:237–46. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- Noori HR, Spanagel R, Hansson AC. Neurocircuitry for modeling drug effects. Addict Biol. 2012;17(5):827–64. doi: 10.1111/j.1369-1600.2012.00485.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Boston: Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- Peris J, Zharikova A, Li Z, Lingis M, MacNeill M, Wu MT, Rowland NE. Brain ethanol levels in rats after voluntary ethanol consumption using a sweetened gelatin vehicle. Pharmacol Biochem Behav. 2006;85(3):562–8. doi: 10.1016/j.pbb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23(1–2):163–78. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Sellström A, Hamberger A. Potassium-stimulated gamma-aminobutyric acid release from neurons and glia. Brain Res. 1977;119(1):189–98. doi: 10.1016/0006-8993(77)90099-3. [DOI] [PubMed] [Google Scholar]
- Shackman JG, Watson CJ, Kennedy RT. High-throughput automated post-processing of separation data. J Chromatogr A. 2004;1040(2):273–82. doi: 10.1016/j.chroma.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci. 2013;13:127–61. doi: 10.1007/7854_2011_170. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-HT2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329(2):625–33. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav. 2002;71(4):735–44. doi: 10.1016/s0091-3057(01)00710-9. [DOI] [PubMed] [Google Scholar]
- van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90(2):135–47. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Vincek AS, Booth RG. New Approach to 4-Phenyl-β-aminotetralin from 4-(3-Halophenyl)tetralen-2-ol Phenylacetate. Tetrahedron Lett. 2009;50:5107–5109. doi: 10.1016/j.tetlet.2009.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Zaniewska M, Kamińska K, Ferraro L, Fuxe K, Filip M. Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self376administration and its extinction in rats. Addict Biol. 2013;18(2):307–24. doi: 10.1111/adb.12031. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Davy S. Dopaminergic modulation of glutamate release in striatum asmeasured by microdialysis. J Neurochem. 1992;58(5):1736–42. doi: 10.1111/j.1471-4159.1992.tb10048.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Watanabe Y, Tanaka M, Kimura M. Serotonin2C receptors in the nucleus accumbens are involved in enhanced alcohol-drinking behavior. Eur J Neurosci. 2012;35(8):1368–80. doi: 10.1111/j.1460-9568.2012.08037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]