Abstract
OBJECTIVE
To evaluate associations between prepregnancy lifestyle factors, psychological distress and adverse pregnancy outcomes among female survivors of childhood cancer.
STUDY DESIGN
We examined pregnancies of 1,192 female participants from the Childhood Cancer Survivor Study. Generalized linear models, adjusted for age at diagnosis, age at pregnancy, parity, and education were used to calculate the odds ratio (OR) and confidence interval (CI) for associations between prepregnancy inactivity, overweight or obese status, smoking status, risky drinking, psychological distress and pregnancy outcomes. Interactions between lifestyle factors, psychological distress, type of cancer and cancer treatment were assessed in multivariable models.
RESULTS
The median age of study participants at the beginning of pregnancy was 28 years (range: 14–45). Among 1,858 reported pregnancies, there were 1,300 singleton live births (310 were preterm), 21 stillbirths, 397 miscarriages, and 140 medical abortions. Prepregnancy physical inactivity, risky drinking, distress and depression were not associated with any pregnancy outcomes. Compared to those who had never smoked, survivors with > 5 pack-years smoking history had a higher risk for miscarriage among those treated with > 2.5 Gy uterine radiation (OR: 53.9; 95% CI: 2.2, 1,326.1) than among those treated with ≤ 2.5 Gy uterine radiation (OR: 1.9; 95% CI: 1.2, 3.0). There was a significant interaction between smoking and uterine radiation (Pinteraction = 0.01).
CONCLUSION
While most lifestyle factors and psychological distress were not predictive of adverse pregnancy outcomes, the risk for miscarriage was significantly increased among survivors exposed to > 2.5 Gy uterine radiation who had a history of smoking.
Keywords: pregnancy, childhood cancer survivors, uterine radiation, smoking, lifestyle
INTRODUCTION
In the United States, approximately 10,450 new cases of childhood cancer are expected to occur among children younger than 15 years of age in 2014; 80% of these children will survive for at least five years.1 Because most of these survivors will reach reproductive age, adverse pregnancy outcomes including preterm birth, stillbirth, and miscarriage are of concern. Overall, when compared to their siblings, female survivors of childhood cancer are not at increased risk for stillbirth or miscarriage,2 but do have an increased risk of preterm birth.3 Previous studies have identified treatment-related risks, reporting an increased risk of preterm birth following ≥ 5 Gray (Gy) of uterine radiation,3 stillbirth following ≥ 10 Gy of uterine and ovarian radiation,4 and miscarriage following abdominal radiation.5, 6 However, the influence of potentially modifiable prepregnancy lifestyle factors such as body mass index (BMI), smoking, heavy alcohol consumption, physical inactivity, and psychological distress on adverse pregnancy outcomes in childhood cancer survivors has not been evaluated.
In the general population, prepregnancy BMI and smoking are associated with adverse pregnancy outcomes;7–14 however, limited evidence exists regarding the influence of prepregnancy heavy alcohol consumption, physical inactivity, and psychological distress. Adult female survivors of childhood cancer have high rates of both underweight and obesity,15 physical inactivity,16 and psychological distress.17 However, survivors are less likely than siblings to report smoking18, 19 and heavy alcohol consumption.20 Lifestyle and psychological factors may explain a portion of the risk for adverse pregnancy outcomes not explained by treatment exposures. If additional risk is observed due to the presence of these factors before pregnancy, health care providers could provide targeted counseling and interventions to help modify lifestyle and psychological status of vulnerable survivors.
The current study was designed to evaluate potential associations between prepregnancy lifestyle factors, psychological distress and adverse pregnancy outcomes among female survivors of childhood cancer.
MATERIALS AND METHODS
Study population
Participants were members of the Childhood Cancer Survivor Study (CCSS) cohort, described in detail previously.21, 22 Briefly, participants were at least 5 year survivors of childhood cancer, diagnosed when younger than age 21 years at one of 26 institutions in North America between 1970 and 1986. The protocol was approved by institutional review boards at all institutions. Consent was obtained from survivors older than 18 years and from parents of survivors younger than 18 years of age. Of 20,691 eligible survivors, 14,358 survivors were enrolled. Study participants completed a baseline questionnaire in 1995 and follow-up questionnaires thereafter (http://www.stjude.org/ccss). The baseline questionnaire gathered information on demographics, cancer type, medications, psychological status, pregnancy history, and lifestyle factors. The medical records of those who consented were abstracted. The follow-up 2000 pregnancy questionnaire gathered information on parent’s age at pregnancy, time to pregnancy, fertility problems, infertility treatment such as medications or in-vitro fertilization, chemotherapy or radiotherapy received by either parents during or a year before pregnancy, antenatal care, substance abuse, medical complications during pregnancy, and dates and outcomes of pregnancies. The follow-up 2003 questionnaire was similar to the baseline questionnaire and in addition collected information on neurocognitive functions, short form-36 health survey, dental and bone health, and post-traumatic stress. The follow-up 2007 questionnaire gathered information similar to the baseline questionnaire. Survivors who reported relapse or second neoplasms before pregnancy were excluded.
For the current study, we restricted our sample to survivors who consented to medical record abstraction for treatment information, and included pregnancies reported by female survivors 14 to 45 years of age who had previously completed a questionnaire reporting lifestyle factors and psychological distress (Figure 1). Non-singleton pregnancies, pregnancies resulting from in-vitro fertilization (IVF), or reported pregnancies without a known outcome, were not included (Figure 1).
Figure 1.
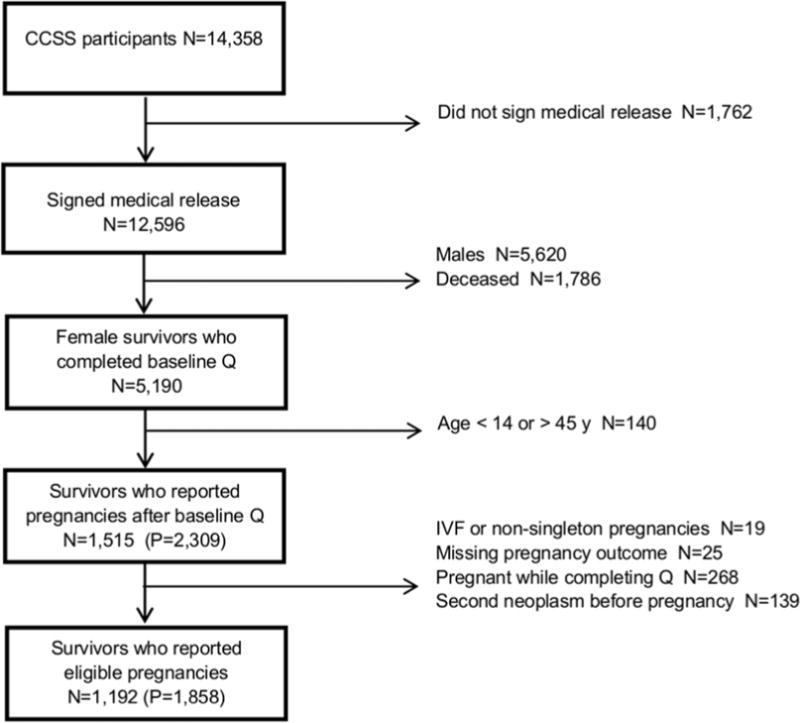
Flowchart illustrating the selection of eligible pregnancies from the Childhood Cancer Survivor Study (Q = questionnaire; IVF = in-vitro fertilization; P = pregnancies)
Adverse pregnancy outcomes
Pregnancy outcomes including preterm birth, stillbirth and miscarriage were obtained from the 2000 and 2007 CCSS questionnaires. Pregnancy outcomes were self-reported by survivors as live birth, stillbirth, miscarriage or medical abortion. We defined preterm birth as a reported gestational age of less than 37 weeks for a live birth. Stillbirth was defined as fetal death occurring after 20 weeks of gestation or later and a miscarriage was defined as fetal death occurring before 20 weeks of gestation. Self-reported pregnancy outcomes of live birth, stillbirth, miscarriage or medical (elective) abortion were further validated by expert review with additional clarification through a telephone interview.22
Independent variables
Variables of interest, including BMI, smoking, alcohol consumption, physical inactivity, and psychological distress were obtained from the baseline and 2003 questionnaires completed prior to pregnancy. Alcohol consumption was only captured on the baseline questionnaire and thus evaluated in separate multivariable models only including pregnancies reported on the 2000 questionnaire. We defined BMI as self-reported weight in kilograms (kg) divided by self-reported height in meters squared (m2) and categorized survivors as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2).23 Pack-years of smoking were calculated by dividing the product of the average number of cigarettes smoked per day and the number of years smoked by twenty. Smoking status was categorized based on pack-years of smoking as 0, 0.1 to 5 or > 5 pack-years (0 was assigned for those who never smoked). Survivors who reported either > 3 drinks/day or > 7 drinks/week were classified as risky drinkers, per the National Institute on Alcohol Abuse and Alcoholism guidelines.24 Survivors were classified as physically inactive if they reported < 150 minutes of moderate or < 60 minutes of vigorous physical activity per week.25,26 Information from the Brief Symptom Inventory (BSI)-18 was used to identify survivors with global psychological distress or depression.27 Survivors with T-score ≥ 63 for the total BSI-18 were classified as having global distress and those with T-score score ≥ 63 for the depression subscale as having depression.28
Cancer and treatment variables were obtained from medical records. Analyses included organ-specific radiotherapy and chemotherapeutic exposures previously reported to influence adverse pregnancy outcomes.2–4, 29 Organ-specific radiation dose was estimated by medical physicists and the sum of all radiation treatments was used as the total radiation dose30 to the uterus, ovaries, and pituitary, which was categorized as ≤ 2.5 Gy and > 2.5 Gy for analysis.3 The alkylating agent score was calculated by dividing the cumulative sum of the tertile scores of all alkylating agents into tertiles and given a score of 1 to 3.3 Anthracycline treatment was also categorized in cumulative dose tertiles. Other variables including race, annual household income, level of educational attainment, marital status, and insurance status, were self-reported by survivors on questionnaires completed prior to pregnancy.
Statistics
Demographic factors and treatment exposures of survivors with eligible pregnancies were examined as frequencies and percentages. To evaluate participation bias, we compared the demographics, treatment characteristics, and exposure distribution of the participants of baseline to 2000 and 2003 to 2007 intervals to the survivors (non-participants) who did not complete 2000 and 2007 questionnaires. Data analysis was performed using SAS software, version 9.2 (SAS Institute., Cary, NC).
To evaluate associations between factors of interest and adverse pregnancy outcomes, multivariable regression models with a generalized estimating equation31 adjustment to account for correlated data from women who had more than one pregnancy were used. The COPY method (10,000 copies of original data) was applied to estimate the maximum likelihood when models did not converge.32 A minimally sufficient set of covariates including age at diagnosis, age at pregnancy, educational attainment, and parity was identified a priori.33 Potential interactions between lifestyle factors or psychological distress and age at start of pregnancy, parity, type of cancer and treatment were evaluated in multivariable models. Results are reported as odds ratios (OR) with 95% confidence intervals (CI). P values < 0.05 were considered significant, except for interaction analyses where we used P < 0.15.34 When interaction terms were significant, ORs for each strata of the significant effect modifier are presented. Our results may be subject to selection bias because not all eligible survivors participated. To assess this potential bias, we performed a sensitivity analyses (see supplement).35
RESULTS
The demographic, treatment, lifestyle, and psychological characteristics of the eligible pregnancies (N=1,858) reported by 1,192 participants are presented in Table 1. Of the 1,192 participants, 722 contributed one eligible pregnancy each and 470 reported at least two eligible pregnancies contributing to the remaining 1,136 eligible pregnancies. The difference between age at pregnancy and age at exposure assessment questionnaires for female survivors was an average of 2.20 years (standard deviation=1.54 years; range: 0 to 7.58 years). We also compared the study participants with non-participants. Participants were more likely to be non-Hispanic white women, have a college education, health insurance, and an annual household income of more than $ 20,000. Among the 1,858 eligible pregnancies reported by the survivors, there were 1,300 live births, 140 medical abortions, 21 stillbirths, and 397 miscarriages. Of the 1,300 live births with available gestational age, 310 were preterm and 959 were delivered full term.
Table 1.
Demographics, treatment characteristics, and exposure distribution of eligible pregnancies sample and comparison between female participants and non-participants
| Eligible pregnancies N=1,858 | Participants N=4,492 | Non-participants N=558 | P | |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| 0–4 | 836 (45.0) | 1,990 (44.3) | 255 (45.7) | 0.47 |
| 5–9 | 455 (24.5) | 965 (21.5) | 126 (22.6) | |
| 10–14 | 363 (19.5) | 891 (19.8) | 110 (19.7) | |
| 15–20 | 204 (11.0) | 646 (14.4) | 67 (12.0) | |
| Age at start of pregnancy (years) | ||||
| 14–20 | 214 (11.5) | – | – | – |
| 21–25 | 435 (23.4) | – | – | – |
| 26–30 | 648 (34.9) | – | – | – |
| 31–35 | 389 (20.9) | – | – | – |
| 35–45 | 172(9.3) | – | – | – |
| Race/ethnicity | ||||
| White, non-Hispanic | 1,576 (84.8) | 3,847 (85.6) | 407 (72.9) | <0.001 |
| Black and Other, non-Hispanic | 196 (10.6) | 428 (9.5) | 105 (18.8) | |
| Hispanic/Latino | 86 (4.6) | 217 (4.9) | 46 (8.2) | |
| Parity | ||||
| Nulliparous | 1,063 (57.2) | – | – | – |
| Multiparous | 795 (42.8) | – | – | – |
| Education level | ||||
| Did not graduate high school | 313 (17.0) | 1,482 (34.8) | 202 (39.4) | <0.001 |
| Graduated from high school | 767 (41.7) | 1,720 (40.3) | 225 (43.9) | |
| Graduated from college | 758 (41.3) | 1,063 (24.9) | 86 (16.7) | |
| Health Insurance | ||||
| Yes | 1,615 (86.9) | 3,963 (89.3) | 441 (81.2) | <0.001 |
| No | 243 (13.1) | 474 (10.7) | 102 (18.8) | |
| Annual household income (US$) | ||||
| < 20,000 | 641 (36.6) | 776 (18.9) | 163 (33.7) | <0.001 |
| ≥ 20,000 | 1,112 (63.4) | 3,336 (81.1) | 321 (66.3) | |
| Cancer diagnosis | ||||
| Leukemia | 741 (39.9) | 1,627 (36.2) | 210 (37.6) | 0.79 |
| Central nervous system | 121 (6.5) | 529 (11.8) | 70 (12.5) | |
| Hodgkin lymphoma | 162 (8.7) | 492 (11.0) | 51 (9.2) | |
| Non-Hodgkin lymphoma | 97 (5.2) | 218 (4.9) | 24 (4.3) | |
| Renal tumor | 249 (13.4) | 487 (10.8) | 61 (10.9) | |
| Neuroblastoma | 166 (8.9) | 368 (8.2) | 50 (9.0) | |
| Soft tissue sarcoma | 161 (8.7) | 384 (8.5) | 51 (9.1) | |
| Bone cancer | 161 (8.7) | 387 (8.6) | 41 (7.4) | |
| Pituitary radiation | ||||
| 0–2.5 Gy | 1,364 (75.3) | 2,852 (65.4) | 329 (63.4) | 0.21 |
| > 2.5 Gy | 446 (24.7) | 1,512 (34.6) | 190 (36.6) | |
| Uterine radiation | ||||
| 0–2.5 Gy | 1,703 (91.9) | 3,867 (88.6) | 470 (91.3) | 0.07 |
| > 2.5 Gy | 149 (8.1) | 497 (11.4) | 45 (8.7) | |
| Ovarian radiation | ||||
| 0–2.5 Gy | 1,666 (91.8) | 3,749 (86.0) | 447 (86.6) | 0.69 |
| > 2.5 Gy | 149 (8.2) | 610 (14.0) | 69 (13.4) | |
| Alkylating agent scorea | ||||
| 0 (no alkylators) | 1,007 (56.7) | 2,329 (56.0) | 278 (54.9) | 0.86 |
| 1 | 415 (23.4) | 919 (22.1) | 109 (21.5) | |
| 2 | 254 (14.3) | 582 (14.0) | 77 (15.2) | |
| 3 | 100 (5.6) | 327 (7.9) | 42 (8.3) | |
| Anthracycline score | ||||
| 0 (no anthracycline) | 1,127 (61.9) | 2,779 (64.0) | 345 (64.5) | 0.35 |
| 1 | 200 (11.0) | 541 (12.4) | 70 (13.1) | |
| 2 | 302 (16.6) | 537 (12.4) | 53 (9.9) | |
| 3 | 191 (10.5) | 487 (11.2) | 67 (12.5) | |
| Physically inactive | ||||
| Yes | 916 (52.3) | 2,646 (60.5) | 353 (65.5) | 0.03 |
| No | 835(47.7) | 1,725 (39.5) | 186 (34.5) | |
| Body mass index (kg/m2) | ||||
| Underweight: <18.5 | 114 (6.7) | 581 (13.5) | 71 (13.5) | 0.13 |
| Normal: 18.5–25 | 1,056 (62.3) | 2,510 (58.4) | 282 (53.6) | |
| Overweight: 25–30 | 321 (18.9) | 750 (17.4) | 105 (20.0) | |
| Obese: ≥30 | 205 (12.1) | 460 (10.7) | 68 (12.9) | |
| Smoking (pack-years) | ||||
| 0 (Never) | 1,286 (75.1) | 3,456 (82.2) | 398 (77.4) | 0.03 |
| 0.1–5 | 293 (17.1) | 471 (11.2) | 74 (14.4) | |
| > 5 | 133 (7.8) | 277 (6.6) | 42 (8.2) | |
| Risky drinkingb | ||||
| Yes | 217 (31.2) | 732 (28.8) | 95 (32.6) | 0.16 |
| No | 479 (68.8) | 1,813 (71.2) | 196 (67.4) | |
| Psychological statusc | ||||
| Global distress | ||||
| Yes | 116 (7.9) | 264 (8.6) | 36 (9.7) | 0.49 |
| No | 1,347 (92.1) | 2,794 (91.4) | 335 (90.3) | |
| Depression | ||||
| Yes | 128 (8.7) | 291 (9.5) | 38 (10.2) | 0.65 |
| No | 1,338 (91.3) | 2,773 (90.5) | 334 (89.8) | |
| Pregnancy outcomes | ||||
| Live birth | 1,300 (70.0) | – | – | – |
| Stillbirth | 21 (1.1) | – | – | – |
| Miscarriage | 397 (21.4) | – | – | – |
| Medical abortion | 140 (7.5) | – | – | – |
| Preterm birth | ||||
| Yes | 310 (24.4) | – | – | – |
| No | 959 (75.6) | – | – | – |
Participants were those who completed 1) both baseline and 2000 questionnaire or
2) both 2003 and 2007 questionnaire
Non-participants were those who completed 1) baseline but not the 2000 questionnaire or
2) 2003 but not the 2007 questionnaire
P value was calculated for chi-squared test
non-platinum alkylators
defined as >3 drinks/day or >7 drinks/week based on the National Institute on Alcohol
Abuse and Alcoholism guidelines based on only 1995–2000 interval
from Brief Symptom Inventory-18 (T score ≥ 63)
Numbers may not equal total because of unanswered questions
The association between lifestyle factors, psychological distress, and adverse pregnancy outcomes after controlling for age at pregnancy, age at diagnosis, educational attainment and parity are shown in Table 2. Physical inactivity, BMI, smoking, and global psychological distress were not associated with preterm birth and stillbirth. The null associations of these factors were also observed in a model that 1) replaced global distress with depression; and, 2) additionally controlled for risky drinking. We did not observe any interaction between lifestyle factors or psychological distress and age, parity, type of cancer, and cancer treatment while evaluating the risk for preterm birth and stillbirth.
Table 2.
Adjusted risk of adverse pregnancy outcomes in female survivors of childhood cancer
| Preterm birth (N=310) |
Stillbirth (N=21) |
Miscarriageb (N=397) |
Risk of Miscarriage by Uterine Radiationc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 0–2.5 Gy (N=1,703) |
> 2.5 Gy (N=149) |
||||||||||
|
|
|||||||||||
| N | Row % | RR (95% CI) | Row % | RR (95% CI) | Row % | RR (95% CI) | Row % | RR (95% CI) | Row % | RR (95% CI) | |
| Physically inactivea | |||||||||||
| Yes | 916 | 22.6 | 0.9 (0.6, 1.2) | 0.7 | 0.5 (0.1, 1.8) | 22.6 | 1.0 (0.8, 1.4) | 22.8 | 1.1 (0.8, 1.5) | 21.1 | 0.5 (0.2, 1.5) |
| No | 835 | 26.2 | 1.0 | 1.4 | 1.0 | 21.3 | 1.0 | 20.8 | 1.0 | 28.1 | 1.0 |
| Body Mass Index (kg/m2) | |||||||||||
| Underweight: <18.5 | 114 | 18.3 | 1.0 (0.5, 2.1) | 1.8 | 1.3 (0.1, 17.9) | 18.4 | 0.8 (0.4, 1.4) | 18.8 | 0.9 (0.5, 1.6) | 15.4 | 0.2 (0.1, 0.7) |
| Normal weight: 18.5–25 | 1,056 | 22.7 | 1.0 | 0.7 | 1.0 | 21.2 | 1.0 | 21.1 | 1.0 | 23.5 | 1.0 |
| Overweight: 25–30 | 321 | 27.9 | 1.3 (0.9, 1.9) | 2.2 | 2.3 (0.5, 10.9) | 20.9 | 0.9 (0.6, 1.3) | 20.6 | 0.8 (0.5, 1.2) | 24.0 | 0.5 (0.1, 1.8) |
| Obese: ≥30 | 205 | 31.5 | 1.1 (0.7, 1.8) | 0.5 | 0.8 (0.1, 7.9) | 28.8 | 1.4 (0.8, 2.1) | 28.6 | 1.3 (0.8, 2.0) | 35.7 | 1.5 (0.4, 6.3) |
| Global distress | |||||||||||
| Yes | 116 | 20.3 | 0.8 (0.4, 1.7) | 1.7 | 1.3 (0.1, 10.9) | 20.9 | 1.5 (0.9, 2.5) | 28.4 | 1.6 (0.9, 2.7) | 28.6 | 0.9 (0.3, 3.0) |
| No | 1,347 | 24.5 | 1.0 | 0.7 | 1.0 | 28.4 | 1.0 | 20.9 | 1.0 | 21.6 | 1.0 |
| Smoking (pack-years) | |||||||||||
| 0 (Never) | 1,286 | 24.8 | 1.0 | 1.2 | 1.0 | 20.3 | 1.0 | 20.3 | 1.0 | 21.2 | 1.0 |
| 0–5 | 293 | 21.4 | 0.7 (0.5, 1.2) | 1.4 | 1.2 (0.3, 5.2) | 21.2 | 1.1 (0.7, 1.6) | 21.3 | 1.1 (0.8, 1.7) | 20.0 | 0.7 (0.3, 1.9) |
| > 5 | 133 | 35.1 | 1.6 (0.8, 3.0) | 0.8 | 1.4 (0.1, 16.1) | 34.6 | 2.2 (1.4, 3.5) | 31.2 | 1.9 (1.2, 3.0) | 87.5 | 53.9 (2.2, 1326.1) |
Risk presented as odds ratio (95% confidence interval); Models adjusted for age at pregnancy, age at diagnosis, educational attainment, and parity
< 60 minutes vigorous or < 150 minutes moderate physical activity per week
In a multivariable model evaluating risk for miscarriage, Pinteraction for smoking*uterine radiation = 0.01
In a multivariable model adjusted for age at pregnancy, age at diagnosis, educational attainment, parity, inactivity, BMI, global distress, and smoking, the risk for miscarriage among those who were exposed to > 2.5 Gy of radiation was 1.2 (95% CI 0.8, 1.9).
The multivariable association between smoking and miscarriage was modified by uterine radiation (Table 2). Compared to those who had never smoked, survivors with > 5 pack-years smoking history had higher risk for miscarriage among those with > 2.5 Gy uterine radiation (OR: 53.9; 95% CI: 2.2, 1,326.1) than among those with ≤ 2.5 Gy of uterine radiation (OR: 1.9; 95% CI: 1.2, 3.0; Pinteraction = 0.01). The interaction between smoking and uterine radiation for risk of miscarriage was also observed after replacing global distress with depression in the multivariable model (Pinteraction = 0.02). Other interaction terms between lifestyle factors or psychological distress and age, parity, type of cancer and cancer treatment were not significant when evaluating the risk for miscarriage. In separate multivariable models, no association was observed between risky drinking and preterm birth, stillbirth, or miscarriage.
In the sensitivity analysis, we calculated the lower and upper confidence bounds of our unadjusted estimates for risk of miscarriage among those who smoked > 5 pack-years and had received > 2.5 Gy uterine radiation (see supplement). We estimated confidence bounds assuming both no association (OR: 1.0) and a 2-fold protective association (OR: 0.5) between smoking > 5 pack-years and miscarriage among non-participants. With miscarriage rate estimates of 30, 25, 20, and 15%, summary OR ranged from 3.7 to 6.3 for the null assumption and 3.0 to 4.4 for the 2-fold protective assumption.
COMMENT
To our knowledge this is the first study to evaluate the associations between prepregnancy lifestyle factors, psychological distress, and adverse pregnancy outcomes in a large cohort of female survivors of childhood cancer. We found no associations between prepregnancy BMI, risky drinking, physical inactivity, or psychological distress, and adverse pregnancy outcomes. However, we observed a positive interaction between prepregnancy smoking and uterine radiation for risk of miscarriage. Female survivors of childhood cancer who smoked > 5 pack-years of cigarettes before pregnancy had higher risk for miscarriage compared to those who never smoked, regardless of radiation exposure. However, risk for miscarriage was particularly high among those who received > 2.5 Gy uterine radiation when compared to those who received ≤ 2.5 Gy of uterine radiation during cancer treatment.
Previous CCSS reports have observed adverse pregnancy outcomes and treatment-related risk factors among female survivors of childhood cancer.2–4 Green et al2 identified a non-significant increased risk for miscarriage among those treated with ovarian radiation (relative risk [RR] = 1.86; 95% CI: 0.82, 4.18) or with ovaries near a radiation field (RR = 1.64; 95% CI: 0.97, 2.78). Signorello et al3 observed that survivors had almost twice the risk for a preterm birth compared to the siblings, and also identified a dose-response association between uterine radiation and risk for preterm birth. Another CCSS study reported that survivors treated with uterine and ovarian radiation > 10 Gy have 9 times the risk for stillbirth and neonatal deaths compared to those treated with no radiation.4 In this study we observed 70% live births, 7.5% medical abortions, 1.1% stillbirths, 21.4% miscarriages, and 24.4% preterm births among female childhood cancer survivors. Previous CCSS reports have observed 70.9% live births, 11.6% medical abortions, 0.7% stillbirths, 25.5% miscarriages,2 and 12.6% preterm births3 among female siblings of childhood cancer survivors.
Although no study has reported an interaction between uterine radiation and prepregnancy smoking on risk for miscarriage, previous studies indicate that a history of abdominal or uterine radiation in female survivors of childhood cancer5, 6 and prepregnancy smoking in the general population12 are independent risk factors for miscarriage. The British Childhood Cancer Survivor Study observed an increased risk for miscarriage among female survivors (4,113 singleton pregnancies) who were treated with abdominal radiation (OR: 1.4; 95% CI: 1.0, 1.9).5 Similarly, a report from the Danish Cancer Registry found a higher risk for miscarriage among the 1479 pregnancies of female survivors who were treated with uterine and ovarian radiation (range: 1 to 40 Gy) when compared to the 5,092 pregnancies among their sisters (Proportion Ratio: 2.8; 95% CI: 1.7, 4.7).6 Elevated risk for miscarriage is reported in the general population among women with history of heavy prepregnancy smoking. In a nested case-control study of prospectively collected data from 1,921 women, Nielsen et al12 observed that the risk for miscarriage increased by 20% for every five cigarettes smoked per day (OR: 1.20; 95% CI: 1.04, 1.39).
The increase in risk for miscarriage among female survivors treated with > 2.5 Gy uterine radiation and a smoking history is likely explained by the combined effects of radiation and smoking on uterine myometrium, endometrium, and vasculature. Uterine radiation during childhood is associated with smaller adult uterine size,36, 37 absence of endometrial growth in response to exogenous estrogen36 and progesterone,37 and impairment of uterine blood flow as measured by Doppler ultrasound.37 Some investigators propose that radiation-induced uterine and abdominopelvic fibrosis restricts uterine expansion thus impairing the maintenance of pregnancy.38, 39 Cigarette smoking influences the uterus by increasing the production of myometrial oxytocin receptors and myometrial contractility, and reducing progesterone synthesis and endometrial vascularity.40, 41 Additionally, smoking also reduces the endothelium-dependent nitric oxide-mediated relaxation in the small arteries of the uterus, which may persist despite smoking cessation during early pregnancy.42 Uterine radiation exposure > 2.5 Gy is likely a result of direct abdominal or pelvic radiotherapy whereas uterine radiation < 2.5 Gy is likely because of exposure to total body, or scatter from spinal, chest, or proximal lower extremity irradiation.
Several limitations need to be considered when interpreting our study findings. First, our results may be subject to selection bias because not all eligible survivors participated. In the sensitivity analyses,42 even if the direction of the association between smoking and miscarriage among non-participants differed from participants, the positive association between smoking > 5 pack-years and miscarriage among survivors exposed to > 2.5 Gy uterine radiation remained. Second, miscarriage is often underestimated because not all pregnancy losses are recognized.43 If this underestimation is unrelated to a survivor’s smoking status then the resulting bias would only produce conservative estimates.44 Third, smoking status of survivors in our study may be underestimated because of the self-reported nature of the assessment.45 However, we believe the magnitude of this bias would be minimal because self-reported cigarette smoking underestimates prevalence of serum cotinine ≥ 15 ng/mL by only 0.6% in general US population.45 Fourth, we may have failed to detect an association between other lifestyle factors and adverse pregnancy outcomes due to small sample size. For instance, we had 53% power to detect an odds ratio of 2.0 for an association between smoking and preterm birth among 1,269 live births with available gestational age.9 Fifth, our results may also be biased due to unmeasured confounders such as other drug abuse, which is associated with both smoking and miscarriage.46 Finally, we did not have information about the lifestyle factors and psychological status during pregnancy or about changes in these risk factors between self-report and conception.
Female survivors of childhood cancer, especially those treated with ovarian or uterine radiation, are more likely to be infertile.29 Therefore, it is vital to identify factors detrimental for normal progress of pregnancy among female survivors. Our findings indicate that smoking more than 5 pack-years of cigarettes before pregnancy increases the risk of miscarriage, particularly among survivors treated with > 2.5 Gy uterine radiation. Given the current evidence about smoking-related adverse health outcomes, female survivors of childhood cancer who smoke and are planning to become pregnant should be provided with tools to promote smoking cessation. In addition, young female survivors of childhood cancer exposed to uterine radiation should be counseled early about this risk to discourage smoking initiation. Future large prospective studies are required to evaluate the null associations we observed between prepregnancy BMI, risky drinking, physical inactivity, or psychological distress, and adverse pregnancy outcomes.
Table 3.
Sensitivity of association between smoking > 5 pack-years (versus 0 pack-years) and miscarriage among those who received > 2.5 Gy uterine radiation (N=121)a because of non-participation.
| Assumed prevalence of miscarriage among non-participants | Observed unadjusted odds ratio of 25.9b among participants (N=121) | Assumed odds ratio among non-participants (N=15)c | Summary odds ratio (N=136)d |
|---|---|---|---|
| 30% | 25.9 | 1.0 | 6.3 |
| 25% | 25.9 | 1.0 | 5.5 |
| 20% | 25.9 | 1.0 | 3.7 |
| 15% | 25.9 | 1.0 | 4.4 |
| 30% | 25.9 | 0.5 | 4.4 |
| 25% | 25.9 | 0.5 | 4.0 |
| 20% | 25.9 | 0.5 | 4.2 |
| 15% | 25.9 | 0.5 | 3.0 |
Out of 149 pregnancies with > 2.5 Gy uterine radiation (see Table 2), 8 had history of > 5 pack-years and 113 had no history of smoking (N=121).
Out of the 8 with history of > 5 pack-years 7 had miscarriages and out of the 113 with no history of smoking 24 had miscarriages. Thus, the observed unadjusted risk of miscarriage among those with history of smoking > 5 pack-years was 25.9.
We assumed a non-participation rate of 11% (15 of 136) based on the observed 558 (11%) non-participants out of 5,050 eligible participants
If exposure-outcome distribution of non-participants were combined with exposure-outcome distribution of participants
Acknowledgments
Funding source: This work was supported by a grant from the National Cancer Institute (U24 CA55727, L.L. Robison, Principal Investigator), and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study location: The study was conducted in Memphis, TN, USA
Author contributions:
Conception and design: P.L. Gawade and K.K. Ness
Development of methodology: All authors
Acquisition of data: All authors
Analysis and interpretation of data: P.L. Gawade, K.K. Ness, W. Leisenring, and L.L. Robison
Writing, review and/or revision of the manuscript: P.L. Gawade and K.K. Ness wrote the first draft and all authors reviewed and revised the manuscript
Administrative, technical, or material support: P.L. Gawade and K.K. Ness
Study supervision: P.L. Gawade and K.K. Ness
The preliminary results of this study were presented at the “13th International Conference on Long-Term Complications of Treatment of Children and Adolescents of Cancer” organized by St. Jude Children’s Research Hospital in Memphis, TN from 13–15th June 2013.
Conflict of interest disclosures: The authors report no conflicts of interest.
Condensation: Childhood cancer survivors exposed to > 2.5 Gy uterine radiation who had a history of smoking had an increased risk of miscarriage.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187:1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 3.Signorello LB, Cohen SS, Bosetti C, et al. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98:1453–61. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Signorello LB, Mulvihill JJ, Green DM, et al. Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet. 2010;376:624–30. doi: 10.1016/S0140-6736(10)60752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reulen RC, Zeegers MP, Wallace WH, et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2239–47. doi: 10.1158/1055-9965.EPI-09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winther JF, Boice JD, Jr, Svendsen AL, Frederiksen K, Stovall M, Olsen JH. Spontaneous abortion in a Danish population-based cohort of childhood cancer survivors. J Clin Oncol. 2008;26:4340–6. doi: 10.1200/JCO.2007.15.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas JS, Fuentes-Afflick E, Stewart AL, et al. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159:58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83:713–20. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Davies GA, Maxwell C, Mcleod L, et al. SOGC Clinical Practice Guidelines: Obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet. 2010;110:167–73. doi: 10.1016/j.ijgo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Han Z, Mulla S, Beyene J, Liao G, Mcdonald SD. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2011;40:65–101. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen A, Hannibal CG, Lindekilde BE, et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet Gynecol Scand. 2006;85:1057–65. doi: 10.1080/00016340600589560. [DOI] [PubMed] [Google Scholar]
- 13.Salihu HM, Sharma PP, Getahun D, et al. Prenatal tobacco use and risk of stillbirth: a case-control and bidirectional case-crossover study. Nicotine Tob Res. 2008;10:159–66. doi: 10.1080/14622200701705431. [DOI] [PubMed] [Google Scholar]
- 14.Torloni MR, Betran AP, Daher S, et al. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. J Matern Fetal Neonatal Med. 2009;22:957–70. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- 15.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer. Cancer. 2005;103:1730–39. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 16.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–94. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol. 2002;20:1608–16. doi: 10.1200/JCO.2002.20.6.1608. [DOI] [PubMed] [Google Scholar]
- 19.Frobisher C, Winter DL, Lancashire ER, et al. Extent of Smoking and Age at Initiation of Smoking Among Adult Survivors of Childhood Cancer in Britain. J Natl Cancer Inst. 2008;100:1068–81. doi: 10.1093/jnci/djn210. [DOI] [PubMed] [Google Scholar]
- 20.Lown EA, Goldsby R, Mertens AC, et al. Alcohol consumption patterns and risk factors among childhood cancer survivors compared to siblings and general population peers. Addiction. 2008;103:1139–48. doi: 10.1111/j.1360-0443.2008.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–27. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO Technical Report Series 894. Geneva: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. (WHO Technical Report Series 894). [PubMed] [Google Scholar]
- 24.National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician’s guide. Bethesda, MD: [Google Scholar]
- 25.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–07. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 26.Services DoHaH, editor. Department of Health and Human Services. 2008 physical activity guidelines for Americans. Washington DC: 2008. [Google Scholar]
- 27.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 28.Derogatis LR. Brief Symptom Inventory (BSI)-18. Administration, scoring and procedures manual. Minneapolis: NCS Pearson, Inc; Number of pages. [Google Scholar]
- 29.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–85. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–52. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Zeger S, Heagerty P, Liang Kung-Yee, Diggle P. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 32.Petersen MR, Deddens JA. A revised SAS macro for maximum likelihood estimation of prevalence ratios using the COPY method. Occup Environ Med. 2009;66:639. doi: 10.1136/oem.2008.043018. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S. Causal diagrams for epidemiologic research. Vol. 10. Philadelphia, PA: Lippincott, Williams Wilkins; 1999. [PubMed] [Google Scholar]
- 34.Selvin S. Statistical analysis of epidemiologic data. New York, NY: Oxford University Press; Number of pages. [Google Scholar]
- 35.Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiologic data. New York, NY: Springer; Number of pages. [Google Scholar]
- 36.Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Muller J, Andersen AN. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand. 2004;83:96–102. doi: 10.1111/j.1600-0412.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 37.Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol. 1992;99:392–4. doi: 10.1111/j.1471-0528.1992.tb13755.x. [DOI] [PubMed] [Google Scholar]
- 38.Li FP, Gimbrere K, Gelber RD, et al. Outcome of pregnancy in survivors of Wilms’ tumor. JAMA. 1987;257:216–9. [PubMed] [Google Scholar]
- 39.Byrne J, Mulvihill JJ, Connelly RR, et al. Reproductive problems and birth defects in survivors of Wilms’ tumor and their relatives. Med Pediatr Oncol. 1988;16:233–40. doi: 10.1002/mpo.2950160403. [DOI] [PubMed] [Google Scholar]
- 40.Dechanet C, Anahory T, Mathieu Daude JC, et al. Effects of cigarette smoking on reproduction. Hum Reprod Update. 2011;17:76–95. doi: 10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- 41.Raine-Fenning NJ, Campbell BK, Kendall NR, Clewes JS, Johnson IR. Quantifying the changes in endometrial vascularity throughout the normal menstrual cycle with three-dimensional power Doppler angiography. Hum Reprod. 2004;19:330–8. doi: 10.1093/humrep/deh056. [DOI] [PubMed] [Google Scholar]
- 42.Andersen MR, Uldbjerg N, Stender S, Sandager P, Aalkjær C. Maternal smoking and impaired endothelium-dependent nitric oxide–mediated relaxation of uterine small arteries in vitro. Am J Obstet Gynecol. 2011;204:177.e1–77.e7. doi: 10.1016/j.ajog.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Wilcox AJ, Weinberg CR, O’connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 44.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 45.West R, Zatonski W, Przewozniak K, Jarvis MJ. Can We Trust National Smoking Prevalence Figures? Discrepancies Between Biochemically Assessed and Self-Reported Smoking Rates in Three Countries. Cancer Epidemiol Biomarkers Prev. 2007;16:820–22. doi: 10.1158/1055-9965.EPI-06-0679. [DOI] [PubMed] [Google Scholar]
- 46.Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and Tobacco Use and the Risk of Spontaneous Abortion. New Engl J Med. 1999;340:333–39. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]


