Abstract
Individual vulnerability to stress-induced relapse during abstinence from chronic heroin exposure is a key feature of opiate addiction, with limited studies on this topic. Arginine vasopressin (AVP) and its V1b receptor, components of the brain stress responsive systems, play a role in heroin-seeking behavior triggered by foot shock (FS) stress in rats. In this study, we tested whether individual differences in the FS-induced heroin-seeking were associated with alterations of AVP and V1b, as well as other stress responsive systems, including pro-opiomelanocortin (POMC), orexin, plasma ACTH and corticosterone, as well as dopamine D2 receptor (D2) and plasma prolactin. Sprague-Dawley rats were subjected to 3-hour intravenous heroin self-administration (SA) and then tested in extinction, FS-induced and heroin priming-induced reinstatements. The rats that self-administered heroin were divided to high and low reinstatement responders induced by FS (H-RI; L-RI). Over SA sessions, both the H-RI and L-RI displayed similar active lever responding, heroin infusion and total heroin intake. Compared to the L-RI, however, the H-RI showed greater active lever responses during stress-induced reinstatement, with higher AVP mRNA levels in medial/basolateral amygdala and lower D2 mRNA levels in caudate putamen. However, heroin priming resulted in similar reinstatement in both groups and produced similarly low POMC and high orexin mRNA levels in hypothalamus. Our results indicate that: 1) enhanced amygdalar AVP and reduced striatal D2 expression may be related to individual vulnerability to stress-induced reinstatement of heroin- seeking; and 2) heroin abstinence-associated alterations of hypothalamic orexin and POMC expression may be involved in drug priming-induced heroin-seeking.
Keywords: arginine vasopressin, POMC, orexin, D2, stress, heroin-seeking behavior
Introduction
There have been numeral animal studies on drug addiction that explored individual propensities to develop addictive behavior, including locomotor responses to novelty, to initial drug challenges or development of behavioral sensitization [e.g., 1, 2]. While these factors could contribute to the initiation and maintenance of drug self-administration, there is limited data exploring the core feature of addiction: individual vulnerability to relapse during abstinence from chronic drug self-administration. In animal studies of drug self-administration, vulnerability for relapse can be assessed using reinstatement procedures [3, 4]. These procedures may represent valid models of drug craving because similar environmental and pharmacological stimuli trigger both drug-seeking behavior in animals and drug craving in humans [4, 5], and may have predictive validity in evaluating anti-craving compounds for use in the treatment of addiction [e.g., 6].
Heroin addiction is a chronic relapsing disease of the brain caused by drug-induced neuroadaptations at the molecular and cellular levels [7, 8]. It is well known that many stress responsive systems in the CNS, including the peptides, their receptors, and stress-related hormones, are involved in drug cravings in humans [9, 10, 11], and drug-seeking behavior triggered by exposure to various stressors in animals [4]. More specifically, in response to stress, hypothalamic arginine vasopressin (AVP) which activates V1b receptors stimulates ACTH secretion from the corticotropes in the anterior pituitary [e.g., 12, 13, 14]. The AVP/V1b receptor system is widely distributed in the CNS, suggesting that it may be mediators of the actions of central stress responsive systems [15]. Indeed, there is a growing body of evidence suggesting that AVP activity in the amygdala is an important element in the neurobiology of stress-related behaviors in rodents [15]. Increased AVP gene expression in the amygdala is associated with early withdrawal from chronic exposure to opiates, and the selective AVP V1b (but not V1a) receptor antagonist attenuates heroin-seeking behavior induced by foot shock stress [16]. These findings suggest that the amygdalar stress-responsive AVP/V1b receptor system may be a critical component of the neural circuitry underlying opiate withdrawal, and the effect of stress on drug-seeking behavior.
Heroin, morphine and other short-acting opiates regulate the activity of endogenous opioid systems, and chronic opiate exposure can cause a relative deficiency in beta-endorphin/mu opioid receptor system [7]. Although early studies reported a lack of effect on beta-endorphin-immunoreactivity levels in many brain regions of rodents [8], studies of pro-opiomelanocortin (POMC) gene expression indicated that chronic opiate exposure significantly decreased POMC mRNA levels in the hypothalamus [e.g., 17, 18]. Another interesting stress-related peptide is orexin: mu opioid receptor agonists (including beta-endorphin) inhibit orexin-producing neurons in the lateral hypothalamus [19]. Acute opiate withdrawal is associated with increased orexin expression and activity in this region [20, 21], which may play an important role in opiate-seeking behaviors [22, 23].
The objective of this study is explore whether individual differences in the vulnerability to drug relapse after comparable histories of drug exposure may be related to individual differences in key stress-responsive neurochemical systems. For this purpose, we specifically designed a set of novel experiments in rats to determine if individual differences in stress-induced heroin-seeking would be associated with differences in the expression of central AVP, POMC, or orexin genes. In the present study, rats were trained to intravenously self-administer heroin for 7 days and, after a 4-day period of extinction, they were tested for reinstatement of lever pressing (heroin –associate lever with saline available only) induced by acute (15-min) intermittent foot shock stress [6, 24]. Following this test, rats were divided into high and low reinstatement responders (H-RI and L-RI) to acute foot shock stress. In order to verify the specificity of the stress-defined H-RI and L-RI groups, all rats were also tested for reinstatement of lever pressing induced by a heroin prime. Nine days after heroin self-administration, specific brain regions were collected to quantify AVP, POMC and orexin gene expressions, and the alterations were analyzed in the H-RI and L-RI groups. Because availability of dopamine D2 receptor (D2) binding and D2 gene expression in the striatum have been associated with cocaine seeking [25, 26], we also determined if the changes in striatal D2 expression, as well as in the stress hormones (ACTH, corticosterone and prolactin), were correlated with vulnerability to stress-induced heroin-seeking.
Material and methods
I. Heroin self-administration, extinction, foot shock- and heroin- induced reinstatement
As mentioned in the self-administration section below, we used an acquisition criterion to identify sub-groups of drug self-administration responders (n=20) and non-responders (n=6) that differed in amount of heroin exposure during self-administration. Within the group of drug self-administration responders, we also observed large individual differences in levels of lever-pressing during the acute foot shock-induced reinstatement session. Thus, we employed the median-split approach [e.g., 27] to further subdivide this group into “high” (H-RI) and “low” (L-RI) stress-reinstatement rats.
1. Subjects
Twenty six adult male out-bred Sprague-Dawley rats (Charles River, QC), weighing 225–250g at the beginning of the experiment, were singly housed and maintained on a reverse light/dark cycle (8:00 hour lights off; 20:00 hour lights on) with free access to food and water except during behavioral testing, which occurred during the dark cycle. All experiments were approved by the Animal Care Committee of the University of Guelph and were carried out in accordance with the recommendations of the Canadian Council on Animal Care.
2. Surgery
Rats were surgically implanted with intravenous silastic catheters (Dow Corning, Midland, MI) in the right jugular vein, under general anesthesia induced by a combination of sodium pentobarbital (18.5 mg/kg IP, MTC Pharmaceutical, Cambridge, ON), morphine (5 mg/kg SC, Ontario Veterinary College, Guelph, ON), and diazepam (1 mg/kg SC, Sabex Inc., Boucherville, QC). Rats were given atropine sulfate (4.5 mg/kg SC, Ontario Veterinary College, Guelph, ON) just before surgery and Depocillin (300 000 IU, 0.1 ml/rat IM, Intervet Canada, Whitby, ON) immediately following surgery. The catheter was secured to the vein with silk sutures and was passed subcutaneously to the top of the skull where it exited into a connector (a modified 22 G cannula; Plastics One, Roanoke, VA) mounted to the skull with jeweler’s screws and dental cement. A plastic blocker was placed over the opening of the connector during the recovery period and at all other times when the rats were not in a self-administration session. To prevent clogging, catheters were flushed every day with 0.1 ml of a saline–heparin solution (0.2 mg/ml Hepalean 1.000 IU, Organon, Toronto, ON). Behavioral testing began after 8 days of recovery from surgery.
3. Apparatus
Twenty-six Plexiglas operant chambers (model ENV-008CT, Med Associates, Georgia, VT) were each enclosed in larger sound-attenuating plywood chambers (model ENV-018M, Med Associates). Each operant box had a house light (28 V) and two levers, one retractable and one stationary, located 10 cm apart and 8 cm above the floor of the box. The retractable lever (active lever) was connected to an infusion pump positioned outside the sound-insulating chamber for the delivery of drugs (Razel Scientific Instruments, Stamford, CT). The stationary lever served to control for baseline, non-reinforced operant behavior; pressing this lever had no consequence (inactive lever), but all presses were recorded. A white light (28V) stimulus located 3 cm above the active lever was illuminated for 30 s at the beginning of the session, and for the duration of each drug infusion (5 s), serving as a discrete cue or conditioned stimulus for drug delivery. Each self-administration chamber was fitted to deliver constant-current, intermittent, inescapable, electric foot shock through a scrambler (model ENV-414, Med Associates) to the grid floor.
4. Procedure
The behavioral experiment consisted of three phases: self-administration, extinction, and reinstatement (see Fig. S1).
(A) Self-administration:
One 3-h self-administration session occurred every day for 7 consecutive days. Rats were transferred from their home cages to the operant chambers and their cannulas attached to infusion lines. Each session began with the activation of the house light, the entry of the retractable lever and the illumination of the light cue for 30 s. If the rat pressed the active lever during this initial presentation of the light cue, it received an infusion of heroin (0.05 mg/kg/infusion) followed by termination of the light. Subsequently, active lever-presses led to heroin infusions, and activation of the light (conditioning stimulus), according to a schedule of continuous reinforcement. Drug was infused in a volume of 150 µl over a 5-s period, and responses during this period were recorded, but did not lead to additional infusion.
During heroin self-administration, we noted individual variability in heroin intake that was not attributable to any obvious technical issue since catheter patency was verified daily by drawing blood. Rather than excluding the “non-responders (NR)”, these rats were tested until the conclusion of the experiment and employed as “heroin control” for the rats that showed clear acquisition. The criterion used to classify the NR was as follows: less than a total of 20 infusions taken on days 5, 6 and 7 of self-administration testing.
An additional control group (n=8) was comprised of rats were trained to self-administer saline. On the subsequent stages of the experiment, these rats were not exposed to foot-shock or to the heroin prime (heroin/stress control group).
(B) Extinction
Forty-eight hours after the last heroin self-administration session, extinction occurred over four 3-h sessions (days 9–12, see Fig. S1). Extinction sessions were identical to the self-administration sessions except that saline was substituted for heroin.
(C) Reinstatement
Twenty-four hours after the last extinction session (day 13), rats were tested for foot shock-induced reinstatement. During this session, rats were exposed to 15 min of intermittent foot shock stress (0.5 mA, 0.5 s ON, a mean OFF period of 40 s) and then immediately monitored for lever-pressing in extinction conditions for 3 hours. Forty-eight hours later (day 15), rats were tested for heroin-induced reinstatement. No additional extinction was given between the two tests. For this second test, rats received an injection of heroin (0.25 mg/kg s.c.), and following a 10-min interval, lever-pressing in extinction conditions was monitored for 3 h. Twenty-four hours following the heroin reinstatement session (day 16, 9 days after the last self-administration session), the experiment was concluded by exposing the rats to 15 min of intermittent foot shock stress (0.5 mA, 0.5 s ON, a mean OFF period of 40 s) in the operant chambers. Shortly after (45 min) this stress “challenge,” rats were sacrificed by decapitation following brief exposure to CO2 (approximately 15 seconds), and specific brain and pituitary regions, as well as plasma were collected for subsequent mRNA and hormone analyses.
As mentioned above, the “heroin/stress control” received saline during self-administration, no foot-shock prior to the first reinstatement test, and saline prior to the second test. Also this group did not receive the stress “challenge” prior to tissue collection. Although other controls (heroin self-administration only, stress reinstatement only, heroin reinstatement only) could have been included, this was the only group necessary for the interpretations of inter-group individual differences in gene expression.
II. Preparation of RNA extracts
Nine days after the last heroin self-administration session, all rats (except the heroin/stress controls) were exposed to the 15-min acute intermittent foot shock stress identical to that used during the acute stress-induced reinstatement session, and 45 min later, the brain tissues and plasma were collected.
Each brain was removed from the skull and placed in a chilled rat brain matrix (ASI Instruments, Houston, Texas). A coronal slice containing the region of interest was removed from the matrix and placed on a chilled petri dish. Dissection was carried out using razor blades and forceps under a dissecting microscope. The brain regions of interest were identified according to The Rat Brain in Stereotaxic Coordinates [28]. The lateral hypothalamus (LH), medial portion of the hypothalamus (MH, include the arcuate nucleus, dorsomedial hypothalamus, and paraventricular nucleus), caudate-putamen (CPu), central amygdal (CeA) and medial/basolateral portions of the amygdala (Me/BLA), ventral tegmental area (VTA), and anterior pituitary (AP) were dissected on ice, homogenized in guanidinium thiocyanate buffer and extracted with acidic phenol and chloroform. After the final ethanol precipitation step, each extract was re-suspended in DEPC-treated H2O and stored at −80°C.
III. Solution hybridization ribonuclease protection-trichloroacetic acid precipitation assay
The protocol for the solution hybridization ribonuclease protection-trichloroacetic acid precipitation has been described in detail in earlier report [21]. A 538-base pair (bp) fragment from the rat POMC cDNA or a 860-bp fragment from the rat dopamine D2 receptor cDNA was cloned into the polylinker region of either pSP64 or pSP65 plasmids (Promega, Madison, WI) in both the sense and antisense orientations. A 531 bp fragment from the rat orexin (or hypocretin) cDNA was cloned into the polylinker region of pBC SK+ (Stratagene, La Jolla, CA). A 502 bp fragment from the rat AVP cDNA or a 1201 bp fragment from the rat V1b receptor cDNA was cloned into the polylinker region of pCR II (Invitrogen, Carlsbad CA). The plasmid pS/E (a pSP65 derivative) was used to synthesize riboprobe for the 18S rRNA to determine total RNA. 33P-labeled cRNA antisense probes and unlabeled cRNA sense standards were synthesized using a SP6, T3 or T7 transcription system. A denaturing agarose gel containing 1.0 M formaldehyde showed that a single full-length transcript had been synthesized from each plasmid.
RNA extracts were dried in 1.5 ml Eppendorf tubes and resuspended in 30 µl of 2 × TESS (10 mM N-Tris[hydroxy-methyl]methyl-2-aminoethane sulfonic acid, pH 7.4; 10 mM ethylenediaminetetraacetic acid [EDTA]; 0.3 M NaCl; 0.5% sodium dodecyl sulfate [SDS]) that contained 150 to 300 K cpm of a probe. Samples were covered with mineral oil and hybridized overnight at 75°C. For RNase treatment, 250 µl of a buffer containing 0.3 M NaCl; 5 mM EDTA; 10 mM Tris-HCl (pH 7.5), 40 µg/ml RNase A (Worthington, Biochemicals, Freehold, NJ) and 2 µg/ml RNase T1 (Calbiochem, San Diego, CA) was added and each sample was incubated at 30°C for 1 hour. Trichloroacetic acid precipitation was effected by the addition of 1 ml of a solution that contained 5% TCA and 0.75% sodium pyrophosphate. Precipitates were collected onto a filter in sets of 24 using a cell harvester (Brandel, Gaithersburg, MD) and were measured in a scintillation counter with liquid scintillant (Beckman, Palo Alto, CA).
The procedure to measure mRNA levels involved a comparison of values obtained from experimental samples (brain extracts) to those obtained for a set of calibration standards. The calibration standards had known amounts of an in vitro sense transcript whose concentration was determined by optical absorbance at 260 nm. The set of calibration standards included those with no added sense transcript and those that contained between 1.25 and 80 pg of the sense transcript [21]. To determine the total attomoles of each mRNA in each extract, the amounts calculated from the standard curves were multiplied by 2.7 for POMC, 1.25 for ppDyn, 3.5 for KOR, 4.3 for orexin, 5.0 for AVP, 2.4 for AVP V1b receptor, and 1.2 for dopamine D2 receptor to correct for the difference in length between the sense transcript and full-length mRNA. A new standard curve was generated each time experimental samples were analyzed and all extracts of a particular tissue were assayed for each mRNA as a group in a single assay.
Total cellular RNA concentrations were measured by hybridization of diluted extracts to a 33P-labeled probe complementary to 18S rRNA at 75°C. The calibration standards for this curve contained 10 µg of E. coli tRNA plus from 0.0 to 40 ng of total RNA from rat brain whose concentration was determined by optical absorbance at 260 nm.
IV. Radioimmunoassays
At the time of decapitation, blood from each rat was collected in tubes, placed on ice, and was spun in a refrigerated centrifuge. Plasma was separated and stored at −40°C for hormonal measurements by radioimmunoassay. Levels of ACTH, corticosterone (CORT) and prolactin immunoreactivity were assayed from unextracted plasma by using kits from MP Biomedicals (Costa Mesa, CA), DiaSorin Inc. (Stillwater, MN), and Amersham Life Science Inc. (US), respectively. All values were determined in duplicate in a single assay.
V. Drugs and doses
Heroin (diaceltymorphine) was obtained from Almat Pharmachem (Concord, Ontario, Canada), dissolved in 0.9% physiological saline and self-administered at a dose of 0.05 mg/kg/infusion. This dose has been shown to induce acquisition of self-administration behavior within 7 days [29].
VI. Statistical analysis
Self-administration, extinction and reinstatement were analyzed using two-way mixed ANOVAs. In case of significant interactions or significant main effects, multiple comparisons were performed using Newman–Keuls post hoc method to identify significant differences between groups. Group differences in mRNA levels of each gene in each brain region and in plasma hormonal levels were analyzed using one-way ANOVA (high reinstatement, low reinstatement and heroin self-administration non-responder groups). To explore possible relationships between gene expressions/stress hormones and individual vulnerability to stress-induced reinstatement, total responses made on the active lever on reinstatement tests and neurochemical measures were also examined by linear regression. The accepted level of significance was p<0.05.
Results
I. Effect of 3-hour heroin self-administration, extinction, foot shock- and heroin-induced reinstatement
1. Self-administration
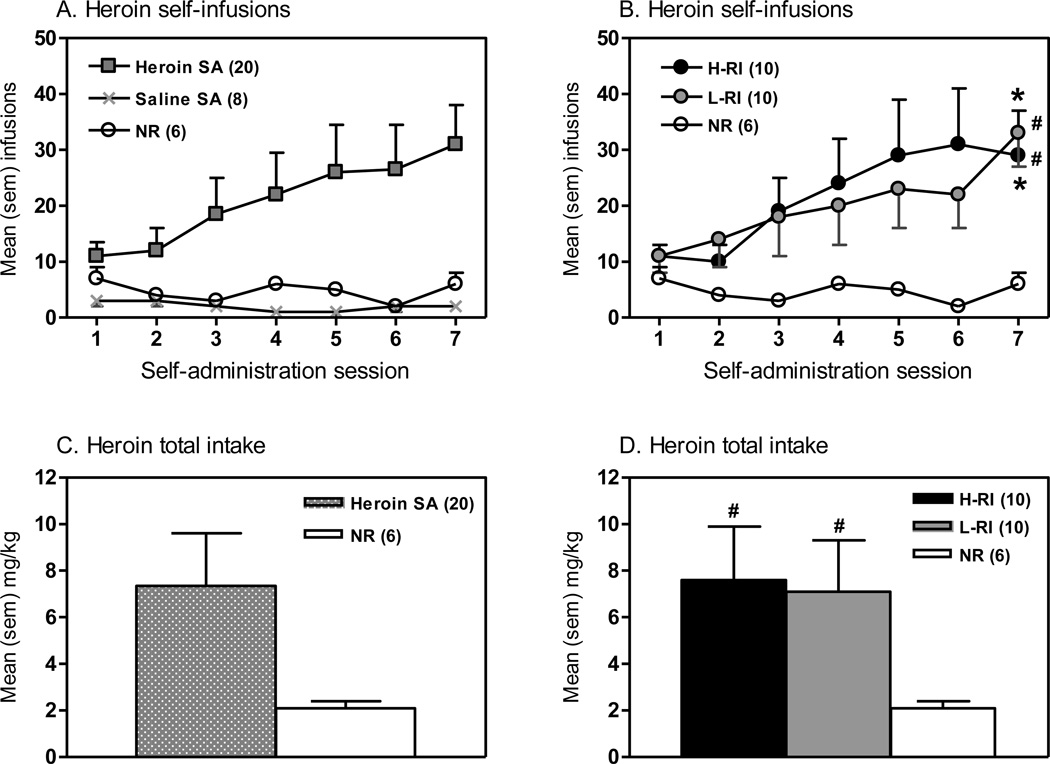
Over the seven sessions of heroin self-administration, heroin infusions significantly increased over the course of the self-administration period in all the rats that self-administered heroin (Fig. 1A), and both the H-RI and L-RI rats displayed similar pattern (Fig. 1B). ANOVA revealed significant main effects of Session [F(6,181) = 6.06, p<0.001] and Group x Session interaction [F(12,181) = 2.05, p<0.05]. Newman-Keuls post hoc tests showed that both the H-RI and L-RI rats took significantly more infusions on Session 7 than Session 1 (p<0.001), with no change in number of infusions in the NR rats. In Session 7, both the H-RI and L-RI rats had significantly more infusions than the NR rats (p<0.05), with no differences between the H-RI and L-RI rats. As shown in Fig. 1C and 1D, both the H-RI and L-RI rats had significantly higher overall heroin intake over 7 days of drug self-administration sessions than the NR rats (p<0.05), with no difference between the two groups (Fig. 1D).
Figure 1.
Comparison of heroin self-administration (SA) behavioral responses. The panels show the mean (sem) actual self-infusions obtained (A, B) and the mean (sem) heroin total intake (mg/kg) (C, D) over the seven 3-h sessions of heroin SA (0.05 mg/kg/infusion, continuous schedule of reinforcement) in all heroin or saline SA rats (A, C) or each of the 3 groups (B, D). Three groups of animals: (1) H-RI, the heroin SA rats with “high” reinstatement induced by acute foot shock stress, n = 10; (2) L-RI, the heroin SA rats with “low” reinstatement induced by acute foot shock stress, n = 10; and (3) NR, the rats below the criterion for acquisition of heroin SA (20 infusions on days 5–7 of SA testing, 0.05 mg/kg/infusion), n = 6. * p<0.001 vs. Session 1 within the group; # p<0.05 vs. NR group.
Both the H-RI and L-RI rats displayed increases in responding on the active lever (i.e. heroin lever) in parallel with decreases in responding on the inactive lever. For the active lever (Fig. S2A), two-way repeated ANOVA revealed a significant main effect of Session [F(6,181) = 6.38, p<0.001]. Newman-Keuls post hoc tests showed that both the H-RI and L-RI rats displayed significantly higher responding on the active lever in Session 7 than that in Session 1 (p<0.001) and Session 2 (p<0.001). The NR rats did not show differences in the active lever responding between Session 7 and Session 1. In Session 7, both the H-RI and L-RI rats responded significantly more on the active lever than the NR rats (p<0.05), with no difference between the H-RI and L-RI rats. On the inactive lever (Fig. S2B), ANOVA revealed a significant main effect of Session [F(6,181) = 5.70, p<0.001]. Newman-Keuls post hoc tests showed that the H-RI rats displayed significantly lower responding on the inactive lever in Session 7 than that in Session 1 (p<0.001). The L-RI or NR rats did not show differences in the inactive lever responding between any Sessions. In Session 7, there were no differences between groups.
2. Extinction
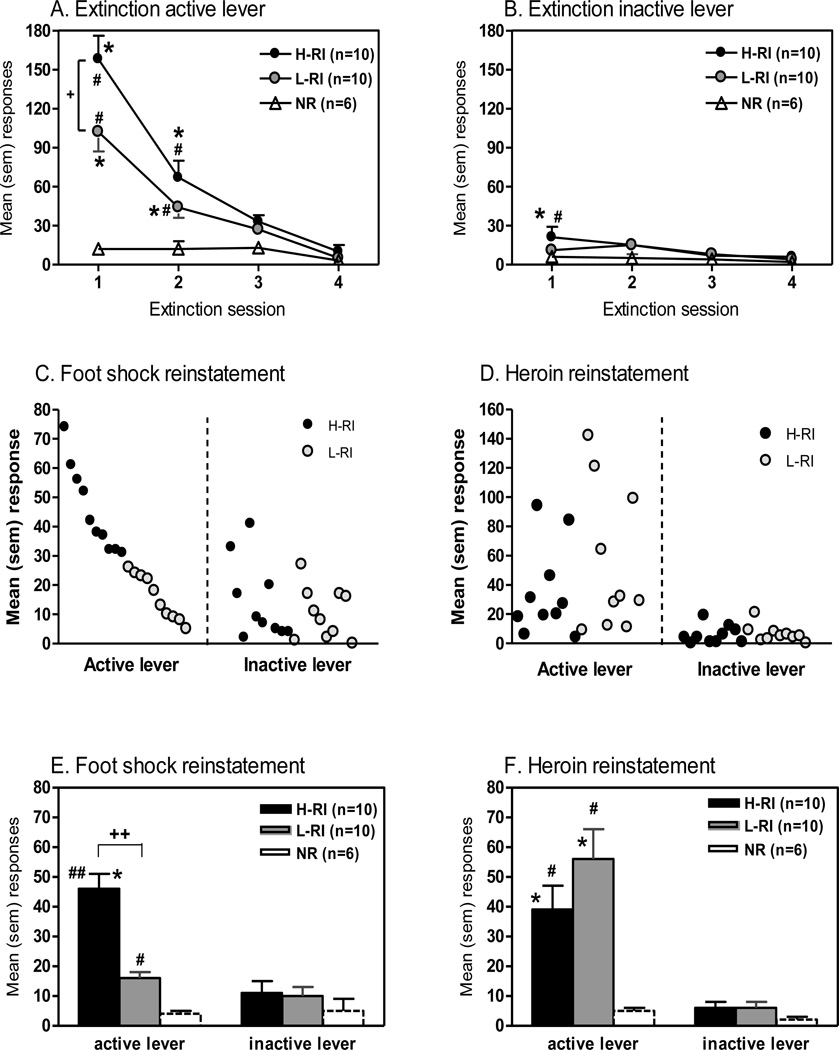
Responding on the active lever during the four extinction sessions is represented in Fig. 2A. ANOVA revealed significant main effects of Session [F(3,103) = 47.6, p<0.001], Group [F(2,103) = 16.3, p<0.001] and Group x Session interaction [F(6,103) = 11.2, p<0.001]. Newman-Keuls post hoc tests showed that both the H-RI and L-RI rats responded significantly more on the active lever in Sessions 1 and 2 than in Session 4 (p<0.001). The NR rats did not show responding differences across Sessions. In Session 1, the H-RI rats responded significantly more than the L-RI and NR rats (p<0.001), and the L-RI rats responded significantly more than the NR rats (p<0.001). In Session 2, both the H-RI and L-RI rats had significantly more responding than the NR rats (p<0.05), with no difference between the H-RI and L-RI groups.
Figure 2.
Comparison of extinction and reinstatement behavioral responses. The same 3 groups of animals: (1) H-RI, the heroin SA rats with “high” reinstatement induced by acute foot shock stress, n = 10; (2) L-RI, the heroin SA rats with “low” reinstatement induced by acute foot shock stress, n = 10; and (3) NR, the rats below the criterion for acquisition of heroin SA, n = 6. The upper panels show the mean (sem) responses on the active lever (A) and inactive lever (B) during the four 3-h extinction sessions. * p<0.001 vs. Session 4 within the group; + p<0.05 vs. L-RI group; # p<0.05 vs. NR group. The middle panels (C, D) show individual responses and the lower panels (E, F) show the mean (sem) responses on both the active and inactive levers following exposure to 15-min intermittent foot shock stress (C, E) and to heroin (0.25 mg/kg, s.c.) (D, F) during the 3-h session of two reinstatement tests. * p<0.05 vs. inactive lever within the group; ++ p<0.001 vs. L-RI group; # p<0.05, ## p<0.01 vs. NR group.
Responding on the inactive lever during the four extinction sessions is represented in Fig. 2B. ANOVA revealed only a significant main effect of Session [F(3,103) = 4.88, p<0.005]. Newman-Keuls post hoc tests showed that the H-RI rats responded significantly more on the inactive lever in Session 1 than in Session 4 (p<0.005). Neither the L-RI nor the NR rats showed responding differences across Sessions. In Session 1, the H-RI rats showed significantly higher responding than the NR rats (p<0.05).
3. Foot shock-induced reinstatement
As shown in Fig. 2C, animals displayed reinstatement of operant responding induced by acute foot-shock stress, with large individual differences. Therefore, using the median-split approach, the animals were divided into the high (H-RI) and low reinstatement (L-RI) groups, and there was approximate 3-fold difference in mean foot shock-induced reinstatement of drug-paired lever responding between the H-RI and L-RI rats (Fig. 2E). ANOVA revealed significant main effects of Group [F(2,51) = 29.9, p<0.001], Lever [F(1,51) = 55.5, p<0.001] and Group x Lever interaction [F(2,51) = 26.0, p<0.001]. Newman-Keuls post hoc tests showed that the H-RI rats responded significantly more on the active lever than the inactive lever during the 3-h session following exposure to 15-min intermittent foot shock stress (p<0.05). Neither the L-RI nor the NR rats showed differences between active and inactive lever responding. Following the acute foot shock stress, both the H-RI and L-RI rats responded significantly more on the active lever than the NR rats (p<0.001 and p<0.05, respectively), and the H-RI rats responded significantly more on the active lever than the L-RI rats (p<0.001) (Fig. 2E).
4. Heroin-induced reinstatement
Forty-eight hours later, the H-RI and L-RI rats were tested for heroin-induced reinstatement, and Fig. 2D show individual reinstatement of operant responding induced by heroin priming. ANOVA revealed a significant main effect of Lever [F(1,51) = 4.89, p<0.05]. Newman-Keuls post hoc tests showed that both the H-RI and L-RI rats responded significantly more on the active lever than the inactive lever during the 3-h session following the heroin priming injection (0.25 mg/kg, s.c.) (p<0.05). The NR rats did not show differences between the active and inactive lever responding. Following the heroin injection, both the H-RI and L-RI rats responded significantly more on the active lever than the NR rats (p<0.05), with no differences between the H-RI and L-RI groups (Fig. 2F).
II. AVP and V1b receptor mRNA levels in the Me/BLA, MH and AP
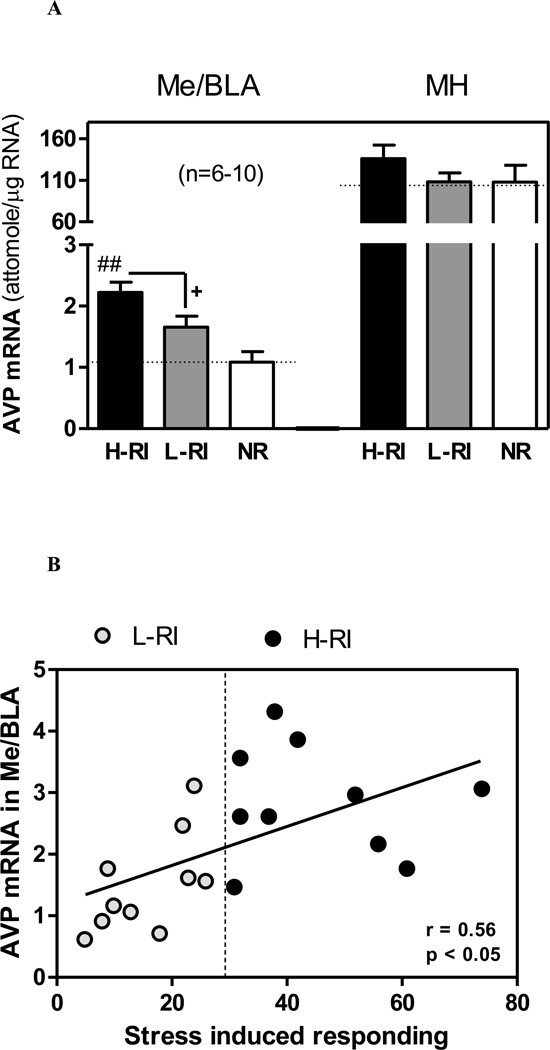
Since both the medial amygdala and paraventricular nucleus in the MH contain AVP-synthesizing neurons, and altered activity of amygdalar AVP as a consequence of stress or opiate withdrawal contributes to anxiety-like and drug-seeking behaviors [16, 30, 31], we examined the mRNA levels of AVP in these two brain regions and of its V1b receptor in the AP. In the Me/BLA, one-way ANOVA revealed a significant effect of the Group [F(2,23) = 8.96, p<0.01] (Fig. 3A). Newman-Keuls post hoc tests showed that the AVP mRNA level in the H-RI rats was significantly higher than that of L-RI rats (p<0.05) and that of NR rats (p<0.01). The presence of individual differences in groups created by median split was confirmed by a significant positive correlation between the Me/BLA AVP mRNA levels and foot shock-induced responding on the active lever during the stress induced reinstatement (r = 0.56, n = 20, p<0.05) (Fig. 3B). When the AVP mRNA levels were analyzed with heroin-induced lever responding in all heroin self-administration rats, no significant correlation was found (data not shown). No group differences in the AVP mRNA in the MH (Fig. 3A) or the V1b mRNA in the AP were found (Table 1).
Figure 3.
(A) Comparison of arginine vasopressin (AVP) mRNA levels in the medial/basolateral amygdala (Me/BLA) and medial hypothalamus (MH). The same 3 groups of animals: (1) H-RI, the heroin SA rats with “high” reinstatement induced by acute foot shock stress; (2) L-RI, the heroin SA rats with “low” reinstatement induced by acute foot shock stress; and (3) NR, the rats below the criterion for acquisition of heroin SA (n=6–10). The dotted line represents basal mean mRNA level in each brain region of rats with saline SA without acute foot shock stress (heroin/stress control, n = 8). The panels show the mean (sem) mRNA levels (attomole/µg total RNA) measured 45 min after 15-min intermittent foot shock stress on day 16 (9 days after the last heroin SA). + p<0.05 vs. H-RI group; ## p<0.01 vs. NR group. (B) Regression of AVP mRNA levels in the Me/BLA and the foot shock stress-induced lever responding during 3-hour reinstatement session in the H-RI and L-RI rats. There was a significant correlation between the AVP mRNA level in the Me/BLA and the stress induced responding in all heroin SA rats, both the H-RI (•) (n = 10) and L-RI (○) animals (n = 10).
Table 1.
Comparison of gene expression levels (attomole/µg total RNA) in selective brain regions and pituitary. Four groups of animals: (1) H-RI, the heroin SA rats with “high” reinstatement induced by acute foot shock stress; (2) L-RI, the heroin SA rats with “low” reinstatement induced by acute foot shock stress; (3) NR, the rats below the criterion for acquisition of heroin SA (20 infusions on days 5–7 of SA testing, 0.05 mg/kg/infusion); and (4) Stress control saline SA rats without acute foot shock stress (n = 6–9). Shown are the mean (sem) mRNA levels (attomole/µg total RNA) measured 45 min after 15-min intermittent foot shock stress on day 16 (9 days after heroin SA), and in the Stress control group. * p<0.05, # p=0.05 vs. L-RI group.
| Gene | Region | Group | |||
|---|---|---|---|---|---|
| H-RI | L-RI | NR | Stress control | ||
| POMC | AP | 488 ± 80 | 472 ± 56 | 536 ± 188 | 500 ± 48 |
| Me/BLA | 3.2 ± 0.31 | 3.4 ± 0.54 | 3.8 ± 0.62 | 3.3 ± 0.71 | |
| D2R | CPu | 1.7 ± 0.13 # | 2.3 ± 0.23 | 2.4 ± 0.39 | 2.5 ± 0.21 |
| AP | 0.27 ± 0.04 | 0.29 ± 0.04 | 0.32 ± 0.05 | 0.31 ± 0.05 | |
| VTA | 0.018 ± 0.003 | 0.019 ± 0.003 | 0.024 ± 0.004 | 0.025 ± 0.005 | |
| V1b | AP | 1.7 ± 0.18 | 1.8 ± 0.12 | 1.9 ± 0.18 | 1.8 ± 0.18 |
Note: AP, anterior pituitary; CPu, caudate putamen; D2R, dopamine D2 receptor; Me/BLA, medial/basolateral amygdala; POMC, pro-opiomelanocortin; V1b, arginin vasopressin V1b subtype receptor; VTA, ventral tegmental area.
III. POMC mRNA levels in the MH, AP and Me/BLA
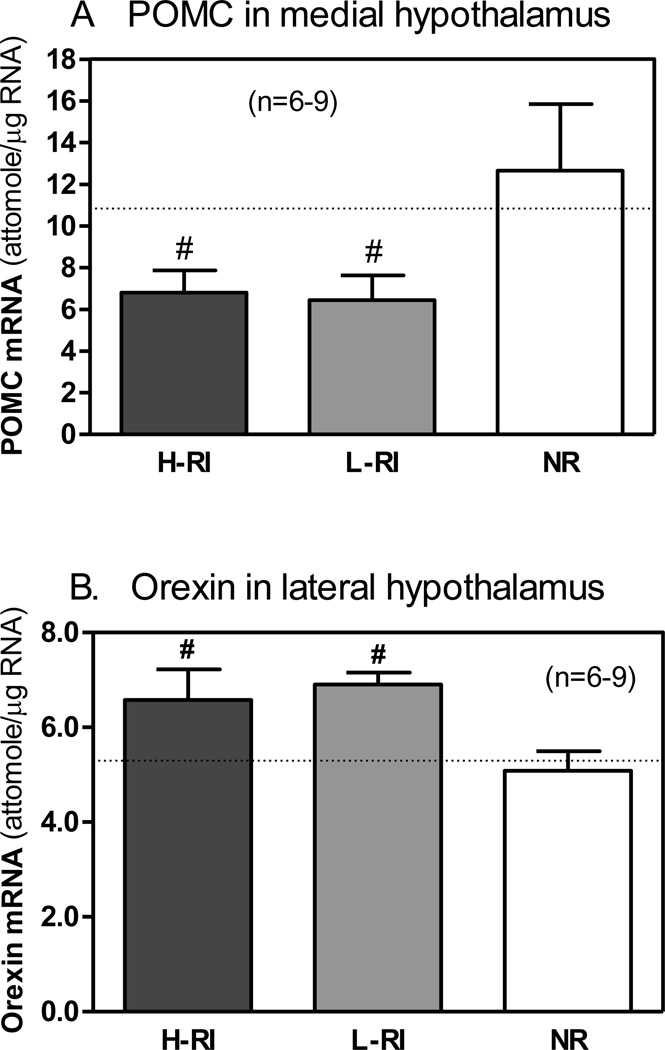
In the MH, one-way ANOVA revealed a significant effect of the Group on the POMC mRNA level [F(2,21) = 3.53, p<0.05] (Fig. 4A). Newman-Keuls post hoc tests showed that the POMC mRNA levels in both the H-RI and L-RI rats were significantly lower than that of NR group (p<0.05).
Figure 4.
Comparison of pro-opiomelanocortin (POMC) mRNA levels in the medial hypothalamus (A) and orexin mRNA levels in the lateral hypothalamus (B). The same 3 groups of animals: (1) H-RI, the heroin SA rats with “high” reinstatement induced by acute foot shock stress; (2) L-RI, the heroin SA rats with “low” reinstatement induced by acute foot shock stress; and (3) NR, the rats below the criterion for acquisition of heroin SA (n = 6–9). The dotted line represents basal mean mRNA level for each gene in each brain region of the rats with saline SA without acute foot shock stress (heroin/stress control, n = 8). The panels show the mean (sem) mRNA levels (attomole/ug total RNA) measured 45 min after 15-min intermittent foot shock stress on day 16 (9 days after the last heroin SA). # p<0.05 vs. NR group.
Since the expression level of the POMC gene in the CeA of rats was very low, and the neuroanatomical distribution of POMC neurons in the Me and BLA is unclear, we measured the POMC mRNA levels in the combined ME and BLA tissues only. In the Me/BLA or PA, no group differences in the POMC mRNA expression were found (Table 1).
IV. Orexin mRNA levels in the LH
One-way ANOVA revealed a marginally significant effect of the Group on the orexin mRNA level [F(2,21) = 3.39, p=0.052] (Fig. 4B). Newman-Keuls post hoc tests showed that the orexin mRNA level in both the H-RI and L-RI rats were significantly higher than that of NR group (p<0.05).
V. Dopamine D2 receptor mRNA levels
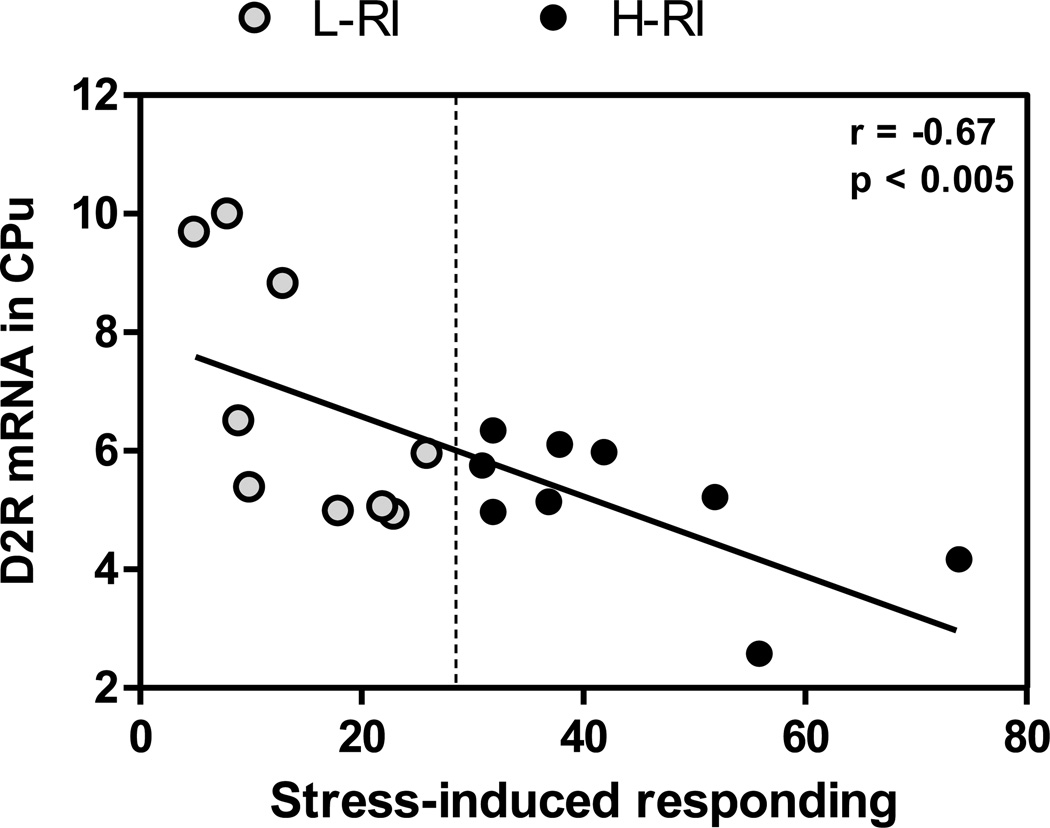
In the CPu, one-way ANOVA just failed to show a significant effect of the Group [F(2,21) = 3.39, p=0.08] (Table 1). However, the presence of individual differences in groups created by median split was confirmed by a significant negative correlation found between the CPu D2 receptor mRNA levels and foot shock-induced responding on the active lever during the stress induced reinstatement in all heroin self-administration rats (r = −0.67, n = 18, p<0.005) (Fig. 5). Since the H-RI rats responded significantly more after foot shock stress than the L-RI rats, a Student t-test was carried out: there was marginally significant difference between the H-RI and L-RI groups [t (16) = 4.40, p = 0.05]. When the D2 mRNA levels were analyzed with heroin-induced lever responding in the H-RI and L-RI rats, no significant correlation was found (data not shown). In the VTA or AP, no significant group differences in the D2 mRNA expression were found (Table 1).
Figure 5.
Regression of dopamine D2 receptor mRNA levels in the caudate putamen (CPu) and foot shock stress-induced lever responding during 3-hour reinstatement session in the H-RI rats (heroin SA rats with “high” reinstatement induced by acute foot shock stress) and the L-RI rats (heroin SA rats with “low” reinstatement induced by acute foot shock stress). There was a significant correlation between the D2 mRNA level in the CPu and the stress induced responding in all heroin SA rats, both the H-RI (•) (n = 9) and L-RI (○) animals (n = 9).
VI. Plasma ACTH and CORT levels
To examine stress responsive hormones, we used one-way ANOVA to compare each group after foot shock stress, and no group differences in plasma ACTH or CORT levels were found. In comparison with stress control group (the stress/heroin control group), however, significant elevations in both plasma ACTH and CORT levels were found in all the H-RI, L-RI and NR groups (p<0.05) after foot shock (Table 2).
Table 2.
Comparison of plasma levels of ACTH (pg/ml), corticosterone (CORT, ng/ml) and prolactin (ng/ml). Four groups of animals: (1) H-RI, the heroin SA rats with “high” reinstatement induced by acute foot shock stress; (2) L-RI, the heroin SA rats with “low” reinstatement induced by acute foot shock stress; (3) NR, the rats below the criterion for acquisition of heroin SA (20 infusions on days 5–7 of SA testing, 0.05 mg/kg/infusion); and (4) Stress control, saline SA rats without acute foot shock stress (n = 6–10). Shown are the mean levels measured 45 min after 15-min intermittent foot shock stress on day 16 (9 days after heroin SA), and in the Stress control group.
| Group | ||||
|---|---|---|---|---|
| H-RI | L-RI | NR | Stress control | |
| ACTH | 442 ± 51# | 375 ± 46# | 426 ± 61# | 219 ± 30 |
| CORT | 653 ± 102# | 723 ± 124# | 528 ± 101# | 204 ± 22 |
| Prolactin | 7.4 ± 0.7#* | 5.4 ± 0.6 | 5.1 ± 0.8 | 3.7 ± 0.5 |
p<0.05 vs. Stress control;
p<0.05 vs. L-RI group.
VII. Plasma prolactin levels
One-way ANOVA of plasma prolactin levels revealed a significant main effect of foot shock stress [F(3,27) = 4.48, p<0.05] (Table 2). Newman-Keuls post hoc tests showed that the prolactin level in the H-RI rats 45 min after foot shock was significantly higher than that in either the stress control rats (p<0.01) or the L-RI rats (p<0.05).
Discussion
I. AVP in the Me/BLA and D2 in the CPu involved in foot shock stress-induced reinstatement
In this study, we tested whether individual differences in stress responsive gene expressions would be associated with stress-induced vulnerability to heroin-seeking behavior. In the behavioral experiment, animals that self-administered heroin reliably displayed reinstatement of operant responding induced by acute foot-shock stress, but large individual differences were noted. Therefore, we used the median-split approach [27] to distinguish between the high (H-RI) and low reinstatement (L-RI) groups. The H-RI and L-RI groups were compared for their behavioral histories, including total heroin intake and active/inactive lever responding during self-administration sessions, and extinction responding, as well as heroin priming-induced reinstatement after the foot shock reinstatement. Finally, the mRNA levels of stress responsive genes in multiple brain regions were analyzed among the H-RI, L-RI and heroin self-administration non-responders (NR) groups.
Although there was no group difference in total heroin intake, or active/inactive lever responding during self-administration, or after heroin-induced reinstatement, the H-RI group displayed more responding on the active lever (and on the inactive lever) during the initial extinction sessions, suggesting an enhanced tendency to respond to drug withdrawal-associated stress, as reflected by elevated stress hormones [e.g., 16, 32]. Following foot shock stress, the responding on the active lever in the H-RI rats was significantly higher (3 fold) than that of the L-RI rats, and the H-RI rats also displayed an elevated AVP mRNA level in the Me/BLA, a brain region known to play an important role in drug-seeking behaviors [33]. This effect seemed to be gene-specific (no effect on POMC mRNA levels in the same region) and region-specific (no effect on AVP mRNA levels in the medial hypothalamus). In a separate pilot experiment, levels of AVP mRNA in the Me/BLA were also compared between the rats that self-administered saline and self-administered heroin without the reinstatement tests by foot shock stress. We found no AVP mRNA alteration in the rats with heroin self-administration and extinction/withdrawal procedures (data not shown), which clearly indicates that this AVP increase required both heroin self-administration and stress to be expressed.
Increased AVP activity might be expected as a result of enhanced AVP gene expression, and may be partially responsible for the drug-seeking action in rats triggered by foot shock stress. In support of this idea, our previous study has demonstrated that selective blockade of V1b receptors blunted stress-induced heroin-seeking behavior [16]. Also, activation of V1b receptor pathways in the amygdala has been found to be an important step in the neurobiology of stress-related behaviors [15, 34] and alcohol dependence in rodent models [35]. Both the Me/BLA and central nucleus of the amygdala receive noradrenergic (NA) projections that are sensitive to stress and to adrenergic manipulations [36, 37]. Also, blockade of beta NA receptors within the central amygdala block stress-induced reinstatement [24]. Thus, although this tentative interpretation requires additional investigation, our data suggests that the NA-AVP interaction within the amygdala may be involved in individual differences in heroin-seeking precipitated by stress.
A generalized motor deficit in the L-RI rats following foot shock stress is unlikely as they displayed similar levels of lever-pressing responses during self-administration sessions and during the heroin-induced reinstatement session. Also, the difference between the H-RI and L-RI groups cannot be caused by differences in amount of heroin consumption: H-RI and L-RI rats had very similar total heroin intake over 7 days of self-administration. To our knowledge, this is the first demonstration that there is a relationship between individual differences in AVP gene expression and a propensity for the heroin-seeking behavior in response to acute stress. A formal test of this hypothesis would require studying the effect of the over-expression of the AVP gene or administration of AVP V1b antagonists into the Me/BLA prior to acute stress-induced reinstatement tests.
Although caution should be used when interpreting the relationships between behavior and mRNA expression (reflecting protein biosynthesis but not function) of any genes, the study of gene expression or peptide data in animals with established drug-seeking vulnerabilities provide useful information about a potential role of individual variations in response to stress-induced relapse. Both reduced availability of D2 receptor binding and decreased D2 gene expression in the striatum have been consistently associated with heroin cravings in humans [e.g., 38, 39] and cocaine seeking in animals [e.g., 25, 26]. In the present study, we observed a down-regulation of CPu D2 mRNA levels in the H-RI rats. Reduced D2 availability resultant from the decreased D2 gene expression could possibly be involved in the seeking action of heroin in the H-RI rats. In support of this concept, correlation analysis between the active lever responding during foot shock-induced reinstatement session and the CPu D2 mRNA levels revealed that the H-RI rats seeking relatively more heroin showed relative lowered CPu D2 mRNA levels.
II. POMC and orexin in the hypothalamus in heroin-induced reinstatement
In earlier studies using morphine pellets [e.g., 17] or heroin administration by experimenters [18], it was found that POMC mRNA in the hypothalamus was decreased after chronic opiate exposure in rats. In the current study, we examined POMC gene expression in two brain regions (the medial hypothalamus and Me/BLA) after 9 days of abstinence from 7 days of heroin self-administration, and found a significant decrease in the POMC mRNA levels in the medial hypothalamus only. These novel results extend the above earlier findings to the heroin abstinence after active intravenous heroin self-administration. Different from the AVP in the Me/BLA, similar decreases in POMC mRNA levels of the hypothalamus were found in both H-RI and L-RI groups, showing a dis-association of the POMC decrease with stress-related heroin reinstatement.
In humans, heroin dependent individuals use heroin also to escape the negative affective and emotional states associated with drug withdrawal [9]. Rats self-administer beta-endorphin into the brain, supporting a role for central beta-endorphin in reinforcement [40]. There is also evidence in animals that the motivational properties of opiates are altered by spontaneous and naloxone-precipitated withdrawal [41, 42, 43]. It is reasonable, therefore, to postulate that the decreased POMC gene expression with concomitant decreases in beta-endorphin peptide biosynthesis and/or release may initiate and/or maintain behaviors leading to opiate seeking, in order to reach normal endogenous opioid tone.
In both the H-RI and L-RI rats, we also measured orexin mRNA levels in the lateral hypothalamus 9 days after heroin self-administration, and found a significant increase in both groups. This is consistent with previous reports showing enhanced orexin gene expression during acute withdrawal from chronic morphine or heroin exposure [20, 21]. Since orexin peptide A has been found to decrease the excitability of brain reward systems in the lateral hypothalamus [44], the alteration of orexin gene expression is likely involved in the negative affective state in heroin withdrawal and abstinence, which may be important in the modulation of drug-seeking behaviors [22, 23].
Conclusion
Region-specific alterations of AVP and D2 gene expression were found in the rats after heroin self-administration, and expression of these genes correlated with individual differences in stress-induced reinstatement of heroin-seeking. In contrast, a decrease in POMC gene expression was found in parallel with an increase in orexin gene expression in the hypothalamus of all rats after heroin self-administration. Our present data suggest that (1) the consequence of a new set point of AVP and D2 gene expressions in response to stress may play an important role in individual vulnerability to acute stress-induced relapse during opiate abstinence; and (2) the alterations of hypothalamic orexin and POMC expression may be involved in drug-seeking behavior induced by a drug prime.
Supplementary Material
Highlights.
individual vulnerability to stress-induced heroin-seeking behavior;
high AVP mRNA levels in amygdala increased stress-induced reinstatement;
low D2 mRNA levels in caudate putamen increased stress-induced reinstatement.
Acknowledgements
This work was supported by NIH NIDA Research Center Grant DA-P50-05130 (MJK), Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK), and NET grant from CIHR (FL). The authors would like to thank Dr. G Aguilera for providing rat AVP and V1b receptor cDNAs; Dr. L de Lecea for rat hypocretin (or orexin) cDNA; Drs. T Nilsen and P Maroney for 18S cDNA; and Dr. J Roberts for rat POMC cDNA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflicts of interest.
Author contributions. Y.Z., F.L. and M.J.K. designed the research; Y.Z., F.L. and E.C. performed the research; Y.Z., F.L. and E.C. analyzed the data. Y.Z., F.L., E.C. and M.J.K. wrote the paper.
References
- 1.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 2.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug-seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 3.Self DW, Nestler EJ. Relapse to drug seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Catapano D, O’Malley S. Stress induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs RA, Tran-Nguyen LTL, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinc- tion/reinstatement model of drug craving. Psychopharmacology. 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 7.Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to bench. Cur Opin Pharmacol. 2009;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Merrer L, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Litvin Y, Piras AP, Pfaff DW, Kreek MJ. Persistent increase in hypothalamic arginine vasopressin gene expression during protracted withdrawal from chronic escalating-dose cocaine in rodents. Neuropsychopharmacology. 2011;36:2062–2075. doi: 10.1038/npp.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roper JA, O’Carroll A, Young WS, III, Lolait SJ. The vasopressin AVPr1b receptor: molecular and pharmacological studies. Stress. 2011;14:98–115. doi: 10.3109/10253890.2010.512376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- 17.Bronstein DM, Przewlocki R, Akil H. Effects of morphine treatment on pro-opiomelanocortin systems in rat brain. Brain Res. 1990;519:102–111. doi: 10.1016/0006-8993(90)90066-k. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Leri F, Ho A, Kreek MJ. Suppression of hypothalamic-pituitary-adrenal axis by acute heroin challenge in rats during acute and chronic withdrawal from chronic heroin administration. Neurochem Res. 2013;38:1850–1860. doi: 10.1007/s11064-013-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28:2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, Dileone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- 22.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 23.Sharf R, Sarhan M, DiLeone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leri F, Zhou Y, Goddard B, Levy A, Jacklin D, Kreek MJ. Steady-state methadone blocks cocaine seeking and cocaine-induced gene expression alterations in the rat brain. Eur Neuropsychopharmacol. 2009;19:238–249. doi: 10.1016/j.euroneuro.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A, Robbins TW, Dalley JW, Everitt BJ, Belin D. Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology. 2013;38:1963–1973. doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deminiere JM, Piazza PV, Le Moal M, Simon H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev. 1989;13:141–147. doi: 10.1016/s0149-7634(89)80023-5. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates (Ed 2) San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 29.Leri F, Stewart J. Drug-induced reinstatement to heroin and cocaine seeking: a rodent model of relapse in poly-drug use. Exp Clin Psychopharmacol. 2001;9:297–306. doi: 10.1037//1064-1297.9.3.297. [DOI] [PubMed] [Google Scholar]
- 30.Salome N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology. 2006;187:237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltier RL, Guerin GF, Dorairaj N, Goeders NE. Effects of saline substitution on responding and plasma corticosterone in rats trained to self-administer different doses of cocaine. J Pharmacol Exp Ther. 2001;299:114–120. [PubMed] [Google Scholar]
- 33.Brown RM, Lawrence AJ. Neurochemistry underlying relapse to opiate seeking behavior. Neurochem Res. 2009;34:1876–1887. doi: 10.1007/s11064-009-9967-y. [DOI] [PubMed] [Google Scholar]
- 34.Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol. 2012;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to foot shock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- 37.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- 38.Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- 39.Zijlstra F, Booij J, van den Brink W, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18:262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Van Ree JM, Smyth DG, Colpaert FC. Dependence creating properties of lipotropin C-fragment (b-endorphin): Evidence for its internal control of behavior. Life Sci. 1979;24:495–502. doi: 10.1016/0024-3205(79)90170-x. [DOI] [PubMed] [Google Scholar]
- 41.Negus S. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 42.Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 43.Cooper ZD, Truong YN, Shi YG, Woods JH. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326:920–929. doi: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.