Abstract
Objectives
To examine the short-term (3 and 6-month), self-reported bleeding and cramping patterns with intrauterine devices (IUDs) and the contraceptive implant, and the association of these symptoms with method satisfaction.
Study Design
We analyzed 3 and 6-month survey data from IUD and implant users in the Contraceptive CHOICE Project, a prospective cohort study. Participants who received a long-acting reversible contraceptive (LARC) method (levonorgestrel intrauterine system (LNG-IUS), copper IUD, or the etonogestrel implant) and completed their 3- and 6-month surveys were included. Univariable and multivariable analyses were performed to examine the association of bleeding and cramping patterns with short-term satisfaction.
Results
Our analytic sample included 5,011 CHOICE participants: 3001 LNG-IUS users, 826 copper IUD users, and 1184 implant users. At 3 months, over 65% of LNG-IUS and implant users reported no change or decreased cramping, while 63% of copper IUD users reported increased menstrual cramping. Lighter bleeding was reported by 67% of LNG-IUS users, 58% of implant users, and 8% of copper IUD users. Satisfaction of all LARC methods was high (≥90%) and significantly higher than non-LARC methods (p<0.001). LARC users with increased menstrual cramping (HR 0.96, 95% CI 0.92 – 0.99), heavier bleeding (HR 0.91, 95% CI 0.87 – 0.96), and increased bleeding frequency (HR 0.92, 95% CI 0.89 – 0.96) were less likely to report being very satisfied at 6 months.
Conclusion
Regardless of the LARC method, satisfaction at 3 and 6 months is very high. Changes in self-reported bleeding and cramping are associated with short-term LARC satisfaction.
Introduction
Long-acting reversible contraception (LARC) methods such as the levonorgestrel-releasing intrauterine system (LNG-IUS), copper IUD, and the subdermal etonorgestrel implant are safe and highly-efficacious in preventing pregnancy, with failure rates similar to sterilization.1 LARC methods are more effective than other reversible contraceptives because they require little effort or maintenance on the part of the user after insertion.2 Although these methods are used widely in parts of Europe and Asia, only 8.5% of American women ages 15–44 who use contraception reported using an IUD or implant in 2009.3
LARC users in several studies have cited increased bleeding, irregular bleeding, and cramping as the most common causes for method discontinuation within the first two years of use.4–9 Short-term changes in bleeding and cramping pattern associated with LARC use have been reported in several studies in Europe, Asia, and Latin America. However, limited data are available regarding the short-term experience of LARC users in the United States. Thus, the purpose of this analysis is to examine the short-term (3- and 6-month) bleeding and cramping patterns with IUDs and the contraceptive implant, and the association of these symptoms with method satisfaction. We hypothesize that decreased amount and frequency of bleeding and a reduction in cramping compared to the previous survey time point will be associated with higher levels of method satisfaction.
Materials and Methods
The Contraceptive CHOICE Project (CHOICE) is a prospective cohort study conducted in St. Louis, Missouri. All contraceptive methods in CHOICE are provided at no cost for 2–3 years. This analysis is focused on LARC users and examines bleeding and cramping patterns, and level of satisfaction with the contraceptive method reported during the 3- and 6-month telephone surveys. The protocol for recruitment, enrollment, and follow-up of participants is detailed by Secura et al10 and was approved by the Human Research Protection Office at Washington University School of Medicine. The methods of CHOICE and this specific sub-analysis are outlined briefly below.
CHOICE enrolled women between 14 and 45 years of age who lived or sought clinical services in the greater St. Louis area and who were interested in a new method of birth control. To be eligible a woman must have been sexually active with a male partner or anticipated sexual activity within six months of enrollment, and did not desire pregnancy within a year of enrollment. Women who had undergone a sterilization procedure or hysterectomy were not eligible.
Women interested in enrolling in CHOICE were provided with a brief scripted introduction to the LARC methods, and then screened for eligibility by a trained staff member. Screening was conducted in person at various recruitment sites or via telephone. Eligible participants were enrolled during an in-person, two-hour enrollment session where additional standardized contraceptive counseling about all reversible methods was provided.11 Written informed consent was obtained from each participant prior to enrollment. LARC methods were inserted at the enrollment site, or at the participant’s clinic, or private practice. Women who did not receive a LARC method on the day of enrollment were provided with a bridge method until pregnancy was ruled-out definitively, and LARC insertion was deemed clinically appropriate. Demographic information, baseline bleeding patterns, and reproductive history were recorded during the enrollment interview. At baseline, women were asked about the number of bleeding and spotting days each month; whether their menses are regular or irregular; and the average cycle length from one menses to the next. Follow-up interviews were conducted at 3 and 6 months, and then every 6 months using a standardized telephone survey. At follow-up, participants were asked if their bleeding and cramping each were the same, better, or worse than three months earlier.
This analysis examines the symptoms and satisfaction reported by CHOICE LARC users 3 and 6 months after enrollment. Thus, only CHOICE participants who had a LNG-IUS, copper IUD, or implant inserted prior to the 3-month interview were included in this analysis. Duration of LARC use at the time of the 3-month and 6-month interviews was calculated as the difference in days between the follow-up interview dates and the date of method insertion. For the 3-month analysis, only those participants who started their method two to four months (60 – 120 days) from the date of 3-month survey were included. Similarly, for the 6-month analysis, only those women who started the method five to seven months (150 – 210 days) from the date of 6-month survey were included. Data from women who discontinued their LARC method due to a pregnancy or desire to get pregnant were excluded from the satisfaction analysis. During the 3-month telephone survey, participants were asked to report changes in the amount and frequency of bleeding, and changes in cramping since insertion of their LARC method. At the 6-month interview, participants were asked to report changes in bleeding and cramping compared to what they experienced at 3 months of use. For our reported results, “heavier” or “lighter” bleeding refers to the volume of flow and “increased” or “decreased” frequency of bleeding refers to the number of days of bleeding.
Finally, at the 3- and 6-month interviews, participants rated their satisfaction with their LARC method as “very satisfied,” “somewhat satisfied,” or “not satisfied.” Participants who discontinued their LARC method before the 3-month or 6-month follow-up interview were coded as “not satisfied” in the analyses except those who discontinued to conceive. For the analyses, we grouped “satisfied” or “very satisfied” together as “satisfied.”
To compare the baseline demographic characteristics and bleeding patterns among our study participants, we used chi-square or Fisher’s exact test for categorical variables, and ANOVA for continuous, normally-distributed variables. Poisson regression with robust variance was conducted to evaluate associations between bleeding and cramping patterns and satisfaction. This approach provides an unbiased estimate of relative risk when the outcome is common (greater then 10%).12,13 All factors significantly associated with satisfaction in the univariable analysis were further evaluated for effect modification and potential confounding. Effect modifications were identified if the corresponding interaction terms were statistically significant. Confounders were identified if there was a greater than 10% change in the relative risk estimate with the potential factor in the model compared to the risk estimate without the potential factor in the model. All the confounders were included in the final multivariable model to evaluate the association between satisfaction and method used, change in bleeding amount, change in bleeding frequency, and change in cramping. Stata 11 (StataCorp, College Station, TX) was used for all statistical analyses. The statistical significance level was set at 0.05.
Results
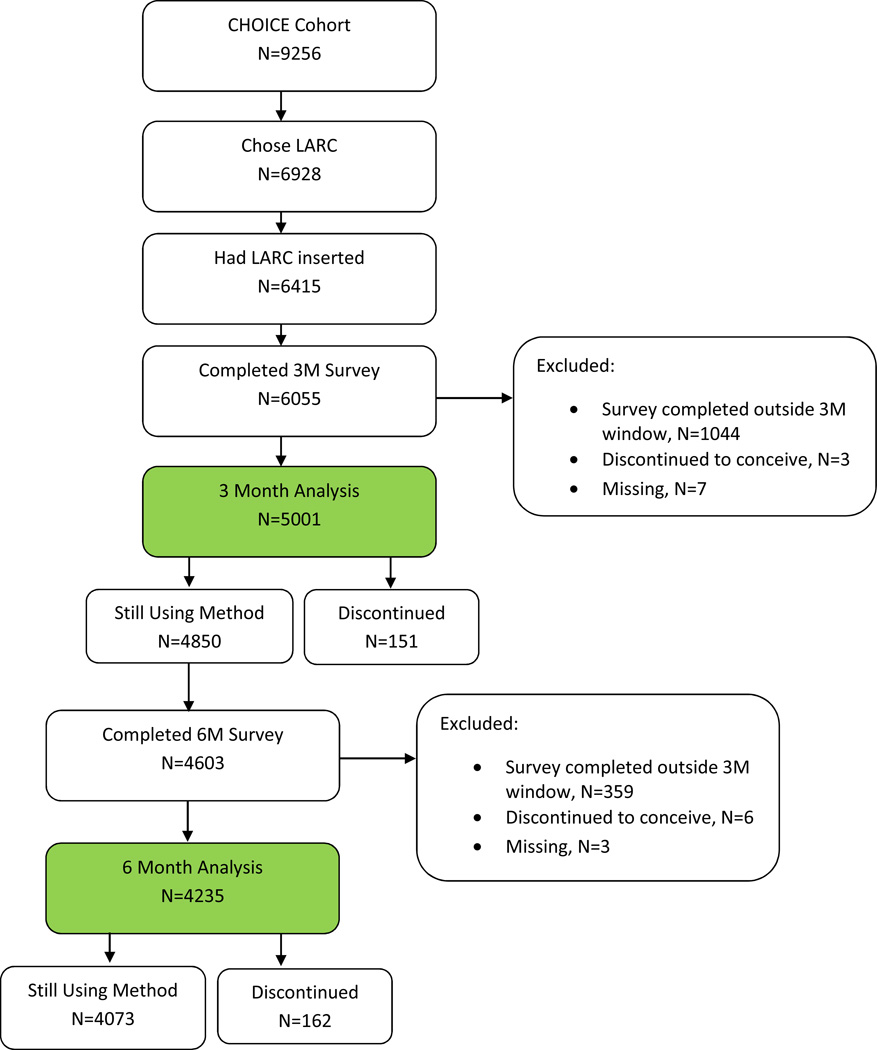
Figure 1 shows the inclusion criteria and analytic sample. Among 9,256 participants enrolled in CHOICE, 75% desired a LARC method. Ninety-three percent (n=6,415) of participants received their LARC method; 1,044 reported method use at the 3-month interview that was less than 2 months (n=850) or greater than 4 months (n=194) and were therefore excluded from this analysis, leaving 5,001 participants in the 3-month analysis. The mean duration of use at the 3-month survey was 86 days. Among 4,850 continuers at 6-month survey, 247 did not finish the 6-month survey and another 359 women reported method use at the 6-month interview that was less than 5 months (n=99) or greater than 7 months (n=260); thus, 4,235 participants were included in the 6-month analyses. The mean duration of use at the 6- month survey was 179 days. We excluded 3 women from the 3-month analyses and 6 women from the 6-month analyses who discontinued their LARC method to conceive. Women who discontinued at 3 months were coded as “not satisfied” for that analysis (n=151) but were not included in the analysis at 6 months. All other LARC users who discontinued for other reasons (n=283) by 3 or 6 months were included in the analyses and were coded as “not satisfied”.
The baseline demographic and reproductive characteristics of the participants stratified by LARC method are shown in Table 1. The mean age was 25.6 years. Implant users were significantly younger than IUD users, and were more likely to be black, single, nulliparous, and to have less than a 12th grade education. Prior to method insertion, implant users were more likely to have shorter cycles (< 21 days) and irregular cycles. LNG-IUS users were more likely to have heavier menstrual bleeding and more commonly experienced dysmenorrhea. Copper IUD users were less likely to report baseline menstrual cramping compared to women who chose the hormonal LARC methods.
Table 1.
Baseline demographic characteristics of LARC users
| All LARC (N=5011) | LNG-IUS (N=3001) | Copper IUD (N=826) |
Implant (N=1184) |
p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Mean | SD | Mean | SD | Mean | SD | Mean | SD | <0.001 | ||
| 25.6 | 6.0 | 26.0 | 5.8 | 27.9 | 6.3 | 23.0 | 5.7 | ||||
| N | % | N | % | N | % | N | % | ||||
| Age Category | <0.001 | ||||||||||
| <18 | 274 | 5.5 | 75 | 2.5 | 10 | 1.2 | 189 | 16.0 | |||
| 18–20 | 713 | 14.2 | 378 | 12.6 | 67 | 8.1 | 268 | 22.6 | |||
| 21–25 | 1859 | 37.1 | 1173 | 39.1 | 264 | 32.0 | 422 | 35.6 | |||
| 26–30 | 1160 | 23.1 | 757 | 25.2 | 230 | 27.8 | 173 | 14.6 | |||
| >30 | 1005 | 20.1 | 618 | 20.6 | 255 | 30.9 | 132 | 11.1 | |||
| Race | <0.001 | ||||||||||
| Black | 2442 | 48.7 | 1419 | 47.3 | 298 | 36.1 | 725 | 61.2 | |||
| White | 2186 | 43.6 | 1376 | 45.9 | 454 | 55.0 | 356 | 30.1 | |||
| Other | 383 | 7.6 | 206 | 6.9 | 74 | 9.0 | 103 | 8.7 | |||
| Ethnicity | <0.001 | ||||||||||
| Hispanic | 265 | 5.3 | 132 | 4.4 | 46 | 5.5 | 87 | 7.3 | |||
| Marital Status | <0.001 | ||||||||||
| Single/never married | 2897 | 57.8 | 1714 | 57.1 | 383 | 46.4 | 800 | 67.6 | |||
| Married/living with a partner | 1738 | 34.7 | 1063 | 35.4 | 350 | 42.4 | 325 | 27.5 | |||
| Separated/divorced/widowed | 373 | 7.4 | 223 | 7.4 | 92 | 11.2 | 58 | 4.9 | |||
| Education level | <0.001 | ||||||||||
| Less than 12th grade | 1727 | 34.5 | 906 | 30.2 | 198 | 24.0 | 623 | 52.7 | |||
| Some College | 2074 | 41.4 | 1316 | 43.9 | 346 | 41.9 | 412 | 34.8 | |||
| College Degree or above | 1208 | 24.1 | 778 | 25.9 | 282 | 34.1 | 148 | 12.5 | |||
| Gravidity | <0.001 | ||||||||||
| 0 | 1331 | 26.6 | 724 | 24.1 | 231 | 28.0 | 376 | 31.8 | |||
| 1 | 1038 | 20.7 | 598 | 19.9 | 122 | 14.8 | 318 | 26.9 | |||
| 2 | 946 | 18.9 | 621 | 20.7 | 148 | 17.9 | 177 | 14.9 | |||
| 3+ | 1696 | 33.8 | 1058 | 35.3 | 325 | 39.3 | 313 | 26.4 | |||
| Parity | <0.001 | ||||||||||
| 0 | 2124 | 42.4 | 1196 | 39.9 | 348 | 42.1 | 580 | 49.0 | |||
| 1 | 1285 | 25.6 | 810 | 27.0 | 169 | 20.5 | 306 | 25.8 | |||
| 2 | 976 | 19.5 | 630 | 21.0 | 170 | 20.6 | 176 | 14.9 | |||
| 3+ | 626 | 12.5 | 365 | 12.2 | 139 | 16.8 | 122 | 10.3 | |||
| Baseline BMI | 0.06 | ||||||||||
| <18.5 | 110 | 2.2 | 64 | 2.2 | 17 | 21 | 29 | 2.5 | |||
| 18.5 – 24.9 | 1880 | 37.9 | 1093 | 36.8 | 352 | 43.0 | 435 | 37.0 | |||
| 25 – 29.9 | 1311 | 26.4 | 810 | 27.3 | 197 | 24.1 | 304 | 25.9 | |||
| 30+ | 1662 | 33.5 | 1001 | 33.7 | 253 | 30.9 | 408 | 34.7 | |||
| Any History of STI – Excluding BV & PID | 0.87 | ||||||||||
| No | 2989 | 59.6 | 1798 | 59.9 | 492 | 59.6 | 699 | 59.0 | |||
| Yes | 2022 | 40.4 | 1203 | 40.1 | 334 | 40.4 | 485 | 41.0 | |||
| Smoking Status | <0.001 | ||||||||||
| Never smoker | 2653 | 53.1 | 1543 | 51.6 | 403 | 48.8 | 707 | 59.9 | |||
| Previous smoker | 1189 | 23.8 | 744 | 24.9 | 220 | 26.7 | 225 | 19.1 | |||
| Current smoker | 1155 | 23.1 | 704 | 23.5 | 202 | 24.5 | 249 | 21.1 | |||
At 3 months, copper IUD users were more likely to report increased cramping (63%), compared to 32% of LNG-IUS users and 13% of implant users (Table 2). More than 70% of copper IUD users experienced heavier bleeding at 3 months compared to baseline. Less than 15% of LNG-IUS or implant users reported heavier bleeding. Approximately two-thirds of LNG-IUS users (67%) and implant users (58%) of reported lighter bleeding volume by 3 months, while only of 8% of copper IUD users reported lighter bleeding compared to baseline. Bleeding volume was heavier in 71% of copper IUD users at 3 months. In terms of bleeding frequency, almost half of LNG-IUS and implant users (42%) reported reduced frequency of bleeding, compared to only 10% of copper IUD users.
Table 2.
Association between bleeding change and satisfaction at 3-month interview
| All LARC | LNG-IUS | Copper IUD | Implant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Satisfied N(%) |
Not satisfied N(%) |
Total | Satisfied N(%) |
Not satisfied N(%) |
Total | Satisfied N(%) |
Not satisfie d N(%) |
Total | Satisfied N(%) |
Not satisfied N(%) |
|
| Cramping | ||||||||||||
| No change | 2081 | 1964(94) | 117(6) | 1,215 | 1146(94) | 69(6) | 230 | 216(94) | 14(6) | 636 | 473(74) | 163(26) |
| Less | 1287 | 1238(96) | 49(4) | 828 | 803(97) | 25(3) | 71 | 64(90) | 7(10) | 388 | 303(78) | 85(22) |
| More | 1617 | 1508(93) | 109(7) | 946 | 885(94) | 61(4) | 520 | 492(95) | 28(5) | 151 | 93(62) | 58(38) |
| Bleeding volume | ||||||||||||
| No change | 1123 | 1031(92) | 92(8) | 616 | 551(89) | 65(11) | 178 | 168(94) | 10(6) | 329 | 252(77) | 77(23) |
| Lighter | 2697 | 2615(97) | 82(3) | 1964 | 1918(98) | 46(2) | 61 | 50(82) | 11(18) | 672 | 512(76) | 160(24) |
| Heavier | 1097 | 998(91) | 99(9) | 367 | 323(88) | 44(12) | 579 | 551(95) | 28(5) | 151 | 86(57) | 65(43) |
| Bleeding frequency | ||||||||||||
| No change | 1379 | 1306(95) | 73(5) | 712 | 661(93) | 51(7) | 399 | 388(97) | 11(3) | 268 | 233(87) | 35(13) |
| Decreased | 1814 | 1759(97) | 55(3) | 1252 | 1212(97) | 40(3) | 79 | 70(89) | 9(11) | 483 | 390(81) | 93(19) |
| Increased | 1734 | 1591(92) | 143(8) | 988 | 927(94) | 61(6) | 339 | 310(91) | 29(9) | 407 | 231(57) | 176(43) |
Cramping, bleeding volume, and bleeding frequency at 6 months, compared to 3 months, is shown in Table 3. Most women using the three LARC methods reported no change in cramping at 6 months compared to 3 months. Sixty-two percent of LNG-IUS users and 45% of implant users experienced lighter bleeding at 6 months compared to 3 months. Seventy-two percent of copper IUD users reported no change in bleeding frequency at 6 months compared to three months, while 55% of LNG-IUS user and 48% of implant users reported decreased frequency of bleeding at 6 months.
Table 3.
Association between bleeding change and satisfaction at 6-month interview
| All LARC | LNG-IUS | Copper IUD | Implant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N |
Satisfied N(%) |
Not satisfied N(%) |
Total N |
Satisfied N(%) |
Not satisfied N(%) |
Total N |
Satisfied N(%) |
Not satisfied N(%) |
Total N |
Satisfied N(%) |
Not satisfied N(%) |
|
| Cramping | ||||||||||||
| No change | 2396 | 2249(94) | 147(6) | 1,355 | 1291(95) | 64(5) | 390 | 373(96) | 17(4) | 651 | 585(90) | 66(10) |
| Less | 1317 | 1236(94) | 81(6) | 879 | 839(95) | 40(5) | 219 | 199(91) | 20(9) | 219 | 198(90) | 21(10) |
| More | 510 | 442(87) | 68(13) | 295 | 258(87) | 37(13) | 103 | 87(84) | 16(16) | 112 | 97(87) | 15(13) |
| Bleeding volume | ||||||||||||
| No change | 1530 | 1424(93) | 106(7) | 753 | 703(93) | 50(7) | 396 | 380(96) | 16(4) | 381 | 341(90) | 40(10) |
| Lighter | 2137 | 2036(95) | 101(5) | 1523 | 1471(97) | 52(3) | 180 | 162(90) | 18(10) | 434 | 403(93) | 31(7) |
| Heavier | 465 | 377(81) | 88(19) | 187 | 149(80) | 38(20) | 135 | 116(86) | 19(14) | 143 | 112(78) | 31(22) |
| Bleeding frequency | ||||||||||||
| No change | 1627 | 1520(93) | 107(7) | 823 | 772(94) | 51(6) | 510 | 486(95) | 24(5) | 294 | 262(89) | 32(11) |
| Decreased | 1930 | 1843(95) | 87(5) | 1360 | 1318(97) | 42(3) | 109 | 95(87) | 14(13) | 461 | 430(93) | 31(7) |
| Increased | 581 | 480(83) | 101(17) | 281 | 234(83) | 47(17) | 94 | 79(84) | 15(16) | 206 | 167(81) | 39(19) |
At both 3 and 6 months, most women (> 90%) were satisfied (either somewhat satisfied or very satisfied) with their LARC method (3 months: 95% LNG-IUS, 94% copper IUD, 94% implant; 6 months: 94% LNG-IUS, 93% copper IUD, 90% implant). A larger percentage of participants using the LNG-IUS (74% at 3 months; 77% at 6 months) and copper IUD (74% at 3 months; 76% at 6 months) reported being “very satisfied” compared to implant users (68% at 3 months; 62% at 6 months; P=0.001). Ethnicity, race, parity, marital status, education level, body mass index (BMI), smoking status and baseline bleeding and cramping patterns were not effect modifiers or confounders in the relationship between contraceptive methods, bleeding and cramping change and method satisfaction
We found similar patterns of predictors of satisfaction at 3 and 6 months in our multivariable analysis. Participants that experienced more cramping (RRadj=0.78, 95% CI 0.72 – 0.85), heavier bleeding volume (RRadj=0.83, 95% CI 0.76 – 0.92), and increased bleeding frequency (RRadj=0.73, 95% CI 0.67 – 0.80) with LARC methods were less likely to be very satisfied with their contraceptive method at 6 months than women who experienced no change. Participants who reported heavier volume of bleeding were also less likely to be satisfied (RR 0.91, 95% CI 0.87 – 0.96).
Comment
Most LARC users in the CHOICE cohort were satisfied with their LARC method three months and six months after method insertion, despite differences experienced in bleeding and cramping patterns. When dichotomized into “satisfied” versus “not satisfied,” 90% of implant users, 93% of copper IUD users, and 95% of LNG-IUS users were satisfied with their method at 6 months. Increased cramping, increased bleeding volume, and increased bleeding frequency were all associated with reduced short-term satisfaction.
At baseline, there were differences in volume and duration of menses, as well as prevalence of dysmenorrhea among users of the different LARC methods. This is likely due to self-selection as participants with heavy menses at baseline were more likely to choose the LNG-IUS. Copper IUD users were more likely to have lighter, less painful menses at baseline probably because of the same selection bias. Although we attempted to control for differences in the comparison groups at baseline, there is potential for bias based on the counseling participants received. Despite the use of a standardized counseling session at the time of method selection, participants were informed of potential method-specific symptoms. 11 Furthermore, at the time of insertion the clinician would again review potential side effects which may have “primed” participants for subsequent reporting of particular symptoms (e.g., if a copper IUD user is told to expect heavier, longer periods). Participants who discontinued a method at either 3 or 6 months were not asked questions about satisfaction, which may impact our results if there were women who were satisfied with their method but discontinued for another reason. An additional limitation is that users who discontinued at 3 months were not included at 6 months, which may affect our results. However, given the large number of women included in the analysis and the relatively small number who discontinued by 3 months, the observed change in satisfaction if discontinuers were included is likely to be small.
The bleeding and cramping patterns reported by CHOICE participants were similar to those reported by IUD users in studies conducted primarily outside the United States. Increased volume of menstrual bleeding after insertion of a copper IUD has been shown in several prior studies.7,14,15 However, few studies have established patterns of changes in bleeding frequency associated with copper IUD use. Hubacher et al report that about 20% of women experience inter-menstrual spotting for a mean of 1 day each cycle during the first year of copper IUD use.15 In our study, the most common bleeding pattern reported in copper IUD users was no change in the frequency of bleeding, suggesting that the many copper IUD users continue to experience regular cycles even during the first 3–6 months of use. A majority of women using the copper IUD reported increased cramping and heavier bleeding by three months; but by 6 months, a smaller percentage of participants (< 50%) reported increased cramping and heavier bleeding compared to baseline.
The LNG-IUS has been associated with decreased amounts of bleeding and cramping within six months of insertion.16,17 At the end of one year of use, the number of bleeding days is significantly reduced for LNG-IUS users, and the number of users with regular menstrual cycles is significantly reduced.18 By 3 months, almost two-thirds of CHOICE LNG-IUS users reported lighter bleeding, 68% reported either no change or less frequent bleeding, and 25% reported reduced cramping. In contrast, prolonged bleeding within the first three months of use among implant users has been reported in previous studies.18,19 This bleeding pattern may account for the lower satisfaction observed among implant users compared to IUD users.
Given the previously established patterns of bleeding and cramping, copper IUD users in this study were counseled to expect increased bleeding and cramping, while users of the LNG-IUS and implant were counseled to expect irregular bleeding during the first few months of use. The majority of users experiencing these expected symptoms remained satisfied with their method. The reported effect size of lighter bleeding on continuation is small, and likely is not clinically significant when compared to those with no change in bleeding.
An important strength of this study is that it examined a large cohort of participants who chose their contraceptive method and were not assigned randomly. Allowing women to choose their contraceptive method is a more realistic appraisal of method satisfaction in clinical practice. The study included only those women who were willing to begin using a new contraceptive method. Thus, the reported satisfaction profile is similar to new users of these methods in a clinical setting in the US. One limitation of this study is the manner in which bleeding and cramping data were collected via telephone survey with general impressions of bleeding and cramping patterns rather than the actual number of days of bleeding. Given the large sample size of CHOICE, it was not feasible to collect daily bleeding diaries. This does limit the capacity of our study to describe actual changes in bleeding and cramping patterns. However, the collected responses reflect participants’ perceptions of symptoms, which could be an important factor in determining our principal outcomes of satisfaction for the LARC methods. Another limitation is that we did not directly assess changes in cramping and bleeding at 6 months compared to baseline; thus, we had to calculate an approximate measure of overall change.
The LNG-IUS, copper IUD, and implant were well-tolerated regardless of differences in baseline demographic characteristics, baseline bleeding patterns, and symptoms experienced during initial use. A large majority of users remain satisfied with their LARC methods at 3 and 6 months. Further examination of symptoms and satisfaction at 12 months of use will provide more information on the potential for widespread, continued use of one or more of these highly effective contraceptive methods in the United States.
Acknowledgments
The Contraceptive CHOICE Project is funded by the Susan T. Buffett Foundation. This publication also was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences and award number K23HD070979 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. Peipert receives research funding from Bayer, Teva, and Merck, and serves on advisory boards for Teva Pharmaceuticals, Bayer, and Watson/Activis. Dr. Madden serves on an advisory board for Bayer Healthcare Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Desai, Diedrich, and Secura and Ms. Zhao report no conflict of interest.
References
- 1.Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 2.Trussell J. Contraceptive failure in the United States. Contraception. 2004;70(2):89–96. doi: 10.1016/j.contraception.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007–2009. Fertil Steril. 2012;98(4):893–897. doi: 10.1016/j.fertnstert.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour D, Korver T, Marintcheva-Petrova M, Fraser IS. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(Suppl 1)(785022368):13–28. doi: 10.1080/13625180801959931. [DOI] [PubMed] [Google Scholar]
- 5.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. [Accessed November 25, 2013];Contraception. 1994 49(1):56–72. doi: 10.1016/0010-7824(94)90109-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8137626. [DOI] [PubMed] [Google Scholar]
- 6.Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. [Accessed November 25, 2013];Int J Womens Health. 2010 2:211–220. doi: 10.2147/ijwh.s6914. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2971735&tool=pmcentrez&rendert ype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lara-Torre E, Spotswood L, Correia N, Weiss PM. Intrauterine contraception in adolescents and young women: a descriptive study of use, side effects, and compliance. J Pediatr Adolesc Gynecol. 2011;24(1):39–41. doi: 10.1016/j.jpag.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.O'neil-Callahan M, Peipert JF, Zhao Q, Madden T, Secura G. Twenty-four-month continuation of reversible contraception. Obstet Gynecol. 2013;122(5):1083–1091. doi: 10.1097/AOG.0b013e3182a91f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunloh DS, Casner T, Secura GM, Peipert JF, Madden T. Characteristics associated with discontinuation of long-acting reversible contraception within the first 6 months of use. Obstet Gynecol. 2013;122(6):1214–1221. doi: 10.1097/01.AOG.0000435452.86108.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115.e1–115.e7. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madden T, Mullersman JL, Omvig KJ, Secura GM, Peipert JF. Structured contraceptive counseling provided by the Contraceptive CHOICE Project. Contraception. 2013;88(2):243–249. doi: 10.1016/j.contraception.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. [Accessed March 2, 2014];Am J Epidemiol. 2003 157(10):940–943. doi: 10.1093/aje/kwg074. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12746247. [DOI] [PubMed] [Google Scholar]
- 13.Zou G. A modified poisson regression approach to prospective studies with binary data. [Accessed March 13, 2014];Am J Epidemiol. 2004 159(7):702–706. doi: 10.1093/aje/kwh090. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15033648. [DOI] [PubMed] [Google Scholar]
- 14.Suvisaari J. Patterns After- Postmenstrual and Postabortal Insertion of a Copper IUD or a Levonorgestrel-Releasing. 1996;7824(96) doi: 10.1016/s0010-7824(96)00189-8. [DOI] [PubMed] [Google Scholar]
- 15.Milsom I, Andersson K, Jonasson K, Lindstedt G, Rybo G. The influence of the Gyne-T 380S IUD on menstrual blood loss and iron status. [Accessed November 25, 2013];Contraception. 1995 52(3):175–179. doi: 10.1016/0010-7824(95)00163-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7587189. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002;65(2):129–132. doi: 10.1016/s0010-7824(01)00302-x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11927115. [DOI] [PubMed] [Google Scholar]
- 17.Suhonen S, Haukkamaa M, Jakobsson T, Rauramo I. Clinical performance of a levonorgestrelreleasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69(5):407–412. doi: 10.1016/j.contraception.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. [Accessed November 25, 2013];Contraception. 1999 60(1):1–8. doi: 10.1016/s0010-7824(99)00053-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10549446. [DOI] [PubMed] [Google Scholar]
- 19.Funk S, Miller MM, Mishell DR, et al. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71(5):319–326. doi: 10.1016/j.contraception.2004.11.007. [DOI] [PubMed] [Google Scholar]