Abstract
Objective(s)
Primary human trophoblasts were previously shown to be resistant to viral infection, and able to confer this resistance to non-trophoblast cells. Can trophoblasts protect non-trophoblastic cells from infection by viruses or other intracellular pathogens that are implicated in perinatal infection?
Study Design
Isolated primary term human trophoblasts were cultured for 72 h. Diverse non-placental human cell lines (U2OS, HFF, TZM-bl, MeWo, and Caco-2) were pre-exposed to either trophoblast conditioned, non-conditioned medium, or miR-517-3p for 24 h. Cells were infected with several viral and non-viral pathogens known to be associated with perinatal infections. Cellular infection was defined and quantified by plaque assays, luciferase assays, microscopy, and/or colonization assays. Differences in infection were assessed by Student's t-test or ANOVA with Bonferroni's correction.
Results
Infection by rubella and other togaviruses, HIV-1, and varicella zoster, was attenuated in cells pre-exposed to trophoblast conditioned medium (p <0.05), and a partial effect by the Ch.19 microRNA miR-517-3p on specific pathogens. The conditioned medium had no effect on infection by Toxoplasma gondii or Listeria monocytogenes.
Conclusion
Our findings indicate that medium conditioned by primary human trophoblasts attenuate viral infection in non-trophoblastic cells. Our data point to a trophoblast-specific antiviral effect that may be exploited therapeutically.
Keywords: C19MC, conditioned medium, miRNA, trophoblasts, viruses
Introduction
Beyond providing a physical barrier between the maternal and fetal vasculature, the placenta governs the exchange of gases, nutrients, and waste products between these two compartments. In the hemochorial placenta, this exchange is regulated primarily by the syncytiotrophoblasts, a layer of multinucleated, terminally differentiated cells that are bathed in the maternal blood and play a critical role in protecting the developing fetus from invading pathogens.1 Despite this defensive barrier, some pathogens are able to invade the fetal environment.
Viral infection of the intrauterine compartment can spread to the fetus and/or the mother. Active maternal viral infections can lead to infection during delivery or to pregnancy loss (either early or late) resulting from systemic spread of the infection.2 Viruses that are transmitted directly to the fetus can result in developmental abnormalities or fetal or neonatal disease. For example, prior to the widespread use of vaccination, fetal infection rates by the rubella virus were nearly 50% during maternal rubella infection in the first trimester of pregnancy and were associated with congenital rubella syndrome, characterized by deafness, cataracts, damage to the central nervous system, and cardiac defects.3 Congenital infection with varicella during pregnancy can lead to spontaneous abortion or neonatal varicella infection, which may result in devastating birth defects known as congenital varicella syndrome.4,5 Venezuelan equine encephalitis virus has been linked to pregnancy complications such as spontaneous abortion.6 HIV tends to be transmitted during vaginal delivery or invasive procedures.7
Beyond the formation of a syncytial physical barrier, mechanisms by which placental trophoblasts influence viral infections are insufficiently understood. We recently demonstrated that primary human trophoblasts (PHT) are resistant to infection by an unrelated panel of viruses.8 Furthermore, viral resistance was conferred to non-trophoblast cells when incubated with conditioned medium from PHT cells. This resistance was mediated, at least in part, by exosomal delivery of miRNAs from the chromosome 19 microRNA cluster (C19MC), which is the largest miRNA cluster in humans unique to primates and almost exclusively expressed in the placenta.9 C19MC miRNAs are highly expressed in exosomes released from PHT cells, and can be found circulating in the plasma of pregnant women.10,11 In cells exposed to PHT-conditioned medium we also observed a strong induction of autophagy, a pro-survival catabolic process where cellular organelles are partly or fully enclosed in cytoplasmic phagosomes, and degraded upon fusion with the lysosomes. Autophagy was also observed in cells transfected with selected miRNA members of the C19MC, and attenuation of autophagy mitigated this antiviral effect.8 Here we expand upon our previous observations and focus on viruses that are pertinent to fetal infection during pregnancy and/or delivery, including rubella virus and other togaviruses, HIV, and varicella zoster virus (VZV). Additionally, we compared these effects to infection by two clinically relevant non-viral perinatal pathogens, Listeria monocytogenes, and Toxoplasma gondii.
Materials and Methods
Study design
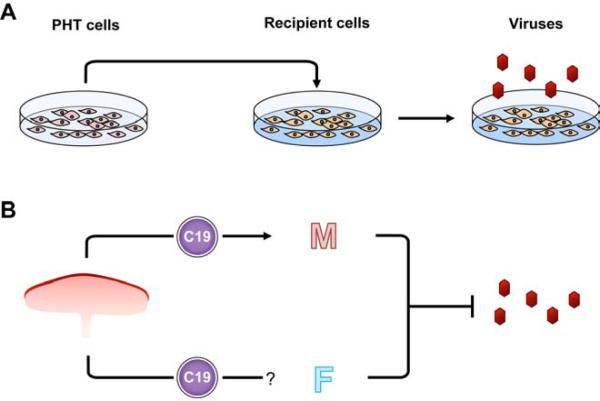
To assess the ability of human trophoblasts to confer pathogen resistance to non-trophoblastic cells, we collected conditioned medium from PHT cells, cultured from 48-72 h, or control non-conditioned medium. This medium was added to non-trophoblast recipient cells for 24 h prior to infection. Pathogens were then added to cells exposed to either medium, and subsequent infection was assessed utilizing the assays listed below. Infection was quantified relative to control conditions. This design is illustrated in Fig. 1.
Figure 1. Experimental design and model.
(A) The medium from primary human trophoblasts (PHT cells) was collected after 48-72 hours, and transferred to nontrophoblast recipient cells 24 h prior to infection. The level of pathogen infection in recipient cells was compared to cells exposed to control medium. See Methods for details. (B) A proposed model for the protective effect of exosome-packaged C19MC miRNAs. Exosomes are transferred from the placenta into the maternal circulation (M), and perhaps into the fetal circulation (F), where they may attenuate viral infection.
Cells
Human osteosarcoma U2OS, human foreskin fibroblast (HFF), melanoma-derived cells (MeWo), and TZM-bl cells12 were cultured in DMEM (Corning, USA) supplemented with 10% FBS (Sigma, USA) and antibiotics. Human epithelial cells (Caco-2 cells, ATCC HTB-37) were cultured in Eagle's MEM containing 20% heat-inactivated FBS and 10 U penicillin and streptomycin. Vero African green monkey kidney cell line, commonly used for assessment of viral infections, were maintained in DMEM supplemented with 5% FBS and antibiotics.
PHT cells were acquired from healthy singleton term placentas, using the procedure previously described13, with modifications.14,15 Cells were maintained in DMEM containing 10% bovine growth serum (HyClone, USA), 20 mM HEPES, and antibiotics at 37°C. Cells were maintained 72 h after plating, with cell quality monitored morphologically and by human chorionic gonadotropin (hCG) levels (ELISA, DRG International, USA) in the medium, which show a characteristic increase as cytotrophoblasts differentiate into syncytiotrophoblasts16.
Conditioned medium preparation
Conditioned medium samples were collected from PHT cultures as previously described, and only medium that demonstrated at least 70% reduction in vesicular stomatitis virus (VSV) infection was used for subsequent infectivity assays.8 Briefly, U2OS cells were exposed to conditioned or non-conditioned PHT medium for 24 h, and infected with VSV at a multiplicity of infection (MOI) of 1 for 6 h or until cytopathic effect was evident. Cells were then lysed with 1mL Qiazol lysis reagent, and infection was quantified by RT-qPCR, as previously described.8 Notably, 65% of the media screened exhibited at least 70% reduction in VSV infection, and thus used for subsequent assays.
Rubella plaque assays
Vero cells were pre-exposed to either conditioned or non-conditioned medium and infected with rubella virus at an MOI of 10. Plaque assays were performed with serial dilution of the virus. One milliliter of each dilution and a control (PBS/1% FBS) were plated on 30 mm plates confluent with Vero cells, and the plates were incubated for 1 h at 37°C. Cells were overlaid with a liquid agar solution (60 mL 0.4% liquid agar, 34 mL 3X MEM, 1 mL FBS, 3 mL 5% NaHCO3, 0.1 mL penicillin/streptomycin and 0.1 mL diethylaminoethanol). The plates were incubated for 7 days at 37°C. After incubation, the agar was remove d, and the plates were stained with crystal violet solution to reveal plaques. Duplicate plaque assays were performed for each infection, and the final titer of each infection was the average of the two plaque assays.
Alphavirus Luciferase assays
Vero cells were pre-exposed to either conditioned or non-conditioned media for 24 h prior to infection. Reporter alphaviruses that expressed a cleavable firefly luciferase in between the capsid and PE2 proteins (as described17) were added to the cells at an MOI of 0.1 for 8 h and lysed with 1X Passive lysis buffer (Promega). Infection was quantified by measuring luciferase expression and normalized to protein level. Alphavirus constructs included eastern equine encephalitis virus (EEEV), Venezuelan equine encephalitis virus (VEEV), chikungunya virus (CHIKV), and sindbis virus (SINV). These virus constructs were also used to infect U2OS cells transfected with miRNA mimics from the C19MC, as described below. These experiments were conducted with an MOI of 1 for EEEV, VEEV, and CHIKV while SINV was used at an MOI of 0.1.
HIV infectivity assay
Utilizing the TZM-bl cell line, a HeLa cell derivative that are CD4+ and expresses CCR5 and CXCR4, as well as a Tat-inducible luciferase reporter, HIV-1 infectivity was quantified on the basis of relative luciferase expression.12,18 These cells, which are permissive to wild type HIV-1, were pre-exposed to PHT-conditioned media or non-conditioned media for 24 h prior to infection. HIV-1 NL4-3 was added at increasing concentrations, indicated in the figure legend. After 48 h, infected cells were washed with PBS and lysed in luciferase lysis buffer (Promega, USA). Lysates (40 μl) were transferred to white 96-well plates, and 50 μl luciferase reagent (Promega) was injected into each well. Luciferase activity was determined by detection of luminescence recorded by Synergy 2 SL luminescence microplate reader (BioTek, VT, USA).
VZV Luciferase assays
VZV infectivity was measured using reporter viruses expressing firefly or renilla luciferase reporter enzymes respectively. Recombinant viruses were developed from a VZV BAC using methods detailed previously.19 The luciferase gene was placed directly downstream of either the immediate early gene encoding the latency associated regulatory protein IE63 or the late VZV ORF9 gene encoding the abundant VZV tegument protein at the native locus, and was expressed as bi-cistronic mRNAs with a T2A ribosome skipping motif (MB Yee and PR Kinchington, manuscript in preparation). VZV permissive HFF cells were pre-exposed to conditioned or non-conditioned media for 24 h. Cells were then infected with 1000 pfu/mL of either VZVORF9Luc or VZVORF63Luc vector for 48 h. Cells were lysed, and luciferase expression was quantified as described above.
Fluorescence microscopy
Toxoplasma gondii infection was quantified using a yellow fluorescent protein (YFP)-tagged RH strain provided by David Roos, University of Pennsylvania, as previously described.20 HFF cell monolayers were cultured in eight-well chamber slides (Nunc Lab-Tek, Thermo-Fisher, USA) at 37°C, 5% CO 2. Either conditioned or non-conditioned medium was added to the cells 24 h prior to infection. Toxoplasma gondii RH-YFP was added at an MOI of 0.5. Forty-eight h post-infection, cells were washed and fixed with 4% paraformaldehyde in PBS and permeabilized with 0.25% Triton X-100 in PBS. Fixed monolayers were then mounted with Vectashield (Vector Laboratories, USA) containing DAPI. Images were captured with an Olympus FluoView 1000 laser scanning confocal microscope. Parasitophorous vacuole number and size, measured by region of interest length (uM), were quantified using Image J software (NIH, USA).
Listeria infectivity assay
Wild type Listeria monocytogenes (DP10403S) was cultured overnight at 37°C in brain and heart infusion (BHI). The following day, bacteria were diluted (1/20) in BHI and grown at 37°C until OD 600 nm reached 0.7–0.8. Bacteria were washed three times with PBS and suspended in MEM at the indicated MOI. Caco-2 cells were plated in 24-well plates at a density of 40,000 cells/well on glass coverslips coated with rat tail collagen. After 24 h, the cell culture medium was replaced with 500 μL conditioned or control medium, in triplicate, for 24 h. Caco-2 cells were washed with MEM and infected with L. monocytogenes at the indicated MOI. The bacterial suspension (500 μL MEM) was added to each well, and the cell culture plate was centrifuged at 1,500 rpm for 2 min at room temperature. Caco-2 cells and bacteria were then co-incubated for 30 min at 37°C, followed by two washes with warm ME M. Caco-2 cells were further incubated for the indicated times in the presence of conditioned or control medium supplemented with 15 μg/mL gentamicin.21,22 Cells were washed three times with warm PBS. A volume of 300 μL PBS containing 0.2% Triton X-100 was added to each well to lyse the Caco-2 cells. Cell lysates were diluted and plated on BHI agar plates to enumerate bacterial colony forming units.
Mimic transfections
Mimics for C19MC miRNAs (miRIDIAN) as well as a non-targeting control miRNA mimic were obtained from Thermo-Fisher USA as previously described.8 U2OS cells were reverse transfected with miRNA mimics or miRNA mimic control (final concentration, 25 nM for each miRNA mimic), using DharmaFECT-1 transfection reagent (Thermo-Fisher) according to the manufacturers’ instructions. Cells were assayed 48 h post-transfection.
Statistics
Experiments were performed at least three times as indicated in the legend of each figure. Data are presented as mean ± SD. Except where specified, Student's t-test was used to determine statistical significance for virus infections when two sets were compared, and one-way analysis of variance (ANOVA), with Bonferroni's correction for post hoc analysis of multiple comparisons, was used to determine statistical significance for reporter gene assays; p <0.05 was considered significant.
Ethical approval
PHT cells were cultured from term or near-term placentas collected by the Obstetrical Specimen Procurement Unit at Magee-Womens Hospital of the University of Pittsburgh Medical Center. Sample collection was conducted according to an exempt protocol approved by the Institutional Review Board of the University of Pittsburgh. All specimens were de-identified.
Results
PHT conditioned medium or miRNA mimics from the C19MC attenuate infection of select members of the Togaviridae family
We found that, when compared to non-conditioned medium, pre-exposure to PHT conditioned medium markedly reduced the infection of Vero cells by rubella virus (Fig. 2A). We expanded our studies to other members of the Togaviridae family and measured the activity of luciferase from reporter viruses to represent the relative infection of a panel of viruses from the alphavirus genus.17 We found that infection by EEEV, VEEV, CHIKV, and SINV was attenuated in cells exposed to conditioned medium (Fig. 2B). Because we had previously shown that PHT-conditioned medium contained relatively high levels of antiviral C19MC miRNAs,8 we tested the antiviral activity of one of these miRNAs, miR-517-3p, shown previously to confer resistance to VSV, VV, and herpes simplex virus-1 (HSV-1). Indeed, we found that transfection of a miR-517-3p mimic significantly reduced infection of only CHIKV and SINV when compared to cells transfected with a scramble control miRNA (Fig. 2C).
Figure 2. PHT conditioned medium or miRNA mimics from the C19MC attenuate infection of select togaviruses.
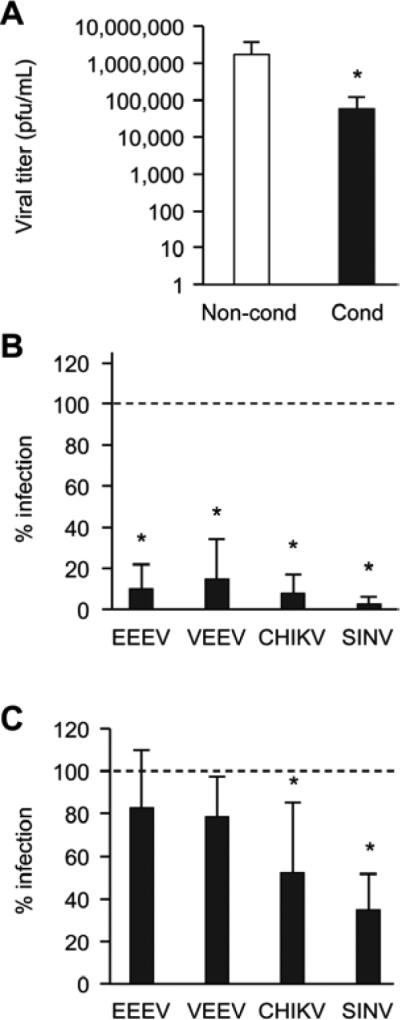
(A) Log scale of Rubella virus titers from cells exposed to non-conditioned or conditioned PHT medium. Data are mean of three independent plaque assays, each run in duplicate. p <0.05 (Student's t test). (B) Activity of alphavirus luciferase reporter constructs. Eastern equine encephalitis virus (EEEV), Venezuelan equine encephalitis virus (VEEV), Chikungunya virus (CHIKV), and Sindbis virus (SINV), expressing luciferase in Vero cells, were exposed to conditioned PHT or control non-conditioned medium. Data are presented as percent infection relative to control and represent a mean of three independent infections, each run in triplicate. p <0.0001 (ANOVA with Bonferroni correction). (C) EEEV, VEEV, CHIKV, and SINV luciferase expression in cells transfected with miR-517-3p or control scrambled mimic. Data are presented as percent infection relative to control and represent a mean of three independent infections, each run in triplicate. p <0.0001 (ANOVA with Bonferroni correction).
PHT-conditioned medium attenuates HIV-1 infection
To determine whether conditioned PHT medium could inhibit HIV-1 infection in nontrophoblastic cells, we used an HIV-1 infectivity assay. We observed a significant reduction in luciferase expression in cells that were exposed to conditioned medium compared to cells exposed to non-conditioned medium (Fig. 3A). To investigate whether or not miR-517-3p was capable of conferring resistance to HIV-1, we transfected TZM-bl cells with scramble control miRNA, miR-517-3p, or miR-720, a non-C19MC miRNA expressed in trophoblasts. We observed a non-significant trend in reduction of HIV-1 reporter activity in cells transfected with miR-517-3p, but not with miR-720 (Fig. 3B).
Figure 3. PHT conditioned medium inhibits HIV infection.
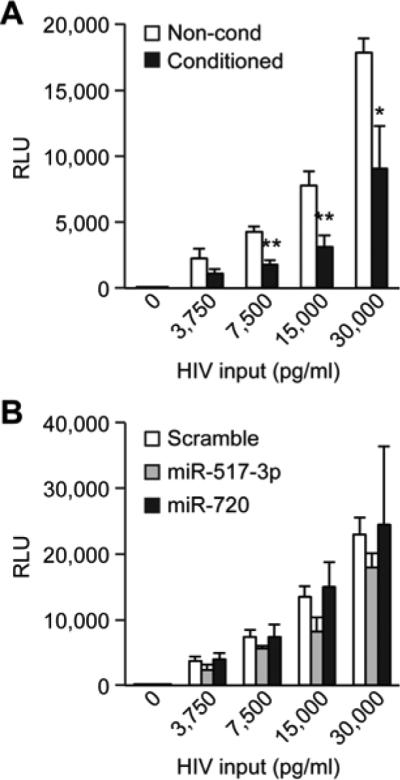
(A) Relative luciferase activity (RLU) of an HIV Tat-inducible reporter in TZM-bl cells exposed to either conditioned PHT or control non-conditioned medium and infected with increasing concentrations of HIV, as described in Methods. Data are representative of three experiments, each performed in triplicate. * denotes p <0.05, and ** denotes p <0.001 (Student's t test). (B) Relative luciferase expression in TZM-bl cells transfected with miR-517-3p, miR-720, or control scrambled mimic and infected with increasing concentrations of HIV. Data are representative of three experiments, each performed in triplicate. None of the differences were statistically significant.
PHT conditioned medium or C19MC miRNA mimics attenuate VZV replication
Using either a VZV reporter virus that expresses luciferase after an immediate early varicella gene (ORF63) or a reporter virus expressing luciferase after a late VZV gene (ORF9), we found that pre-exposure to PHT conditioned medium only impacted the late viral gene reporter, but did not significantly impact expression of the immediate early viral reporter (Fig. 4A). We next assessed the effect of miR-517-3p on VZV infection. Because of inefficient transfection of HFF cells with miRNA mimics, we transfected VZV permissive MeWo cells23 with miR-517-3p, miR-720 (non-C19MC control), or scrambled miRNA mimics. We found that transfection of miR-517-3p, but not the control miRNAs, significantly reduced luciferase activity due to infection with VZVORF9 (Fig. 4B), albeit to a lesser extent than PHT conditioned medium.
Figure 4. PHT conditioned medium and miRNA mimics from the C19MC attenuate infection of VZV after initiation of infection and immediate early gene expression.
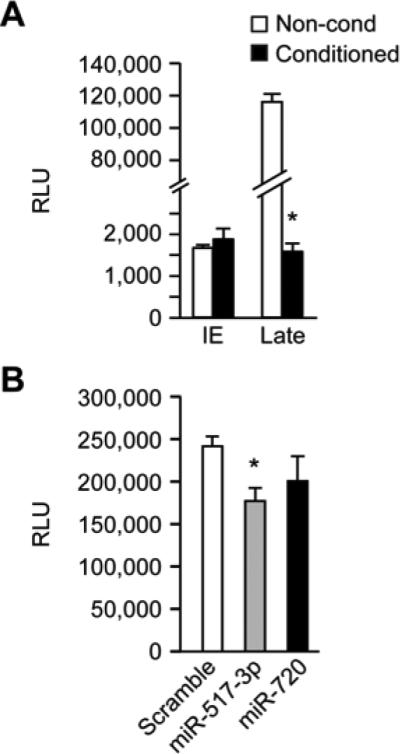
(A) Relative luciferase activity (RLU) from cells infected with either immediate early (IE) reporter virus, or “late” ORF9 reporter virus, exposed to either non-conditioned or conditioned PHT medium. Data are representative of three independent experiments, each performed in triplicate. p <0.001 (Student's t test. (B) Relative luciferase activity (RLU) in MeWo cells transfected with either miR-517-3p, miR-720, or scrambled mimic and infected with VZV_ORF9_Luc. Data are representative of three independent experiments, each performed in triplicate. p <0.05 (ANOVA with Boneferroni correction).
PHT conditioned medium has no effect on infection by the non-viral perinatal pathogens Toxoplasma gondii or Listeria monocytogenes
To determine whether conditioned PHT medium could inhibit T. gondii infection, we exposed HFF cells to either PHT conditioned or non-conditioned medium for 24 h and determined infection by YFP-expressing T. gondii.20 We found that PHT conditioned medium had no effect on T. gondii infection (Fig. 5A) quantified by vacuole number and size (Fig. 5B-C). We next tested whether PHT conditioned medium could confer resistance to L. monocytogenes in Caco-2 cells. Bacterial burden was measured after 1.5, 5, and 10 h time points. Our results showed a lack of consistent reduction in L. monocytogenes burden (Fig. 6).
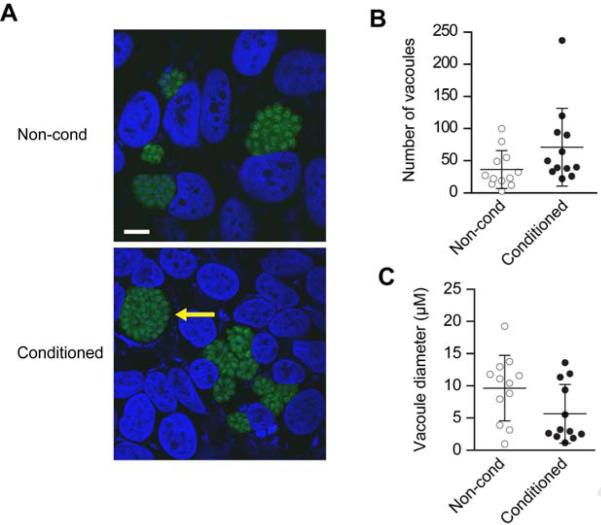
Figure 5. PHT conditioned medium has no effect on Toxoplasma gondii infection.
(A) Representative micrographs showing fluorescent parasites within vacuoles in U2OS cells exposed to non-conditioned or conditioned PHT media. Arrow denotes parasitophorous vacuole. Scale bar =10 μM. (B) Vacuole number, or (C) vacuole size in cells exposed to conditioned or non-conditioned media. Quantities are the average of four fields per sample and were assessed via three independent experiments. None of the differences were statistically significant.
Figure 6. PHT conditioned medium has no effect on Listeria monocytogenes replication.
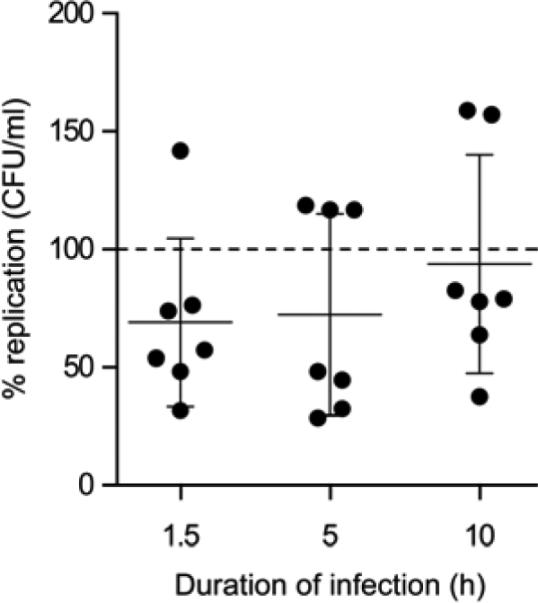
Colony forming units for L. monocytogenes after infection of 1.5, 5, and 10 h, as detailed in Methods. Data are presented as percent replication of bacteria in cells exposed to conditioned medium compared to that of bacteria in cells exposed to non-conditioned medium. Data presented are the mean of three independent experiments. None of the differences were statistically significant.
Comment
Principle findings and their meaning
We recently demonstrated that PHT cells are resistant to a diverse panel of viruses, including coxsackie virus B, polio virus, VSV, vaccinia virus, HSV-1, and cytomegalovirus. Importantly, we found that this resistance could be conferred to non-trophoblast cells by exposing them to PHT conditioned medium and, to a lesser degree, to C19MC miRNAs.8 Here we expanded our experiments to include viruses that are associated with human perinatal infection and to assess the effect of PHT conditioned medium on non-viral pathogens.1,24 We first tested rubella, a togavirus that crosses the placenta by hematogenous dissemination and causes congenital rubella syndrome.3 We then broadened our studies to include other members of the togavirus family. The Old world alphaviruses, including CHIKV and SINV, cause febrile and arthritogenic disease in humans, while the New world alphaviruses, including EEEV and VEEV, can cause acute encephalitis in humans.25-27 Infection by all four alphaviruses was significantly attenuated in cells pre-exposed to PHT conditioned medium. Interestingly, transfecting cells with miR-517-3p attenuated infection by the Old world, but not the New world, alphaviruses.
We also found that PHT conditioned medium attenuates infection by HIV-1, which is transmitted to the fetus predominantly during delivery,28 with less frequent antenatal transmission during invasive procedures such as amniocentesis.29 In addition, we found that pre-exposure to PHT conditioned medium significantly reduced cell infection by VZV, the causative agent of chickenpox and a significant perinatal pathogen which causes the rare but devastating congenital varicella syndrome.30 VZV gene expression occurs in a cascade, with immediate early, early, and late viral genes being expressed in sequence. Interestingly, our data suggest that the effect of PHT conditioned medium is observed only on late viral genes during VZV infection. We previously observed inhibition of early gene expression in other large DNA genome viruses, vaccinia virus and HSV-1, suggesting that the underlying mechanism of inhibition may depend on the viral life cycle and on the timing of infection during pregnancy.
We found no effect of PHT conditioned medium on infection by the protozoan Toxoplasma gondii, which is an important perinatal pathogen, that causes direct fetal organ damage.31 Similarly, PHT conditioned medium had no consistent effect on infection by L. monocytogenes, a Gram-positive facultative intracellular pathogen that can cause miscarriage, stillbirth, or neonatal meningioencephalitis.32 As syncytiotrophoblasts were previously shown to be resistant to colonization by T. gondii or by L. monocytogenes,33,34 our findings may indicate that PHT-conditioned medium induces a primarily antiviral state, while other mechanisms may drive resistance to bacterial or protozoan intracellular pathogens.
Strengths and weaknesses, and implications of findings
Our results, presented here and in our previous work,8 indicate that the conditioned medium from PHT cells is broadly antiviral, inhibiting infection by viruses known to cause perinatal infection in humans. This inhibition was observed for RNA and DNA viruses. Previously we showed that viral inhibition was, in part, due to induction of autophagy, as recipient cells exposed to PHT medium demonstrated increased autophagy with internalizing virions localizing to autophagosomes. Further, the antiviral effect was mitigated when autophagy was blocked.8,35 We previously showed that PHT conditioned medium contains high levels of miRNAs from the C19MC family that correspond to the expression level of these miRNAs in PHT cells.10 Interestingly, our data show that the effect of C19MC miRNAs is markedly weaker than that of PHT conditioned medium8, and not all viruses tested were sensitive to C19MC-associated miRNAs previously shown to exert antiviral effects on VSV and HSV1. It is possible that expression of additional members of the C19MC miRNAs, or the entire miRNA cluster, might be needed for a more potent antiviral effect. In addition, a more effective miRNA delivery system (e.g., via exosomes) might potentiate the antiviral effect.8,36 This might be particularly relevant to VZV, in which infection might be enhanced by the initial stages of autophagy, but impaired by the complete, more effective autophagy process, which includes protein degradation.37
Our data do not rule out additional mechanisms for the anti-viral effect of trophoblastic conditioned medium. We previously reported 8 that cells rendered incapable of responding to type1 interferon retain their anti-viral activity upon exposure to conditioned medium. Nonetheless, PHT conditioned medium may contain non-classical interferons or additional factors that contribute to the antiviral state and exhibit an additive or synergistic effect with the miRNAs. Importantly, the lack of effect on infection with either Listeria monocytogenes or Toxoplasma gondii suggests that the PHT conditioned medium and, to an extent, the C19MC miRNAs stimulate a selective viral-specific response in non-trophoblast cells. Given the diversity of the viruses tested, it is possible that this response may inhibit viral infection at different points of the viral life cycle. Future studies have been designed to address the mechanisms underlying the antiviral effect and elucidate the targets of the C19MC and other antiviral factors potentially present in PHT conditioned media.
Condensation.
Primary human trophoblasts confer resistance to clinically relevant viruses linked to perinatal infections in non-trophoblast cells, but not to intracellular bacteria or parasites.
Acknowledgements
We thank David Roos (Department of Biology, University of Pennsylvania) for T. gondii related reagents. We thank Elena Sadovsky and Judy Ziegler for technical assistance, Lori Rideout for assistance in preparing the manuscript, and Bruce Campbell for editing (Magee-Womens Research Institute, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh). We note that Dr Coyne and Dr Sadovsky contributed equally to this work.
Study funding: NIH T32AI049820, T32AI060525 training grants (DWT), NIH R01AI083383 (WBK); NIH R01AI057083 (TES); NIH P30EY08098, NIH R01NS064022 and Research to Prevent Blindness Inc (PRK); NIH R01AI081759 and the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award (CBC); *Pew Scholarship in the Biomedical Sciences and R21AI093906 (JB)*; NIH R01HD075665 (CBC and YS); and NIH R01HD065893 and R21HD071707 (YS).
Glossary
- PHT
primary human trophoblasts
- siRNA
small inhibitory RNA, used to reduce mRNA and protein expression
- MicroRNA (miRNA)
small non-coding RNAs approximately 19-24 nucleotide long, known to block translation or degrade mRNA
- Scramble siRNA
non-targeting siRNA (control)
- C19MC
chromosome 19 miRNA cluster of miRNA. This is the largest miRNA cluster in humans. It is primate specific, and is almost exclusively expressed in the placenta
- Conditioned medium
cell culture medium that has been incubated with specific cells, and may contain factors released by these cells.
- Plaque assays
A standard method to determine virus concentration by examining the number of plaque-forming units of viruses in a petri dish
- Luciferase assays
A molecular assay to determine protein activity, used to indirectly quantify viral proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: CBC and YS, who contributed equally to this work, are named inventors on a pending patent application describing the use of C19MC microRNAs as therapeutics.
Presentation: Data was presented, in part, at the 32nd Annual Meeting of the American Society for Virology in State College, PA, in July 2013.
References
- 1.Robbins JR, Bakardjiev AI. Pathogens and the placental fortress. Curr Opin Microbiol. 2012;15:36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivas SK, Ma Y, Sammel MD, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 3.Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, Green MF, editors. Creasy & Resnik's Maternal Fetal Medicine: Principles and Practice. Elsevier Saunders; Philadelphia: 2014. [Google Scholar]
- 4.Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548–51. doi: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith CK, Arvin AM. Varicella in the fetus and newborn. Semin Fetal Neonatal Med. 2009;14:209–17. doi: 10.1016/j.siny.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Rivas F, Diaz LA, Cardenas VM, et al. Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J Infect Dis. 1997;175:828–32. doi: 10.1086/513978. [DOI] [PubMed] [Google Scholar]
- 7.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 8.Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110:12048–53. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortolin-Cavaille ML, Dance M, Weber M, Cavaille J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009;37:3464–73. doi: 10.1093/nar/gkp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donker RB, Mouillet JF, Chu T, et al. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18:417–24. doi: 10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–29. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 12.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DM, Johnson RD, Smith SD, Anteby EY, Sadovsky Y. Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol. 1999;180:896–902. doi: 10.1016/s0002-9378(99)70661-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–72. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- 16.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31:781–4. doi: 10.1016/j.placenta.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun C, Gardner CL, Watson AM, Ryman KD, Klimstra WB. Stable, High-Level Expression of Reporter Proteins from Improved Alphavirus Expression Vectors To Track Replication and Dissemination during Encephalitic and Arthritogenic Disease. J Virol. 2014;88:2035–46. doi: 10.1128/JVI.02990-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erazo A, Yee MB, Banfield BW, Kinchington PR. The alphaherpesvirus US3/ORF66 protein kinases direct phosphorylation of the nuclear matrix protein matrin 3. J Virol. 2011;85:568–81. doi: 10.1128/JVI.01611-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubbels MJ, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob Agents Chemother. 2003;47:309–16. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dramsi S, Cossart P. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect Immun. 2003;71:3614–8. doi: 10.1128/IAI.71.6.3614-3618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dramsi S, Dehoux P, Lebrun M, Goossens PL, Cossart P. Identification of four new members of the internalin multigene family of Listeria monocytogenes EGD. Infect Immun. 1997;65:1615–25. doi: 10.1128/iai.65.5.1615-1625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980;101:1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- 24.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–8. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336:1867–74. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 26.Johnson KM, Martin DH. Venezuelan equine encephalitis. Adv Vet Sci Comp Med. 1974;18:79–116. [PubMed] [Google Scholar]
- 27.Ryman KD, Klimstra WB. Host responses to alphavirus infection. Immunol Rev. 2008;225:27–45. doi: 10.1111/j.1600-065X.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 28.Rouzioux C, Costagliola D, Burgard M, et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. Am J Epidemiol. 1995;142:1330–7. doi: 10.1093/oxfordjournals.aje.a117601. [DOI] [PubMed] [Google Scholar]
- 29.Mandelbrot L, Jasseron C, Ekoukou D, et al. Amniocentesis and mother-to-child human immunodeficiency virus transmission in the Agence Nationale de Recherches sur le SIDA et les Hepatites Virales French Perinatal Cohort. Am J Obstet Gynecol. 2009;200:160, e1–9. doi: 10.1016/j.ajog.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 30.Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163–5. doi: 10.1155/S1064744994000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beazley DM, Egerman RS. Toxoplasmosis. Semin Perinatol. 1998;22:332–8. doi: 10.1016/s0146-0005(98)80022-0. [DOI] [PubMed] [Google Scholar]
- 32.Benshushan A, Tsafrir A, Arbel R, Rahav G, Ariel I, Rojansky N. Listeria infection during pregnancy: a 10 year experience. Isr Med Assoc J. 2002;4:776–80. [PubMed] [Google Scholar]
- 33.Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012;80:418–28. doi: 10.1128/IAI.05899-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6:e1000732. doi: 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9:2173–4. doi: 10.4161/auto.26558. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: Placenta-specific microRNAs in exosomes - Good things come in nano-packages. Placenta. 2014;35S:S69–S73. doi: 10.1016/j.placenta.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grose C, Yu X, Cohrs RJ, Carpenter JE, Bowlin JL, Gilden D. Aberrant virion assembly and limited glycoprotein C production in varicella-zoster virus-infected neurons. J Virol. 2013;87:9643–8. doi: 10.1128/JVI.01506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]