Abstract
Although considerable research has shown that inflammation leads to social withdrawal more generally, it is also possible that inflammation leads to social approach when it comes to close others. Whereas it may be adaptive to withdraw from strangers when sick, it may be beneficial to seek out close others for assistance, protection, or care when sick. However, this possibility has never been explored in humans nor have the neural substrates of these behavioral changes. Based on the role of the ventral striatum (VS) in responding to: (1) the anticipation of and motivation to approach rewarding outcomes and (2) viewing social support figures, the VS may also be involved in sickness-induced approach toward support figures. Thus, the goal of the present study was to examine whether inflammation leads to a greater desire to approach support figures and greater VS activity to viewing support figures. To examine this, 63 participants received either placebo or low-dose endotoxin, which safely triggers an inflammatory response. Participants reported how much they desired to be around a self-identified support figure, and viewed pictures of that support figure while undergoing an fMRI scan to assess reward-related neural activity. In line with hypotheses, endotoxin (vs. placebo) led participants to report a greater desire to be around their support figure. In addition, endotoxin (vs. placebo) led to greater VS activity to images of support figures (vs. strangers) and greater increases in inflammation (IL-6 levels) were associated with greater increases in VS activity. Together, these results reveal a possible neural mechanism important for sickness-induced social approach and highlight the need for a more nuanced view of changes in social behavior during sickness.
Keywords: inflammation, social support, close relationships, functional magnetic resonance imaging, cytokines, social approach
As part of the innate immune response, an organism will exhibit a multitude of symptoms, termed “sickness behavior,” in response to infection or illness. Symptoms of sickness are triggered by the release of proinflammatory cytokines, which act as chemical messengers to signal the brain to change behavior. The most commonly observed inflammatory-induced change in social behavior has been withdrawal from others. Thus, animal research has shown that an acute inflammatory challenge leads to reduced social exploration of others (Bluthe, Michaud, Kelley, & Dantzer, 1996; Bluthe et al., 1994, b; Marvel, Chen, Badr, Gaykema, & Goehler, 2004). Similarly, humans exposed to an experimental inflammatory challenge report increased feelings of social disconnection (Eisenberger, Inagaki, Mashal, & Irwin, 2010) and greater threat-related neural activity to negatively-valenced pictures of unknown others (Inagaki, Muscatell, Cole, Irwin, & Eisenberger, 2012). Though unpleasant in the short-term, changes in social behavior such as social withdrawal are thought to be adaptive responses in promoting rest and recuperation from illness or infection (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Hart 1988).
Despite this literature linking inflammation and social withdrawal, animal models have shown that, under certain circumstances, animals will engage in more rather than less social behavior during sickness (Aubert, 1999; Hennessy, Deak, & Schiml, 2014). This is particularly true when given the chance to affiliate with a familiar other. For instance, after being injected with lipopolysaccharide (LPS), which elicits an inflammatory response, rats spend more time huddling with familiar cage-mates as compared to responses of placebo-injected controls (Yee & Prendergast, 2010). Increases in affiliative social behavior during sickness have also been observed in non-human primates. At a relatively low dose, LPS-treated rhesus monkeys (vs. saline-treated control monkeys) show significantly more close social contact with cage-mates and, at the higher dose, proximal social contact (defined as passively sitting near a companion) is positively correlated with levels of interleukin-6 (IL-6), an inflammatory cytokine and wellknown mediator of sickness behavior (Dantzer, 2001; Willette, Lubach, & Coe, 2007). Thus, depending on the target of the social behavior, sickness can lead to increased approach toward others.
In fact, increasing interactions with close, supportive individuals may confer a survival advantage should those close individuals provide care and protection to the sick (Cole, 2006; Hennessy et al., 2014). In other words, just as it may be important to withdraw from strangers or signs of threat during sickness, it may be just as important to approach close others in order to obtain care. Indeed, sickness increases social approach behavior toward close others in young children, such that infants or children who are sick become more clingy, spend more time in proximity with their caregivers, and become more upset following separation from their caregivers (Ainsworth, 1973; Bowlby, 1988; Mikulincer & Shaver, 2007). However, the effect of inflammation on the motivation to approach support figures has not yet been explored in humans.
In addition, the neural regions underlying motivations to approach loved ones during times of sickness are currently unknown. Results from studies of the neurobiology of close social relationships suggest that regions related to reward processing, especially the ventral striatum (VS), underlie feelings of social connection in close relationships (Aron, Fisher, Mashek, Strong, Li, & Brown, 2005; Acevedo, Aron, Fisher, & Brown, 2011; Inagaki & Eisenberger, 2013). For instance, reminders of close others in the neuroimaging environment, such as loving messages from close others (Inagaki & Eisenberger, 2013) or pictures of a loved one (Acevedo et al., 2011; Strathearn, Fonagy, Amico, & Montague, 2009; Strathearn, Li, Fonagy, & Montague, 2008) robustly activate the VS. In addition, the VS is particularly sensitive to the motivation to approach highly pleasing rewards such as money or sweet tastes (Berridge, Robinson, & Aldridge, 2009; Knutson & Cooper, 2005). Thus it appears as if the VS is sensitive both to the motivation to approach rewards as well as close support figures and therefore may be associated with social approach during sickness as well.
The current study assessed the effect of an experimentally induced inflammatory challenge on the motivation to approach a support figure. Based on results from the animal literature, we expected inflammation (vs. placebo) to lead to a greater self-reported desire to be around support figures. We also investigated whether inflammation altered neural activity in a key reward-related brain region in response to viewing photographs of a social support figure, but not to photographs of an unknown stranger. We hypothesized that individuals exposed to an inflammatory challenge (vs. a placebo) would show greater neural activity in the VS in response to viewing pictures of their support figure, but would show no differences in response to viewing pictures of a stranger. Finally, we explored the association between endotoxin-induced changes in the proinflammatory cytokines, IL-6 and TNF-α, and VS activity to viewing support figures with the hypothesis that increases in cytokines would be associated with greater VS activity.
Methods
Overview
Detailed descriptions of similar methods have been published elsewhere (Eisenberger et al., 2010; Eisenberger, Inagaki, Rameson, Mashal, & Irwin, 2009; Inagaki et al., 2012), but are summarized here. Participants were deemed eligible to participate after being evaluated for psychiatric conditions (via the Structure Clinical Interview for DSM Axis I Disorders; First, Spitzer, Gibbon, & Williams, 2012), scanner-safety (claustrophobia and for the females, pregnancy), and general health (vitals, BMI, blood draw). Following screening, eligible participants were contacted and asked to send digital photographs of a self-identified support figure for the scanner task. On the day of the experimental session, participants were randomly assigned to receive low dose endotoxin, which safely triggers an inflammatory response, or placebo. Approximately two hours after injection, when the inflammatory response begins to peak (Eisenberger et al., 2009, 2010), all participants were asked about their desire to be around their support figure and then underwent an fMRI scan where they viewed images of their support figure and a sex, race, age and expression matched stranger (see below for more details). Hourly blood draws were taken throughout the experimental protocol to assess levels of inflammation (at baseline prior to endotoxin/placebo administration and then approximately every hour over a total time of six and a half hours after endotoxin/placebo administration). Cytokine analyses for the current study focused on the baseline time point and the post-scan time point because this second time point was closest to when the fMRI task was collected and because our prior work has shown sustained increases in cytokines (relative to baseline) at this time (Eisenberger et al., 2009, 2010).
Participants
115 participants (69 females, M age = 24.17, SD = 6.61) were randomly assigned to receive low dose endotoxin (0.8 ng/kg of body weight, O:113; n = 61) or placebo (0.9% saline; n = 54) administered as an IV bolus over a 30–60 second period through a catheter placed in the non-dominant forearm. Of this sample, 52 participants were not run through the support figure task due to logistical constraints (i.e. some participants failed to respond to email requests for pictures of a support figure, last minute scheduling changes did not allow sufficient time to collect pictures, the reserved scanning time would end before we were able to acquire data for this task). These constraints left a sample of 63 participants (M age = 24.25, SD = 6.56, n endotoxin = 32 (18 females), n placebo =31 (16 females)) who completed the support figure task. The ethnic breakdown of this sample was as follows: 39.7% Caucasian, 33.3%, Asian/Pacific Islander, 17.5% Latino, 6.3% Other, and 3.2% African American. All procedures were run in accordance with UCLA’s Institutional Review Board.
Pre-session ratings
Prior to the experimental session, eligible participants were emailed and asked to send the experimenters two digital photographs of a support figure. Specifically they were instructed to send pictures of someone they could go to for help or for comfort (for example, a family member, a close friend, or a significant other). Additionally, participants rated whether they could “really count on this person to help them feel better when they are feeling generally down-in-the-dumps” and how much they can “rely on this person for help if they have a serious problem” on a 1–7 scale, with 1 corresponding to “not at all” and 7 corresponding to “a lot”. Overall ratings on these two measures were high (M = 6.46, SD = .84 for “really count on this person” and M = 6.52, SD = .95 for “rely on this person”), indicating that they were in fact support figures. No differences in these ratings were found between those in the endotoxin condition and those in the placebo condition (p’s > .55).
Behavioral assessments
Motivation to approach support figure
Approximately two hours after injection, when the inflammatory response begins to peak (Eisenberger et al., 2009, 2010), participants reported on their desire to be around their support figure by answering whether they felt “like being around this person right now” on a 1–7 scale, anchored by “not at all” and “a lot.” One outlier was removed from the final analyses (from the endotoxin condition, evaluated as greater than 3 SD’s below the mean of the entire sample) and one participant failed to complete this item (from the placebo condition). Thus, the motivation to approach a support figure is based on a sample of 61 participants (n endotoxin = 31; n placebo = 30).
fMRI paradigm
To assess ventral striatum activity to a support figure, participants viewed images of their support figure as well as a sex, race, age, and expression matched (because most participants provided images in which their support figures were smiling) stranger interspersed with blocks of serial subtraction as a distraction task to decrease any carryover effects from viewing the support figure. This design was modified from other neuroimaging studies assessing neural activity to close relationship partners (Aron et al., 2005; Acevedo et al., 2011). A total of sixteen 12-second blocks separated by a 1-second fixation crosshair were presented with 4 blocks each for the support figure and the stranger and 8 blocks of serial subtraction (e.g., count back by 7’s from 1753). All images were standardized to the same black and white standard resolution.
fMRI Data Acquisition
Imaging data were acquired on a Siemens 3 Tesla “Tim Trio” MRI scanner housed at UCLA’s Staglin IMHRO Center for Cognitive Neuroscience. Foam padding was placed around the participants’ heads for comfort and to constrain head movement. A high-resolution T1-weighted echo-planar imaging volume (spin-echo, TR = 5000 ms; TE = 33 ms; matrix size 128 × 128; 36 axial slices; FOV = 20 cm; 3-mm thick, skip 1mm) and T2-weighted, matchedbandwidth anatomical scan (slice thickness = 3 mm, gap = 1 mm, 36 slices, TR = 5000 ms, TE = 34 ms, flip angle = 90°, matrix = 128×128, FOV = 20 cm) were acquired for each participant followed by a single functional scan, lasting 3 minutes, 42 seconds (echo planar T2* weighted gradient-echo, TR = 2000 ms, TE = 25ms, flip angle = 90°, matrix size 64 × 64, 36 axial slices, FOV = 20 cm; 3-mm thick, skip 1mm).
Plasma levels of cytokines
Whole blood samples were collected in pre-chilled EDTA tubes. After collection, the samples were centrifuged at 4°C, plasma was harvested into multiple aliquots, and then stored in a −70°C freezer until the completion of the study.
Using a Bio-Plex 200 (Luminex) Instrument, Bio-Plex software v4.1, and a 5-parameter logistic curve fit, plasma levels of IL-6 and TNF-α were quantified by means of high sensitivity bead-based multiplex immunoassays (Performance High Sensitivity Human Cytokine, R& D Systems, Minneapolis, MN). This R&D Systems multiplex assay has been shown to have excellent intra- and inter-assay reproducibility for these two analytes in a recent temporal stability study of circulating cytokine levels (Epstein et al., 2013), and very strong correlations (r ≥ .94) across a wide range of concentrations with high sensitivity ELISA kits from the same manufacturer (Breen, Perez, Olmstead, Eisenberger, & Irwin, 2014). All multiplex assays were performed on plasma samples diluted 2-fold according to the manufacturer’s protocol, and all calculated concentrations generated by the BioPlex Manager software were included in data analyses. Due to the strength of the parent study design (Eisenberger et al., 2009, 2010), which utilized repeated measures of cytokine values over seven time points for each subject, each time point was evaluated in a single determination. Every subject demonstrated the expected profile of change of cytokine concentrations over time, based on these previous studies (Eisenberger et al., 2009, 2010).
Paired samples from each subject (baseline and the post-scan time point) were assayed on the same 96-well plate; multiplex assays were chosen for the analyses because of the large dynamic range necessary to evaluate both low physiologic (baseline) and very high post-endotoxin (post-scan) cytokine concentrations in the same assay. The ranges of detection for IL-6 and TNF-α were 0.2–3800 pg/mL and 0.8–3100 pg/mL, respectively, and no samples exceeded the upper limit of detection for either analyte. The mean intra-assay CV% of the standards was < 8% for IL-6 and TNF-α; the inter-assay CV% of an internal laboratory quality control sample was ≤ 13% for both analytes.
Statistical Analyses
Behavioral Assessments
To evaluate between-group differences in the effect of endotoxin vs. placebo on cytokine levels (IL-6 and TNF-α) we ran repeated measures analyses of variance (ANOVA) in SPSS. Cytokine values for the 63 participants (out of the full sample of 115) run through the current task were log-transformed to evaluate changes from baseline to post-fMRI scan. Selection of the time point for these analyses is guided by: 1) the fact that the task used here always occurred at the end of the scanning session, which was closer in time to the cytokine assessment that was taken after the scan and 2) our prior work (Eisenberger et al., 2009, 2010) showing that IL-6 and TNF-α responses peak 2–3 hours post administration. The full temporal profile for these cytokines are reported separately (Moieni, Irwin, Jevtic, Olmstead, Breen, & Eisenberger, under review).
In order to assess the effect of an inflammatory challenge on the desire to be around the support figure, data were analyzed with a one-way ANOVA with condition (endotoxin vs. placebo) as the between-subjects factor.
In addition, to assess any potential sex differences in the current results, sex was included as an independent variable. However no significant main effects or interactions emerged when looking at the self-reported desire to be around the support figure, the neural data, or the cytokine data (p’s > .08).
fMRI Data
The preprocessing stream followed the DARTEL (Diffeomorphic Anatomical Registration Through Exponential Lie Algebra) procedure in SPM8 (Wellcome Department of Imaging Neuroscience, London) and involved realignment to correct for head motion, normalizing the T2-weight matched bandwidth to warp the images into Montreal Neurologic Institute (MNI) space (resampled at 3×3×3mm) and spatial smoothing using an 8mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. First-level effects were estimated using the general linear model to investigate neural activity to each of the image types (support figure and stranger) compared to blocks of serial subtraction. Random effects analyses of the group were then computed using the first-level contrast images for each participant.
ROI analyses
Due to the a-priori hypothesis about the effect of inflammation on VS activity to a support figure, analyses were constrained to an anatomical ROI focusing on the VS. The VS ROI was structurally defined by combining the left and right caudate and putamen from the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002) of the Wakeforest University Pickatlas (Maldjian, Laurienti, Burdette, & Kraft, 2003) and then constraining the regions at −10 <×< 10, 4 < y < 18, −12 < z < 0 based on coordinates showing increased VS activity to the anticipation of reward (Knutson, Fong, Bennett, Adams, & Hommer, 2003; Knutson, Rick, Wimmer, Prelec, & Loewenstein, 2007). ROI analyses were run in Marsbar (http://marbar.sourceforge.net) and thresholded at p < .05. Based on the hypothesis that ventral striatum activity would be greatest to the support figure in the endotoxin participants, greater mean activity in the VS was expected when comparing conditions (endotoxin vs. placebo) for the support figure vs. stranger contrast. Post-hoc analyses examined each target separately (support figure and stranger, each compared to the serial subtraction condition) in order to assess the direction of the effects. Two outliers (greater than 3 SD’s from the entire sample mean, one from the placebo condition, one from the endotoxin condition) were removed from the imaging data leaving a final imaging sample of 61 (n endotoxin = 31, n placebo = 30).
Correlations between VS ROI activity and changes in cytokines from baseline to post-scan (separately for IL-6 and TNF-α) were run for subjects in the endotoxin condition to assess the relationship between individual differences in the magnitude of the inflammatory response and neural activity to the support figure (vs. serial subtraction). In addition, we explored other correlations within the endotoxin subjects, including: 1) the correlation between VS activity and the desire to be around the support figure and 2) the correlation between changes in cytokines from baseline to post-scan and the desire to be around the support figure.
Results
Behavioral results
Replicating previous work using a similar paradigm (Eisenberger et al., 2009; 2010), endotoxin (vs. placebo) led to significantly greater increases in IL-6 (F(1, 61) = 210.66, p < .001). Specifically, there was a bigger increase in IL-6 from baseline (M = 3.09 pg/mL, SD = 4.73 (values reported here are raw values)) to post-scan (M = 144.34 pg/ml, SD = 142.30) for the endotoxin participants (t(31)= 17.21, p < .001) than for the placebo participants (baseline: M = 2.00 pg/ml, SD = 1.82, post-scan: M = 2.84, SD = 1.83; t(30)= 3.58, p < .01). Endotoxin (vs. placebo) also led to significantly greater increases in TNF-α (F(1, 62) = 509.19, p < .001). Participants in the endotoxin condition showed an increase in TNF-α from baseline (M = 8.22 pg/mL, SD = 8.31) to post-scan (M = 175.63, SD = 119.38, t(31) = 24.30, p < .001) whereas participants in the placebo condition did not (baseline: M = 6.65, SD = 1.60, post-scan: M = 7.12, SD = 2.11; t(30)=1.32, p = .20).
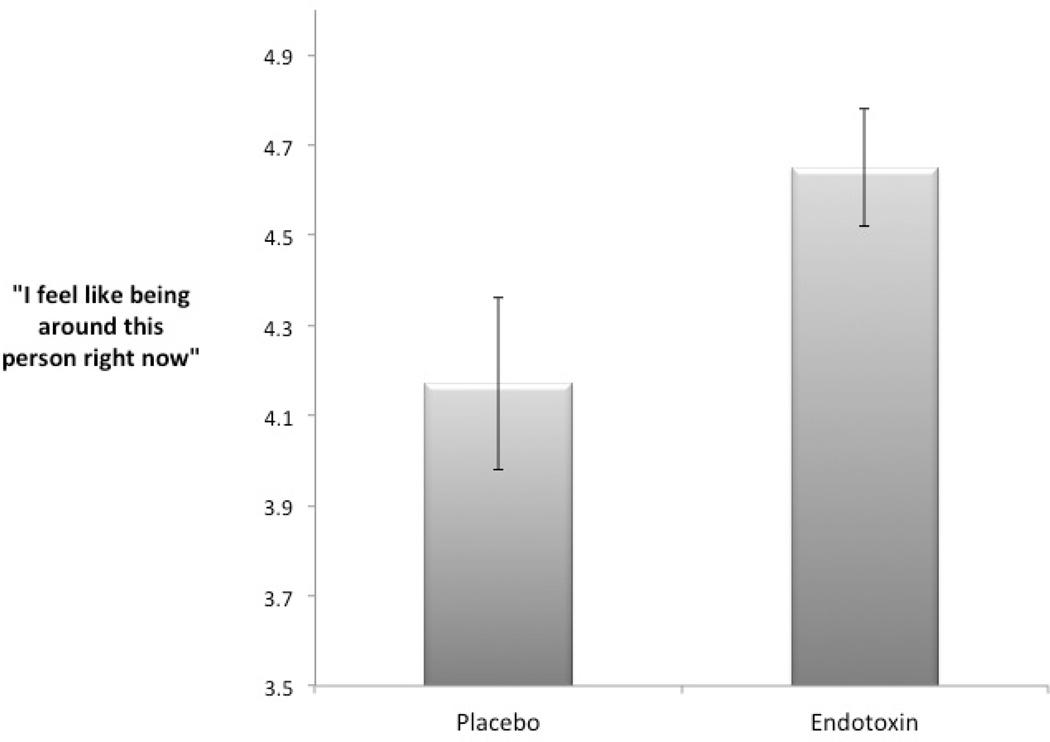
In line with the hypothesis that inflammation would increase the motivation to approach support figures, endotoxin led to a greater self-reported desire to be around the support figure (M = 4.65, SD = .71), compared to placebo (M = 4.17, SD = 1.05; F(1, 59) = 3.49, p = .04, see Fig. 1.).
Fig. 1.
Self-reported desire to be around the support figure. Endotoxin led participants to report a greater desire to be around the support figure compared to those who were administered placebo.
Effect of condition on VS activity to a support figure
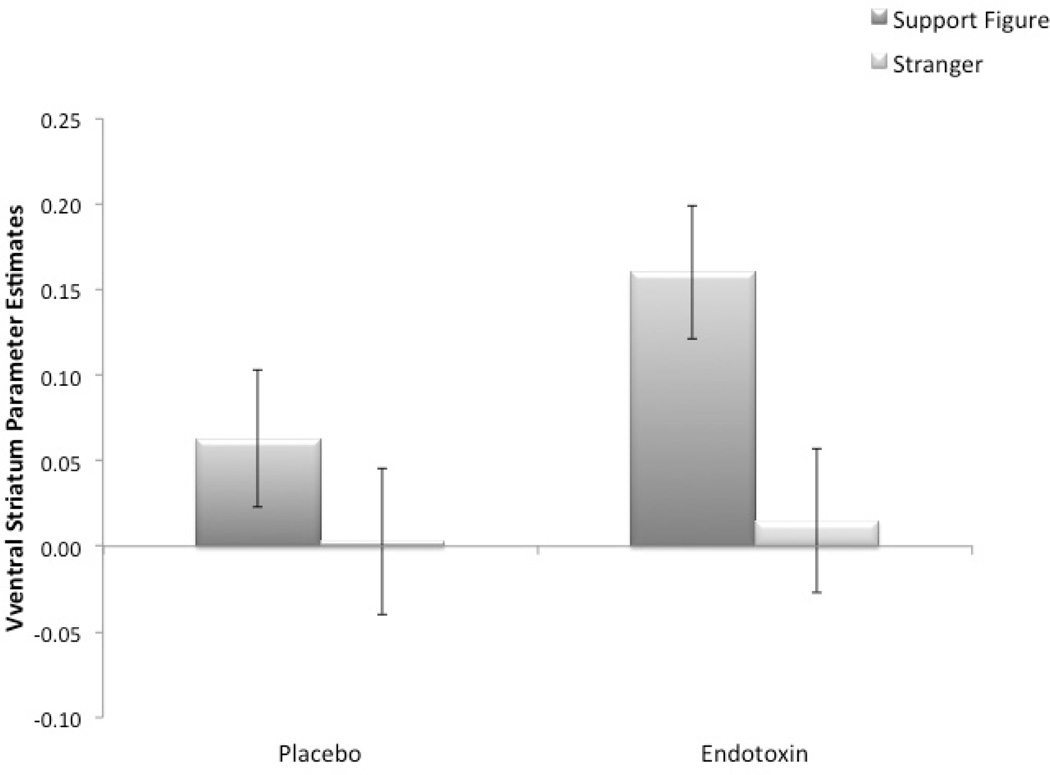
In order to assess whether endotoxin (vs. placebo) led to greater VS activity to a support figure compared to a stranger, we ran a two-sample t-test comparing neural activity during endotoxin vs. placebo for the contrast: support figure vs. stranger. This is equivalent to testing the interaction between condition (endotoxin vs. placebo) and target (support figure vs. stranger). As hypothesized, compared to placebo, endotoxin led to greater VS activity for the support figure vs. stranger contrast (t(59) = 1.31, p = .10). Even though the interaction was marginally significant, given the a priori hypothesis that VS activity to support figures would be greatest in the endotoxin participants, further analyses were conducted to assess which condition was driving the interaction. As expected, participants in the endotoxin, compared to the placebo, condition displayed heightened VS activity to viewing images of their support figure (vs. serial subtraction, t(59) = 1.66, p = .05, see Fig. 2). However, there was no effect of condition on VS activity to viewing images of strangers (vs. serial subtraction, t(59) = .16, p = .44). Similarly, breaking down the interaction by condition, for those in the endotoxin condition, there was significantly greater VS activity to viewing support figures compared to viewing strangers (F(1, 30) = 10.43, p = .003); however, for those in the placebo condition, there was no difference (F(1, 29) = 1.89, p = .18). That is, VS activity to viewing support figures (vs. strangers) was only heightened in the endotoxin-exposed participants.
Fig. 2.
Parameter estimates from ventral striatum (VS) region of interest (ROI) during support figures and strangers for endotoxin and placebo participants. Endotoxin (vs. placebo) led to increased VS activity to images of support figures (compared to serial subtraction). Error bars reflect standard errors.
Correlations between outcomes in the endotoxin condition
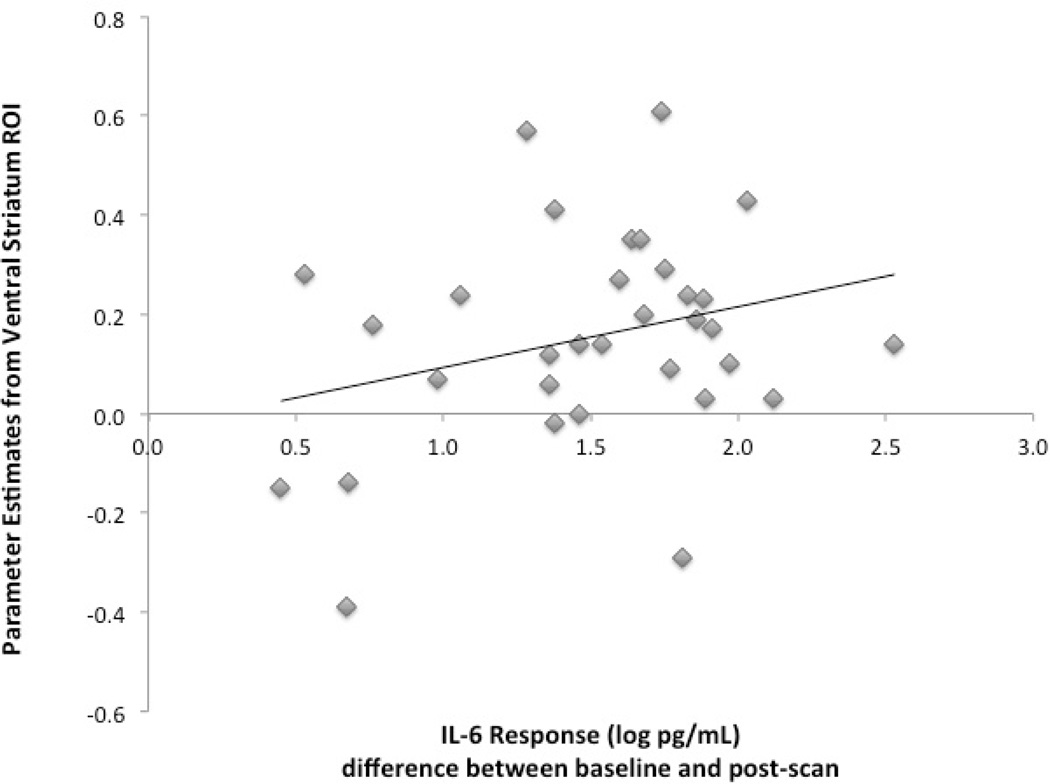
To assess whether the VS might be a mechanism of the motivation to approach a support figure during sickness, we examined correlations between cytokine changes, VS activity, and self-reported motivation to approach a support figure among participants in the endotoxin condition. There was a marginally significant positive correlation between IL-6 and VS activity (r = .28, p = .06, see Fig. 3). That is, increases in IL-6 from baseline to post-scan were associated with increased VS activity to viewing images of support figures (vs. serial subtraction). The association between VS activity and TNF-α followed the same pattern, but was not significant (r = .23, p = .11). However, there was no association between VS activity to viewing images of a support figure (vs. a stranger) and the desire to be around the support figure (r = −.01, p = .48), and there were no associations between changes in inflammatory activity and self-reported desire to be around the support figure (p’s > .08).
Fig. 3.
Relation between inflammatory response to the endotoxin as measured by log-transformed IL-6 increases from baseline to post-scan and ventral striatum (VS) activity when viewing support figures (vs. serial subtraction) in participants exposed to endotoxin. IL-6 responses to endotoxin were positively correlated with parameter estimates from the VS ROI.
Discussion
Although a prominent symptom of sickness behavior is social withdrawal, individuals may respond differently to social support figures -- approaching, rather than withdrawing, from them during times of sickness. In support of this hypothesis, endotoxin (vs. placebo) led to a greater reported desire to be around a support figure and greater ventral striatum activity to images of a support figure. Furthermore, increases in IL-6 responses (from baseline to post-scan) were associated with increased VS activity to the support figure in the participants exposed to endotoxin, suggesting that increases in inflammation may be driving the motivation to approach. This is the first study to show an increased motivation to approach support figures during sickness using an experimental inflammatory challenge paradigm in humans.
These results lend support to recent theorizing about changes in social behavior due to inflammation (Cole, 2006; Hennessy et al., 2014). While there is a substantial body of work showing that inflammation produces social withdrawal behavior (Eisenberger et al., 2009; 2010; Reichenberg et al., 2001), these data suggest that a more nuanced view of changes in social behavior is warranted. Thus, both withdrawal from distant others and approach toward supportive others may help support rest and recovery, the proposed function of changes in social behavior due to sickness (Dantzer, 2001). In other words, while withdrawing from most people may allow a sick person to conserve his/her energy, recovery may also be facilitated by approaching a support figure who can offer care while a person is in a more vulnerable state. Indeed, the longstanding literature on social support suggests that support is most helpful during times of need, such as during sickness (Cohen & Wills, 1985; House, Landis, & Umberson, 1988; Uchino, 2004). Furthermore, data from the present study are consistent with results from animal research, which has shown that during times of heightened inflammation, animals will spend more time huddling with familiar cage-mates (Yee & Prendergast, 2010) and maintain closer physical contact with companions (Willette et al., 2007). Moreover, it is largely consistent with animal research showing a direct relationship between greater levels of IL-6 and more time spent with close others (Willette et al., 2007). Taken together, these converging lines of animal and human work suggest that sickness may motivate people to approach close others while withdrawing from distant others.
The heightened ventral striatum response in endotoxin-exposed participants is interesting given previous work on the effect of inflammation on reward-related neural activity. In humans, experimental inflammatory challenges (vs. placebo) lead to reduced, rather than increased, VS to monetary reward (Capuron et al., 2012; Eisenberger et al., 2010). Similarly, healthy individuals who report higher anhedonic symptoms, a major symptom of inflammatory disorders such a depression (Miller, Maletic, & Raison, 2009), show reduced reward activity to a monetary reward task. Though not tested here, it could be the case that inflammation, in the acute setting, differentially alters reward processing depending on the target. That is, inflammation may heighten reward-related activity to rewards that are potentially helpful during sickness, such as social support figures, and dampen responding to those that are of less immediate utility, such as money. However, the mechanism that underlies this differential response is not yet known.
Relatedly, support figures in this study were constrained to only those who were perceived as highly close to and supportive of the participant (the average pre-session ratings of supportiveness were near ceiling with a mean of 6.49 on a 1–7 scale). However, it is possible that inflammation may have different effects on the desire to be around individuals with whom we are less close, but who may still be able to provide protection and care or supportive individuals who do not look approachable (e.g. support figures who look angry). Future work examining other potential targets of interaction during times of sickness may help fine-tune the current results to help us understand who, when, and under what circumstances VS activity signals approach toward others during sickness. Furthermore, future studies could more formally test whether a “supportive” picture of the support figure (i.e. smiling) vs. an “unsupportive” picture of a support figure is important for altering approach motivations or whether behavior is more affected by the reminder of a supportive figure in general.
A limitation of the current results is the lack of an association between the self-reported desire to be around the support figure and the neural and inflammatory measures among those participants exposed to endotoxin. These non-significant findings may be due to the fact that the self-report measure was collected nearly 2 hours before the neural (VS) and inflammatory measures. Indeed, the neural and inflammatory data, which were collected closer in time were more highly correlated (VS activity and IL-6). Future studies will be needed to more carefully interrogate these relationships.
To conclude, the findings presented here provide the first evidence that an experimental inflammatory challenge can increase the desire to approach a support figure, and that an acute episode of inflammation leads to heightened activity in a key reward-related region (i.e., the VS) in response to viewing pictures of a loved one. These data suggest that the effect of inflammation on the desire to approach or withdraw from others may depend on the nature of the relationship with these individuals.
Acknowledgements
The authors extend their thanks to the Staglin IMHRO Center for Cognitive Neuroscience, the staff and support of UCLA’s Clinical and Translational Science Institute (CTSI: UL1TR000124), and the participants in the study. In addition, this work was supported by R01-AG034588; R01-AG026364; R01 CA160245-01; R01-CA119159; R01 HL095799; R01 DA032922-01; P30-AG028748 to MRI, and the Cousins Center for Psychoneuroimmunology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Social Cognitive Affective Neuroscience. 2012;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MDS. The development of infant-mother attachment. In: Caldwell BM, Ricciuti HN, editors. Review of child development research. Vol. 3. Chicago, IL: University of Chicago Press; 1973. pp. 1–94. [Google Scholar]
- Aron A, Fisher H, Mashek D, Strong G, Li H, Brown L. Reward, motivation and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;93:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Aubert A. Sickness and behavior in animals: a motivational perspective. Neuroscience and Biobehavioral Reviews. 1999;23:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy attenuates behavioral effects of interleukin-1 injected peripherally, but not centrally. Neuroreport. 1996;7:1485–1488. doi: 10.1097/00001756-199606170-00008. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, et al. Lipopolysaccharide induces sickness behavior in rats by a vagal mediated mechanism. Comptes Rendus de L’Academie des Sciences. Serie III, Sciences de la vie. 1994;317:499–503. 18. [PubMed] [Google Scholar]
- Bowlby J. A secure base: Parent-child attachment and healthy human development. New York, NY: Basic Books; 1988. [Google Scholar]
- Breen EC, Perez C, Olmstead R, Eisenberger NI, Irwin MR. Comparison of Multiplex Immunoassays and ELISAs for the Determination of Circulating Levels of Inflammatory Cytokines. Brain, Behavior, and Immunity. 2014;40:e39. [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Cole SW. The complexity of dynamic host networks. In: Deisboeck TS, Kresh JY, editors. Complex Systems Science in BioMedicine. New York: Kluwer Academic; 2006. pp. 605–629. [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: Mechanisms and implications. Annals of the New York Academy of Sciences. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection. Brain Behavior & Immunity. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Birmann BM. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Review. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml PA. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain, Behavior, and Immunity. 2014;37:15–20. doi: 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Eisenberger NI. Shared neural mechanisms underlying “social warmth” and physical warmth. Psychological Science. 2013;24:2272–2280. doi: 10.1177/0956797613492773. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;15:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain, Behavior, and Immunity. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Boosting attachment security to promote mental health, prosocial values, and inter-group tolerance. Psychological Inquiry. 2007;18:139–156. [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Social and affective responses to inflammation: the role of sex differences. (under review). [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague RM. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and physical health: Understanding the health consequences of relationships. New Haven, CT: Yale University Press; 2004. [Google Scholar]
- Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain, Behavior, and Immunity. 2007;21:807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JR, Prendergast BJ. Sex-specific regulation of inflammatory responses and sickness behaviors. Brain, Behavior, and Immunity. 2010;24:942–951. doi: 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]