Abstract
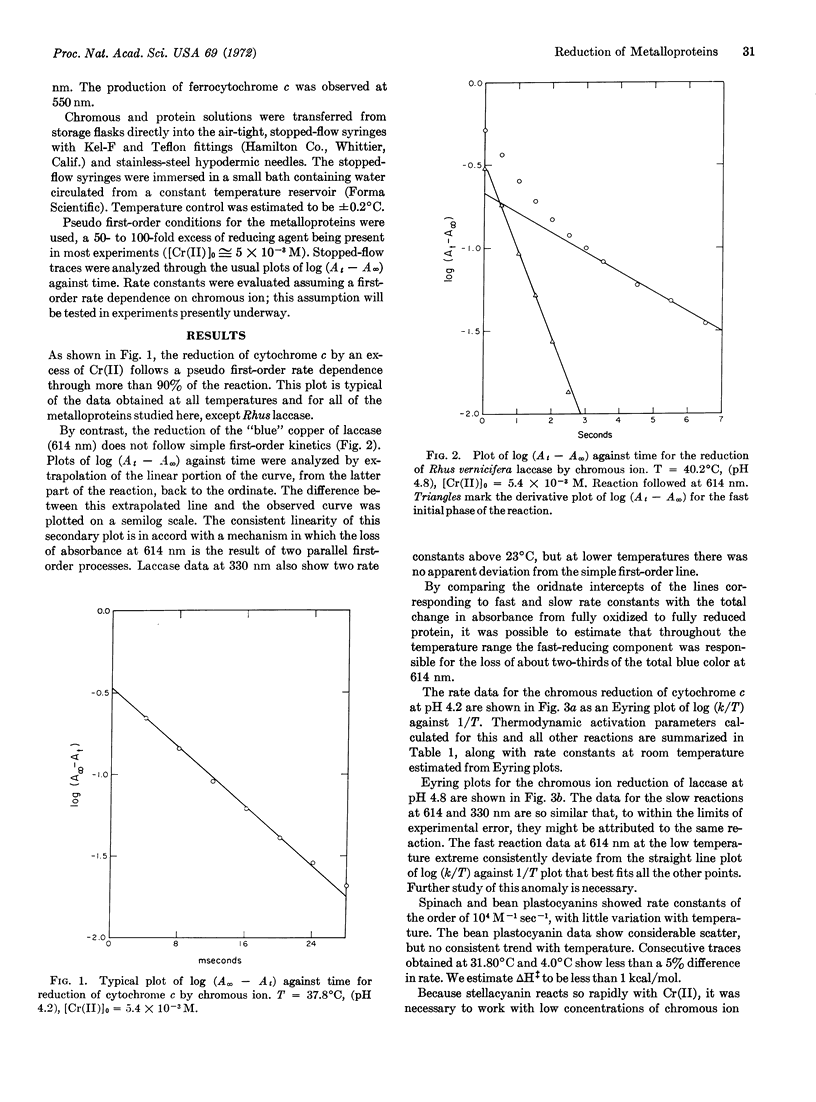
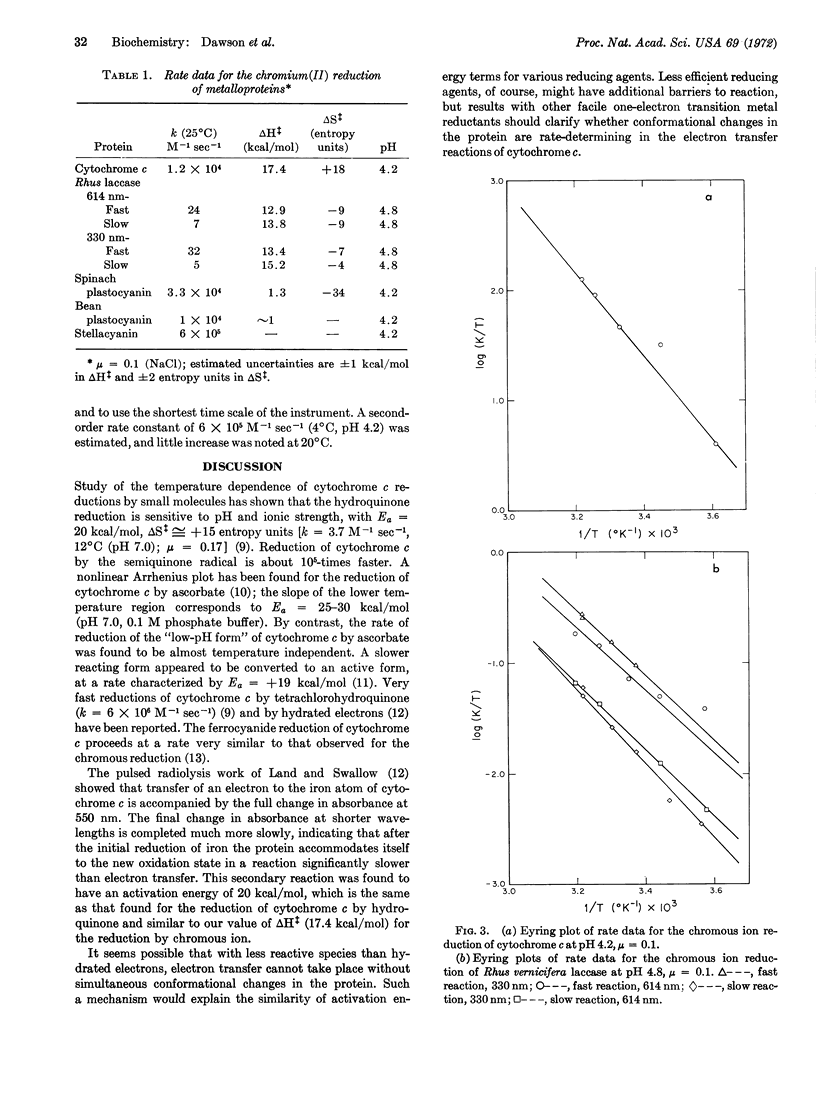
The reduction of Cu(330) in Rhus vernicifera laccase by chromous ion is 30% faster than reduction of Cu(614) at room temperature [pH 4.8, μ = 0.1 (NaCl)], and two parallel first-order paths, attributed to heterogeneity of the protein, are observed at both wavelengths. The reactions of stellacyanin, spinach and French-bean plastocyanins, and cytochrome c with chromous ion under similar conditions are faster than that with laccase by factors of 102 to 104, and are first order in protein concentration. Comparison of rates and activation parameters for the reduction of “blue” copper in laccase, stellacyanin, and the two plastocyanins indicates that reduction of the Cu(614) site in laccase may occur by intramolecular electron transfer from one of the Cu(330) sites. Our value of ΔH‡ (17.4 kcal/mol) for the chromous ion reduction of cytochrome c is consistent with a mechanism in which major conformational changes in the protein must accompany electron transfer.
Keywords: laccase, cytochrome c, plastocyanins, temperature, rate constants
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt K. G., Parks P. C., Czerlinski G. H., Hess G. P. On the elucidation of the pH dependence of the oxidation-reduction potential of cytochrome c at alkaline pH. J Biol Chem. 1966 Sep 25;241(18):4180–4185. [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Fee J. A., Malkin R., Malmström B. G., Vänngård T. Anaerobic oxidation-reduction titrations of fungal laccase. Evidence for several high potential electron-accepting sites. J Biol Chem. 1969 Aug 10;244(15):4200–4207. [PubMed] [Google Scholar]
- Greenwood C., Palmer G. Evidence for the existence of two functionally distinct forms cytochrome c manomer at alkaline pH. J Biol Chem. 1965 Sep;240(9):3660–3663. [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- Kowalsky A. A study of the mechanism of electron transfer in cytochrome c. Chromium as a probe. J Biol Chem. 1969 Dec 25;244(24):6619–6625. [PubMed] [Google Scholar]
- Land E. J., Swallow A. J. One-electron reactions in biochemical systems as studied by pulse radiolysis. V. Cytochrome c. Arch Biochem Biophys. 1971 Jul;145(1):365–372. doi: 10.1016/0003-9861(71)90049-x. [DOI] [PubMed] [Google Scholar]
- Makino N., Ogura Y. Oxidation-reduction titrations of copper ions in Rhus-laccase. Properties of two types of copper ions in the molecule. J Biochem. 1971 Jan;69(1):91–100. doi: 10.1093/oxfordjournals.jbchem.a129463. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. Two forms of copper (II) in fungal laccase. Biochim Biophys Acta. 1968 Feb 1;156(1):67–76. doi: 10.1016/0304-4165(68)90105-0. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G. R. The reduction of cytochrome c by hydroquinone. Can J Biochem Physiol. 1963 Jan;41:231–237. [PubMed] [Google Scholar]


