Abstract
Cognitive-affective mechanisms related to the maintenance of smoking among trauma-exposed individuals are largely unknown. Cross-sectional data from trauma-exposed treatment-seeking smokers (n = 283) were utilized to test a series of multiple mediator models of trauma exposure and smoking, as mediated by the sequential effects of negative affect and affect-modulation smoking motives. The sequential effects of both mediators indirectly predicted the effect of greater trauma exposure types on nicotine dependence, a biochemical index of smoking, perceived barriers to smoking cessation, and greater withdrawal-related problems during past quit attempts. Negative affect and affect-modulation motives for smoking may contribute to the trauma-smoking association.
Keywords: trauma, smoking, nicotine dependence, negative affect, negative reinforcement, PTSD
Exposure to traumatic events (with or without posttraumatic stress disorder [PTSD]) is a relatively common phenomenon1) associated with a host of negative physical and mental health outcomes, including –but not limited to -increased risk for cardiovascular diseases (e.g., heart failure, stroke), pulmonary diseases (e.g., bronchitis, asthma), higher body mass index/obesity, sleep problems, higher rates of depression, PTSD, and substance use2–4. Rates of cigarette smoking, in particular among trauma-exposed individuals, are nearly double the rates of non-psychiatric individuals 5, and these smokers tend to smoke more heavily and have higher levels of nicotine dependence than non-trauma-exposed smokers 6,7.
Prospective studies suggest that trauma exposure is associated with the onset and maintenance of cigarette smoking. For example, in a large, non-clinical nationally representative sample of adolescents, exposure to various types of traumatic events (e.g., interpersonal violence, physical assault, child sexual or physical assault) was predictive of increased likelihood of daily smoking after sixth grade8. Additionally, following a major disaster, an Australian-based study found 13.2% of the trauma-exposed individuals either initiated smoking or resumed or increased cigarette consumption within the four years after the event9; and increases in tobacco smoking were associated with experiencing a greater number of traumatic events, regardless of PTSD symptom severity9. Most of the available literature examining trauma-exposure and smoking has specifically examined the impact of PTSD. For example, trauma-exposed college students with PTSD, relative to those students with no trauma-exposure history, demonstrated linear increases in cigarette smoking during college years after controlling for general negative affect10. Trauma-exposed daily smokers with PTSD have also been found to differ from non-psychiatric smokers in terms of reporting greater motivation to quit smoking, although they report more failed prior quit attempts and more severe withdrawal quit problems while trying to quit11. Indeed, PTSD also appears to impair cessation success6. A recent study utilizing time sampling methodology found that smokers with PTSD had shorter time to lapse after a quit attempt relative to smokers without PTSD, and that those with PTSD were more likely to report negative affect as a situational factor (antecedent) to lapse12.
Theoretical models of smoking posit that cigarette smoking may be motivated by attempts to modulate general negative affect, which may be particularly elevated among subpopulations, such as those who have been trauma-exposed13,14. In fact, smokers with PTSD hold stronger beliefs about the negative affect reduction properties of smoking11,15, and among trauma-exposed smokers, such beliefs mediate the relation between posttraumatic stress symptom severity and cigarette smoking16. In line with this perspective, smokers with PTSD are also more likely than smokers without PTSD to smoke in response to stressful situations17, and smoking appears to temporarily relieve distress among smokers with PTSD18. Experimental work with trauma-exposed smokers with PTSD has documented that, compared to smokers who were able to remain abstinent more than one week, smokers who relapsed within one week tended to react with more negative affect and increased posttraumatic stress symptoms in response to stressful stimuli19.
Available work suggests negative affect is likely an important process in the re-initiation, maintenance, and relapse of smoking among trauma-exposed smokers with and without PTSD20. For instance, the number of trauma exposure types among daily smokers without PTSD has been found to be predictive of greater coping-oriented smoking motivation21. This work is bolstered by prospective research that found that, among trauma-exposed female veteran smokers seeking treatment for trauma-related disorders, increases in negative affect symptoms were associated with increases in smoking, and that decreases in negative affect were associated with longitudinal decreases in smoking22.
Extant work suggests at least two important avenues for further exploration. First, available data indicate that trauma exposure, even in the absence of psychiatric diagnosis (e.g., PTSD), is associated with significant smoking-related outcomes (e.g., initiation, smoking rate, cessation difficulty;9. Yet, there is relatively little empirical work addressing trauma-exposed smokers in general, or the mechanisms underlying these associations. Second, two inter-related explanatory processes are theorized to, at least in part, underlie such trauma-smoking associations -- state/trait levels of negative affect (i.e., non-specific general distress) and negative affect reduction smoking motives. Theoretically, smokers with trauma exposure histories may have higher levels of general negative affect and in turn, may result in greater reliance on smoking to manage negative affect states, which may account for higher smoking rates and greater quit difficulties. This process is likely sequential in that heightened negative affect precedes the coping-oriented smoking motives18, however this sequential meditational model has not yet been empirically evaluated.
The aim of the current study was to examine the associations between trauma exposure, as indexed by the number of different trauma types experienced21, in relation to various aspects of smoking, and as a function of negative affect and affect-regulatory smoking motives. Specifically, among a sample of trauma-exposed treatment-seeking smokers, the association between trauma exposure severity and criterion smoking measures was evaluated. Further, the mediational effects of general negative affect and negative affect-management motives on this association were evaluated. Criterion smoking measures employed included: nicotine dependence, expired carbon monoxide (CO), perceived barriers for quitting smoking, and number of problems quitting during past cessation attempts. It was hypothesized that, among trauma-exposed smokers, experiencing more trauma exposure types would impact smoking by (first) increased general negative affect and (second) increased smoking for affect-regulatory purposes (negative affect reduction). Thus, the indirect effects of the proposed mediators (negative affect and smoking motives; in this order) are theorized to sequentially impact smoking processes. It was not expected that either mediator alone would indirectly impact smoking processes.
Method
Participants and Procedures
Treatment-seeking adult daily smokers were recruited from the community to participate in a large randomized controlled trial examining the efficacy of two smoking cessation interventions (clinicaltrials.gov #NCT01753141). All participants were recruited from two different academic outpatient specialty (anxiety/health) treatment clinics (at the University of Vermont and Florida State University). Inclusion criteria for the parent study included daily cigarette use (average ≥ 8 cigarettes per day for at least 1 year), age between 18–65, and reported motivation to quit smoking of at least 5 on a 10-point scale. Exclusion criteria included inability to give informed consent, current use of smoking cessation treatment, past-month suicidality, and history of psychotic-spectrum disorders. Participants in the current study were selected from the larger parent study on the basis of having endorsed at least one DSM-IV-TR23 PTSD Criterion A1 trauma exposure per the study objectives.1 Baseline assessment data (prior to the intervention) were analyzed for purposes of this study.
The sub-sample of trauma-exposed smokers (n = 283 of 472 in the total baseline sample; Mage = 38.3, SD = 13.10; 47.3% female) self-identified race as White (86.9%), African-American (7.8%), Hispanic (2.5%), Asian (0.7%), and other (2.1%). Participants were generally well-educated (78.1% completed at least part of college) and were primarily never married (38.5%) or married/cohabitating (36.4%). Participants reported an average of 3.0 (SD = 1.78) lifetime traumatic events. The average age of initiating regular daily smoking was 14.7 years (SD = 3.63) and the smoking rate in the past week was 17.3 (SD = 10.45) cigarettes per day. Current (past year) Axis I disorders were prevalent among 46.3% of the sample and were as follows: social phobia (14.8%), specific phobia (9.5%), PTSD (7.4%), major depressive disorder (7.1%), generalized anxiety disorder (6.4%), alcohol use disorder (6.0%), cannabis use disorder (6.0%), panic disorder with/without agoraphobia (4.9%), dysthymia (4.9%), obsessive-compulsive disorder (3.2%), other substance use disorder (2.5%), anxiety disorder not otherwise specified (2.1%), other mood disorder (1.8%), and anorexia nervosa (0.4%). Please see Table 1 for descriptive details on the sample in terms of smoking and trauma–related variables.
Table 1.
Descriptive statistics of sample characteristics
| Descriptive Overview | Mean (SD) Or n (%) |
Females (n = 134) |
Males (n = 149) |
t value or x2 |
|---|---|---|---|---|
| Trauma History | ||||
| # trauma exposure types n (%) | 3.0 (1.78) | 3.0 (1.65) | 3.0 (1.89) | .102 |
| Trauma Types n (%) | ||||
| Serious accident, fire, explosion | 175 (61.8) | 75 (56.0) | 100 (67.1) | 3.713* |
| Natural disaster | 118 (41.7) | 46 (34.3) | 72 (48.3) | 5.683* |
| Non-sexual assault/known | 95 (33.6) | 48 (35.8) | 47 (31.5) | .579 |
| Non-sexual assault/stranger | 88 (31.1) | 27 (20.1) | 61 (40.9) | 14.233** |
| Sexual assault/known | 45 (15.9) | 34 (25.4) | 11 (7.4) | 17.076** |
| Sexual assault/stranger | 42 (14.8) | 35 (26.1) | 7 (4.7) | 25.616** |
| Military combat or war zone | 19 (6.7) | 1 (0.7) | 18 (12.1) | 14.471** |
| Sexual contact/child | 78 (27.6) | 49 (36.6) | 29 (19.5) | 10.338** |
| Imprisonment | 36 (12.7) | 11 (8.2) | 25 (16.8) | 4.667* |
| Torture | 16 (5.7) | 6 (4.5) | 10 (6.7) | .660 |
| Life threatening illness | 77 (27.2) | 37 (27.6) | 40 (26.8) | .021 |
| Other event | 59 (20.8) | 31 (23.1) | 28 (18.8) | .806 |
| PDS-Total | 8.0 (9.48) | 10.2 (10.38) | 6.0 (8.15) | −3.708** |
| Smoking History | ||||
| Age onset | 14.7 (3.63) | 15.6 (3.21) | 14.8 (3.98) | .491 |
| Years smoking | 20.1 (13.09) | 20.8 (12.71) | 19.5 (13.43) | −.819 |
| Cigarettes per day | 17.3 (10.45) | 16.1 (8.39) | 18.5 (11.90) | 1.932 |
| FTND-Total | 5.2 (2.36) | 5.0 (2.30) | 5.3 (2.42) | .822 |
| Expired CO at baseline | 20.3 (12.57) | 18.9 (12.85) | 21.5 (12.22) | 1.692 |
| Tobacco medical probs. n (%) | 93 (32.9) | 46 (34.3) | 47 (31.5) | 1.116 |
| Number prior quit attempts | 3.6 (2.40) | 3.6 (2.45) | 3.5 (2.37) | −.522 |
| Number prior withdrawal sx | 7.6 (3.87) | 7.7 (3.72) | 7.5 (4.02) | −.305 |
| BCS-Total | 23.8 (11.10) | 26.8 (11.22) | 21.2 (10.32) | −4.386** |
| Other Psychiatric Variables | ||||
| Any current Axis I Dx n (%) | 131 (46.3) | 71 (53.0) | 60 (40.3) | 4.589* |
| Number of Axis I diagnoses | 0.8 (1.17) | 1.0 (1.31) | 0.6 (1.00) | 2.444* |
| Cannabis use/30 days n (%) | 151 (53.4) | 68 (50.7) | 83 (55.7) | .697 |
| Global Assess. Functioning | 74.1 (10.85) | 72.3 (11.39) | 75.8 (10.07) | −2.703** |
| Mediator Variables | ||||
| PANAS-NA | 18.7 (7.12) | 20.2 (7.73) | 17.4 (6.24) | −3.370** |
| RFS-NA | 3.4 (0.82) | 3.7 (0.78) | 3.2 (0.79) | −5.392** |
Note:
p < .05;
p < .01;
Trauma types from the PDS: (a) serious accident, fire, explosion, (b) natural disaster, (c) non-sexual assault by a family member, (d) non-sexual assault by a stranger, (e) sexual assault by a family member or someone you know, (f) sexual assault by a stranger, (g) military combat or war zone, (h) sexual contact when you younger than 18 with someone who was 5 or more years older than you, (i) imprisonment, (j) torture, (k) life-threatening illness, (l) other traumatic event.
After providing written informed consent, participants were administered a clinical diagnostic assessment of Axis I psychopathology and completed a biochemical verification of smoking status (via expired carbon monoxide breath sample) as well as a computerized self-report assessment battery. All participants provided informed consent and the study protocol was approved by the Institutional Review Boards at both universities.
Measures
Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/ Non-Patient Version;24)
Axis I psychopathology was assessed with the SCID-I/NP. Interviews were administered by doctoral level graduate students or highly trained post-baccalaureate clinical research assistants with diagnostic assessment experience; interviewers were supervised by independent doctoral-level psychologists. All SCID assessments were checked by two independent doctoral-level raters for ensure diagnostic accuracy (no discrepancies were noted; 100% reliability). These data were used in the current study for descriptive purposes and to document rates of PTSD among the sample. A summary variable (total number of current, past year, Axis I disorders) was calculated and entered as a covariate in the analyses. Additionally, the clinician-rated global assessment of functioning score (GAF; range 0–100 with higher scores indicating better functioning) was examined for descriptive purposes.
Posttraumatic Diagnostic Scale (PDS;25)
The PDS is a four-part, 49-item self-report assessment that corresponds to the DSM-IV-TR symptoms of PTSD. Respondents indicate if they have experienced any of 12 traumatic event types, including an “other” category and indicate which event was most disturbing. The PDS also assesses the severity of 17 PTSD symptoms, as experienced in the past month, rated on a frequency scale, ranging from 0 (“not at all” or “only one time”) to 3 (“five or more times a week/almost always”), based on the most disturbing traumatic event endorsed. The 17 symptom ratings are summed to create a total severity score (range 0 – 51). A total sum score was calculated based on the number of traumatic exposure event types (observed range 1–9). The PDS has strong psychometric properties, including excellent internal consistency, good test-retest reliability, and convergent validity25. Internal consistency in the current study was excellent (α = .93).
Medical History Form
A medical history checklist was used to assess medical-based exclusionary criteria for the current study. A composite variable was computed for the present study as an index of tobacco-related medical problems. Items in which participants indicated having ever been diagnosed (heart problems, hypertension, respiratory disease and asthma; all coded 0 = no, 1 = yes) were summed and a total score was created (observed range from 0 – 3), with higher scores reflecting the occurrence of multiple markers of tobacco-related disease.
Marijuana Smoking History Questionnaire (MSHQ;26)
The MSHQ is a 40-item measure that assesses cannabis use history and patterns of use. One item was used in the current study to determine status of marijuana use in the past 30 days: “Please rate your marijuana use in the past 30 days” (Responses range from 0 = No use, 4 = Once a week, to 8 = More than once a day). This item was dichotomously coded to reflect a marijuana use status variable (0 = No use; 1 = Past 30-day use).
Positive and Negative Affect Scale (PANAS;27)
The PANAS is a self-report measure that requires participants to rate the extent to which they experience each of 20 different feelings and emotions (e.g., nervous, interested) based on a Likert-scale that ranges from 1 (“Very slightly or not at all”) to 5 (“Extremely”). The measure yields two factors, negative and positive affect, and has strong documented psychometric properties27. The negative affect scale was used in the current study as the first mediator variable. Internal consistency was good (Cronbach’s α = .91).
Reasons for Smoking (RFS;28)
The RFS is a 23-item self-report measure that assesses motivations for smoking. Participants are asked to rate their tendency to smoke in each of the circumstances listed, rated on a 5-point Likert scale (1 = never to 5 = always). The psychometric properties of this scale, including measures of factor structure, internal consistency, and test-retest reliability, are well established. In the present study, the negative affect reduction subscale (RFS-NA; e.g., ‘‘When I feel uncomfortable or upset about something, I light up a cigarette”) was used as the second mediator variable. Internal consistency for the RFS-NA subscale was good (Cronbach’s α = .89).
Fagerström Test for Nicotine Dependence (FTND;29)
The FTND is a 6-item scale that assesses gradations in tobacco dependence. Scores range from 0–10, with higher scores reflecting high levels of physiological dependence on nicotine. The FTND has adequate internal consistency, positive relations with key smoking variables (e.g., saliva cotinine, and high test-retest reliability29,30). Internal consistency was found to be acceptable in the current sample (Cronbach’s α = .65).
Carbon Monoxide
Biochemical verification of smoking status was completed by Carbon Monoxide (CO) analysis of breath samples. Expired air CO levels were assessed using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc.). Baseline CO breath samples were used as a criterion variable in the current study, as a complementary index of smoking behavior.2
Barriers to Cessation Scale (BCS;31)
The BCS is a self-report assessment of perceived barriers associated with quitting smoking. Specifically, the BCS is a 19-item measure on which respondents indicate, on a 4-point Likert scale (0 = Not a barrier or not applicable to 3 = Large barrier), the degree to which they identify with each listed barriers (e.g., “Weight gain,” “Friends encouraging you to smoke,” “Fear of failing to quit”). Scores are summed and a total score is derived. The BCS has strong psychometric properties, including content and predictive validity, internal consistency, and reliability31; α = .89 in the present sample.
Smoking History Questionnaire (SHQ;32)
The SHQ is a self-report questionnaire used to assess smoking history, pattern, and retrospective quit history. In the present study, the SHQ was employed to describe the sample on smoking history and patterns of use and then to create a sum composite score of withdrawal-related problem symptoms experienced during past quit attempts. A total of 16 possible problems and one other category are listed, in which respondents indicate whether or not each problem was experienced during prior quit attempts and the severity of each symptom. Symptoms included: weight gain, increased eating, digestive problems, nausea, headaches, drowsiness, depression/low mood, fatigue, insomnia, difficulty concentrating, heart pounding/sweating, decrease heart rate, irritability, restlessness, anxiety, cravings, or other symptoms. All items endorsed were summed to create a variable of number of withdrawal-related symptoms experienced during prior quit attempts (observed range 0–17).
Data Analytic Strategy
Preliminary descriptive analyses were conducted to examine the bivariate associations between number of trauma exposure types and all study variables. Primary analyses included four sequential mediator path models to examine the impact of negative affect reduction smoking motives (RFS-NA; M2) through general negative affect (PANAS-NA; M1) as mediators of the relations between number of trauma exposure types (number of traumatic event exposure types) and the criterion variables (nicotine dependence, expired CO, perceived barriers to smoking cessation, and number of prior quit problems). The statistical strategy utilized 33,34 allows for estimation and significance testing of the indirect effect, through bootstrapping. Bootstrapping generates an empirical representation of the sampling distribution of the indirect effect, from which a confidence interval can be generated33. The current analytic approach allows for examination of two mediators, in a causal or sequential fashion, while simultaneously testing the indirect effects of each mediator independently35. That is, the model estimates each specific indirect effect (path a1*b1 and path a2*b2); however, in order to test for sequential mediation effects, the indirect effect of path a1*a3*b2 was also estimated and tested for statistical significance.
Gender, tobacco-related medical problems, past 30-day cannabis use status (per MSHQ), and number of Axis I disorders were included as covariates in all models given the theoretical impact of these variables on trauma-exposure and smoking processes (e.g.,21,36). Analyses were conducted using PROCESS, a conditional process modeling program that utilizes an ordinary least squares-based path analytical framework to test for both direct and indirect effects35. The 95-percentile confidence intervals (CI) for R2 indices were obtained analytically; the CIs for the specific and conditional indirect effects were estimated with bootstrap analyses (10,000 resamples; as recommended,33,34,37).
Results
Bivariate correlations
A greater number of trauma types was significantly and positively associated with negative affect (r = .206, p < .0001) and nicotine dependence (r = .161, p = .007). Negative affect (PANAS-NA) and negative affect reduction smoking motives (RFS-NA) were significantly inter-related (r = .383, p < .001). Negative affect was also associated with greater number of Axis I diagnoses (r = .387, p < .0001), barriers to smoking cessation (r = .374, p < .0001), and number of quit problems (r = .141, p = .018). Negative affect reduction smoking motives were also associated with greater number of Axis I diagnoses (r = .185, p = .002), nicotine dependence (r = .326, p < .0001), barriers to smoking cessation (r = .546, p < .0001), and number of quit problems (r = .230, p < .0001). Nicotine dependence was associated with expired CO (r = .376, p < .0001), perceived barriers to smoking cessation (r = .167, p = .005) and number of prior quit problems (r = .134, p = .025). Perceived (BCS-Total) and actual quit problems (number of prior quit problems) were significantly correlated (r = .333, p < .0001); expired CO was not significantly associated with perceived barriers or prior quit problems.
Path Mediation Analyses
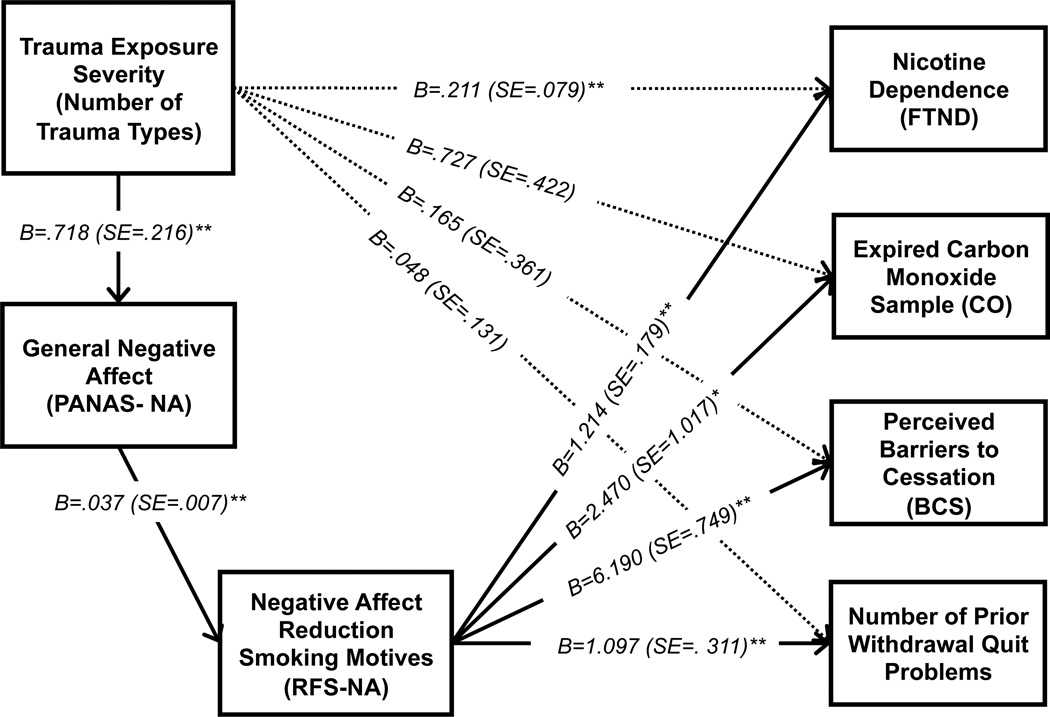
Four path analyses were conducted in order to test the impact of trauma exposure on each criterion variable through negative affect (M1) and negative affect reduction smoking motives (M2). Please see Figure 1 for a visual presentation of results. For these analyses, a Bonferroni corrected alpha (.05/4 planned analyses = .0125) was used to control the family-wise Type I error rate. The number of trauma exposure types directly predicted significant variance in negative affect (b = .718, t = 3.315, p = .001) and negative affect predicted significant variance in affect-regulatory smoking motives (b = .037, t = 5.425, p < .0001). The specific total effect of number of trauma exposure types was only significant for nicotine dependence (b = .211, t = 2.659, p = .008), but not the other criterion variables. Next, the significance of the full model including both mediators were examined for each criterion variable, in addition to the conditional direct and indirect effects of the mediators on the outcome variables.
Figure 1.
Path model for the effect of trauma exposure severity on various smoking processes, through the effects of negative affect and affect-regulatory smoking motives.
Notes: *p<.05, **p<.01; The model for each criterion variable was tested separately. B=Unstandardized regression coefficients; SE=standard error. Covariates in the model are not presented for ease of viewing. Notes: The total effects (‘path c’) of trauma exposure severity on outcomes are presented on the dashed lines. Direct effects of predictor and mediators variables are presented on solid lines. The conditional indirect effects (product of direct effects) were tested for sequential mediation, and are presented in text. All conditional indirect effects were significant.
With regard to trauma exposure types predicting nicotine dependence, the full model including both proposed mediators was significant [R2 = .169, F(7, 275) = 8.006, p < .0001), with male gender (b = −.695, t = −2.519, p = .012) being a significant covariate. The conditional direct effect of smoking motives on nicotine dependence was significant (b = 1.214, t = 6.796, p < .0001). Regarding the test of the indirect (mediational) effects, number of trauma types was predictive of higher nicotine dependence indirectly through the sequential effect of greater negative affect and negative affect reduction smoking motives (b =.033; SE = .015, CI95% =.010, .070), but not indirectly through either specific mediator alone.
Next, the number of trauma exposure types was tested in terms of expired CO. The full model of number of trauma exposure types on expired CO with both mediators was non-significant [R2 = .048, F(7, 275) = 1.989, p = .057), but male gender (b = −3.306, t = −2.104, p = .036) was a significant covariate and the conditional direct effect of smoking motives on expired CO was significant (b = 2.470, t = 2.428, p = .0156); although considered non-significant at the more conservative corrected alpha level. The indirect effects were estimated: having a greater number of trauma exposure types was predictive of higher expired CO levels, which indirectly occurred through the sequential effect of negative affect and negative affect reduction smoking motives (b = .066, SE = .041, CI95% = .013, .191), but not indirectly through either specific mediator alone.
The full model with trauma exposure types predicting perceived barriers to cessation including the mediators was significant [R2 = .339, F(7,275) = 20.153, p < .0001]. Here, there was a significant conditional direct effect of motives on barriers to cessation (b = 6.190, t = 8.267, p < .0001). The indirect effects of greater number of trauma exposure types was predictive of greater perceived barriers to smoking cessation indirectly through the sequential effect of negative affect and negative affect reduction smoking motives (b = .166, SE = .076, CI95 = .050, .362). In addition, the indirect effect of negative affect alone was also significant (b = .206, SE = .096, CI95% = .063, .456).
Last, the full model of trauma exposure with the mediators predicted significant variance in number of prior quit problems [R2 = .067, F(7, 275) = 2.833, p = .007], with the conditional direct effect of smoking motives significantly predicting this outcome (b = 1.097, t =3.533, p = .0005). The test of the conditional indirect effects revealed that having exposure to a higher number of trauma event types predicted a greater number of withdrawal-related quit problems in the past, which occurred indirectly through greater negative affect and negative affect reduction smoking motives (b = .029, SE = .016, CI95 =.008, .075), but not indirectly through either specific mediator alone.
Specificity Analyses
To strengthen the interpretation of the mediational models, four alternative models were tested by reversing the two proposed mediators to test if the opposite sequential indirect effects were significant. Tests of the indirect effects were estimated based on 10,000 bootstrapped re-samples. Results of the alternative path models were non-significant when negative affect reduction smoking motives (RFS-NA) was entered as M1 and negative affect (PANAS-NA) was entered as M2 for nicotine dependence (b = −.005, CI95% = −.017, .002), expired CO (b = −.017, CI95% = −.093, .007), perceived barriers to smoking cessation (b = .026, CI95% = −.008, .089), and number of withdrawal-related prior quit problems (b = .004, CI95% = −.002, .022).
Discussion
The current study examined the direct and indirect effects of trauma exposure and various aspects of smoking as a function of the combined sequential effects of negative affect and affect-regulatory smoking motives. After controlling for gender, tobacco-related health problems, past-month cannabis use, and number of psychological disorders, a greater number of types of trauma exposure was only directly related to greater levels of nicotine dependence. This pattern of findings suggests that trauma history may not relate in a direct manner with all smoking processes, as has been theorized in integrative models of smoking-PTSD12.
As hypothesized, the sequential indirect effects of negative affect and affect-regulatory smoking motives were consistently found to explain the effect of trauma exposure on all four smoking criterion variables. These data suggest that for trauma-exposed daily cigarette smokers who have experienced more types of trauma exposure, those with higher levels of negative affect are more vulnerable to coping-oriented smoking motivation, which may, in part, account for greater reliance on smoking. Specifically, smoking reliance was indexed by higher levels of nicotine dependence, expired CO, greater perceived barriers for quitting, and more withdrawal symptoms while quitting in the past. These findings are broadly in line with theoretical models of smoking, trauma, and PTSD co-occurrence12.
Additionally striking, although not the primary focus of the current study was the prevalence of past-month cannabis use among this sample of trauma-exposed smokers (52.3%). This finding is perhaps not surprising given literature documenting the association between trauma-exposure, PTSD, and cannabis use36, and emotional factors related to co-use of cigarettes and cannabis38. Moreover, it is worth noting that male gender emerged as a significant covarying factor in models of smoking rate (expired CO breath sample) and nicotine dependence; however, no other theoretically-relevant factors appeared to predict significant variance in the statistical models beyond the primary predictor and mediator variables.
Clinically, the present findings suggest that it may be helpful to address negative mood states and affect regulation motives among trauma-exposed smokers seeking smoking cessation treatment. Specifically, it appears important to assess trauma history, the functional relationship between trauma symptoms and smoking (i.e., identifying antecedent, response, and outcome), in terms of reliance (nicotine dependence) and quit processes (believing smoking will be hard, experiencing intense withdrawal during prior quit attempts). In particular, it appears that cognitive restructuring to address maladaptive beliefs for smoking is warranted, in addition to skill-building to enhance alternative emotion coping skills. It is also likely important to utilize pharmacological intervention (e.g., nicotine replacement therapy, varenicline) in order to address the greater physiological dependence on nicotine and tendency to experience withdrawal. For example, among trauma-exposed smokers, integrating mood management skills training via psychoeducation, cognitive restructuring, and behavioral activation with smoking cessation treatment may facilitate greater success in reducing smoking behavior and cessation success, relative to standard smoking cessation treatment. Notably, there has been some progress in this domain, with integrated smoking-PTSD protocols being associated with greater cessation success39,40.
There are limitations associated with the current study. First, the data were cross-sectional, which precludes the ability to establish causal relations between the tested variables. We attempted to examine temporal associations between both mediators by sequentially modeling the effect of negative affect as occurring prior to smoking motives; however, prospective investigations should be conducted to further strengthen these findings. Second, specific data were unavailable on the onset of initial trauma exposure relative to smoking. Thus, it is unknown whether smoking temporally preceded initial trauma exposure. Third, the trauma exposure measure utilized in this study provided data on the type of lifetime trauma exposures, but did not assess the frequency of each of the exposures. It may be useful in future studies to also assess the quantity as well as type of lifetime trauma exposure as it relates to smoking. Fourth, there was insufficient data to conduct analyses on smokers with PTSD. The posttraumatic stress symptoms in this sample were in the mild range (see Table 1), suggesting that even among individuals with sub-threshold PTSD symptoms, negative affect and regulatory affective smoking motives are significantly related to smoking. To further gauge the clinical significance of the current findings for smokers with PTSD, it would be important for future work to replicate this model with smokers demonstrating higher levels of posttraumatic stress symptoms expression and/or PTSD. Finally, our sample consisted of community-recruited, treatment-seeking, trauma-exposed daily cigarette smokers with a moderate smoking rate. Future studies may benefit by sampling from lighter and heavier smoking populations as well as clinical populations to ensure the generalizability of the results to the general smoking population.
Overall, the current findings further explicate the association between trauma exposure and smoking, and identify two specific mechanisms that interplay to maintain smoking among trauma-exposed smokers. Future work is needed to explore the extent to which trauma-exposed smokers with symptoms of posttraumatic stress may benefit from targeted psychosocial strategies aimed at decreasing PTSD and negative affect with the goal of long term abstinence from smoking.
Acknowledgment
Ms. Farris is supported by pre-doctoral National Research Service Award (F31-DA035564). Dr. Beckham is supported by grant# 2K24DA016388, the Durham, NC Veterans Affairs Medical Center, and Veterans Affairs Clinical Sciences Research and Development. Dr. Vujanovic is supported, in part, by a National Institutes of Health/UT Health Science Center Clinical and Translational Sciences Award (KL2TR000370-07) and a National Institute on Drug Abuse Research Center of Excellence award (P50 DA09262) granted to the Center for Neurobehavioral Research on Addictions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government, and the funding sources had no other role than financial support.
This work was supported by the National Institute of Mental Health grant (R01-MH076629-01A1) awarded to Drs. Michael J. Zvolensky and Norman B. Schmidt.
Footnotes
In the current study, only those participants indicating that they experienced, witnessed, or were confronted with a traumatic event that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others (i.e., met criteria for a DSM-IV-TR defined criterion A1 trauma) were included in the sample. This definition is also consistent with the DSM-541 definition of a criterion A trauma (i.e., not limited to experiencing the fear, horror, or helplessness in response to the event.) Of note, the sample included individuals with all levels of posttraumatic stress, including clinical and non-clinical (mild) symptomology. It is important to note that the 12.0% of the sample met criteria for current PTSD.
Inhalation of other tobacco products or cannabis can impact expired CO levels. Given the high rate of past-month cannabis in the current sample, a between-groups comparison of past month cannabis versus non-cannabis users in terms of expired CO was conducted – no significant group differences were noted [t(281) = .687, p = .493].
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
Contributor Information
Samantha G. Farris, University of Houston, Department of Psychology, Fred J. Heyne Building, Suite 104, Houston, TX 77204 USA. Tel: +1 (713) 743-8056; sgfarris@uh.edu.
Michael J. Zvolensky, University of Houston, Department of Psychology, Fred J. Heyne Building, Suite 104, Houston, TX 77204 USA and The University of Texas MD Anderson Cancer Center, Department of Behavioral Science, 1155 Pressler Street, Houston, TX 77030 USA. mjzvolen@central.uh.edu
Jean C. Beckham, Durham Veteran's Affairs Medical Center (116B) DVAMC, 508 Fulton Street, Durham, NC 27705. beckham@duke.edu
Anka A. Vujanovic, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, Center for Neurobehavioral Research on Addictions, 1941 East Road, Houston, TX 77054 USA. anka.a.vujanovic@uth.tmc.edu
Norman B. Schmidt, Florida State University, Department of Psychology, 1107 West Call Street, Tallahassee, FL 32306 USA. schmidt@psy.fsu.edu
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiat. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Boscarino JA. Posttraumatic Stress Disorder and Physical Illness: Results from Clinical and Epidemiologic Studies. In: Yehuda R, McEwen B, editors. Biobehavioral stress response: Protective and damaging effects. New York, NY US: New York Academy of Sciences; 2004. pp. 141–153. [DOI] [PubMed] [Google Scholar]
- 3.Schnurr PP, Green BL. Understanding relationships among trauma, posttraumatic stress disorder, and health outcomes. In: Schnurr PP, Green BL, editors. Trauma and health: Physical health consequences of exposure to extreme stress. Washington, DC US: American Psychological Association; 2004. pp. 247–275. [Google Scholar]
- 4.Spitzer C, Barnow S, Völzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: Findings from the general population. Psychosom Med. 2009;71(9):1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- 5.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA-J Am Med Assoc. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 6.Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. J Cogn Psychot. 2008;22(4):346–365. [Google Scholar]
- 7.Hapke U, Schumann A, Rumpf H-J, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis. 2005;193(12):843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- 8.Roberts ME, Fuemmeler BF, McClernon FJ, Beckham JC. Association between trauma exposure and smoking in a population-based sample of young adults. J Adolescent Health. 2008;42(3):266–274. doi: 10.1016/j.jadohealth.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parslow RA, Jorm AF. Tobacco use after experiencing a major natural disaster: Analysis of a longitudinal study of 2063 young adults. Addiction. 2006;101(7):1044–1050. doi: 10.1111/j.1360-0443.2006.01481.x. [DOI] [PubMed] [Google Scholar]
- 10.Read JP, Wardell JD, Vermont LN, Colder CR, Ouimette P, White J. Transition and change: Prospective effects of posttraumatic stress on smoking trajectories in the first year of college. Health Psychol. 2013;32(7):757–767. doi: 10.1037/a0029085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, Bernstein A. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. J Anxiety Disord. 2008;22(7):1214–1226. doi: 10.1016/j.janxdis.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine Tob Res. 2013;15(6):1122–1129. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 14.Weaver TL, Etzel JC. Smoking patterns, symptoms of PTSD and depression: Preliminary findings from a sample of severely battered women. Addict Behav. 2003;28(9):1665–1679. doi: 10.1016/j.addbeh.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Calhoun PS, Levin HF, Dedert EA, Johnson Y, Beckham JC. The relationship between posttraumatic stress disorder and smoking outcome expectancies among U.S. military veterans who served since September 11, 2001. J Trauma Stress. 2011;24(3):303–308. doi: 10.1002/jts.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruska B, Bernier J, Kenner F, et al. Examining the relationships between posttraumatic stress disorder symptoms, positive smoking outcome expectancies, and cigarette smoking in people with substance use disorders: A multiple mediator model. Addict Behav. 2013 doi: 10.1016/j.addbeh.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Beckham JC, Feldman ME, Vrana SR, et al. Immediate Antecedents of Cigarette Smoking in Smokers With and Without Posttraumatic Stress Disorder: A Preliminary Study. Exp Clin Psychopharm. 2005;13(3):219–228. doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- 18.Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addict Behav. 2007;32(12):2900–2915. doi: 10.1016/j.addbeh.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calhoun PS, Dennis MF, Beckham JC. Emotional reactivity to trauma stimuli and duration of past smoking cessation attempts in smokers with posttraumatic stress disorder. Exp Clin Psychopharm. 2007;15(3):256–263. doi: 10.1037/1064-1297.15.3.256. [DOI] [PubMed] [Google Scholar]
- 20.Cook JW, McFall MM, Calhoun PS, Beckham JC. Posttraumatic stress disorder and smoking relapse: A theoretical model. J Trauma Stress. 2007;20(6):989–998. doi: 10.1002/jts.20275. [DOI] [PubMed] [Google Scholar]
- 21.Feldner MT, Babson KA, Zvolensky MJ, et al. Posttraumatic stress symptoms and smoking to reduce negative affect: An investigation of trauma-exposed daily smokers. Addict Behav. 2007;32(2):214–227. doi: 10.1016/j.addbeh.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Helstrom AW, Bell ME, Pineles SL. Feeling better and smoking less: The relationship between trauma symptoms and smoking over time. Cognitive Ther Res. 2009;33(2):235–240. [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and statistical manual of mental disorders – (4th ed. text revision) Arlington, VA: American Psychiatric Publishing; 2000. [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCIDI/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- 25.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psych Assess. 1997;9(4):445–451. [Google Scholar]
- 26.Bonn-Miller MO, Zvolensky MJ. An evaluation of the nature of marijuana use and its motives among young adult active users. Am J Addiction. 2009;18(5):409–416. doi: 10.3109/10550490903077705. [DOI] [PubMed] [Google Scholar]
- 27.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 28.Ikard FF, Green DE, Horn D. A scale to differentiate between types of smoking as related to the management of affect. Int J Addict. 1969;4:649–659. [Google Scholar]
- 29.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Brit J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 30.Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 31.Macnee CL, Talsma A. Development and testing of the barriers to cessation scale. Nurs Res. 1995;44(4):214–219. [PubMed] [Google Scholar]
- 32.Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111(1):180–185. [PubMed] [Google Scholar]
- 33.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. [Google Scholar]
- 34.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Ins C. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY US: Guilford Press; 2013. [Google Scholar]
- 36.Bonn-Miller MO, Vujanovic AA, Feldner MT, Bernstein A, Zvolensky MJ. Posttraumatic stress symptom severity predicts marijuana use coping motives among traumatic event-exposed marijuana users. J Trauma Stress. 2007;20(4):577–586. doi: 10.1002/jts.20243. [DOI] [PubMed] [Google Scholar]
- 37.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 38.Bonn-Miller MO, Zvolensky MJ, Johnson KA. Uni-morbid and co-occurring marijuana and tobacco use: Examination of concurrent associations with negative mood states. J Addict Dis. 2010;29(1):68–77. doi: 10.1080/10550880903435996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldner MT, Smith RC, Monson CM, Zvolensky MJ. Initial evaluation of an integrated treatment for comorbid PTSD and smoking using a nonconcurrent, multiple-baseline design. Behav Ther. 2013;44(3):514–528. doi: 10.1016/j.beth.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 40.McFall M, Saxon AJ, Thompson CE, et al. Improving the Rates of Quitting Smoking for Veterans With Posttraumatic Stress Disorder. Am J Psychiat. 2005;162(7):1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]