Abstract
Background
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide. The rise in metabolic syndrome has contributed to this trend. Adipokines such as adiponectin are associated with prognosis in several cancers, but have not been well studied in HCC.
Methods
We prospectively enrolled 140 patients with newly diagnosed or recurrent HCC with Child-Pugh A or B cirrhosis. We examined associations between serum adipokines, clinico-pathological features of HCC, and time to death. We also examined a subset of tumors with available pathology for tissue adiponectin receptor (AR) expression by immunohistochemistry.
Results
Median age of subjects was 62 years; 79% were men, 59% had underlying hepatitis C, and 36% were diabetic. Adiponectin remained a significant predictor of time to death (HR: 1.90; 95% CI: 1.05–3.45, p=0.03) in a multivariable adjusted model that included age, alcohol history, CP class, stage and serum AFP level. Cytoplasmic AR expression (AR-1 and 2) in tumors trended higher in those with higher serum adiponectin levels and in those with diabetes mellitus, but the association was not statistically significant.
Conclusions
In this hypothesis-generating study, we found serum adiponectin level to be an independent predictor of overall survival in a diverse cohort of HCC patients.
Keywords: cancer biology, Hepatocellular carcinoma
Introduction
The incidence of hepatocellular carcinoma (HCC) has tripled in the United States over the last 30 years. This is thought to be at least partly due to metabolic syndrome, which has the highest population-attributable risk for HCC in the United States [1–3]. Patients with features of metabolic syndrome, including obesity, insulin resistance, and diabetes, also have worsened outcomes from several kinds of cancer, including HCC [4, 5].
We and others have found that features of the metabolic syndrome may contribute to more aggressive tumor phenotypes, both in animal models and in patients. For instance, both diet and genetic obesity have been shown to promote HCC development in mouse models [6]. Prior work by our group also suggests an association between increased body mass index (BMI) and vascular invasion in tumors in patients with HCC [7, 8].
There has been growing interest in the relationship between adipokines, such as adiponectin, and cancer. Adiponectin is a hormone that is usually inversely correlated with body mass index and percent body fat. High levels typically predict lower incidences of several types of solid tumors [9, 10], and better prognosis [11].
In this prospective study of patients with HCC, we examined serum adiponectin, leptin, HOMA-IR (homeostasis model assessment-estimated insulin resistance) levels, and clinico-pathologic features of HCC, to evaluate preliminary relationships between adipokines and HCC outcome.
Expression levels of the two adiponectin receptor forms (adiponectin R1 and R2) have also been associated with both physiological and pathological states including obesity and insulin resistance [12]. Reduced AR expression has been implicated in the pathogenesis of NASH and development of cirrhosis [13, 14], so we also examined a subset of subjects for AR expression in tumor and surrounding tissue.
Methods
Study Population
From 2008–2012, 140 adult patients 18 years and older with newly-diagnosed or recurrent HCC at Columbia University Medical Center were recruited for this prospective study and followed until December 2012. Subjects had histologically proven HCC or met AASLD criteria for HCC diagnosis [15]. We excluded patients who had any previous malignancy within the last five years, those that had undergone liver transplant, and those that had received any systemic cancer therapy. Those with Child Pugh C disease and/or clinically significant ascites were also excluded. The protocol was approved by the Columbia University Institutional Review Board, and all subjects signed written informed consent.
Procedures
Subjects completed an epidemiologic questionnaire and underwent a physical exam including weight, height, waist and hip measurements. Fasting morning blood samples were collected at the time of enrollment. Laboratory analyses included a complete blood count, basic metabolic/hepatic panels, hemoglobinA1c, lipid panel, hepatitis screen, AFP, ferritin, transferrin, ceruloplasmin, alpha-1 antitrypsin phenotype, antinuclear antibody, anti-smooth-muscle antibody, and anti-mitochondrial antibody to define the underlying etiology of HCC.
An additional 30 mL fasting blood sample was collected at the same blood draw for adipokine evaluation. Whole blood, plasma, serum and buffy coat were divided into aliquots and frozen at (−) 70 degrees, then run in batches at the Irving Institute’s Biomarker Core at Columbia University. Specimens were de-identified with a unique study number and barcode, and results were forwarded to the study coordinator for entry into a secure database. Laboratory personnel were blinded to study hypotheses and outcomes.
Measures
Our main exposures of interest were serum levels of adipokines. We used a radioimmunoassay to measure baseline levels of leptin (RIA Linko, St Charles, MO, quantification limit 0.5 ng/mL, inter-assay precision 4.6%, intra-assay precision 5%), adiponectin (RIA Millipore, Billerica, MA, quantification limit 1 ng/mL, inter-assay precision 6.9%, intra-assay precision 6.2%) and insulin (RIA Siemens, Deerfield Il, quantification limit 2 ulU/mL, inter-assay precision 5.3%, intra-assay precision 4.3%). HOMA-IR was used as a measure of insulin resistance and was calculated as fasting insulin (mU/L) × plasma glucose (mmol/L)/22.5 [16]. All samples were run in duplicate, and the median value was used. Our primary outcome of interest was time to death from diagnosis. Mortality data were obtained from medical records and confirmed with the National Death Index.
The following covariates were used in the analysis: age (≥ or < median), gender, race/ethnicity, etiology of liver disease (HBV, HCV, alcohol, DM), BMI (Kg/m2), Child-Pugh class, metastasis (yes/no), BCLC stage, Milan criteria (within/outside), and serum AFP levels (≥ or < median). Those who self-reported alcohol use of more than two standard drinks per day (28 oz) consistently for over a year at any point in their lives were classified as having alcohol-related disease. Diabetes was defined as having a fasting glucose greater or equal to 126 mg/dL, or being on drug therapy for diabetes. NASH was not formally assessed because of difficulties assessing it pathologically in the setting of cirrhosis, and because not all subjects had available pathology for review. Since waist to hip ratio (WHR) has often been shown to better estimate mortality risks due to general and abdominal obesity, we conducted separate analyses assessing WHR (≥ or < median) [17]. Variations in circulating levels of serum adipokines have been shown to play a role in liver fibrogenic processes [18, 19], therefore in an exploratory analysis in a subset of patients (54%) for whom pathology data were available, we also assessed the association between Batts-Ludwig stage of liver fibrosis (I–IV) and serum adipokine levels [20].
Immunohistochemistry
Tissue sections from 17 patients were evaluated for adiponectin R1 and R2 expression by immunohistochemistry. Four groups were selected randomly from cases with available formalin-fixed paraffin-embedded tissue based on low and high levels of serum adiponectin (≥ or < median) and degree of fibrosis assessed by Batts-Ludwig stage. After antigen retrieval in ULTRA Cell Conditioning Solution (Ventana Medical Systems, Tucson, Ariz), slides from all cases were stained on an autostainer (Ventana Benchmark Ultra; Ventana Medical Systems) for AR1 (dilution 1:100, rabbit monoclonal antibody; Abcam, Cambridge, MA) and AR2 (374–386, rabbit polyclonal antibody; dilution 1:100; Phoenix Pharmaceuticals, Burlingame, CA). Slides were examined by a liver pathologist blinded to clinical and laboratory values, who scored the expression levels of adiponectin R1 and R2 in both tumor and surrounding tissue as either positive or negative based on presence or absence of staining.
Statistical Analysis
Descriptive statistics including mean levels and ranges were used to summarize baseline adipokine levels. Associations between serum adiponectin, leptin, HOMA-IR (all as categorical variables using median values as cut-offs) and covariates including etiology and clinico-pathologic variables (all as categorical variables) were analyzed using chi-squared tests.
Our preliminary data showed a 3-fold increased risk of death for those with higher than median adiponectin levels (>12,000 ng/mL) in univariate and multivariate models (adjusted for stage, AFP, age, and Child-Pugh class). Our pilot data suggested a median survival of 290 days for the high adiponectin group, and we conservatively assumed a median survival of 600 days for the lower adiponectin group. We would then have had 83% power to identify a survival difference of about 300 days at p=0.05, with 40 patients in each group using Kaplan-Meier analyses. With a sample size of 140 patients, we planned to adjust for additional covariates, including BMI, etiology of liver disease, age, race/ethnicity, stage, and tumor features.
Kaplan-Meier plots and Cox proportional hazard models were used to determine the association between variables of interest and time to death. We defined cut-offs for low and high levels for our main exposure variables (adiponectin, leptin and HOMA-IR) using quartiles of baseline serum levels. Variables that were clinically relevant and showed statistical significance in univariable analysis were included in the multivariable Cox proportional hazards models. Subjects contributed person-time for survival analyses from the date of enrollment to the date of death/date of censoring. Subjects were censored if they were lost to follow-up or at the end of study period (December 31, 2012). There were no significant differences in baseline adipokine levels for the 18 patients that were lost to follow-up. Further, excluding these participants did not lead to any significant change in the hazard ratios in unadjusted and multivariable adjusted Cox proportional hazard models.
In additional analyses, we examined the relationship between insulin resistance (using HOMA-IR) and adipokine level (as continuous variables) using Pearson correlation coefficients. Since the ratio of adiponectin to leptin has been used as an index to evaluate insulin resistance [21], we assessed adiponectin-leptin ratio as an independent predictor of survival in patients with HCC. Finally, to further evaluate the predictive value of these markers, we tested for interaction and calculated separate hazard ratios stratified by baseline subject and tumor characteristics using medians as cut-offs to define high and low levels of adiponectin, leptin and HOMA-IR. All tests of statistical significance were two sided, and analyses were done using SAS, Version 9.3 (SAS Institute Inc., Cary, NC).
Results
The median age of study participants was 62 years. 79% were men, 49% were non-Hispanic white, 59% had hepatitis C, and 36% were diabetic. 64% were Child-Pugh class A and 44% were within the Milan criteria at enrollment. Mean (median, and range ) baseline serum levels were 14.1 (7.8, 1.6–89.3) ng/mL for leptin, 16540.7 (13,050, 850.0–82,400.0) ng/mL for adiponectin and 5.5 (3.2, 0.35–67.60) for HOMA-IR, respectively. Median follow-up was 8 months and median survival time was 18 months. During the follow-up period, 76% of patients received loco-regional therapy, 19% underwent resection, 15% received sorafenib, and 20% underwent a liver transplant.
Subject and tumor characteristics stratified by low (< median) and high (≥ median) levels of serum leptin, adiponectin and HOMA-IR are summarized in Table 1. High adiponectin levels (≥13,050 ng/mL) were associated with female gender (p=0.003), being hepatitis C positive (p=0.002), and being Child-Pugh class B (p<0.001). High leptin (p=0.003) and HOMA-IR (p=0.03) levels were significantly associated with a higher degree of liver fibrosis. Interestingly, adiponectin level was not significantly associated with liver fibrosis (p=0.29).
Table 1.
Subject and Tumor Characteristics According to Low (<Median) and High (≥Median) Levels of Serum Leptin, Adiponectin and HOMA-IR.
| Missing n (%) | Leptin (ng/mL) (n=140) | Adiponectin (ng/mL) (n=140) | HOMA-IR (n=139) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | <7.9 | ≥7.9 | P-value | <13,050 | ≥13,050 | P-value | <3.2 | ≥3.2 | P-value | |
| Age | 0 (0%) | 0.85 | 0.23 | 0.44 | ||||||
| ≥ 62.0 | 35 (50%) | 36 (51%) | 32 (46%) | 39 (56%) | 32 (46%) | 37 (53%) | ||||
| < 62.0 | 35 (50%) | 34 (49%) | 38 (54%) | 31 (44%) | 37 (54%) | 33 (47%) | ||||
| Gender | 0 (0%) | 0.01 | 0.003 | 0.27 | ||||||
| Male | 61 (87%) | 49 (70%) | 62 (89%) | 48 (69%) | 52 (75%) | 58 (83%) | ||||
| Female | 9 (13%) | 21 (30%) | 8 (11%) | 22 (31%) | 17 (25%) | 12 (17%) | ||||
| Hepatitis B virus | 0 (0%) | 0.24 | 0.02 | 0.45 | ||||||
| Positive | 13 (19%) | 8 (11%) | 16 (23%) | 5 (7%) | 12 (17%) | 9 (13%) | ||||
| Negative | 57 (81%) | 62 (89%) | 54 (77%0 | 65 (93%) | 57 (83%) | 61 (87%) | ||||
| Hepatitis C virus | 0 (0%) | 0.17 | 0.002 | 0.33 | ||||||
| Positive | 45 (64%) | 37 (53%) | 32 (46%) | 50 (71%) | 43 (62%) | 38 (54%) | ||||
| Negative | 25 (36%) | 33 (47%) | 38 (54%) | 20 (29%) | 26 (38%) | 32 (46%) | ||||
| Diabetes mellitus | 0 (0%) | 0.01 | 0.29 | <0.001 | ||||||
| Positive | 18 (26%) | 32 (46%) | 28 (40%) | 22 (31%) | 13 (19%) | 37 (53%) | ||||
| Negative | 52 (74%) | 38 (54%) | 42 (60%) | 48 (69%) | 56 (81%) | 33 (47%) | ||||
| Alcohol history | 0 (0%) | 1.00 | 1.00 | 0.74 | ||||||
| Positive | 19 (27%) | 19 (27%) | 19 (27%) | 19 (27%) | 18 (26%) | 20 (29%) | ||||
| Negative | 51 (73%) | 51(73%) | 51 (73%) | 51(73%) | 51 (74%) | 50 (71%) | ||||
| BMI at enrollment (Kg/m2) | 0 (0%) | <0.001 | 0.80 | 0.005 | ||||||
| < 25 | 33 (47%) | 8 (11%) | 19 (27%) | 22 (31%) | 27 (39%) | 14 (20%) | ||||
| 25–30 | 28 (40%) | 29 (41%) | 28 (40%) | 29 (41%) | 29 (42%) | 27 (39%) | ||||
| ≥ 30 | 9 (12%) | 33 (47%) | 23 (33%) | 19 (27%) | 13 (19%) | 29 (41%) | ||||
| Waist to hip ratio | 30 (21%) | 0.12 | 0.25 | 0.06 | ||||||
| ≥ 0.95 | 22 (42%) | 33 (57%) | 31 (55%) | 24 (44%) | 22 (41%) | 33 (59%) | ||||
| < 0.95 | 30 (58%) | 25 (43%) | 25 (45%) | 30 (56%) | 32 (59%) | 23 (41%) | ||||
| Child-Pugh class | 0.25 | <0.001 | 0.35 | |||||||
| A | 3 (2%) | 47 (68%) | 40 (59%) | 58 (83%) | 29 (43%) | 44 (64%) | 42 (60%) | |||
| B | 22 (32%) | 28 (41%) | 12 (17%) | 38 (57%) | 25 (36%) | 26 (37%) | ||||
| Metastasis | 0 (0%) | 0.11 | 0.28 | 0.39 | ||||||
| Positive | 11 (16%) | 5 (7%) | 6 (9%) | 10 (14%) | 9 (13%) | 6 (9%) | ||||
| Negative | 59 (84%) | 65 (93%) | 64 (91%) | 60 (86%) | 60 (87%) | 64 (91%) | ||||
| Within Milan criteria | 0 (0%) | 0.06 | 0.61 | 0.81 | ||||||
| Positive | 25 (36%) | 36 (51%) | 29 (41%) | 32 (46%) | 31 (44%) | 30 (43%) | ||||
| Negative | 45 (64%) | 34 (49%) | 41 (59%) | 38 (54%) | 38 (55%) | 40 (57%) | ||||
| BCLC Stage | 0 (0%) | 0.17 | 0.73 | 0.33 | ||||||
| 0, A | 24 (34%) | 32 (46%) | 27 (39%) | 29 (41%) | 25 (36%) | 31 (44%) | ||||
| B, C | 46 (66%) | 38 (54%) | 43 (61%) | 41 (59%) | 44 (64%) | 39 (56%) | ||||
| AFP | 0 (0%) | 0.31 | 0.73 | 0.44 | ||||||
| ≥ 47.9 ng/mL | 38 (54%) | 32 (46%) | 36 (51%) | 34 (49%) | 37 (54%) | 33 (47%) | ||||
| < 47.9 ng/mL | 32 (46%) | 38 (54%) | 34 (49%) | 36 (51%) | 32 (46%) | 37 (53%) | ||||
| Batts-Ludwig Stage | 65 (46%) | 0.003 | 0.29 | 0.03 | ||||||
| I–III | 17 (53%) | 9 (21%) | 16 (40%) | 10 (29%) | 17 (47%) | 9 (23%) | ||||
| IV | 15 (47%) | 34 (79%) | 24 (60%) | 25 (71%) | 19 (53%) | 30 (77%) | ||||
Abbreviations. BMI: Body mass index; BCLC: Barcelona Clinic Liver Cancer; AFP: Alpha-Fetoprotein; HOMA-IR: Homeostasis model of assessment - insulin resistance; HCC: Hepatocellular Carcinoma.
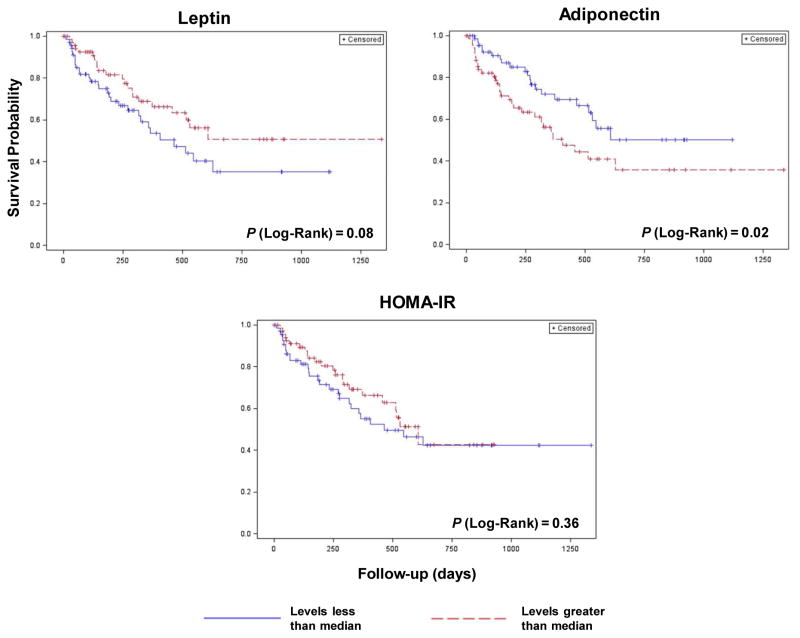
In univariable analysis, a serum adiponectin level greater than median were significantly associated with survival (HR: 1.83; 95% CI: 1.05–3.19, p=0.03). Leptin (HR: 0.62; 95% CI: 0.35–1.08, p=0.09) and HOMA-IR (HR: 0.77; 95% CI: 0.44–1.34, p=0.36) were not (Table 2 and Figure 1). Adiponectin remained a significant predictor of time to death (HR: 1.90; 95% CI: 1.05–3.45, p=0.03) in a multivariable adjusted model that included age, alcohol history, CP class, Milan criteria and serum AFP level (Table 3). However, the hazard ratio for adiponectin was attenuated when we replaced Milan with BCLC stage (HR: 1.34; 95% CI: 0.73–2.45, p=0.35) or included surgical treatment (HR: 1.71; 95% CI: 0.93–3.17, p=0.09) in the above model. Surprisingly, history of alcohol intake was a significant predictor of better overall survival in both unadjusted and multivariable-adjusted model. This may be because history of alcohol intake was associated with better prognostic variables like lower stage at baseline.
Table 2.
Univariable Cox Proportional Hazards Model for Time to Death
| Factor | Reference | HR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Age (years) | ≥ 62.0 | < 62.0 | 0.90 | 0.52–1.56 | 0.71 |
| Gender | Male | Female | 0.98 | 0.51–1.88 | 0.97 |
| Race/ethnicity | Non-Hispanic Black | Non-Hispanic White | 0.71 | 0.27–1.86 | 0.49 |
| Hispanic | Non-Hispanic White | 0.95 | 0.48–1.89 | 0.89 | |
| Hepatitis B virus | Positive | Negative | 0.62 | 0.26–1.45 | 0.27 |
| Hepatitis C virus | Positive | Negative | 1.10 | 0.62–1.95 | 0.73 |
| BMI at enrollment (Kg/m2) | ≥ 25 | < 25 | 0.69 | 0.39–1.24 | 0.22 |
| ≥ 30 | < 30 | 0.70 | 0.36–1.34 | 0.28 | |
| Waist to hip ratio | ≥ 0.95 | < 0.95 | 1.24 | 0.65–2.37 | 0.50 |
| Diabetes Mellitus | Positive | Negative | 1.47 | 0.85–2.55 | 0.17 |
| Alcohol history | Positive | Negative | 0.41 | 0.18–0.91 | 0.03 |
| Child-Pugh class | B | A | 1.89 | 1.08–3.32 | 0.03 |
| Metastasis | Positive | Negative | 2.87 | 1.53–5.40 | 0.001 |
| Within Milan criteria | Negative | Positive | 6.62 | 2.97–14.71 | <0.001 |
| BCLC stage | B,C | 0,A | 2.91 | 1.52–5.55 | 0.001 |
| AFP (ng/mL) | ≥ 47.9 | < 47.9 | 2.26 | 1.27–4.02 | 0.005 |
| Surgery | Yes | No | 0.10 | 0.04–0.28 | <0.001 |
| Leptin (ng/mL) | ≥ 5.2 | < 5.2 | 0.71 | 0.40–1.27 | 0.25 |
| ≥ 7.9 | < 7.9 | 0.62 | 0.35–1.08 | 0.09 | |
| ≥ 19.9 | < 19.9 | 0.65 | 0.33–1.28 | 0.22 | |
| Adiponectin (ng/mL) | ≥ 8450 | < 8450 | 1.84 | 0.89–3.80 | 0.09 |
| ≥ 13050 | < 13050 | 1.83 | 1.05–3.19 | 0.03 | |
| ≥ 21075 | < 21075 | 1.72 | 0.97–3.05 | 0.06 | |
| HOMA-IR | ≥ 0.77 | < 0.77 | 0.86 | 0.45–1.59 | 0.63 |
| ≥ 3.20 | < 3.20 | 0.77 | 0.44–1.34 | 0.36 | |
| ≥ 6.41 | < 6.41 | 1.01 | 0.53–1.90 | 0.96 |
Abbreviations, HR: Hazard ratio; CI: Confidence interval; BMI: Body mass index; BCLC: Barcelona Clinic Liver Cancer; AFP: Alpha-fetoprotein; HOMA-IR: Homeostasis model of assessment - insulin resistance.
Figure 1.
Kaplan-Meier Plots for Time to Death
Table 3.
Multivariable Cox Proportional Hazards Model for Time to Death
| Factor | Reference | HR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Age (years) | ≥ 62.0 | < 62.0 | 1.16 | 0.66–2.03 | 0.62 |
| Alcohol history | Positive | Negative | 0.35 | 0.15–0.84 | 0.02 |
| Child-Pugh class | B | A | 2.18 | 1.28–3.71 | 0.004 |
| Within Milan criteria | Negative | Positive | 7.49 | 3.22–17.41 | <0.001 |
| AFP (ng/mL) | ≥ 47.9 | < 47.9 | 1.14 | 0.61–2.14 | 0.68 |
| Adiponectin (ng/mL) | ≥ 13050 | < 13050 | 1.90 | 1.05–3.45 | 0.03 |
Abbreviations, HR: Hazard ratio; CI: Confidence interval; AFP: Alpha-fetoprotein.
In additional analyses, the relationship between insulin resistance (using HOMA-IR) and adipokine level (as continuous variables) was examined. A significant positive correlation was found between leptin levels and HOMA-IR (Pearson correlation coefficient (r) =0.49; p<0.001), while correlations between HOMA-IR and adiponectin (r=−0.11; p=0.19) were not significant. Every standard deviation increase in adiponectin-leptin ratio was associated with a hazard ratio of 1.38 (p=0.002). This association remained significant (HR=1.38; p=0.01) after further adjusting for other predictors including age, alcohol history, Child-Pugh class, BCLC stage and serum AFP levels.
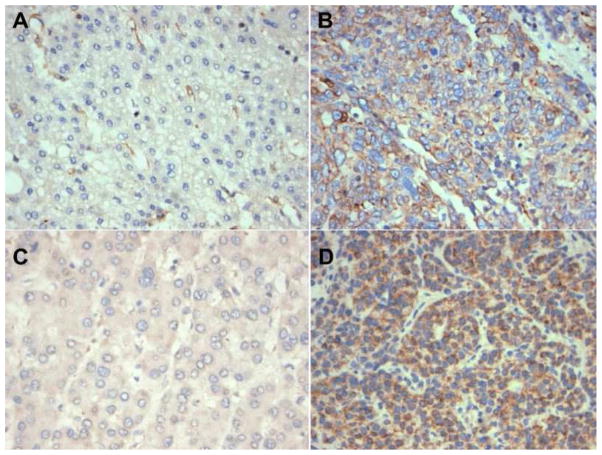
Expression levels of adiponectin R1 or R2 receptors were assessed for 17 tissue sections from subjects containing both tumor and non-neoplastic liver parenchyma (Figure 2). Tumors of patients with higher adiponectin levels were more likely to stain positive for both AR1 (p=0.08) and AR2 (p=0.15) receptors. Staining was predominantly cytoplasmic. AR1 and AR2 expression did not significantly correlate with degree of fibrosis or other clinical or pathological covariates. In general, no significant staining heterogeneity was noted in individual samples. There were also no significant differences in staining pattern or intensity of the background cirrhotic liver for AR1 or AR2 receptors.
Figure 2.
Adiponectin R1 and R2 expression in HCC by immunohistochemistry. Panels A and C show cases with negative adiponectin R1 and R2 reactivity. In comparison, panels B and D demonstrate strong staining for adiponectin R1 and R2, respectively.
Discussion
In this observational study of 140 HCC patients, we found that serum adiponectin level was independently associated with worsened overall survival even after adjusting for important clinical covariates. In a subset of patients with available pathology, adiponectin receptor staining in tumors trended with peripheral adiponectin levels, although these results were not statistically significant likely due to small sample sizes.
Adiponectin is a protein derived from adipocytes which regulates fat and glucose metabolism.[22] Levels are usually lower in those who are obese and diabetic, and are typically increased in those with hepatic fibrosis. [23] In most studies, adiponectin levels predict a better prognosis in cancer patients. [11, 24–29] In contrast, some studies have reported high adiponectin levels to be associated with poor prognosis or more aggressive pathology. For instance, in gastric cancer patients, increased adiponectin levels were associated with higher tumor grade (P=0.02) and more poorly differentiated tumors. [25] Similarly, in prostate cancer, serum adiponectin levels were found to be higher in locally advanced relative to organ-confined disease. [30]
In hepatocellular carcinoma, relationships between adiponectin levels and clinical features of disease are complex. Cell culture and animal models suggest that adiponectin inhibits leptin-induced proliferation of HCC via blockade of downstream pathways including STAT-3, AKT, and M-TOR. [31] Adiponectin also leads to suppression of liver tumor growth and metastasis in mice by inhibiting angiogenesis. [32] Similarly, in a study of human HCC, low adiponectin levels were associated with worsened histological grade of HCC. [33]
In other human studies, however, adiponectin may reflect increased risk of HCC.[34, 35], [36] Elevated adiponectin expression may also correlate with worsened outcome, as noted by a report from Taiwan which suggested that high cytoplasmic staining of HCC tumors with adiponectin was associated with recurrence and worsened survival, possibly via upregulation of AKT. This finding persisted after controlling for BMI and fasting blood sugar. However, this study did not assess peripheral levels of adiponectin. [37].
The median values seen for adipokines here seemed relatively comparable to some other populations in the literature, including those with chronic liver disease and cases with pancreatic cancer compared with controls, although our ranges did seem a bit broader. [9][38]We speculate that this may be due to the significant heterogeneity of our patient population, particularly with respect to tumor stage, nutritional status, and degree of underlying liver dysfunction.
Possible mechanisms that could explain our results include a possible angiogenic role and/or an anti-apoptotic effect of adiponectin, particularly in the setting of high levels of glucose [39–41]. In a study of 609 patients with type II diabetes, higher adiponectin levels were also associated with worsened overall survival. This and other studies showed a high correlation between total and HMW adiponectin, so we chose to present total adiponectin here [42].
Another possible explanation for our results is that adiponectin levels may be increased in cirrhosis, and with increasing stages of fibrosis.[43, 44] Cirrhosis is thought to lead to a state of adiponectin resistance, with downregulation of adiponectin receptors in liver tissue and decreased clearance of adiponectin. [45–48]. However, we did not find a statistically significant association between liver fibrosis and peripheral adiponectin levels. Although higher adiponectin levels were seen in those with CP B disease, adjusting for CP class did not significantly affect adiponectin’s association with overall survival.
Adiponectin and adiponectin receptor expression have been shown to be reduced in patients with NASH [13]. Obesity can lead to decreased adiponectin levels as well as downregulation of adiponectin receptors leading to insulin resistance. [49, 50] In contrast, cachexia has been associated with increases in adiponectin, even when controlling for BMI. [51] In the elderly, higher adiponectin levels also correlate with mortality, are associated with frailty, and can be modified by exercise. [52, 53] High adiponectin levels have also been shown to be associated with poor outcomes in patients in the ICU, independent of BMI or markers of inflammation. [54] These results highlight the multiple competing effects regulating adiponectin and AR levels in these patients. Interestingly, in our study, subjects self-reported weights which were generally lower several years prior to diagnosis (data not shown), making cachexia related to illness less likely in our population, since we excluded those with clinically significant ascites.
There are several strengths of our study including its prospective nature, and use of REMARK guidelines as its basis.[55] Limitations include a relatively small sample size, assessment of adiponectin levels at only one time point, and variety of treatments given before and after enrollment. Although this heterogeneity may be considered a weakness, it also suggests the potential broad applicability of adiponectin as a prognostic biomarker.
Based on our results, we believe that adiponectin deserves further validation as a simple and practical biomarker to assess outcomes in patients with HCC, particularly in those with features of the metabolic syndrome. There are multiple variables which could have opposing effects on adiponectin levels, including metabolic syndrome, cirrhosis, cachexia, and frailty; separating these out in individual patients and clarifying mechanisms for these associations need further examination. Modulation of adiponectin pathways in those with elevated levels of adiponectin as a potential mechanism to alter disease course (potentially via AKT pathway modulation) also deserves further study.
Supplementary Material
Impact.
Understanding how adipokines affect HCC outcome may help develop novel treatment and prevention strategies.
Acknowledgments
Supported by NIH K23 CA149084-01A1 and the Steven J. Levinson Medical Research Foundation (To ABS)
Footnotes
Presented in Part at GI ASCO, 2012, San Francisco, California, #205
None of the authors has a financial conflict of interest relevant to this manuscript.
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welzel TM, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108(8):1314–21. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115(24):5651–61. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel AB, et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94(5):539–43. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel AB, et al. Obesity and microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2010;28(10):1063–9. doi: 10.3109/07357907.2010.483500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao Y, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105(2):95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sher DJ, et al. Relationship between serum adiponectin and prostate cancer grade. Prostate. 2008;68(14):1592–8. doi: 10.1002/pros.20823. [DOI] [PubMed] [Google Scholar]
- 11.Duggan C, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. 2011;29(1):32–9. doi: 10.1200/JCO.2009.26.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchida A, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279(29):30817–22. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 13.Kaser S, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54(1):117–21. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumeier M, et al. Adiponectin and its receptors in rodent models of fatty liver disease and liver cirrhosis. World J Gastroenterol. 2006;12(34):5490–4. doi: 10.3748/wjg.v12.i34.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 53(3):1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Carmienke S, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67(6):573–85. doi: 10.1038/ejcn.2013.61. [DOI] [PubMed] [Google Scholar]
- 18.Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15(2):91–101. doi: 10.1016/j.pathophys.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Ding X, et al. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166(6):1655–69. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig J, et al. Advances in liver biopsy diagnosis. Mayo Clin Proc. 1994;69(7):677–8. doi: 10.1016/s0025-6196(12)61347-0. [DOI] [PubMed] [Google Scholar]
- 21.Oda N, et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57(2):268–73. doi: 10.1016/j.metabol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Cnop M, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 23.Hui CK, et al. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol. 2007;47(2):191–202. doi: 10.1016/j.jhep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Tworoger SS, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92(4):1510–6. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 25.Seker M, et al. The association of serum adiponectin levels with histopathological variables in gastric cancer patients. Med Oncol. 2010;27(4):1319–23. doi: 10.1007/s12032-009-9382-x. [DOI] [PubMed] [Google Scholar]
- 26.Cust AE, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92(1):255–63. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- 27.Guadagni F, et al. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009;29(8):3321–7. [PubMed] [Google Scholar]
- 28.Ferroni P, et al. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res. 2007;27(1B):483–9. [PubMed] [Google Scholar]
- 29.Wei EK, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97(22):1688–94. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 30.Housa D, et al. Adiponectin as a potential marker of prostate cancer progression: studies in organ-confined and locally advanced prostate cancer. Physiol Res. 2008;57(3):451–8. doi: 10.33549/physiolres.931156. [DOI] [PubMed] [Google Scholar]
- 31.Sharma D, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 52(5):1713–22. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man K, et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res. 2010;16(3):967–77. doi: 10.1158/1078-0432.CCR-09-1487. [DOI] [PubMed] [Google Scholar]
- 33.Sumie S, et al. Total and high molecular weight adiponectin and hepatocellular carcinoma with HCV infection. PLoS One. 6(11):e26840. doi: 10.1371/journal.pone.0026840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aleksandrova K, et al. Inflammatory and metabolic biomarkers and risk of liver and bilary tract cancer. Hepatology. 2014 doi: 10.1002/hep.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arano T, et al. Serum level of adiponectin and the risk of liver cancer development in chronic hepatitis C patients. Int J Cancer. 2011;129(9):2226–35. doi: 10.1002/ijc.25861. [DOI] [PubMed] [Google Scholar]
- 36.Chen MJ, et al. The promoting effect of adiponectin in hepatocellular carcinoma. J Surg Oncol. 2012;106(2):181–7. doi: 10.1002/jso.23059. [DOI] [PubMed] [Google Scholar]
- 37.Wang SN, et al. Increased adiponectin associated with poor survival in hepatocellular carcinoma. J Gastroenterol. 2013 doi: 10.1007/s00535-013-0898-7. [DOI] [PubMed] [Google Scholar]
- 38.Chen CL, et al. Plasma adipokines and risk of hepatocellular carcinoma in chronic hepatitis B virus-infected carriers: a prospective study in taiwan. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1659–71. doi: 10.1158/1055-9965.EPI-14-0161. [DOI] [PubMed] [Google Scholar]
- 39.Shibata R, et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279(27):28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 40.Xiao X, et al. Adiponectin protects endothelial cells from the damages induced by the intermittent high level of glucose. Endocrine. 2011;40(3):386–93. doi: 10.1007/s12020-011-9531-9. [DOI] [PubMed] [Google Scholar]
- 41.Nepal S, et al. Globular adiponectin inhibits ethanol-induced apoptosis in HepG2 cells through heme oxygenase-1 induction. Biochem Pharmacol. 2012;84(7):974–83. doi: 10.1016/j.bcp.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Singer JR, et al. Adiponectin and all-cause mortality in elderly people with type 2 diabetes. Diabetes Care. 2012;35(9):1858–63. doi: 10.2337/dc11-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tacke F, et al. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol. 2005;42(5):666–73. doi: 10.1016/j.jhep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 44.Salman TA, et al. Study of adiponectin in chronic liver disease and cholestasis. Hepatol Int. 2010;4(4):767–74. doi: 10.1007/s12072-010-9216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbetta S, et al. Fibrosis is associated with adiponectin resistance in chronic hepatitis C virus infection. Eur J Clin Invest. 2011;41(8):898–905. doi: 10.1111/j.1365-2362.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 46.Tietge UJ, et al. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287(1):E82–9. doi: 10.1152/ajpendo.00494.2003. [DOI] [PubMed] [Google Scholar]
- 47.Derbala M, et al. Adiponectin changes in HCV-Genotype 4: relation to liver histology and response to treatment. J Viral Hepat. 2009;16(10):689–96. doi: 10.1111/j.1365-2893.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 48.Spyridopoulos TN, et al. Low adiponectin levels are associated with renal cell carcinoma: a case-control study. Int J Cancer. 2007;120(7):1573–8. doi: 10.1002/ijc.22526. [DOI] [PubMed] [Google Scholar]
- 49.Cohen SS, et al. Serum adiponectin in relation to body mass index and other correlates in black and white women. Ann Epidemiol. 2011;21(2):86–94. doi: 10.1016/j.annepidem.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 51.Araujo JP, et al. Adiponectin is increased in cardiac cachexia irrespective of body mass index. Eur J Heart Fail. 2009;11(6):567–72. doi: 10.1093/eurjhf/hfp046. [DOI] [PubMed] [Google Scholar]
- 52.Hozawa A, et al. Relationship Between Serum Adiponectin Levels and Disability-Free Survival Among Community-Dwelling Elderly Individuals: The Tsurugaya Project. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glr191. [DOI] [PubMed] [Google Scholar]
- 53.Markofski MM, et al. Exercise Training Modifies Ghrelin and Adiponectin Concentrations and Is Related to Inflammation in Older Adults. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch A, et al. Serum adiponectin upon admission to the intensive care unit may predict mortality in critically ill patients. J Crit Care. 26(2):166–74. doi: 10.1016/j.jcrc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 55.McShane LM, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067–72. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.