Abstract
During the past century, discoveries of microorganisms as causes of infections and antibiotics as effective therapeutic agents have contributed to significant gains in public health in many parts of the world. Health agencies worldwide are galvanizing attention toward antibiotic resistance, which is a major threat to public health (Centers for Disease Control and Prevention [CDC], 2013; World Health Organization [WHO], 2014). Some life scientists believe that we are approaching the post-antibiotic age (Davies & Davies, 2010). The growing threat of antimicrobial resistance is fueled by complex factors with biological, behavioral and societal aspects. This primer provides an overview of antibiotic resistance and its growing burden on public health, the biological and behavioral mechanisms that increase antibiotic resistance, and examples of where health communication scholars can contribute to efforts to make our current antibiotic drugs last as long as possible. In addition, we identify compelling challenges for current communication theories and practices.
Keywords: Antibiotic Resistance, Evolution, Microbiology, Transdisciplinary
What is an antibiotic drug?
Antibiotics are chemical substances that kill bacteria or slow bacterial growth; they are naturally produced by fungi and other microorganisms. An antibiotic drug refers to the synthesized medicine that is used to treat bacterial infections. In any given bacterial infection, there is a large population (e.g., millions or more) of bacteria in the human body. The term “antimicrobial” broadly refers to drugs that are used to treat infections caused by a variety of microorganisms (e.g., bacteria, viruses, fungi, parasites such as malaria).
When were antibiotics first used?
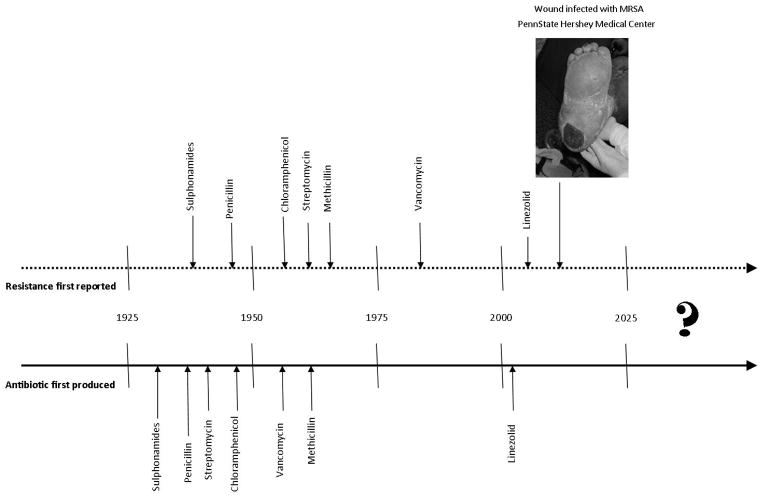
During the past century, scientific discoveries of microorganisms as causes of infections and antibiotics as effective therapeutic agents have contributed to enormous gains in public health (Davies & Davies, 2010). The first antibiotic widely distributed in the US was penicillin in the mid-1940s and a clinical bacterium isolate resistant to penicillin was documented shortly after (Miller, 1947). From the 1940s to the 1960s, 20 new classes of antibiotics were created (Coates, Halls, & Hu, 2011), and resistant strains appeared within five years of each (CDC, 2013, see Figure 1). In the early 1960s, research and development of antibiotics by pharmaceutical companies stalled (Davies & Davies, 2010); since 1962, only two new classes have reached the market (Coates et al., 2011). Use and distribution of antibiotics, however, increased.
Figure 1.
Historical timeline depicting the introduction of antibiotics and detected resistance.
In the past 60 years, millions of metric tons of antibiotics have been produced and distributed worldwide in various products (e.g., toiletries, cleaning products) for many purposes (Davies & Davies, 2010), including veterinary use (75% of the antibiotic market in the US; Fauci & Marston, 2014). People are exposed to antibiotics through their water (Venkatesan & Halden, 2014) and toiletries, in addition to what they take as prescriptions. In 2010, 258 million courses of oral antibiotics were dispensed (833 prescriptions per 1000 persons; Hicks & Taylor, 2013). Importantly, 50% of prescribed antibiotics are unnecessary (CDC, 2013). For example, although antibiotics are ineffective against viruses, nearly 75% of US adults seeking treatment for acute bronchitis, generally caused by a virus, are prescribed antibiotics (Fauci & Marston, 2014).
What does antibiotic resistance mean?
Antibiotic resistance refers to genetic changes in bacteria that reduce or eliminate an antibiotic’s ability to destroy it (CDC, 2013). Drug resistance happens in almost every antimicrobial drug, not just antibiotics, and almost all pathogens and parasites, not just bacteria. Whenever drugs are used against viruses, fungi, single-celled animals (e.g., malaria parasites), and multi-celled animals (e.g., lice, parasitic worms), drug resistance almost always evolves and undermines drug efficacy (zur Wiesch, Kouyos, Engelstadter, Regoes, & Bohnoeffer, 2011); this is referred to as antimicrobial resistance.
How widespread is antibiotic resistance?
Some life scientists believe that we are approaching the post-antibiotic age (Davies & Davies, 2010). Approximately 50% of tested infections for E. coli, K. pneumonia, and S. aureus showed resistance to commonly used antibiotics (WHO, 2014). In the US alone, over 2 million people acquire antibiotic-resistant infections and at least 23,000 people die as a direct result (CDC, 2013). The agencies producing these estimates acknowledge that they underestimate the problem. Resistant infections can be difficult to detect in clinical settings (CDC, 2013; WHO, 2014). Still, in 2011, one in every 25 inpatients in US acute care hospitals had a hospital-acquired infection, and 9700 of these infections were methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (Magill et al., 2014). Of particular concern are gram-negative pathogens, such as Pseudomonas aeruginosa (associated with diseases such as pneumonia; Peleg & Hooper, 2010), because “they are becoming resistant to nearly all drugs that would be considered for treatment” (p. 22, CDC, 2013). Health agencies around the globe are galvanizing attention toward antibiotic resistance, because it is considered a major threat to public health (CDC, 2013; World Health Organization [WHO], 2014).
What causes antibiotic resistance?
Several factors determine the useful lifetime of an antibiotic drug. The first is the time until resistance initially arises. Bacteria with genes providing antibiotic resistance are found naturally in microbial populations: they can arise de novo because of random mutations in a bacterium or as a method of surviving antibiotics produced by competing bacteria (D’Costa, McGrann, Hughes, & Wright, 2006).
Once the gene is present in a population, by mutational accident or by virtue of history, bacteria can acquire the resistance genes by descent, as we inherit our genes from our parents or, unlike us, from non-relatives (Step 4 in Figure 2). This phenomenon of lateral gene transfer—the acquisition of a gene from another un-related bacterium—often involves plasmids or other mobile genetic elements that can rapidly be exchanged between bacteria (Davies & Davies, 2010). Importantly, these genetic elements can be exchanged between members of different species of bacteria. This phenomenon has profound implications for antibiotic preservation. To see why, consider the following illustration.
Figure 2.
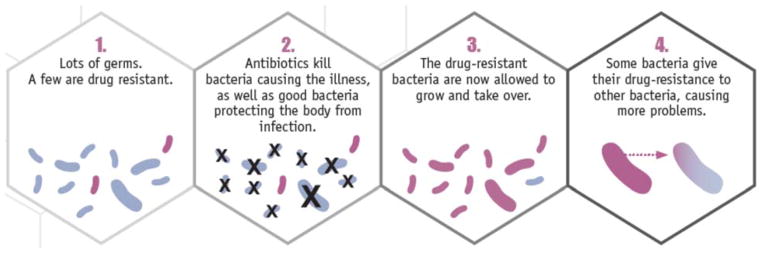
How exposure to antibiotics creates resistance (CDC, 2013, p. 14).
Imagine Susan has been infected with Bacteria X, which lives in the human gut and is targeted by Drug Y. Six months earlier Susan took Drug Y for a different infection, which may or may not have been due to Bacteria X. Bacteria X is not the only bacteria in Susan’s system. Millions of bacteria thrive in healthy humans, performing many helpful functions; many of these “helpful” bacteria were also killed when she took Drug Y, but the ones that were resistant to Drug Y survived. During Susan’s new infection, the Bacteria X multiplying in Susan’s system laterally transferred genes with the Drug-Y-resistant helpful bacteria nearby; thus Susan’s Bacteria X infection becomes resistant to Drug Y.
Although antibiotic resistance is a natural adaptation, human use of antibiotic drugs exacerbates its appearance and spread (Fauci & Marston, 2014) through evolution by natural selection. Several steps are involved (Fig. 2). Although individual bacteria in a bacterial infection may vary from each other in many ways, the evolution of drug resistance begins when at least one bacterium is resistant to an antibiotic drug (i.e., drug-resistant), such as Drug Y. When Drug Y enters the system (e.g., after taking an antibiotic), the drug acts disproportionately against the bacteria without resistance (i.e., drug-sensitive bacteria, Step 2 in Figure 2). The drug-sensitive bacteria die, while some drug-resistant bacteria continue to replicate. This replication advantage can be substantial: resistant bacteria not only survive the drug treatment, but they also have fewer competitors for resources, because the drug-sensitive bacteria have been removed (Step 3 in Figure 2). Thus, drug-resistant bacteria can dominate the bacterial population in an infection, causing drug treatments to fail.
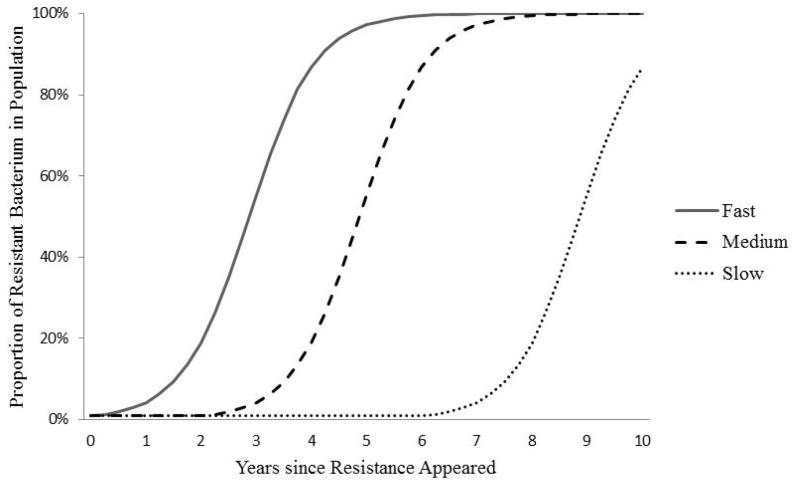
If Drug-Y-resistant Bacteria X are transmitted to another person, then they may spread through the entire host population. If the Drug-Y-resistant bacteria are transmitted to a susceptible host taking Drug Y, the resistant bacteria gain the advantage again, and may continue to spread, thus becoming more common in the overall Bacteria X population in humans. In the simplest case, the spreading resistance looks like the curve shown in Figure 3. The curve’s shape depends on the proportion of infected people who get treated with Drug Y, and the dose and length of exposure to the drug in treated people. All else equal, the more people that are treated with Drug Y and the more aggressive the treatment (e.g., longer courses at high concentrations), the faster resistance spreads, because the drug treatment itself confers the selective advantage on the resistant bacteria.
Figure 3.
Possible diffusion curves for Bacterium X resistant to Drug Y in the population of Bacteria X
How do we slow the evolution of antibiotic resistance?
To slow the evolution of antibiotic resistance, we must reduce the two determinants of the speed of the evolutionary process: (1) the probability a resistance gene will first appear in a disease-causing organism (by natural mutation or lateral gene transfer), and (2) the strength of the reproductive advantage conferred on that resistance by drug use.
One method to reduce the probability that resistance will first arise in an otherwise drug-sensitive pathogen population is antibiotic stewardship. Every administered dose of antibiotic drugs selects for resistance in the harmless bacteria that naturally occur on or in us, which can be a potential source for the lateral transfer of resistance genes to bacterial infections. The lower our antibiotic use, the fewer of those genes will reside in us, or in our environment, thereby reducing the likelihood that pathogens acquire resistance from them. Thus it is critical that people take antibiotics only when necessary. Ideally, people would take only narrow-spectrum antibiotics, which precisely target problematic bacteria with little collateral effect on harmless or useful bacteria that reside on or in us. The first rule of resistance management is to take antibiotics only when absolutely necessary. The second rule is to choose those that cause the smallest amount of selection for resistance in non-target organisms.
Two major methods by which to slow spread are to reduce the number of infections and reduce antibiotic use (CDC, 2013). Whenever an infection is prevented, one less person may be prescribed drugs. Infections can be prevented by vaccines, hygiene and sanitation, as well as by isolation during infectivity. These steps can be challenging to implement; even in hospitals, it is a challenge to implement routine hand washing.
Reducing antibiotic use slows the spread of resistance genes once they emerge in pathogen populations. Prescribing antibiotic drugs at all sets up an interesting conundrum. If we can, in fact, destroy every bacterium X in a person with a treatment of drug Y (i.e., all are drug-sensitive), then we could prevent any bacterium from acquiring resistance in the first place (dead bacteria cannot mutate or receive resistance genes by lateral transfer). However, if resistant bacteria are already present, the more aggressively we kill the drug-sensitive bacteria, the stronger the evolutionary advantage we confer on any existing, drug-resistant bacteria. When full eradication is not guaranteed, then, biologically we can reduce the selection pressure to evolve resistance by reducing antibiotic use (e.g., treating for fewer days; Huijben et al., 2013). Hence the conundrum: aggressive treatment may help to prevent resistance from appearing in the first place, but at the cost of maximizing selection in favor of any existing resistance (Read et al., 2011). Balancing these opposing forces for resistance management is an area of controversy in the life sciences (e.g., Ankomah & Levin, 2014). This biological uncertainty presents a nontrivial conundrum for influencing prescription behavior, because it may create mixed messages about how long to take antibiotic drugs. The best resistance management solution will likely vary with the details of the bacteria, drug, and clinical and epidemiological circumstances (Read et al. 2011). As the science resolves, the complexities in designing health messages and conveying them to clinicians, patients, and the general public, will increase.
What is the role of health communication in antibiotic resistance?
Antibiotic use and misuse are present globally, which increases the likelihood of evolution and spread of antibiotic resistance (Fauci & Marston, 2014), as well as the global level of action needed to slow it. The CDC (2013) has outlined four strategies to slow antibiotic resistance: (1) prevent infections, (2) increase surveillance, (3) improve antibiotic stewardship, and (4) develop new drugs and diagnostic tests. Health communication scholars can assist with the behavioral goals for preventing infections, such as promoting vaccination for seasonal influenza and proper hand hygiene in public and private settings (Barnett & Linder, 2014). Equally the suboptimal adherence to hand-hygiene among healthcare providers (Pittet & Boyce, 2001) demands attention.
Mass media campaigns, such as the “Get Smart” campaign, have shown reductions in the unnecessary use of antibiotics, particularly in pediatrics (Gonzales et al., 2008), an important area because prescription rates in the US are highest among children under 10 (Hicks & Taylor, 2013). Yet, persons 65 or older have similarly high rates (Hicks & Taylor, 2013) and are the most likely to receive broad-spectrum agents (Gahbauer, Gonzales, & Guglielmo, 2014). Older adults have received little attention and may be a promising group for future interventions.
Clinicians and the public likely vary in their beliefs, attitudes, behaviors, and barriers to change. Clinicians, for example, vary in their knowledge of antibiotic resistance and perceived barriers to changing their prescription behaviors (Ackerman, Gonzales, Stahl, & Metlay, 2013). The public may not know that non-essential use of antibiotics is harmful to themselves and others. They may not know of its presence either: a large survey showed that only 52% of the public were aware of antibiotic use in animal feed (M’ikanatha, Dewar, Rankin, & Lautenbach, 2007). Germ-warfare metaphors used to describe the relationship between humans and bacteria (Humans-good/microbes-bad; Lederberg 2000) may produce misperceptions about antibiotic resistance. We may need to segment target audiences, based on shared beliefs, attitudes, fears, behaviors or barriers, to provide different, targeted interventions.
At the policy level, attempts around the world to ban nontherapeutic uses of antibiotics have been difficult to employ, much less the widespread, unregulated distribution of antibiotics in countries with bans in place (Davies & Davies, 2010). Researchers need theoretical guidance on how to prepare systems (communities, states, nations) for the uptake and diffusion of new or different beliefs and practices. Furthermore, researchers need theoretical guidance on which parts of health messages are retained and which parts change as these messages spread through communication networks. Historically, social science disciplines have never enjoyed the funding and resources of the life sciences, and in the life sciences, resources have been skewed toward new drugs and diagnostics, instead of how to evolution-proof existing drugs. We need theoretical guidance on how to make large-scale changes with limited resources, and how to monitor and adapt to changes created by communication shocks to the existing system.
Conclusion
Antibiotic resistance is a major health problem, as drugs that were once highly efficacious no longer cure bacterial infections. In addition to greater morbidity, disability, and mortality, antibiotic-resistant infections contribute to other costs: they require prolonged and costlier treatments, extended hospital stays, and additional medical visits (WHO, 2014). The viability of many areas of medicine is threatened. Antibiotics are involved in cancer treatments, organ transplantation, general surgery, and a range of therapies against autoimmune diseases (CDC, 2013). The growing threat of antimicrobial resistance is fueled by complex biological, behavioral and societal factors. Transdisciplinary collaborations (Parrott & Kreuter, 2011) involving social, biological, medical, and public health scholars are needed to address the growing health burdens imposed by drug-resistant infections.
Acknowledgments
We want to thank Amanda Applegate, Madisen Quesnell, Lydia Glick for feedback on earlier versions of this primer, and Sameh Boktor for assistance with figures. This project was supported by Awards P50 DA010075 from the National Institute on Drug Abuse (NIDA; RS) and R01-GM089932 from the National Institute for General Medicine (NIGM; AR).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the views of NIDA, NIGM, or the National Institutes of Health.
Contributor Information
Rachel A. Smith, The Pennsylvania State University, Department of Communication Arts & Sciences
Nkuchia M. M’ikanatha, Pennsylvania Department of Health
Andrew F. Read, The Pennsylvania State University, Department of Biology
References
- Ackerman SL, Gonzales R, Stahl MS, Metlay JP. One size does not fit all: Evaluating an intervention to reduce antibiotic prescribing for acute bronchitis. BMC Health Services Research. 2013;13:1–9. doi: 10.1186/1472-6963-13-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankomah P, Levin BR. Exploring the collaboration between antibiotics and the immune response in the treatment of acute self-limiting infections. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1400352111. online first. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. Journal of the American Medical Association. 2014;311:2020–2022. doi: 10.1001/jama.2013.286141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2013. 2013 Retrieved from http: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- Coates AR, Halls G, Hu Y. Novel classes of antibiotics or more of the same? British Journal of Pharmacology. 2011;163:184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science. 2006;311:374–377. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Marston HD. The perpetual challenge of antimicrobial resistance. Journal of the American Medical Association. 2014 doi: 10.1001/jama.2014.2465. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Gahbauer AM, Gonzales ML, Guglielmo BJ. Patterns of antibacterial use and impact of age, race/ethnicity, and geographic region on antibacterial use in an outpatient medicaid cohort. Pharmacotherapy. 2014 doi: 10.1002/phar.1425. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Corbett KK, Wong S, Glazner JE, Deas A, Leeman-Castillo B, Flores E, Kafadar K. “Get smart Colorado”: Impact of a mass media campaign to improve community antibiotic use. Medical Care. 2008;46:597–605. doi: 10.1097/MLR.0b013e3181653d2e. [DOI] [PubMed] [Google Scholar]
- Hicks LA, Taylor TH. U.S. Outpatient Antibiotic Prescribing, 2010. New England Journal of Medicine. 2013;368:1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- Hornik R, Woolf KD. Using cross-sectional surveys to plan message strategies. Social Marketing Quarterly. 1999;5:34–41. doi: 10.1080/15245004.1999.9961044. [DOI] [Google Scholar]
- Huijben S, Bell AS, Sim DG, Tomasello D, Mideo N, Day T, Read A. Aggressive chemotherapy and the selection of drug resistant pathogens. PLoS Pathog. 2013;9(9):e1003578. doi: 10.1371/journal.ppat.1003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Infectious history. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- Magill SS, Edwards JR, Bamberg W, et al. Fridkin MD. Multistate point-prevalence survey of health care-associated infections. New England Journal of Medicine. 2014;370:1198–208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikanatha N, Dewar RF, Rankin SC, Lautenbach E. Prevalence of antibiotic prescriptions and attitudes toward antibiotic-free food. Poster presented at the annual meeting of The Infectious Diseases Society of America; San Diego, CA. 2007. [Google Scholar]
- Miller CP. Development of bacterial resistance to antibiotics. Journal of the American Medical Association. 1947;135:749–751. doi: 10.1001/jama.1947.02890120003002. [DOI] [PubMed] [Google Scholar]
- Parrott RL, Kreuter MW. Multidisciplinary, interdisciplinary, and transdisciplinary approaches to health communication: Where do we draw the lines? In: Thompson TL, Parrott R, Nussbaum JF, editors. The Routledge handbook of health communication. 2. New York, NY: Routledge; 2011. pp. 3–17. [Google Scholar]
- Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. New England Journal of Medicine. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D, Boyce JM. Hand hygiene and patient care: pursuing the Semmelweis legacy. Lancet Infectious Diseases. 2001;1:9–20. doi: 10.1016/S1473-3099(09)70295-6. [DOI] [Google Scholar]
- Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proceedings of the National Academy of Science USA. 2011;108:10871–10877. doi: 10.1073/pnas.1100299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. American Community Survey, 2012. 2012 Retrieved from http://factfinder2.census.gov.
- Venkatesan AK, Halden RU. Wastewater treatment plants as chemical observatories to forecast ecological and human health risks of manmade chemicals. Scientific Reports. 2014;4:1–7. doi: 10.1038/srep03731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Antimicrobial resistance: Global report on surveillance. Geneva, Switzerland: Author; 2014. [Google Scholar]
- Zur Wiesch PA, Kouyos R, Engelstadter J, Regoes RR, Bonhoeffer S. Population biological principles of drug-resistance evolution in infectious diseases. Lancet Infectious Diseases. 2011;11:236–247. doi: 10.1016/S1473-3099(10)70264-4. [DOI] [PubMed] [Google Scholar]