Abstract
Vasoactive intestinal peptide (VIP) and pituitary adenylyl cyclase-activating polypeptide (PACAP) are two structurally-related neuropeptides with widespread expression in the central and peripheral nervous systems. Although these peptides have been repeatedly shown to exert potent anti-inflammatory actions when administered in animal models of inflammatory disease, mice deficient in VIP and PACAP were recently shown to exhibit different phenotypes (ameliorated and exacerbated, respectively) in response to experimental autoimmune encephalomyelitis (EAE). Therefore, elucidating what are the specific immunoregulatory roles played by each of their receptor subtypes (VPAC1, VPAC2, and PAC1) is critical. In this study, we found that mice with a genetic deletion of VIPR2, encoding the VPAC2 receptor, exhibited exacerbated (MOG35-55)-induced EAE compared to wild type mice, characterized by enhanced clinical and histopathological features, increased proinflammatory cytokines (TNF-α, IL-6, IFN-γ (Th1), and IL-17 (Th17)) and reduced anti-inflammatory cytokines (IL-10, TGFβ, and IL-4 (Th2)) in the CNS and lymph nodes. Moreover, the abundance and proliferative index of lymph node, thymus and CNS CD4+CD25+FoxP3+ Tregs were strikingly reduced in VPAC2-deficient mice with EAE. Finally, the in vitro suppressive activity of lymph node and splenic Tregs from VPAC2-deficient mice was impaired. Overall, our results demonstrate critical protective roles for PACAP and the VPAC2 receptor against autoimmunity, promoting the expansion and maintenance of the Treg pool.
Keywords: Experimental autoimmune encephalomyelitis, neuroimmunology, autoimmunity, inflammation, neuropeptide, VPAC2 receptor, regulatory T cell
1. Introduction
Vasoactive intestinal peptide (VIP) and pituitary adenylyl cyclase-activating polypeptide (PACAP), are two secretin family neuropeptides widely expressed in the central, autonomic, sensory, and enteric nervous systems, acting through the G protein-coupled receptors VPAC1, VPAC2 and PAC1 (1). Whereas PACAP binds with similar high affinity to all three of these receptors, VIP binds only VPAC1 and VPAC2. Each of these receptors is strongly coupled through Gαs protein to adenylyl cyclase, leading to cAMP production and subsequent activation of PKA (protein kinase A PKA) and in some cases, EPACs (exchange protein directly activated by cAMP, also known as cAMP-GEF). However, a few other parallel signaling pathways have been described. For example, they also couple with Gαi and Gαq proteins that regulate signaling molecules as diverse as PKC, PLC, PLD and SRC, and elevate the intracellular secondary messengers IP3, DAG and Ca2+ (1). Activation of different signaling pathways seems to be dependent on the cellular type/activation status and in the case of PAC1, of the receptor splice variant expressed.
The expression and actions of VIP, PACAP and their receptors in preganglionic and postganglionic neurons of the sympathetic nervous system (SNS) (2-8) suggest that they may function in the autonomic regulation of stress responses, including those associated with inflammation (9, 10). In this regard, VIP-immunoreactive fibers, presumably arising from sympathetic neurons, have been demonstrated in lymph nodes, spleen and thymus of rats (11), and nearly all immune cell types express one or more receptor subtypes (1, 12). For example, macrophages express constitutively VPAC1 and PAC1 receptors, and when exposed to inflammatory stimulus, express VPAC2 (13). Moreover, VPAC1 is expressed constitutively on resting CD4+ T cells, and upon anti-CD3/CD28 activation, downregulates VPAC1 and upregulates VPAC2 (14).
Although VIP and PACAP exert multiple immunomodulatory properties, they are mostly regarded as anti-inflammatory peptides (12, 15-17). In this respect, VIP and PACAP inhibited the lipopolysaccharide (LPS)-induced production of TNF-α, IL-6, and several chemokines in macrophage cultures, increasing the production of IL-10 (18-20). In addition, VIP and PACAP promote Th2 over Th1 responses. Although studies have suggested that these peptides can directly enhance Th2 cell proliferation and survival (21-23), they also act indirectly to affect Th balance by modulating the expression of co-stimulatory molecules and Th-polarizing cytokines on antigen-presenting cells (APCs) (13, 24-26). In fact, human and murine dendritic cells differentiated in vitro in the presence of VIP exhibited a “tolerogenic” phenotype and promoted the generation of regulatory T (Treg) cells in vivo and in vitro (27, 28).
In vivo, VIP and PACAP were show to exhibit therapeutic activity in several murine experimental models of human autoimmune disease (29-32). For example, administrations of VIP and PACAP have been shown to ameliorate the clinical and pathological manifestations of acute, chronic, and relapsing-remitting experimental autoimmune encephalomyelitis (EAE) murine models of multiple sclerosis (MS) (33, 34). These therapeutic effects were associated with an inhibition of the proinflammatory cascade, a blockade of Th1 vs Th2 responses and the expansion of CD4+CD25+Foxp3+ Tregs (35). In addition, our recent studies subjecting PACAP-deficient (KO) mice to MOG35-55-induced EAE demonstrated a protective role for the endogenous source of this peptide and revealed a function for PACAP as an endogenous modulator of Treg expansion (36, 37).
Although the aforementioned studies suggest that VIP and PACAP might be able to expand Tregs, high-affinity receptor subtype-specific agonists may constitute more desirable agents than the peptides themselves. Our present study utilizing VPAC2-deficient mice suggests an involvement of VPAC2 receptors in the pathogenesis of MOG-induced EAE through modulation of T cell responses.
1. Methods
1.1. EAE induction
Eight to 12-week old VPAC2 KO (38) and WT C57BL/6 mice were bred in the UCLA animal facilities. FoxP3EGFP mice were kindly donated by Dr. Talal Chatila (Boston Children's Hospital) (39). All protocols were approved by the UCLA Animal Committee.
EAE was induced by subcutaneous immunization in the flanks with 100μg of MOG35–55 (GLBiochem) in CFA containing 5mg/ml Mycobacterium tuberculosis H37Ra (Difco) as described (36). In addition, mice received intraperitoneally 200ng of pertussis toxin (List Biological Laboratories) on days 0 and 2 post-immunization. Clinical signs of EAE were scored daily from 0 to 4 (0, asymptomatic, 1, tail limpness, 2, wobbling gait, 3, hind limb paralysis and 4, moribund/dead). For histological studies, spinal cords were fixed in 4% paraformaldehyde (PFA) and paraffin-embedded following standard procedures. Seven-μm sections were stained with Hematoxylin-eosin/luxol fast blue and histopathology was scored from 0 to 4 according to the level of immune cell infiltration/demyelination as described (36).
2.2. Cell suspension preparation
Cell suspensions were prepared from lymph nodes, spleens or thymi by tapping the organs through a 40μm mesh. For CNS mononuclear cell isolation, tissues were minced and digested with DNAse I (0.1mg/ml, Worthington) and collagenase IV (0.1mg/ml, Roche). After a 40/80% percoll (GE Healthcare) gradient centrifugation (500g, 30 min), mononuclear cells were removed from the interface.
2.3. RNA extraction and Real time RT-PCR
RNA was isolated from thymi, lymph nodes and spinal cords with Trizol (Sigma) and retrotranscribed with the Iscript kit from Bio-Rad. Real time quantitative PCRs were performed using iQ SYBR Green Supermix (Bio-Rad). Primers are listed in Supplementary Table 1. Amplification was performed as follows: initial denaturation at 95°C for 5 min, 40 cycles of 95°C for 25 secs followed by 60°C for 35 secs, and 72°C for 35 secs for IFNγ, IL-4; 96°C for 20 secs, 60°C for 30 secs, and 72°C for 20 secs for IL-6, IL-10, IL-17A, IL-23p19; 96°C for 20 secs, 62°C for 30 secs, and 72°C for 20 secs for TNFα; 95°C for 1 min, 60°C for 1 min and 72°C for 1 min for Foxp3, 96°C for 20 secs, 60°C for 1 min secs, and 72°C for 10 secs for VPAC2 with a final elongation at 72°C for 10 min for all. The housekeeping gene HPRT was amplified for standardizationof assay conditions. Amplicon sequencing and melting curve analysis confirmed primer specificity. Fold increase vs no EAE control was calculated by the 2–ΔΔCt calculation.
Tregs were purified from thymi of FoxP3EGFP mice for VPAC2 mRNA expression measurement, by CD25 magnetic enrichment (StemCell technologies) and FACS sorting for CD4+CD8−CD25+FoxP3-EGFP+ cells using PerCP-Cy5.5-CD4 and PE-CD8 antibodies (eBioscience).
2.4. Antigen recall assays
Lymph node cells were cultured at 1 × 106 cells/ml in complete medium (RPMI 1640 containing 25mM HEPES, 2mM L-glutamine, 1% penicillin/streptomycin and 2% FBS) with MOG or OVA (10μg/ml). Cytokines in 48 hour supernatants were measured with ELISA kits from Peprotech (IFNγ, IL-10) and eBioscience (IL-17, TGFβ) following the manufacturer's protocols. For proliferation assay, after 2 days of culture, 1μCi/well of [3H]-thymidine was added for 18 additional hours. Incorporated radioactivity was measured on a β-scintillation counter (Beckman).
2.5. Flow cytometry
For Th profile analysis, cells were incubated for 4 hours in complete medium containing PMA (50ng/ml, Sigma), ionomycin (1mg/ml, Sigma), brefeldin (3μg/ml, eBiosciences) and monensin (2μM, eBiosciences). Cells were stained with FITC-anti-CD4, fixed in 2% PFA, and permeabilized with PBS/0.2% Tween 20. Then, cells were incubated with PE-anti-IFNγ, PerCP-Cy5.5-anti-IL-17 and APC-anti-IL-4 (eBioscience) in permeabilization buffer.
For Treg measurement, a staining kit from eBioscience was used. Briefly, cells were incubated with PerCP-Cy5.5-anti-CD4 and PE-anti-CD25 (lymph nodes, CNS) or PerCP-Cy5.5-anti-CD4 and PE-anti-CD8 (thymus). Then, cells were treated with fixation/permeabilization buffer overnight, and incubated with APC-anti-Foxp3 and FITC-anti-Ki67 (for proliferation).
Samples were acquired with a FACScalibur cytometer (BD Biosciences), and analyzed using Weasel software (Walter and Eliza Hall Institute).
2.6. Treg isolation and expansion ex vivo
Tregs from WT or KO mice were isolated as CD4+CD25+ by magnetic bead systems based on negative selection of CD4+ cells and positive selection of CD25+ cells (StemCell Technologies).
For Treg expansion, IL-2 and CD3/CD28 MACSiBead™ Particles (Miltenyi Biotech) were used according to the manufacturer except that the optimal bead concentrations (3:1 bead-to-cell ratio) were also reduced by 1/2 and 1/4. Tregs (1 × 105 cells/well in 96 well-plates) were cultured in complete medium. Cells were counted on culture days 3 and 5, and IL-10 and TGFβ ELISAs were performed on day 5 supernatants. For thymidine incorporation assay, 1 × 105 cells were collected on days 3 and 5 and cultured for 24 hours with IL-2/anti-CD3/CD28. One μCi/well of [3H]-thymidine was added to each well for 18 additional hours and thymidine incorporation was determined.
2.7. Treg suppressive assay
CD4+CD25+ Tregs and CD4+CD25− Teffs were isolated by magnetic separation as above. APCs were obtained by preparation of a syngenic splenocyte suspension, T cell depletion with anti-CD4 and anti-CD8 microbeads (StemCell Technologies), and treatment with mitomycin C (50μg/ml). WT Teff (5 × 104) and APCs (2 × 105) were cultured in 96-well plates with titrated numbers of WT or KO mice Tregs (Treg:Teff = 1:1, 1:2, 1:4, 1:8) in complete medium with IL-2 (50U/ml) and with MOG (20μg/mL). Proliferation was measured 48 hours after culture as above.
2.8. Statistical analyses
ANOVA and Student's t-test were used to assess significance using GraphPad 4.0.
2. Results
2.1. VPAC2 KO mice exhibited exacerbated clinical, histopathological and immunological features of EAE
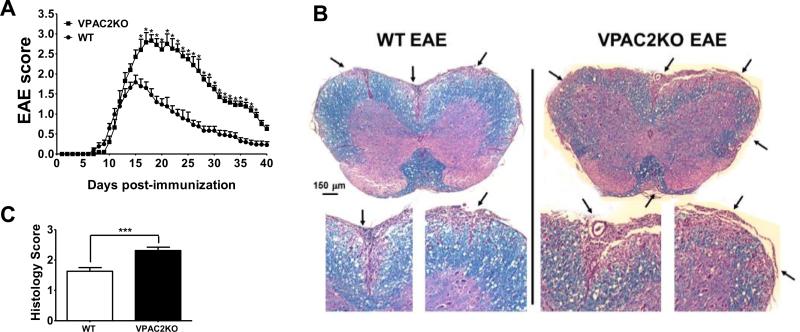
We subjected WT and VPAC2-deficient (VPAC2 KO) mice to MOG35-55-induced EAE, and found that KO mice developed exacerbated and more prolonged clinical disease course than WT animals (Figure 1A). In fact, whereas WT mice reached the peak of the disease on day 15 with a score of 1.79 ± 0.18, the average clinical score of VPAC2 KO mice continued increasing until day 18, when they reached their maximum score of 2.83± 0.15. From this time point, the clinical scores of VPAC2 KO mice were consistently higher than those in WT mice. In correlation with the clinical scoring, VPAC2 KO spinal cords exhibited a higher degree of immune cell infiltration and demyelination than WT (Figure 1B, C).
Figure 1. VPAC2 deficient mice exhibit exacerbated EAE.
EAE was induced by subcutaneous immunization of C57BL/6 wild type (WT) and VPAC2 KO mice (n=8/group) with 100μg of MOG35-55 in CFA/Mycobacterium tuberculosis. In addition, mice received i.p. 200ng of Pertussis toxin on days 0 and 2 post-immunization. A) EAE symptoms were scored from 0 to 4 as described in Materials and Methods; B) Micrographs of spinal cords from WT and VPAC2 KO mice 30 days post-immunization stained with H&E and Luxol Fast Blue, and C) histopathological scores (see Materials and Methods). Results shown are representative of three independent experiments. *P<.05, ***P<.001 (Student's t-test).
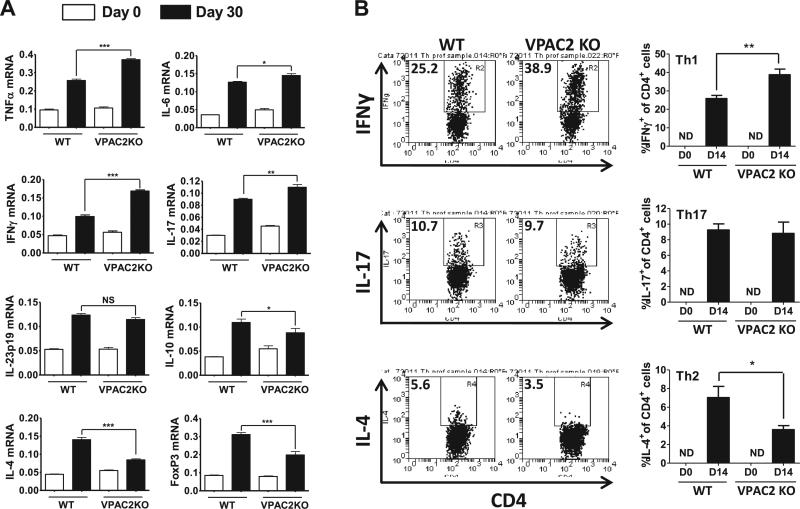
The robust inflammatory response in the CNS during EAE is believed to be orchestrated primarily by encephalitogenic Th1 and Th17 cells. By quantitative real time RT-PCR, we found that VPAC2 KO spinal cords exhibited higher mRNA expression of the pro-inflammatory cytokines TNF-α , IL-6, IFNγ (Th1), IL-17A (Th17), but similar levels of IL-23p19 (Th17-promoting) compared to WT mice 30 days post-EAE immunization, in agreement with the higher clinical and histopathological scores of the KO mice (Figure 2A). However, levels of the anti-inflammatory cytokines IL-4 (Th2), IL-10 (Th2/Treg) and FoxP3 mRNA (a Treg marker) were reduced compared to those in WT animals (Figure 2A). In addition to the cytokine expression analysis, we identified different Th cell subsets by flow cytometry in the CNS on day 14 according to their prototype cytokines. We found significantly higher Th1 but lower Th2 proportions in VPAC2 KO than in WT mice (Figure 2B). However, the proportions of IL-17 producing T cells (Th17) did not differ between the two groups of mice.
Figure 2. Th1/Th17 profiles are enhanced in the CNS of VPAC2 KO mice compared to WT mice.
A) Real time PCR analysis of cytokine mRNA expression in the spinal cord 30 days post-immunization; B) Flow cytometry analysis of IFNγ (Th1), IL-17 (Th17) and IL-4 (Th2) cell subsets in the CNS on day 14 post-EAE. Representative FACS plots are shown on the left. Y axis represents the percentage of CD4+ cells that are IFNγ+, IL-17+ or IL-4+, respectively. ND = not detected. Results shown are representative of three independent experiments of n = 8 mice/group. *P<.05, **P<.01, ***P<.001 (Student's t-test).
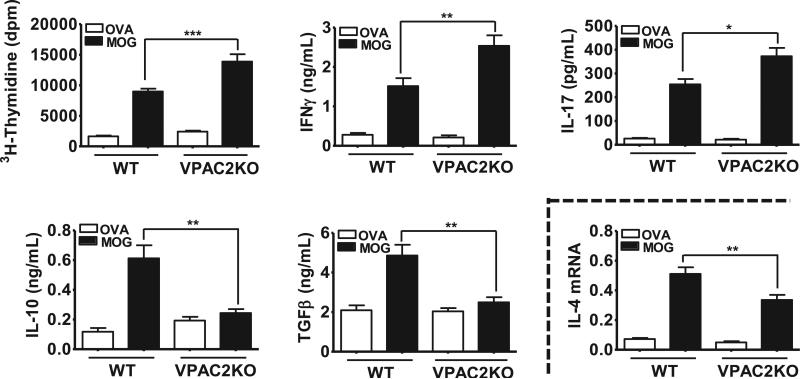
To evaluate the impact of VPAC2 deletion on Th antigen-specific actions, we examined the ability of lymph node T cells from MOG-immunized WT vs VPAC2 KO mice to respond to a MOG re-challenge ex vivo 14 days post-EAE (Figure 3). We found that MOG-driven proliferation and IFNγ (Th1) and IL-17 (Th17) productions were higher in VPAC2 than in WT mice, whereas the antigen-specific inductions of IL-10 and TGFβ, two Treg-associated anti-inflammatory cytokines, were completely blocked in KO mice. The Th2-specific IL-4 cytokine in our cultures was undetectable by ELISA, but IL-4 mRNA levels were diminished in lymph node culture extracts from mutant vs. WT mice (Figure 3). Overall, these results demonstrate that the absence of VPAC2 results in increased Th1 and Th17 and reduced Th2 and Treg responses to MOG, which may contribute to the elevated inflammation in MOG-treated KO mice.
Figure 3. MOG-induced proinflammatory cytokine production and proliferation in lymph node cultures is upregulated in VPAC2 KO mice 14 days after EAE.
Cells (1 × 106 cells/mL) were cultured in complete RPMI 1640 medium with MOG (10 μg/mL) or ovalbumin (OVA) (10 μg/mL). Two days later, cytokine concentrations in supernatants were determined by ELISA and [3H]-thymidine was added for thymidine incorporation assay (see Materials and Methods). Results shown are representative of three independent experiments of n = 8 mice/group. *P<.05, **P<.01, ***P<.001 (Student's t-test).
2.2. VPAC2 KO mice exhibited reduced Treg abundance and proliferation
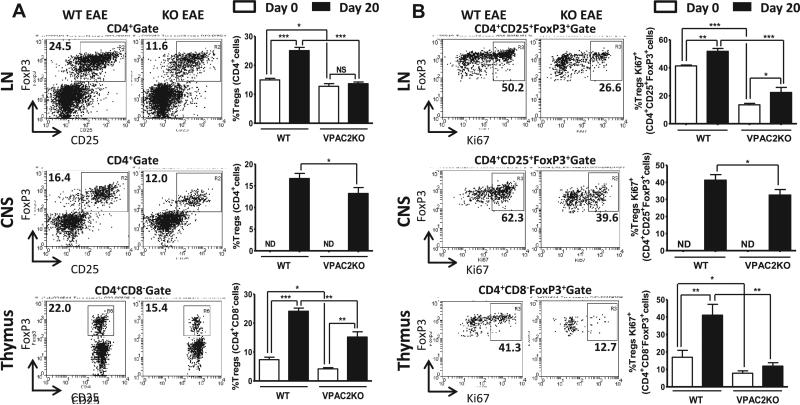
The stronger T cell proliferation and decreased Treg-related cytokine production in VPAC2 KO mice suggested that the abundance and/or suppressive activity of Tregs might be reduced in these mice. To test this hypothesis, we studied the proportions of Tregs as CD4+CD25+FoxP3+ in lymph nodes and CNS, and as CD4+CD8−FoxP3+ in thymus in naïve and MOG-immunized mice, by flow cytometry. The latter was examined on day 20 after MOG administration, when Tregs are expected to have expanded and be most active (40). In naive mice, the proportion of thymic and lymph node Tregs was significantly lower in VPAC2 KO compared to WT mice (Figure 4A). The proportions of FoxP3+ Tregs in all three tissues increased in WT mice after EAE immunization as expected, but these increments were markedly blunted in mice lacking VPAC2 (Figure 4A). To determine potentially-impaired mechanisms regulating the expansion of FoxP3+ Tregs in KO mice, we studied the proliferative rate of these cells using Ki67 as a mitotic marker (Figure 4B). FACS analysis on day 20 showed a blockade of thymic Treg proliferation in receptor-deficient mice. Likewise, we observed a proliferative impairment in lymph node and CNS Tregs in VPAC2 KO compared to WT mice. A reduction in Treg Ki67 staining was also observed in VPAC2 KO naïve animals (Figure 4B), suggesting a defect in the basal proliferation of these cells.
Figure 4. The abundance and proliferative rate of Tregs is significantly reduced in VPAC2 KO mice.
Lymph nodes (LN), CNS and thymus were harvested from WT and VPAC2 KO mice on days 0 and 20 after MOG-immunization. A) Treg abundance was assessed by FACS using CD4, CD25 and Foxp3 antibodies for lymph nodes and CNS, and CD4, CD8 and Foxp3 for thymus; In addition to these markers, Ki67 was used to determine Treg proliferation (B). Representative FACS plots are shown on the left panels. Results shown are representative of three independent experiments of n = 8 mice/group. *P<.05, **P<.01, ***P<.001 (Student's t-test).
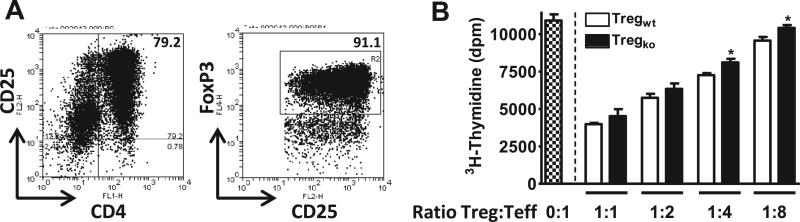
The above data strongly imply that VPAC2 receptor activation is critically-required for proper Treg production during homeostasis and inflammation. It has been previously reported that Tregs isolated from VIP-treated mice with EAE exhibit enhanced suppressive function (35). To investigate a specific involvement of the VPAC2 receptor in modulating Treg function, titrated numbers of Tregs from WT and receptor-deficient mice were incubated with MOG and fixed quantities of responder Teff cells and APCs from spleen and lymph nodes from MOG-immunized WT mice. The purity of Tregs was on average ~80% as assessed by flow cytometry, and 90-95% of these cells expressed Foxp3 (Figure 5A). Although both WT and KO Tregs reduced MOG-specific Teff proliferation, VPAC2 KO Tregs were less efficient than WT Tregs, significantly at low Treg:Teff ratios (1:4 and 1:8) (Figure 5B).
Figure 5. VPAC2 KO Tregs exhibit reduced in vitro suppressive activity.
The Treg suppressive activity of CD4+CD25+ Tregs from WT vs VPAC2 KO spleens and lymph nodes over WT Teff cell proliferation was determined by [3H]-thymidine incorporation assay. (A) Purity of isolated Tregs prior to culture was determined by flow cytometry. Representative plots of CD4+CD25+ cells and Foxp3+ cells (gated on CD4+CD25+ cells) are shown. (B) Tregs and Teffs at different ratios (Treg:Teff = 0:1, 1:1, 1:2, 1:4, 1:8) were co-cultured with WT APCs in complete RPMI 1640 medium with IL-2 (50U/ml) and MOG (20μg/mL). Proliferation was measured 48 hours after culture by thymidine incorporation assay (see Materials and methods). Results shown are representative of three independent experiments of n = 6 mice/group. In addition, cultures were performed in triplicate. *P<.05 (Student's t-test).
2.3. Dynamic changes in VPAC2 receptor gene expression in murine thymus during EAE, and enrichment in FoxP3+ Tregs
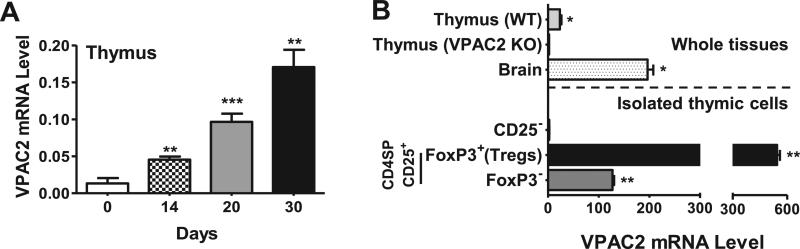
Our results demonstrated that the global deletion of VPAC2 has a significant impact on Tregs in the thymus, a site of de novo production of natural Tregs. We found that VPAC2 mRNA expression in total thymic extracts of naïve WT mice was strongly upregulated after MOG-induced EAE (Figure 6A). To investigate if VPAC2 receptors are expressed within the Treg subpopulation, we purified Tregs from thymi of FoxP3EGFP mice (39) as CD4+CD8−CD25+FoxP3EGFP+ cells. Real time RT-PCR analysis revealed a strong enrichment of VPAC2 transcripts in the thymic FoxP3EGFP+ Treg cell fraction compared to total thymus and to thymic cell subpopulations that were not FoxP3EGFP+ (Figure 6B). This result indicates a high likelihood that the VPAC2 receptor is expressed on Tregs.
Figure 6. VPAC2 mRNA in WT thymus is upregulated during EAE and is enriched in FoxP3+ Tregs.
A) The expression of VPAC2 in whole thymi of WT mice on days 0, 14, 20, and 30 after MOG administration was determined by real time RT-PCR. Results shown are representative of two independent experiments of n = 6 mice/group. B) Thymic cells from FoxP3GFP+ transgenic mice were purified using anti-CD25 magnetic particles (according to StemCell Technology's instructions) and then sorted by FACS to obtain CD4+CD8−Foxp3GFP+ cells. RNA was prepared and reversed transcribed along with equal amounts of RNA from whole WT and VPAC2 receptor KO thymi, WT brain (positive control), and Foxp3GFP- thymic populations resultant from the magnetic and FACS sorting isolations. Real time RT-PCR was performed, using HPRT as a housekeeping gene. Results shown are representative of two independent experiments with n = 3. For all experiments, *P<.05, **P<.01, ***P<.001 (Student's t-test).
2.4. Tregs from VPAC2 KO mice exhibit impaired proliferation ex vivo
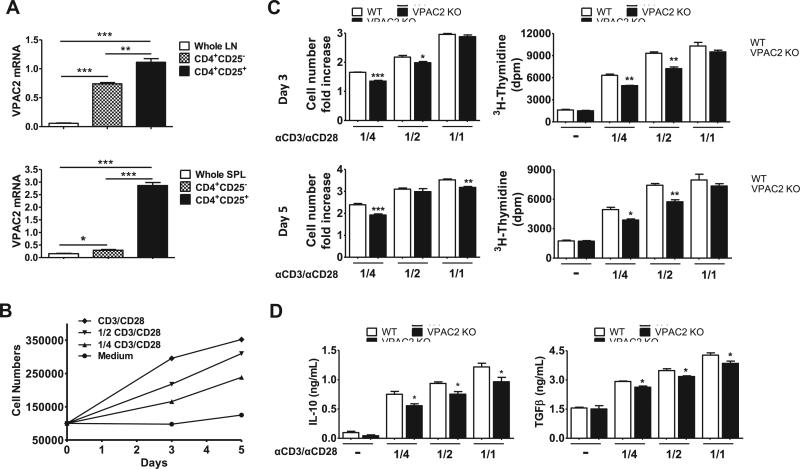
VPAC2 mRNA transcripts were also significantly enriched in Tregs isolated from lymph nodes and spleens of naïve WT mice (Figure 7A). We thus compared the ability of pooled lymph node and splenic Tregs from WT vs. VPAC2 KO to expand ex vivo in the presence of IL-2 (2000U/ml) and manufacturer-recommended and serial dilutions of anti-CD28/CD3 MACSiBead™ particles. Reducing concentrations of anti-CD28/CD3 beads to 1/2 and 1/4 of that recommended resulted in diminished expansion of WT Tregs (Figure 7B). Using this assay, we found that VPAC2 KO Tregs exhibited lower fold increases in cell numbers compared to WT Tregs (Figure 7C, left panels). As another measurement of Treg proliferation, the rate of 3H-thymidine incorporation was determined. For this, cells obtained from day 3 and 5 cultures were expanded in the same conditions for an additional period of 2 days, and the rate of 3H-thymidine incorporation in the last 18 hours was measured (Figure 7C, right panels). Based on this parameter, we found that the proliferative rate of VPAC2 KO Tregs was lower than that in WT Tregs. In addition, we measured the levels of IL-10 and TGFβ, two cytokines that are produced by Tregs and involved in their suppressive activity, after 5 days of expansion (Figure 7D). The levels of these cytokines were diminished in VPAC2 KO cultures. These data suggests that Tregs from MOG-induced VPAC2 KO mice exhibit an intrinsic defect in ability to expand ex vivo.
Figure 7. Tregs from VPAC2 KO mice exhibit reduced expansion ex vivo.
A) VPAC2 receptor gene expression in naïve WT whole axillary lymph nodes (LN) and spleens (SPL) vs CD4+CD25+ Tregs and CD4+CD25− cells isolated by magnetic separation. Results shown are representative of two independent experiments of n = 3. B) Expansion of naïve WT Tregs in the presence of IL-2 (2000U/ml) and different concentrations of CD3/CD28 MACSiBead™ Particles according to the manufacturer's recommendations (undiluted, 1/2, 1/4). C) Fold increase in cell numbers and [3H]-thymidine incorporation of WT vs VPAC2 KO Tregs after 3 and 5 days (upper and lower panels, respectively) of culture. D) IL-10 and TGFβ levels in the supernatants were determined by ELISA in the end of the culture (day 5). For all cultures (B, C, D) results shown are representative of four independent experiments with cultures performed in triplicate. For all experiments, *P<.05, **P<.01, ***P<.001 (Student's t-test).
3. Discussion
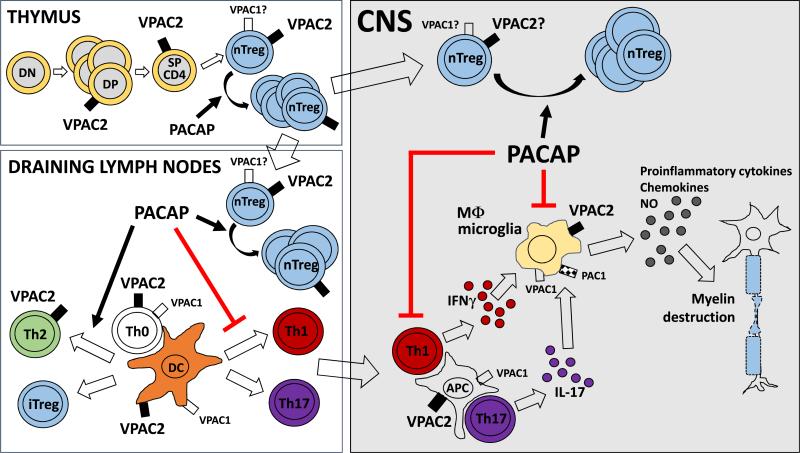
We found that mice lacking the VIP/PACAP receptor VPAC2 developed enhanced EAE, with exacerbated inflammatory Th1/Th17 responses and a remarkable reduction in Th2 and Treg cells. The immunological phenotype of VPAC2 KO mice in response to EAE induction correlates with the well-known anti-inflammatory actions of its ligands VIP and PACAP (12). Similarly, in previous work, we found that PACAP-deficient mice were more sensitive to EAE induction, with qualitatively similar Th/Treg alterations to those in VPAC2 KO mice. Nevertheless, VIP KO mice were unexpectedly resistant to develop clinical EAE (41). Therefore, investigating which receptor modulates the opposing PACAP and VIP KO clinical phenotypes is key to unravel the role of this peptide/receptor system in the pathogenesis of EAE, and to potentially design receptor targeted therapies. Our findings in these ligand and receptor KO mice suggest that the PACAP/VPAC2 signaling pathway may play a protective role in autoimmune disease through mechanisms summarized in Figure 8 (37).
Figure 8. PACAP/VPAC2 interactions in autoimmune neuro-inflammation: Inferences from gene knockout studies in the MOG EAE model.
This scheme represents the potential actions of PACAP/VPAC2 signaling on two critical immune cell types during EAE pathogenesis: antigen presenting cells (APCs) and T lymphocytes. VPAC2 receptors are expressed on APCs (macrophages (Mφ) and dendritic cells (DC)), and on activated T CD4+ lymphocytes. In addition, VPAC2 receptor gene transcripts have been demonstrated in resting thymocytes (DP=double positive and SP= single positive), and, as shown here, are enriched in thymic, splenic and lymph node Tregs. VPAC1 receptors, on the other hand, are constitutively expressed on both APCs and T CD4+ cells, but are rapidly down-regulated in T cells upon activation. PAC1 receptors appear to be much more restricted in the immune system, with gene expression observed only in macrophages. Pharmacological studies indicate that all three receptors exert primarily anti-inflammatory actions. Our gene knockout studies point to a critical requirement for PACAP and VPAC2 signaling in mediating and/or maintaining nTreg expansion in the thymus, draining lymph nodes and CNS during EAE. Whether or not this effect is mediated exclusively through direct actions on Tregs or indirect through APCs or other cells remains to be elucidated. In addition, the studies show that signaling through the VPAC2 receptor is involved in the modulation of Th profiles, by promoting Th2 vs. Th1/Th17 responses in draining lymph nodes and the CNS. Finally, pharmacological studies indicate that PACAP through VPAC2 may potentially inhibit the proinflammatory activity of Mφ/microglia in the CNS.
Evidence using pharmacological approaches has suggested that the VPAC1 receptor mediates the immunoregulatory properties of VIP and PACAP with stronger activity than VPAC2, decreasing the interest in this latter receptor. Nonetheless, a specific although mild immunomodulatory role of VPAC2 has been demonstrated in different in vitro and in vivo experimental models. For example, the VPAC2 agonist Ro25-1553 significantly decreased the production of TNFα and IL-12 by LPS-stimulated peritoneal macrophages and human monocytes in vitro (20, 42), and was partially beneficial in mouse LPS-induced endotoxemia (20). These studies were performed by administration for a short period of time of receptor analogs. Here, we demonstrate that the long-term absence of VPAC2 results in remarkable enhanced inflammation when mice are challenged to EAE, and particularly to a strong impairment in the Treg compartment. Moreover, it has been recently reported that VPAC2 KO mice exhibited exacerbated acute dextran sodium sulphate-induced colitis, with increased levels of the proinflammatory IL-1β, IL-6 and metalloprotease (MMP)-9, despite no differences of Treg proportions in the spleen, mesenteric lymph nodes and Peyer's patches were found in this model (43). Besides its potential involvement during innate immunity, an important role for VPAC2 receptor in the induction of Th2 responses has been demonstrated (22). For example, VPAC2 signaling on T cells upregulated the expression of the Th2-associated transcription factors c-Maf and JunB in vitro and the production of the respective target genes IL-4 and IL-5, leading to a Th2-type phenotype (22). Moreover, VPAC2 KO mice exhibited enhanced cutaneous delayed-type hypersensitivity in response to hapten, in association with increased Th1 vs Th2 splenocyte responses to in vitro anti-CD3/CD28 stimulation (44), whereas mice with enforced expression of VPAC2 in TCD4+ cells exhibited depressed delayed-type hypersensitivity, and a heightened allergic state (45). The present studies demonstrate a critical role for VPAC2 in both Th polarization and Treg expansion in the MOG EAE model (Figure 8).
Interestingly, prior data implicated the VPAC1 receptor in the production of the inducible form of Tregs (iTregs) in the murine model of relapsing-remitting EAE (35). In addition, VIP was reported to promote tolerogenic dendritic cells which induce the generation of iTregs, an effect that was VPAC1 dependent (27). It is noteworthy, however, that natural Tregs (nTregs), and not iTregs, are produced in the acute MOG C57BL/6 EAE model employed in our studies (40). Thus, the current findings implicating VPAC2 receptor involvement in nTreg expansion cannot be extrapolated to iTregs, or vice-versa. Moreover, although our studies imply a critical requirement for VPAC2 in nTreg expansion, they do not argue against any involvement of VPAC1 in the expansion of these cells. Interestingly, it has been shown that VPAC2 gene expression predominates over that of VPAC1 in the thymus, the site of de novo nTreg production (46). In that study, VPAC2 was found to be present in non-stimulated double positive and single positive human thymic T cells. We found that VPAC2 mRNA transcripts were highly enriched in mouse thymic Tregs, and that VPAC2 expression in the thymus increased as EAE progressed. Similarly, we found that this receptor was expressed in Tregs from naïve C57BL/6 mice lymph nodes and spleen. The presence of VPAC2 on Tregs suggests that VIP and PACAP may act directly on these cells to modulate their expansion and/or functionality.
Several lines of investigation have demonstrated the importance of Tregs in the pathogenesis of EAE. In fact, Tregs accumulate in the CNS during the recovery phase of the disease, and their elimination prior to immunization abrogated the natural recovery from EAE (40, 47). It has been also demonstrated in MOG35-55-induced EAE that the thymus is critically required for the observed increase of Tregs in the periphery (48). Thus, the striking reduction in Treg frequency in the thymus of VPAC2 KO mice prior to and after MOG-immunization, may contribute to the decrease in their peripheral numbers. Although several mechanisms may account for the reduction in Tregs in VPAC2 KO mice, we found that their proliferative activity was significantly reduced. In addition, we found an impairment in their in vitro suppressive activity. Although the molecular mechanisms for such effects remain to be elucidated, it has been suggested that cAMP, one of the main signal transducers of VPAC2, is essential to maintain Treg suppressive activity (49-51).
Deficits in Treg frequency, proliferation potential and suppressive activity have been described in patients with relapsing-remitting MS (52). In this line, the use of agents that reestablish or increase Tregs numbers or their immunosuppressive function has proven beneficial in EAE and MS. In fact, the strong amelioration of PLP-induced EAE by VIP administration in SJL mice was associated with an enrichment of Tregs in the lymph nodes and the CNS (35). Some of the currently used drugs for the treatment of MS such as glatiramer acetate or interferon beta elevate Treg suppressive activity and/or numbers in patients (53-55). Nevertheless, the efficiency of these treatments is not optimal.
4. Conclusion
A critical step towards the development of new therapeutical protocols in autoimmune diseases like MS based on the VIP/PACAP/receptor system is the design of highly-specific and stable agonists to minimize undesirable side effects. Our results highlight not only the in vivo immunological relevance of VPAC2 in the pathogenesis of EAE, but also its potential as a novel target to control inflammation and enhance Treg expansion and functionality.
Supplementary Material
Highlights.
Loss of VPAC2 receptor results in exacerbated EAE
Th1 and Th17 responses were enhanced and Th2 diminished in VPAC2 KO mice with EAE
VPAC2 KO mice exhibited impaired Treg expansion in thymus, lymph nodes and CNS
VPAC2 plays a protective role from autoimmunity maintaining the Treg population
Acknowledgements
This work was supported by National Multiple Sclerosis Society RG4859, TA3048_A_1 and National Institutes of Health (NIH) HD04612.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 2.Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- 3.Pettersson LM, Heine T, Verge VM, Sundler F, Danielsen N. PACAP mRNA is expressed in rat spinal cord neurons. J Comp Neurol. 2004;471:85–96. doi: 10.1002/cne.20015. [DOI] [PubMed] [Google Scholar]
- 4.Tanida M, Shintani N, Morita Y, Tsukiyama N, Hatanaka M, Hashimoto H, Sawai H, Baba A, Nagai K. Regulation of autonomic nerve activities by central pituitary adenylate cyclase-activating polypeptide. Regul Pept. 2010;161:73–80. doi: 10.1016/j.regpep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 5.May V, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem. 1995;65:978–987. doi: 10.1046/j.1471-4159.1995.65030978.x. [DOI] [PubMed] [Google Scholar]
- 6.Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–3954. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- 7.Braas KM, Schutz KC, Bond JP, Vizzard MA, Girard BM, May V. Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons. Peptides. 2007;28:1856–1870. doi: 10.1016/j.peptides.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard BM, Keller ET, Schutz KC, May V, Braas KM. Pituitary adenylate cyclase activating polypeptide and PAC1 receptor signaling increase Homer 1a expression in central and peripheral neurons. Regul Pept. 2004;123:107–116. doi: 10.1016/j.regpep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinger DL, Lorton D, Horn L, Brouxhon S, Felten SY, Felten DL. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18:1139–1149. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 12.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 13.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol. 1999;96:167–181. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 14.Lara-Marquez M, O'Dorisio M, O'Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 15.Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J. 2004;18:1325–1334. doi: 10.1096/fj.03-1440hyp. [DOI] [PubMed] [Google Scholar]
- 16.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Rey E, Varela N, Chorny A, Delgado M. Therapeutical approaches of vasoactive intestinal peptide as a pleiotropic immunomodulator. Curr Pharm Des. 2007;13:1113–1139. doi: 10.2174/138161207780618966. [DOI] [PubMed] [Google Scholar]
- 18.Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001;167:966–975. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- 19.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999;162:1707–1716. [PubMed] [Google Scholar]
- 20.Delgado M, Gomariz RP, Martinez C, Abad C, Leceta J. Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol. 2000;30:3236–3246. doi: 10.1002/1521-4141(200011)30:11<3236::AID-IMMU3236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J. 2002;16:1844–1846. doi: 10.1096/fj.02-0248fje. [DOI] [PubMed] [Google Scholar]
- 22.Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- 23.Voice JK, Dorsam G, Chan RC, Grinninger C, Kong Y, Goetzl EJ. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul Pept. 2002;109:199–208. doi: 10.1016/s0167-0115(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 24.Delgado M, Sun W, Leceta J, Ganea D. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999;163:4213–4223. [PubMed] [Google Scholar]
- 25.Delgado M, Leceta J, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999;163:3629–3635. [PubMed] [Google Scholar]
- 26.Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- 27.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107:3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 30.Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–3189. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- 31.Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 32.Abad C, Juarranz Y, Martinez C, Arranz A, Rosignoli F, Garcia-Gomez M, Leceta J, Gomariz RP. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11:674–684. doi: 10.1097/01.mib.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- 33.Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, Ueda R, Suzumura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36:318–326. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- 36.Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan YV, Abad C, Wang Y, Lopez R, Waschek JA. Pituitary adenylate cyclase activating peptide deficient mice exhibit impaired thymic and extrathymic regulatory T cell proliferation during EAE. PLoS One. 2013;8:e61200. doi: 10.1371/journal.pone.0061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harmar AJ. An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol. 2003;15:335–338. doi: 10.1046/j.1365-2826.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- 39.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 40.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, Feng JM, Campagnoni AT, Waschek JA. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewit D, Gourlet P, Amraoui Z, Vertongen P, Willems F, Robberecht P, Goldman M. The vasoactive intestinal peptide analogue RO25-1553 inhibits the production of TNF and IL-12 by LPS-activated monocytes. Immunol Lett. 1998;60:57–60. doi: 10.1016/s0165-2478(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 43.Yadav M, Huang MC, Goetzl EJ. VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol. 2011;267:124–132. doi: 10.1016/j.cellimm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, West KM, Morrison CF, Harmar AJ. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 2001;98:13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voice JK, Dorsam G, Lee H, Kong Y, Goetzl EJ. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 2001;15:2489–2496. doi: 10.1096/fj.01-0671com. [DOI] [PubMed] [Google Scholar]
- 46.Lara-Marquez ML, O'Dorisio MS, Karacay B. Vasoactive intestinal peptide (VIP) receptor type 2 (VPAC2) is the predominant receptor expressed in human thymocytes. Ann N Y Acad Sci. 2000;921:45–54. doi: 10.1111/j.1749-6632.2000.tb06950.x. [DOI] [PubMed] [Google Scholar]
- 47.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Fang L, Song S, Guo TB, Liu A, Zhang JZ. Thymic regulation of autoimmune disease by accelerated differentiation of Foxp3+ regulatory T cells through IL-7 signaling pathway. J Immunol. 2009;183:6135–6144. doi: 10.4049/jimmunol.0901576. [DOI] [PubMed] [Google Scholar]
- 49.Vang AG, Housley W, Dong H, Basole C, Ben-Sasson SZ, Kream BE, Epstein PM, Clark RB, Brocke S. Regulatory T-cells and cAMP suppress effector T-cells independently of PKA-CREM/ICER: a potential role for Epac. Biochem J. 2013;456:463–473. doi: 10.1042/BJ20130064. [DOI] [PubMed] [Google Scholar]
- 50.Lahl K, Mayer CT, Bopp T, Huehn J, Loddenkemper C, Eberl G, Wirnsberger G, Dornmair K, Geffers R, Schmitt E, Buer J, Sparwasser T. Nonfunctional regulatory T cells and defective control of Th2 cytokine production in natural scurfy mutant mice. J Immunol. 2009;183:5662–5672. doi: 10.4049/jimmunol.0803762. [DOI] [PubMed] [Google Scholar]
- 51.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 53.Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J Neuroimmunol. 2009;216:113–117. doi: 10.1016/j.jneuroim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Korporal M, Haas J, Balint B, Fritzsching B, Schwarz A, Moeller S, Fritz B, Suri-Payer E, Wildemann B. Interferon beta-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells. Arch Neurol. 2008;65:1434–1439. doi: 10.1001/archneur.65.11.1434. [DOI] [PubMed] [Google Scholar]
- 55.Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M. Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study. J Neuroimmunol. 2010;218:120–124. doi: 10.1016/j.jneuroim.2009.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.