Abstract
Polyphenolic compounds (anthocyanins, flavonoid glycosides) in berries prevent the initiation, promotion, and progression of carcinogenesis in rat’s digestive tract and esophagus, in part, via anti-inflammatory pathways. Angiogenesis has been implicated in the pathogenesis of chronic inflammation and tumorigenesis. In this study, we investigated the anti-inflammatory and anti-angiogenic effects of black raspberry extract (BRE) on two organ specific primary human intestinal microvascular endothelial cells, (HIMEC) and human esophageal microvascular endothelial cells (HEMEC), isolated from surgically resected human intestinal and donor discarded esophagus, respectively.
HEMEC and HIMEC were stimulated with TNF-α/IL-1β with or without BRE. The anti-inflammatory effects of BRE were assessed based upon COX-2, ICAM-1 and VCAM-1 gene and protein expression, PGE2 production, NFκB p65 subunit nuclear translocation as well as endothelial-leukocyte adhesion. The anti-angiogenic effects of BRE were assessed on cell migration, proliferation and tube formation following VEGF stimulation as well as on activation of Akt, MAPK and JNK signaling pathways.
BRE inhibited TNF-α/IL-1β-induced NFκB p65 nuclear translocation, PGE2 production, up-regulation of COX-2, ICAM-1 and VCAM-1 gene and protein expression and leukocyte binding in HEMEC but not in HIMEC. BRE attenuated VEGF-induced cell migration, proliferation and tube formation in both HEMEC and HIMEC. The anti-angiogenic effect of BRE is mediated by inhibition of Akt, MAPK and JNK phosphorylations.
BRE exerted differential anti-inflammatory effects between HEMEC and HIMEC following TNF-α/IL-1β activation whereas demonstrated similar anti-angiogenic effects following VEGF stimulation in both cell lines. These findings may provide more insight into the anti-tumorigenic capacities of BRE in human disease and cancer.
INTRODUCTION
Low toxicity of natural foods is an alluring possibility for treatment of inflammatory disease, as well as cancer (Xue et al., 2001) (La Vecchia and Tavani, 1998) (Stoner and Wang, 2013). Berries, in particular black raspberries (BR), contain high amounts of antioxidant polyphenols, including ellagic acid and anthocyanins (Kahkonen and Heinonen, 2003) (Noda et al., 2002). (Kahkonen and Heinonen, 2003) Modulation of cyclooxygenase-2 (COX-2), interleukins (ILs) and nuclear factor kappa B (NFκB) expression by black raspberries have been reported (Chen et al., 2006b) (Seeram et al., 2001) (Madhusoodhanan et al., 2010). Anthocyanins, the most abundant and active constituents in black raspberries with potent inhibitory activity are; cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-(2G-xylosylrutinoside which are responsible for its chemopreventive activity (Hecht et al., 2006) (Wang and Stoner, 2008) (Wang et al., 2009). Furthermore, the role of anthocyanins in eliminating free radicals and increased radical-absorbing capability of cells has been shown (Yi et al., 2010) (Furuno et al., 2002). Suppression of carcinogen-induced esophageal and colon cancer in rats, inhibition of intestinal tumors in ApcMin/+ mice and treatment of UV-induced skin tumors in rats by freeze-dried black raspberries have been reported (Stoner and Wang, 2013) (Wang et al., 2012) (Fodde et al., 1994) (Velcich et al., 2002) (Duncan et al., 2009) in addition to its benefits and its antioxidant effects on coronary heart disease and chronic diseases (Grassi et al., 2009). However, the effect of BRE on cytokine and chemokine (e.g. TNF-α, IL-1β) induced human microvascular endothelial activation and up-regulation of inflammatory pathways, which are characteristic of inflammatory bowel disease and esophagitis, has not been elucidated.
Microvascular endothelial cells (ECs) of different organs possess distinct structural, phenotypic, and functional characteristics. Established tissue-specific molecular libraries of ECs demonstrate the attributes of ECs with their organotypic characteristics (Nolan et al., 2013). These libraries identify how adhesion molecules, transcription factors, angiogenic factors, and chemokines are expressed in selected and precise combinations by ECs of each organ and how specifically they react with other cells in tissue micro-environment (Nolan et al., 2013). In inflammatory processes of numerous organs, exposure of ECs to a various stimuli, including cytokines and chemokines provoke distinct reactions in ECs (Molema, 2010) (Muller et al., 2002). Even though the analyses of in vitro studies are limited, but they point to the EC heterogeneity in vivo (Borsum et al., 1982).
In inflammatory bowel disease [IBD; Crohn’s Disease (CD) and ulcerative colitis (UC)] activation of microvascular endothelial cells and adhesion of circulating immune cells requires for the initiation and maintenance of the inflammation and angiogenesis (Binion and Rafiee, 2009). Angiogenesis, the formation of new blood vessels is a crucial mechanism in cancer progression an anti-angiogenic therapy in animal models of IBD has shown beneficial effects (Binion and Rafiee, 2009). To better understand the pathogenesis of chronic inflammatory disease and cancer, it is imperative to characterize the mechanisms underlying microvascular dysfunction (Binion and Rafiee, 2009). The effect of black raspberry extract on microvascular endothelial function, specifically on their ability to regulate leukocyte adhesion during inflammatory activation and angiogenesis is currently undefined.
In this study, we set out to investigate the anti-inflammatory effect of BRE on TNF-α/ IL-1β activated human esophageal microvascular endothelial cells (HEMEC) and human intestinal microvascular endothelial cells (HIMEC) with respect to NFκB activation, cell adhesion molecules expression, COX-2 gene and protein expression and PGE2 production. Furthermore, we determined the anti-angiogenic effect of BRE on VEGF-induced angiogenesis in both HIMEC and HEMEC. We demonstrated that BRE exerts differential anti-inflammatory effects on HEMEC and HIMEC, being a strong anti-inflammatory agent affecting HEMEC, without any effect on HIMEC. However, BRE demonstrated to be a strong anti-angiogenic agent affecting both HIMEC and HEMEC. These findings add to our knowledge of the anti-inflammatory and anti-tumorigenic capacities of BRE and its use in human disease and cancer.
MATERIALS AND METHODS
Reagents
Endothelial Cell Growth Supplement (ECGS) was from Upstate Cell Signaling Solutions (Temecula, CA). RPMI 1640 medium, Fetal Bovine Serum (FBS), MCDB-131 medium, and PSF (penicillin/streptomycin/fungizone) obtained from Invitrogen (Carlsbad, CA). Human plasma fibronectin was from Chemicon International (Temecula, CA). Porcine heparin was from Sigma-Aldrich (St. Louis, MO). ICAM-1 and VCAM-1 ELISA kits were from R&D Systems (Minneapolis, MN). PGE2 ELISA kit was from Cayman Chemical (Ann Arbor, Michigan). Antibodies against COX2 and NFκB p65 were from Cell Signaling Technology (Danvers, MA). HRP tagged secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). RNeasy RNA Extraction Kit was from Qiagen (Valencia, CA). iScript cDNA Synthesis Kit, SYBR Green Master Mix and electrophoresis reagents were from Bio-Rad (Hercules, CA). Oligonucleotides and primers were from IDT (Integrated DNA Technologies, Coralville, IA). Matrigel™ was from BD Biosciences (Bedford, MA). Unless otherwise indicated all other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO).
Isolation of HIMEC and HEMEC
The Institutional Review Board of the Medical College of Wisconsin approved the use of human tissue and all experimental procedures. Primary human intestinal microvascular endothelial cells (HIMEC) were isolated from uninvolved (disease free n=10) surgical specimens from inflammatory bowel disease patients [IBD; Crohn’s Disease (CD) n=5 and ulcerative colitis (UC) n=5] as described previously (Binion et al., 1997). HEMEC cultures were generated from normal donor tissue (n=7) from people who were involved in car (n=4), motorcycle (n=2) and boat crush (n=1) as described previously (Rafiee et al., 2003). Endothelial cultures were recognized by modified lipoprotein uptake (Dil-ac-LDL, Life Technologies, Grand Island, NY) and expression of Factor VIII associated antigen (von Willebrand) microscopically, all experiments were carried out using the cells at passages 8 12 (Binion et al., 1997) (Rafiee et al., 2003).
Black Raspberry Extract Treatment
Enriched anthocyanins fraction of BRE ethanol extract (1 mg concentration) provided by Dr. Gary D. Stoner (Medical College of Wisconsin) was diluted in DMSO. The molar concentrations of 3 major components of BRE at 100ug/ml used in this study are: 4.3 mM cyanidin-3-O-glucoside, 1.26 mM cyanidin-3-O-rutinoside, and 5.6 mM cyanidin-3-O-(2G-xylosylrutinoside. Endothelial monolayers were pre-treated with BRE (100 ug/ml) for 2 h, and then activated with TNF-α and IL-1β (100 U/ml each) or stimulated with VEGF (100ng/ml) for the indicated time points. BRE demonstrated no toxicity at the dosage used in this study and cell survival was not affected.
RNA preparation and Real-time PCR
Using Qiagen’s RNeasy Plus Mini Kit, RNA isolation was done according to manufacturer’s instructions. Reverse transcription was done with 1 μg of RNA using Bio-Rad’s iScript cDNA synthesis kit for RT-PCR. Real-time PCR was done using Bio-Rad’s SYBR Green Master Mix, 2 μl of cDNA, and 250 nM primers in 25 μl reactions as previously described (Rafiee et al., 2010a). Real-time data was analyzed with Bio-Rad’s iQ5 software. Primer sequences were as follows: ICAM-1 forward 5’- CAA TGT GCT ATT CAA ACT GCC C -3’ and reverse 5’- CAG CGT AGG GTA AGG TTC TTG -3’; VCAM-1 forward 5’- TGT TGA GAT CTC CCC TGG AC -3’ and reverse 5’- CGC TCA GAG GGC TGT CTA TC-3’; COX2 forward 5’- CAA ATC CTT GCT GTT CCC ACC CAT -3’ and reverse 5’- AGT CAC GTT TGA TGG CTT CC -3’; GAPDH forward 5’-TGC ACC ACC AAC TGC TTA GC-3’ and reverse 5’-GGC ATG GAC TGT GGT CAT GAG-3’; β-actin forward 5’- CAC TCT TCC AGC CTT CCT TC -3’ and reverse 5’- GGT GTA ACG CAA CTA AGT CAT AG -3’. All experiments were verified three times.
Assessment of CAM expression by ELISA
Culture media of activated HEMEC and HIMEC with or without BRE treatment (from above) were collected after 24h. Expression of cell adhesion molecules ICAM-1 and VCAM-1 were determined by ELISA assay according to the manufacturer protocol. Data from triplicate wells were expressed as the means of pg/ml. A nonspecific monoclonal antibody at equal concentrations and incubation conditions was used as control (Binion et al., 2009).
Endothelial-leukocyte adhesion assay
Adhesion assays were performed as previously described (Rafiee et al., 2010a) (Binion et al., 2009). Briefly, ECs were seeded onto fibronectin-coated 24-well plate (0.5 × 105 cells/well). Activation and treatment was carried as above and after 24–48 h the cells were rinsed, and co-cultured with U937 leukocytes like cells (1×106/ml) for 1h at 37°C in a 5% CO2 incubator. Non-adherent cells were removed and rinsed three times with PBS. Using a modified Wright’s stain (Diff-Quik Stain; Baxter Scientific, McGraw, IL) adhered cells were fixed, stained and counted in 10 high-power fields (X20). Data was expressed as number of adherent leukocytes/mm2 (Rafiee et al., 2010a) (Binion et al., 2009).
Western Blot Analysis
SDS-PAGE and Western blot analysis were performed using specific antibodies to COX2, NFkB (p65), Akt and MAPK as described previously (Rafiee et al., 2010a) (Binion et al., 2009).
PGE2 production in HEMEC and HIMEC culture medium
Confluent cell monolayers were assayed as previously described (Ogawa et al., 2003) (Binion et al., 2008). Briefly, culture media from TNF-α/IL-1β-activated cells with or without BRE were used to measure PGE2 production. Using a commercial ELISA kit, the concentration of PGE2 in the culture supernatant was determined following the manufacturer protocol. Experiments were carried out in triplicate and results are shown as mean pg/ml (SD).
Immunofluorescence Staining
Immuno-staining was performed using NFkB p65 subunit antibody and a secondary antibody FITC- conjugated and using 4,6-diamidino-2-phenylindole (DAPI) for nuclear staining as described previously (Rafiee et al., 2010a) (Binion et al., 2008). Using Olympus BX-40 microscope and a Leica DFC 300FX camera coverslips were visualized.
Cell Migration assay
Using microscopic wounding assay effect of BRE on VEGF-induced endothelial growth and migration was quantified by measuring endothelial migration across a leading edge in vitro as described previously (Rafiee et al., 2004) (Rafiee et al., 2010b). Briefly, confluent monolayer of HEMEC and HIMEC were scraped and wiped along a straight line, and the remaining monolayer was stimulated with VEGF (100ng/ml) with or without BRE (100μg/ml for 2 h) then incubated at 37°C for indicated time pointe. The migration of endothelial cells across the demarcation line was monitored using an inverted microscope. At each time point (24, 48, and 72 h), 10 random fields using an ocular grid were counted in a blinded fashion. Experiments were repeated in 3 independent cell cultures. Data were expressed as cells/mm2 and each condition was assessed in triplicate and expressed as a mean ±S.D.
Cell Proliferation Assay
Cellular DNA synthesis was assessed by [3H]thymidine uptake as described previously (Ogawa et al., 2003) (Binion et al., 2008) (Rafiee et al., 2010b). Briefly, endothelial cells (1×104) were seeded in 24-well plates and grown in media containing 10% FBS for 24 h, then the media was replaced and the cells were incubated in media containing 1% FBS for another 12 h. Next, the cells with or without BRE (100 μg/ml) pretreatment (2h) were stimulated with VEGF (100 ng/ml) and incubated for 24h. After that 1μCi/ml of [3H]thymidine (Perkin Elmer, Waltham, MA) was added to cells and incubated for another 5 h. Then, the cells were washed twice with PBS and fixed with 5% (v/v) trichloroacetic acid for 10 min on ice. Using NaOH (0.25 N) cells were lysed and DNA was released from precipitated material. The supernatants were quantified in a Beckman Liquid Scintillation System. Each condition was assessed in triplicate and data expressed as a mean ±S.D.
Cell Survival Assays
HEMEC and HIMEC were grown to 70% confluence then stimulated with VEGF (100ng/ml) with or without BRE (100μg/ml for 2 h) pretreatment or were left untreated at 37°C for 10 days. After 10 days, the cells were stained with Trypan blue and five random high-power fields were counted using an ocular grid as previously described (Rafiee et al., 2004) (Binion et al., 2008).
Matrigel™ In Vitro-Tube Formation Assay
ECs tube formation in Matrigel™ was carried on as described previously (Rafiee et al., 2004) (Binion et al., 2008) (Rafiee et al., 2010b). Briefly, HEMEC and HIMEC were grown to 70% confluence then stimulated with VEGF (100ng/ml) with or without BRE (100μg/ml for 2 h) pretreatment or were left untreated at 37°C for 10 days. After 10 days, the cells were stained with Trypan blue and five random high-power fields were counted using an ocular grid as previously described (Rafiee et al., 2004) (Binion et al., 2008).
Statistical analysis
Analysis of variance was performed using StatView for Macintosh. P≤ 0.05 was considered significant, and data shown are mean ± S.E.
RESULTS
BRE attenuates CAMs expression in TNF-α/IL-1β-activated HEMEC but not HIMEC
To test the hypothesis that BRE would inhibit the pro-inflammatory effect of TNF-α/IL-1β by attenuating the CAM expression, we examined the effect of BRE on ICAM-1 and VCAM-1 gene and protein expression in resting and TNF-α/IL-1β-activated HEMEC and HIMEC. As expected, expression of ICAM-1 and VCAM-1 in resting ECs was low but upon TNF-α/IL-1β activation was considerably increased in both cell lines (Binion et al., 1997) (Binion et al., 2009). The level of ICAM-1 and VCAM-1 gene expression in TNF-α/IL-1β activated HEMEC significantly was decreased by BRE pretreatment without having any effect on resting HEMEC (Fig. 1 A & C). However, ICAM-1 and VCAM-1 gene expression in activated HIMEC was not affected by BRE (Fig. 1 B & D). Interestingly, BRE alone modestly increased the level of ICAM-1 and VCAM-1 mRNA expression in resting cells and to higher extends in TNF-α/IL-1β-activated HIMEC (Fig. 1 B & D). Results obtained by ELISA in culture media were in agreement with the gene expression, which revealed that BRE inhibited ICAM-1 and VCAM-1 in HEMEC, but not in HIMEC (Fig 1 E & F).
Fig 1. Effect of BRE on CAM expression in HEMEC and HIMEC.
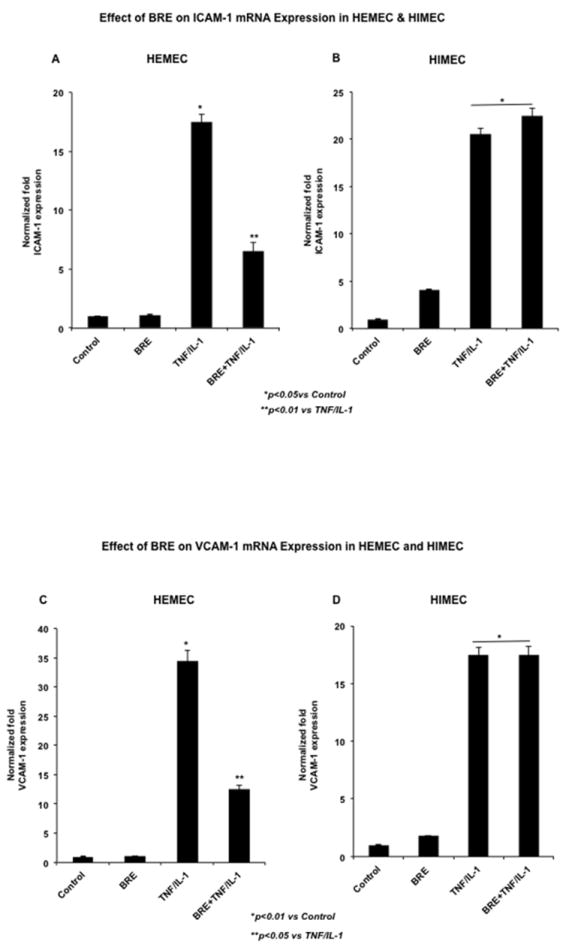
BRE pretreatment of TNF-α/IL-1β activated cells significantly decreased the level of ICAM-1 and VCAM-1 gene expression in HEMEC without having any effect on resting cells (A & C). BRE did not block the ICAM-1 and VCAM-1 gene expression in activated HIMEC, and slightly increased the expression of these genes in HIMEC (B & D). ELISA assay in culture media further support the inhibitory effect of BRE in HEMEC, but not in HIMEC (E & F). Values are means ±SD of 3 independent experiments. An asterisk denotes a significant difference in mean intensity between control and treated cells.
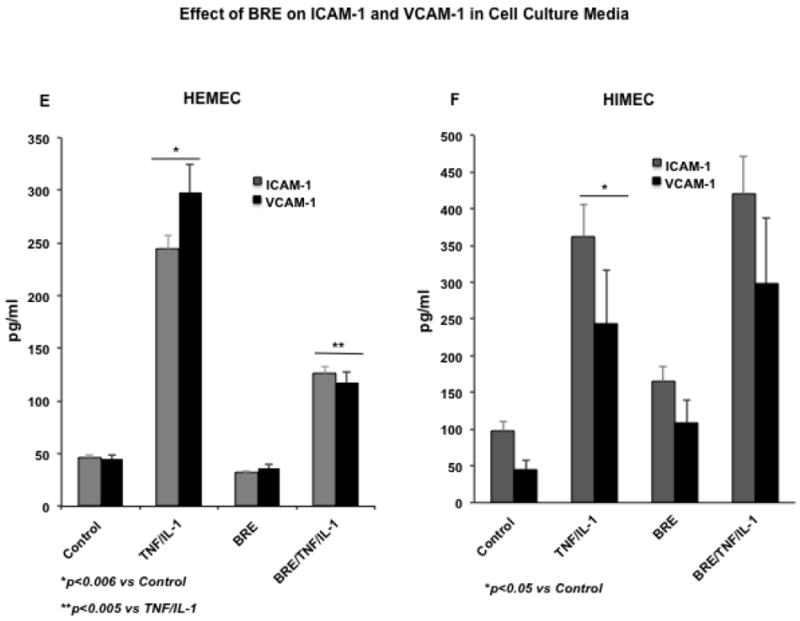
BRE reduces leukocyte adhesion in TNF-α/IL-1β-activated HEMEC but not HIMEC
The effect of BRE on endothelial function in vitro was assessed using co-cultures of endothelial cells with U937 monocyte-like cells (Binion et al., 1997) (Binion et al., 2009). As shown in (Fig. 2 A), control HEMEC adhered to low level of U937 (a) and BRE alone did not affect the resting HEMEC (b). However, TNF-α/IL-1β activation of HEMEC noticeably increased the level of U937 binding (c), and BRE pretreatment effectively inhibited the binding of U937 to TNF-α/IL-1β-activated HEMEC (d). Fig. 2 B control HIMEC bound low levels of U937 (e). BRE treatment modestly increased the leukocyte binding in resting HIMEC (f), which was significantly increased by TNF-α/IL-1β-activation (g) and enhanced the U937 binding to a higher degree in TNF-α/IL-1β-activated HIMEC (h). Quantification of ECs-leukocyte binding show a significant increase in TNF-α/IL-1β HEMEC and HIMEC, which was decreased by BRE only in HEMEC (Fig 2 C & D). These findings suggested that the anti-inflammatory effect of BRE on HEMEC at least in part, is through modulation of CAM expression and leukocyte binding in activated HEMEC.
Fig 2. Effect of BRE on leukocyte adhesion in HEMEC and HIMEC.
As expected, both HEMEC and HIMEC tightly adhere to U937 cells, which was increased with TNF-α and IL-1β activation. 2A). a- resting HEMEC bound low levels of U937, b-BRE had no affect on resting HEMEC, c- TNF-α/IL-1β activation increased HEMEC-U937 adhesion, d- BRE inhibited U937 binding to TNF-α/IL-1β-activated HEMEC. B). e- resting HIMEC bound low levels of U937, f- BRE treatment modestly increased the leukocyte binding in resting HIMEC, g- TNF-α/IL-1β- increased HIMEC-U937 adhesion, h- BRE treatment of TNF-α/IL-1β HIMEC drastically enhanced the U937 binding. C & D represent the quantification of enhanced leukocyte binding by TNF-α/IL-1β in both HEMEC and HIMEC, which was decreased by BRE in HEMEC, but not HIMEC. Experiments were done in triplicate, and the data are expressed as means ± SD. An asterisk denotes a significant difference in mean intensity between control and treated cells.
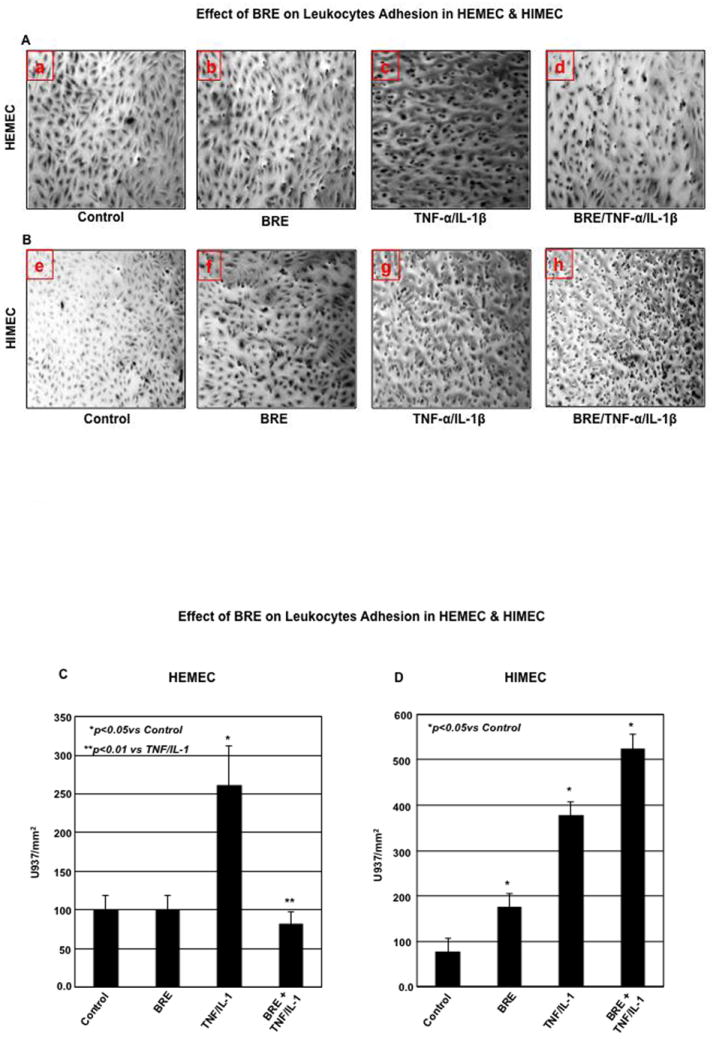
BRE diminishes COX2 and PGE2 in TNF-α/IL-1β-activated HEMEC but not HIMEC
Next, we examined the effect of BRE on COX-2 gene and protein expression. Increased expression of COX-2 mRNA in both HEMEC and HIMEC was evident after 12h in TNF-α/IL-1β activated cells. BRE effectively inhibited COX2 mRNA expression in HEMEC, but had no inhibitory effect on HIMEC (Fig. 3 A & B). Likewise, TNF-α/IL-1β enhanced COX-2 protein expression in both HEMEC and HIMEC after 24 h. However, similar to COX2 gene, BRE treatment eradicated the COX2 protein expression in HEMEC, but did not affect COX-2 protein expression in HIMEC. NS398 (1 μM), a specific inhibitor of COX2 (Zikri et al., 2009) (Baek et al., 2007) completely inhibited COX2 expression (Fig. 3 C & D). β-actin served as loading control in these experiments.
Fig 3. Effect of BRE on COX2 expression in HEMEC and HIMEC.
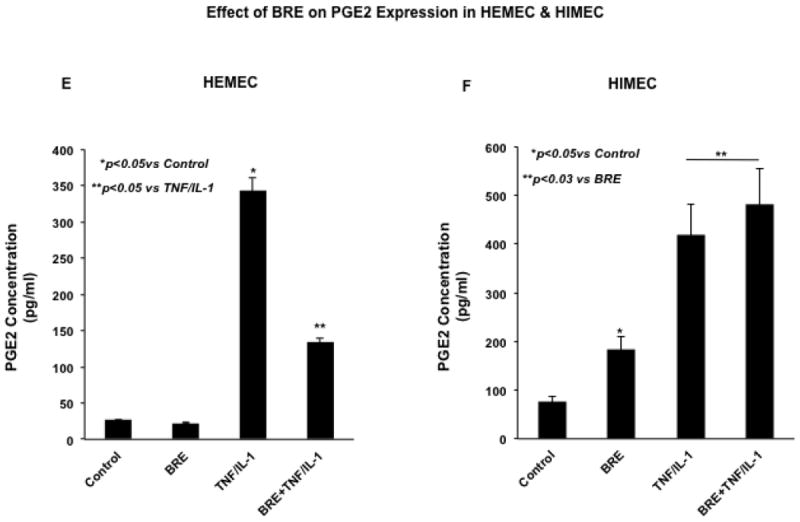
A & B- pretreatment of HEMEC with BRE effectively inhibited the COX2 mRNA expression whereas had no inhibitory effect on HIMEC. C & D- Similarly, BRE eradicated COX2 protein expression in HEMEC, but had no effect on HIMEC. β-actin was used as an internal control in these experiments. E & F- ELISA assay demonstrates that increased prostaglandin E2 (PGE2) production by TNF-α/IL-1β was inhibited by BRE in HEMEC, where as BRE had no inhibitory effect on PGE2 production in activated HIMEC. All experiments were done in triplicate, and the data are expressed as means ± SD. An asterisk denotes a significant difference in mean intensity between the control cells and treatment. Western blots are representative of 3 individual experiments.
In agreement with the effect of TNF-α/IL-1β on enhanced level of COX-2 mRNA and protein expression, the prostaglandin E2 (PGE2) production was significantly increased in both HEMEC and HIMEC and BRE inhibited PGE2 production in HEMEC, but had no effect on HIMEC (Fig. 3 E & F). Similarly, NS398 (1 μM) inhibited PGE2 production in TNF-α/IL-1β endothelial cells, denoting that PGE2 production is reliant on COX-2 activity (data not shown).
BRE inhibits NFκB actbivation in TNF-α/IL-1β-activated HEMEC but not HIMEC
Involvement of NFκB in CAM expression following cytokines activation in ECs has been reported (Rafiee et al., 2003) (Ogawa et al., 2005). Thus we determined to investigate the effect of BRE on NFκB activation in TNF-α/ IL-1β-activated HEMEC and HIMEC. As shown in (Fig. 4 A BRE completely inhibited NFκB activation and reduced the amounts of phosphorylated IκB in HEMEC. In contrast, BRE had no inhibitory effect on either NFκB or IκB on TNF-α/IL-1β activated HIMEC (Fig 4 B). Immunofluorescence staining confirmed the inhibition of NFκB subunit p65 nuclear translocation upon the BRE treatment in HEMEC and but not in HIMEC (Fig. 4 C & D).
Fig 4. Effect of BRE on NFκB activation in HEMEC and HIMEC.
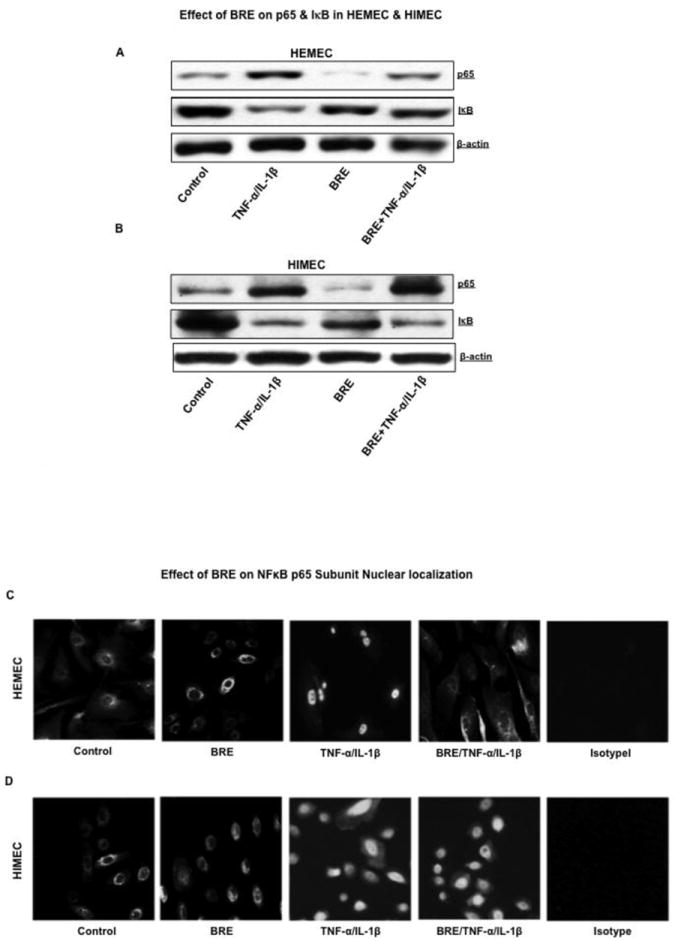
A- BRE inhibited the nuclear translocation p65 of NFκB subunit in TNF-α/IL-1β activated HEMEC. Phosphorylated IκB was detected in cytosolic portion of the cells. B- BRE was not a potent inhibitor of either NFκB or IκB in HIMEC. C & D- These findings were confirmed by immunofluorescence staining of TNF-α/IL-1β activated HEMEC and HIMEC with or without BRE.
Together these results suggest that BRE is an effective anti-inflammatory agent in suppressing TNF-α/IL-1β-induced activation of human esophageal microvascular endothelial cells but not that of human intestinal microvascular endothelial cells.
BRE inhibits cell migration, proliferation, survival and tube formation in VEGF stimulated HEMEC and HIMEC
Increased cell migration, survival, proliferation and tube formation in endothelial cells by VEGF has been demonstrated (Heidemann et al., 2003) (Rafiee et al., 2004) (Binion et al., 2008) (Rafiee et al., 2010b) (Otterson et al., 2012). Anti-angiogenic capacity of black raspberry has been also reported (Liu et al., 2005) (Mallery et al., 2008). To further characterize the anti-angiogenic effect of BRE on HEMEC and HIMEC, we treated the cells with BRE (100 μg/ml) for 2h before VEGF (100 ng/ml) stimulation and the in vitro angiogenesis assays measuring cell migration, proliferation and tube formation were performed. BRE was not toxic at the dosage used in this study.
Effect of BRE on HEMEC and HIMEC migration
Endothelial cells migration was determined in a wounded monolayer, with cell expansion across a leading edge. As expected, VEGF (100ng/ml) was a potent angiogenic stimulator of both HEMEC and HIMEC and drastically increased the cell migration compared to control cells (Fig 5 A & B). Pretreatment of endothelial cells with BRE (100ug/ml) for 2h followed by VEGF (100 ng/ml) abolished the angiogenic effect of VEGF and cells migrated similar to untreated control and BRE alone treated cells.
Fig 5. Effect BRE on VEGF-induced migration, proliferation and tube formation in endothelial cells.
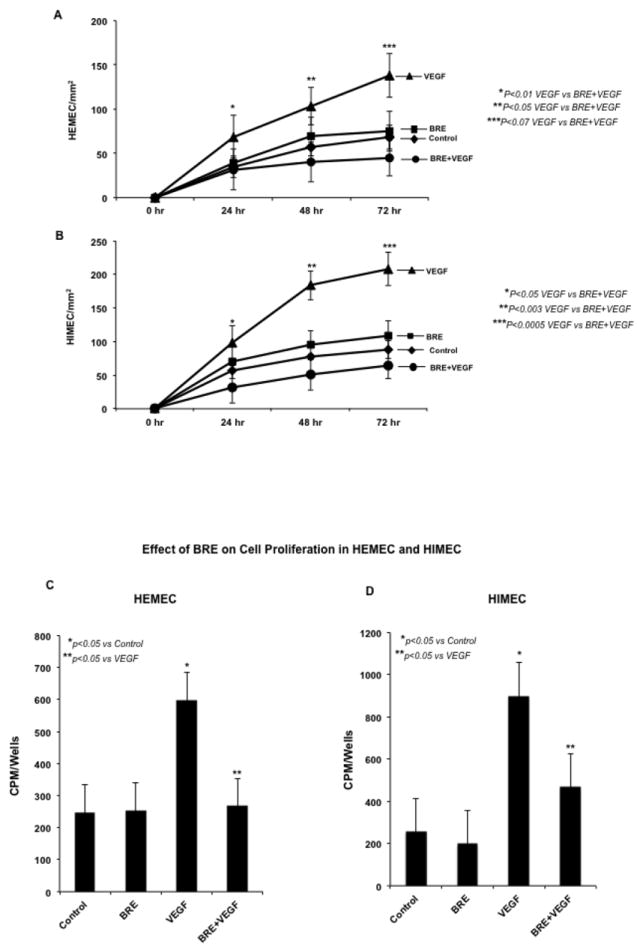
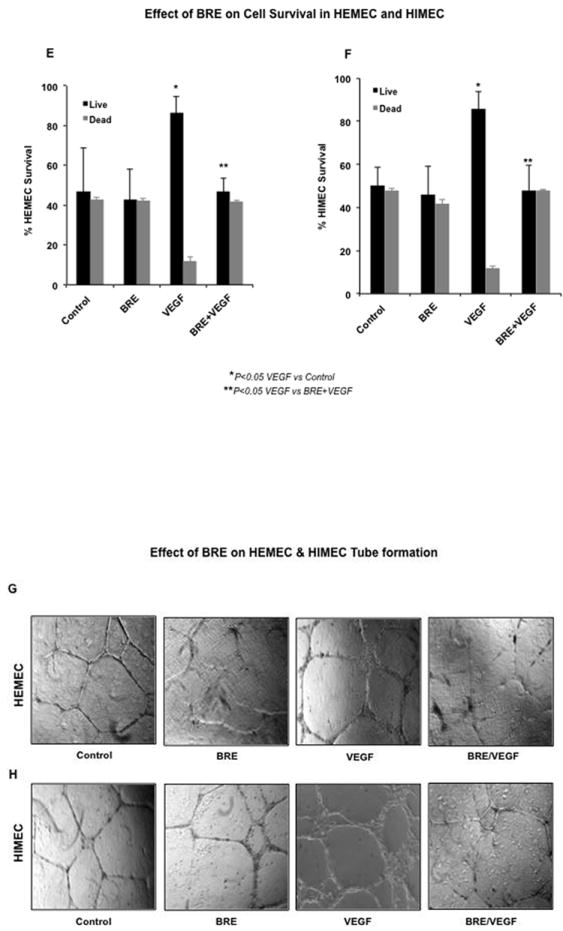
A & B- VEGF exerted a potent angiogenic effect on HEMEC and HIMEC significantly increased the endothelial cells migration across a leading edge. Pretreatment of HEMEC and HIMEC with BRE followed by VEGF stimulation, inhibited the cell migration in a time dependent manner, dropping overall rates of migration to rates similar to control cells. Endothelial cells treated only with BRE migrated across the leading edge similar to control cells. C & D- Proliferation of VEGF stimulated endothelial cells was determined by cellular DNA synthesis and [3H]thymidine uptake. As seen in C and D [3H]thymidine uptake was significantly increased in VEGF stimulated cells and BRE pretreatment inhibited the cell proliferation ultimately to basal levels. Endothelial cells treated only with BRE migrated across the leading edge similar to control cells. E & F Effect of BRE on HEMEC and HIMEC cell survival was determined by Trypan blue exclusion. By 10 days, VEGF stimulation considerably increased both HEMEC and HIMEC survival. BRE pretreatment of the cells followed by VEGF stimulation notably reduced the cell survival as compared to control cells after10 days. Cell survival with BRE alone was same at rate as control cells. G & H- Angiogenic activity of VEGF on HEMEC and HIMEC was assessed by capillary tube formation in Matrigel. Photomicrographs demonstrate that VEGF increased the number of capillary-like tubes in both HEMEC and HIMEC and BRE pretreatment completely inhibited the formation of capillary-like structures induced by VEGF. Assays were done in triplicate experiments, and the data are shown as mean cpm/wells. *P≤ 0.05 was consider to be significant.
Effect of BRE on HEMEC and HIMEC proliferation
An essential step in endothelial angiogenesis is cell cycle re-entry and DNA replication. Therefore, we performed in vitro studies to evaluate the effect of BRE on HEMEC and HIMEC proliferation by measuring [3H]thymidine uptake. VEGF stimulation of endothelial cells after 24h significantly increased [3H]thymidine uptake and BRE pretreatment of both HEMEC and HIMEC considerably inhibited the [3H]thymidine uptake in VEGF stimulated cells (Fig 5 C & D). There were no measurable changes in cells treated with BRE alone compared to control untreated cells.
Effect of BRE on HEMEC and HIMEC survival
Next, we determined the effect of BRE on endothelial cell survival after 10 days by enumeration of adherent and viable cells with Trypan blue exclusion. The increased surviving number of HEMEC and HIMEC stimulated with VEGF was significantly greater compared with control cells. Pretreatment of the cells with BRE (100μg/ml for 2 h) followed by VEGF stimulation decreased the cell survival. There were no detectable changes beyond the baseline from the BRE alone treated cells (Fig. 5 E & F).
Effect of BRE on HEMEC and HIMEC in vitro tube formation
We assessed endothelial in vitro tube formation using the Matrigel™ assay. HEMEC and HIMEC were seeded onto an extracellular matrix (Matrigel™) with or without BRE and VEGF for 24h at 37°C. VEGF stimulation of HEMEC and HIMEC resulted in increased number of endothelial tubes formed in Matrigel. Pretreatment of HEMEC and HIMEC with BRE followed by VEGF stimulation resulted in inhibition of endothelial tube formation, as evidenced by isolated cell clumps with few thin sprouting capillaries (Fig 5 G & H).
BRE inhibits VEGF-induced Akt phosphorylation in endothelial cells
Considering the significant role of the PI3K/Akt pathway in endothelial cell survival and proliferation (Rafiee et al., 2010a) (Binion et al., 2009), we evaluated the effect of BRE on Akt phosphorylation in HEMEC and HIMEC. VEGF-induced Akt phosphorylation in both cell lines as early as 15 min and BRE pre-treatment of the cells resulted in inhibition of Akt phosphorylation (Fig. 6 A & B), similar to the PI3k/Akt specific inhibitor LY294002. These findings suggest that BRE inhibits angiogenesis by PI3k/Akt inhibition in both HEMEC and HIMEC.
Fig 6. Effect of BRE on VEGF-induced Akt phosphorylation in endothelial cells.
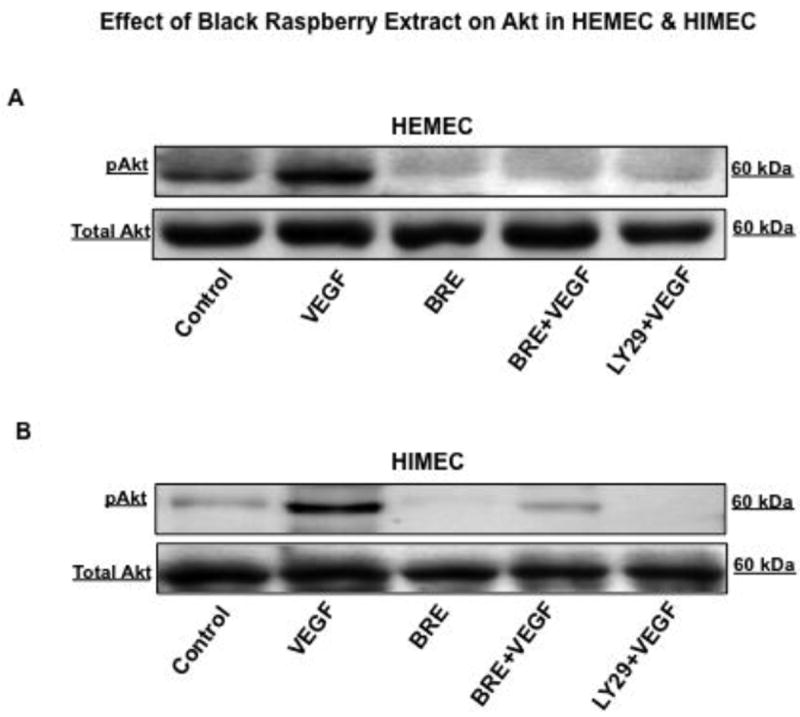
A & B- VEGF induced Akt phosphorylation in both HEMEC and HIMEC was inhibited by BRE, similar to the PI3k/Akt specific inhibitor, LY294002.
BRE inhibits VEGF-induced ERK and JNK phosphorylation in endothelial cells
Finally, the effect of BRE on VEGF-induced activation of MAPKs (p44/42, p38 and JNK) in HEMEC and HIMEC was investigated. VEGF stimulation of HEMEC and HIMEC led to phosphorylation and activation of p44/42 MAPK, p38 MAPK and JNK in both cell lines (Binion et al., 2008). As shown in Fig. 7 A & B, phosphorylation of p44/42 MAPK and JNK by VEGF was significantly decreased by BRE pretreatment of HEMEC and HIMEC, whereas, BRE had no effect on p38 MAPK.
Fig 7. Effect of BRE on VEGF-induced MAPKs phosphorylation in endothelial cells.
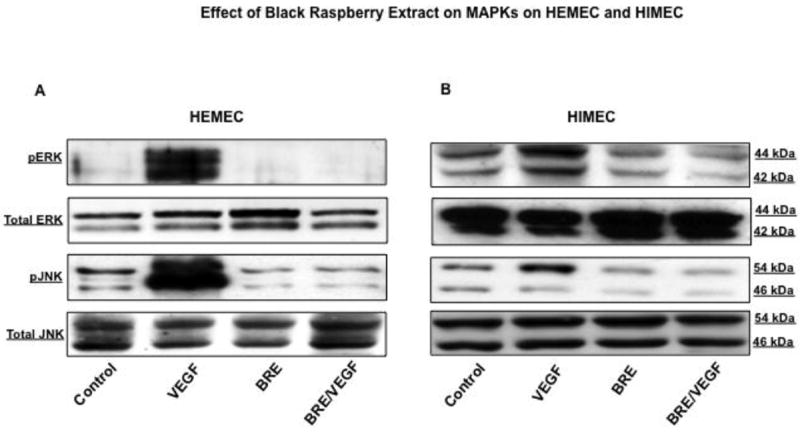
A & B- BRE inhibited the p44/42 MAPK and JNK phoshorylation in VEGF-activated HEMEC and HIMEC.
DISCUSSION
The anti-angiogenic and anti-inflammatory effects of dietary foods in the prevention and treatment of inflammatory diseases and cancer are reported (Liu et al., 2005) (Chen et al., 2006a) (Mallery et al., 2008) (Stoner and Wang, 2013) In the present study we demonstrate the effect of black raspberry extract on both inflammatory and angiogenic activation of primary human microvascular endothelial cells of esophagus (HEMEC) and intestine (HIMEC). We show that; 1) BRE inhibited COX2 expression, PGE2, production, NFκB activation, ICAM-1 and VCAM-1 expression and leukocyte binding in TNF-α/IL-1β-activated HEMEC. 2) BRE did not inhibit the expression of COX2, PGE2 production and NFκB activation and had no inhibitory effect on ICAM-1, VCAM-1 and leukocyte binding in TNF-α/IL-1β-activated HIMEC. 3) Interestingly, BRE alone modestly (Aird, 2007) increased the expression of COX2, ICAM-1, VCAM-1 and leukocyte binding in resting HIMEC and to higher degree in TNF-α/IL-1β activated HIMEC; 4) BRE treatment of VEGF stimulated HIMEC and HEMEC, inhibited cell migration, growth, proliferation and in vitro capillary tube formation. These findings signify the importance of organ-specific endothelial cells and demonstrate the differential anti-inflammatory effect of BRE between esophageal and intestinal microvasculature and reveal the anti-angiogenic properties of BRE on endothelial’s function.
Endothelial cells from various organ are considerably different from one another in structure and function (Aird, 2007). Within each organ, microvascular endothelial cells are distinct and are compelled to fulfill the certain requirements of that specific organ. However, the importance of organ-specific microvascular endothelial cells and the molecular markers defining these endothelial cells still remains poorly understood (Aird, 2007) (Nolan et al., 2013). Analysis of tumor-specific markers (Seaman et al., 2007) and in vitro reactions of tissue-specific endothelium to various stimuli (Muller et al., 2002) imply that endothelial cells are heterogeneous cells. It has been shown that organ-specific microvascular endothelial cells support the tissue homeostasis by expressing cytokines, growth factors and angiogenic factors (Butler et al., 2010) (Doan et al., 2013) (Himburg et al., 2012) (Ding et al., 2012) (Hooper et al., 2009).
Inflammation is marked by accumulation of mucosal immune cells and release of pro-inflammatory cytokines and chemokines (Bouma and Strober, 2003). It has been shown that TNF-α and IL-1β by affecting, the epithelial and endothelial cells function contribute to the pathogenesis of inflammatory bowel disease (Crohn’s disease and ulcerative colitis) IBD as well as esophagitis (Andoh et al., 2008) (Choo and Roh, 2013). TNF-α and IL-1β induces inflammatory responses in vascular endothelium, which results in enhanced expression of cell adhesion molecules such as ICAM-1 and VCAM-1 (Andoh et al., 2008) (Choo and Roh, 2013) (Binion et al., 2009). These major cytokines also mediate the trans-migration of leukocytes to the endothelium leading to endothelial dysfunction and tissue damage. Activation of microvascular endothelial cell by TNF-α and IL-1β lead to increase COX-2 expression and PGE2 production (Turini and DuBois, 2002) while NFκB activation is associated with transcriptional regulation of ICAM-1 and VCAM-1 genes (Jayakumar et al., 2014). In order to determine specific mechanisms that underlie the possible anti-inflammatory effect of BRE, we used our organ specific human primary endothelial cells for modeling the endothelial-leukocyte interaction. As expected we confirmed that TNF-α/IL-1β activation of HEMEC and HIMEC, markedly increased the expression of COX-2, PGE2 production, ICAM-1 and VCAM-1 expression, clearly enhances leukocyte binding and activate NFκB in both endothelial cell lines (Binion et al., 2009) (Binion et al., 2008) (Binion et al., 1997). In HEMEC, BRE significantly attenuated these cytokine-mediated inflammatory responses, indicating the potential role of BRE in prevention and treatment of esophageal inflammatory disease, confirming reported data that associate the black raspberry with down-regulation of COX-2 and inducible nitric oxide synthase (iNOS) in rat esophagus (Chen et al., 2006b) (Lechner et al., 2008).
In contrast to HEMEC, BRE exerted no anti-inflammatory effects and clearly did not attenuate TNF-α/IL-1β-mediated leukocyte-endothelial binding in HIMEC. BRE was found to enhance CAM expression and leukocyte-endothelial adhesion in HIMEC. These findings suggest that BRE do not exert the same anti-inflammatory effects in intestinal inflammatory disease as in the esophagus and may not be beneficial in preventing and treatment of intestinal inflammation such as IBD. On the other hand, Montrose and colleagues recently reported a protective role for BRE during ulcerative colitis in a mouse model of DSS-induced colonic injury (Montrose et al., 2011). This protection was credited to the ability of BRE to suppress both IκBα phosphorylation and COX-2 expression in epithelial cells (Montrose et al., 2011). As shown in our study, similar effects of BRE could not be reproduced in tissue-specific human intestinal endothelial cells (HIMEC) in vitro, indicating differential anti-inflammatory effects of BRE between epithelial and endothelial cells. Interestingly though, in Montrose and colleagues study the protective effect of BRE occurred with no direct effect on inflammatory cell infiltration into the colonic mucosa (Montrose et al., 2011). This phenomenon can be explained by our current finding, that BRE failed to inhibit TNF-α/IL-1β-mediated leukocyte-endothelial binding in HIMEC. Thus, intestinal endothelial cells are activated and able to recruit inflammatory cells to the colonic mucosa, despite the presence of BRE. With regard to the anti-inflammatory effect of BRE, it becomes apparent that the endothelial cells of colonic mucosa behave differently than epithelial cells and esophageal endothelial cells. The exact reason for this differential response is currently unknown but surely depicts major distinct differences between tissue-specific endothelial cells, which may originate either from the phase of embryogenesis or from the later cellular adjustment to the surrounding tissue properties. It has been shown that anthocyanins [cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-(2G-xylosylrutinoside)] were the most active constituents in freeze-dried black raspberries to attenuate the effect N-nitroso-methylbenzylamine (NMBA)–induced esophageal tumors in rat by down-regulating NFκB and AP1 (Hecht et al., 2006) (Wang et al., 2009). The preclinical data indicate that BRE merits further evaluation for chemoprevention efficacy in IBD patients. A possible failure of BRE diets to demonstrate clinical benefits in patients with IBD might be attributed to the absence of anti-inflammatory effects on the intestinal endothelial cells.
The importance of endothelial cells in tissue inflammatory responses through regulated interaction and recruitment of inflammatory cells are well study (Binion et al., 1997) (Binion et al., 2009). In addition to leukocyte recruitment, endothelial cell angiogenesis has emerged as an additional vascular mechanism contributing to chronic inflammation (Danese et al., 2006) (Danese et al., 2007) (Binion and Rafiee, 2009). It has been shown that increased tissue vasculature will enhance inflammatory responses associated with endothelial activation (Binion and Rafiee, 2009) (Hussain and Harris, 2007). While the angiogenesis, formation of new vessels jointly with chronic inflammation contributes to oncogenesis (Binion and Rafiee, 2009) (Hussain and Harris, 2007). Therefore, we set to examine the effect of BRE on the angiogenic properties of HEMEC and HIMEC. As expected stimulation of endothelial with VEGF increased cell proliferation, migration and tube formation confirming already previously reported data (Morales-Ruiz et al., 2000) (Rafiee et al., 2004) (Rafiee et al., 2010b). BRE strongly inhibited VEGF-induced cell proliferation, migration and tube formation in both HEMEC and HIMEC, supporting the notion that BRE possess a strong anti-angiogenic capacity. In addition, the anti-angiogenic effect of BRE was strongly associated with the downstream inhibition of PI3K/Akt and MAPK signaling pathways. These signaling pathways play a central role in malignant transformation, tumor chemo-resistance, and invasiveness by promoting cell survival, proliferation, growth, migration, and angiogenesis (Yang et al., 2004) (Zhang et al., 2004) (Stoner et al., 2006) (Slattery et al., 2013). PI3K/Akt and MAPK signaling pathways have been associated with the anti-carcinogenic activities of anthocyanins and anthocyanin-rich extracts, like BRE, reported in cell culture models and in animal model tumor systems (Wang and Stoner, 2008). Indeed, in animal models of both esophageal and colorectal cancer black raspberry extract has been shown to have a protective and beneficial effects (Stoner et al., 2006) (Wang and Stoner, 2008) (Wang et al., 2009) (Bi et al., 2010). In this study, we observed strong anti-angiogenic effects of BRE with respect to endothelial function and further enhanced our knowledge of the mechanisms of BRE-mediated tumor prevention.
In summary, we report here direct effects of BRE on endothelial function. By using primary endothelial cell lines from human esophagus (HEMEC) and intestine (HIMEC), we observed strong anti-angiogenic capacity for BRE by demonstrating its inhibitory effect to significantly abolish VEGF-mediated endothelial proliferation, migration and tube formation. In addition, we revealed a differential anti-inflammatory effect of BRE between human esophageal and gut endothelial cells suggesting that BRE might be more suitable for protection of esophageal inflammation and tumorigenesis than that of the intestine. Collectively, these data highlight the complexity and importance of the endothelium and demonstrate that endothelial cells derived from different organs possess significant differences in their function. The present findings also suggest that BRE functions through novel vascular mechanisms in addition to its known effects on other cellular functions such as proliferation and apoptosis. Further investigations in defining the therapeutic effect of BRE in the treatment of chronic inflammatory bowel disease, with respect to vascular function, are warranted.
Acknowledgments
The authors are thankful for the generous gift of fluorescence microscope from Levy family (Gerald, Ellin, Douglas, and Patti Levy).
FUNDING SUPPORT: This work was supported by the Medical College of Wisconsin’s Digestive Disease Center (PR), National Center for Advancing Translational Sciences Grant 8UL1 TR-000055, NIH Grant 5U19-AI067734 (MFO), Zablocki VA Medical Center research support and in part by Laura Gralton on behalf of Daniel and Laura Gruber Charitable Lead Trust #3
Abbreviations
- HIMEC
Human Intestinal Microvascular Endothelial Cells
- HEMEC
Human Esophageal Microvascular Endothelial Cells
- BRE
Black Raspberry Extract
- IBD
Inflammatory Bowel Disease
- CD
Crohn’s Disease
- UC
Ulcerative Colitis
- CAM
Cell Adhesion Molecule
- ICAM-1
Intercellular Adhesion Molecule-1
- VCAM-1
Vascular Cell Adhesion Molecule-1
- NFκB
Nuclear factor kappa B
- COX2
Cyclooxygenase 2
- PGE2
Prostaglandin E2
- ELISA
enzyme-linked immunosorbent assay
- PCR
polymerase chain reaction
Footnotes
Authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Andoh A, et al. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154–61. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JY, et al. Selective COX-2 inhibitor, NS-398, suppresses cellular proliferation in human hepatocellular carcinoma cell lines via cell cycle arrest. World J Gastroenterol. 2007;13:1175–81. doi: 10.3748/wjg.v13.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, et al. Black raspberries inhibit intestinal tumorigenesis in apc1638+/- and Muc2-/- mouse models of colorectal cancer. Cancer Prev Res (Phila) 2010;3:1443–50. doi: 10.1158/1940-6207.CAPR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binion DG, et al. Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin. Am J Physiol Gastrointest Liver Physiol. 2009;297:G259–68. doi: 10.1152/ajpgi.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binion DG, et al. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut. 2008;57:1509–17. doi: 10.1136/gut.2008.152496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binion DG, Rafiee P. Is inflammatory bowel disease a vascular disease? Targeting angiogenesis improves chronic inflammation in inflammatory bowel disease. Gastroenterology. 2009;136:400–3. doi: 10.1053/j.gastro.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Binion DG, et al. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- Borsum T, et al. Alterations in the protein composition and surface structure of human endothelial cells during growth in primary culture. Atherosclerosis. 1982;44:367–78. doi: 10.1016/0021-9150(82)90011-9. [DOI] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, et al. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006a;66:2853–9. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, et al. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis. 2006b;27:2301–7. doi: 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- Choo BK, Roh SS. Berberine protects against esophageal mucosal damage in reflux esophagitis by suppressing proinflammatory cytokines. Exp Ther Med. 2013;6:663–670. doi: 10.3892/etm.2013.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese S, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–73. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Danese S, et al. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut. 2007;56:855–62. doi: 10.1136/gut.2006.114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FJ, et al. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev Res (Phila) 2009;2:665–72. doi: 10.1158/1940-6207.CAPR-08-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994;91:8969–73. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno K, et al. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull. 2002;25:19–23. doi: 10.1248/bpb.25.19. [DOI] [PubMed] [Google Scholar]
- Grassi D, et al. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des. 2009;15:1072–84. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- Hecht SS, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide induced NFkappaB and AP-1 activity. Carcinogenesis. 2006;27:1617–26. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann J, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- Himburg HA, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2:964–75. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–74. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- Jayakumar T, et al. Brazilin Ameliorates High Glucose-Induced Vascular Inflammation via Inhibiting ROS and CAMs Production in Human Umbilical Vein Endothelial Cells. Biomed Res Int. 2014;2014:403703. doi: 10.1155/2014/403703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkonen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem. 2003;51:628–33. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Tavani A. Fruit and vegetables, and human cancer. Eur J Cancer Prev. 1998;7:3–8. [PubMed] [Google Scholar]
- Lechner JF, et al. Effects of a black raspberry diet on gene expression in the rat esophagus. Nutr Cancer. 2008;60(Suppl 1):61–9. doi: 10.1080/01635580802393118. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. Black raspberry extract and fractions contain angiogenesis inhibitors. J Agric Food Chem. 2005;53:3909–15. doi: 10.1021/jf048585u. [DOI] [PubMed] [Google Scholar]
- Madhusoodhanan R, et al. Effect of black raspberry extract in inhibiting NFkappa B dependent radioprotection in human breast cancer cells. Nutr Cancer. 2010;62:93–104. doi: 10.1080/01635580903191494. [DOI] [PubMed] [Google Scholar]
- Mallery SR, et al. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008;68:4945–57. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molema G. Heterogeneity in endothelial responsiveness to cytokines, molecular causes, and pharmacological consequences. Semin Thromb Hemost. 2010;36:246–64. doi: 10.1055/s-0030-1253448. [DOI] [PubMed] [Google Scholar]
- Montrose DC, et al. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis. 2011;32:343–50. doi: 10.1093/carcin/bgq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ruiz M, et al. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–6. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- Muller AM, et al. Comparative study of adhesion molecule expression in cultured human macro- and microvascular endothelial cells. Exp Mol Pathol. 2002;73:171–80. doi: 10.1006/exmp.2002.2446. [DOI] [PubMed] [Google Scholar]
- Noda Y, et al. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002;50:166–71. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- Nolan DJ, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–19. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, et al. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol. 2005;288:C272–81. doi: 10.1152/ajpcell.00406.2003. [DOI] [PubMed] [Google Scholar]
- Ogawa H, et al. Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett. 2003;554:88–94. doi: 10.1016/s0014-5793(03)01110-4. [DOI] [PubMed] [Google Scholar]
- Otterson MF, et al. EUK-207 protects human intestinal microvascular endothelial cells (HIMEC) against irradiation-induced apoptosis through the Bcl2 pathway. Life Sci. 2012;91:771–82. doi: 10.1016/j.lfs.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee P, et al. Modulatory effect of curcumin on survival of irradiated human intestinal microvascular endothelial cells: role of Akt/mTOR and NF-{kappa}B. Am J Physiol Gastrointest Liver Physiol. 2010a;298:G865–77. doi: 10.1152/ajpgi.00339.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee P, et al. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun Signal. 2004;2:3. doi: 10.1186/1478-811X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee P, et al. Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1277–92. doi: 10.1152/ajpgi.00484.2002. [DOI] [PubMed] [Google Scholar]
- Rafiee P, et al. Thalidomide inhibits inflammatory and angiogenic activation of human intestinal microvascular endothelial cells (HIMEC) Am J Physiol Gastrointest Liver Physiol. 2010b;298:G167–76. doi: 10.1152/ajpgi.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman S, et al. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–54. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram NP, et al. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–9. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- Slattery ML, et al. Dietary influence on MAPK-signaling pathways and risk of colon and rectal cancer. Nutr Cancer. 2013;65:729–38. doi: 10.1080/01635581.2013.795599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner GD, et al. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer. 2006;54:33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner GD, Wang LS. Chemoprevention of esophageal squamous cell carcinoma with berries. Top Curr Chem. 2013;329:1–20. doi: 10.1007/128_2012_343. [DOI] [PubMed] [Google Scholar]
- Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Wang LS, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila) 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, et al. Gene-Diet Interactions on Colorectal Cancer Risk. Curr Nutr Rep. 2012;1:132–141. doi: 10.1007/s13668-012-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–90. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, et al. Inhibition of cellular transformation by berry extracts. Carcinogenesis. 2001;22:351–6. doi: 10.1093/carcin/22.2.351. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Yi L, et al. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett. 2010;584:583–90. doi: 10.1016/j.febslet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang HM, et al. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem. 2004;279:22539–47. doi: 10.1074/jbc.M314337200. [DOI] [PubMed] [Google Scholar]
- Zikri NN, et al. Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr Cancer. 2009;61:816–26. doi: 10.1080/01635580903285148. [DOI] [PMC free article] [PubMed] [Google Scholar]


