Abstract
The interactions between B7 molecules and CD28-family receptors are crucial in the regulation of adaptive cellular immunity. In cancer, the aberrant expression of co-inhibitory B7 molecules has been attributed to reduced anti-tumor immunity and cancer immune evasion, prompting the development of cancer therapeutics that can restore T cell function. Murine tumor models have provided significant support for the targeting of multiple immune checkpoints involving CTLA-4, PD-1, ICOS, B7-H3 and B7-H4 during tumor growth, and clinical studies investigating the therapeutic effects of CTLA-4 and PD-1 blockade have shown exceptionally promising results in patients with advanced melanoma and other cancers. The expression pattern of co-inhibitory B7 ligands in the tumor microenvironment has also been largely correlated with poor patient prognosis, and recent evidence suggests that the presence of several B7 molecules may predict the responsiveness of immunotherapies that rely on pre-existing tumor-associated immune responses. While monotherapies blocking T cell co-inhibition have beneficial effects in reducing tumor burden, combinatorial immunotherapy targeting multiple immune checkpoints involved in various stages of the anti-tumor response has led to the most substantial impact on tumor reduction. In this review, we will examine the contributions of B7- and CD28-family members in the context of cancer development, and discuss the implications of current human findings in cancer immunotherapy.
Keywords: B7 family, CD28 family, Co-stimulation, Co-inhibition, Cancer immunotherapy, Immune evasion
INTRODUCTION
The immune system plays a critical role in the protection of the host against pathogens and cancer while maintaining tolerance to self and innocuous environmental antigens. Based on the recognition of foreign antigens, the adaptive immune response orchestrates a variety of effector functions such as CD8+ T cell cytotoxicity and CD4+ T helper responses. The regulation of T cell activity is largely achieved during activation, where two signals are required. First, T cell receptors must specifically engage peptides presented by major histocompatibility complexes (MHCs) on antigen presenting cells (APCs); secondly, co-stimulatory CD28 receptors on T cells must bind B7-1 and B7-2 ligands expressed on APCs to prevent anergy. Upon T cell activation, CTLA-4 receptors are induced and outcompete CD28 for B7-1 and B7-2 ligands, thereby preventing excessive T cell expansion (1). This provides a key checkpoint in the regulation of T cell immunity. Subsequently, the upregulation of other T cell co-stimulatory or co-inhibitory receptors modulates the nature and duration of the response, and helps maintain peripheral tolerance (Fig. 1).
Figure 1.
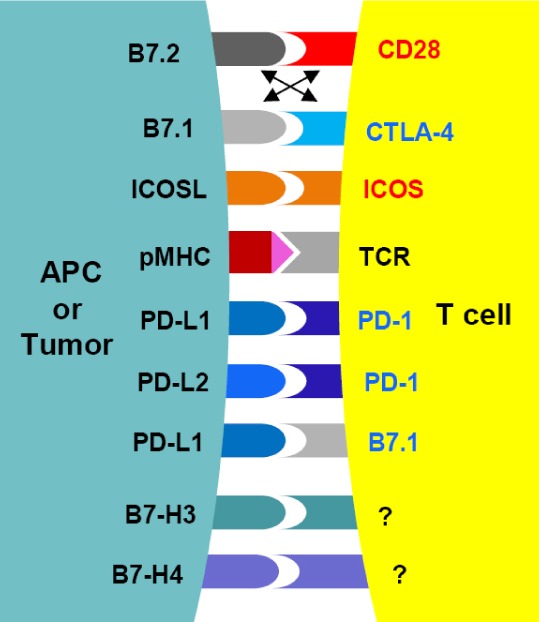
Interactions between co-stimulatory and co-inhibitory B7 and CD28 family members. B7 family ligands belong to the immunoglobulin superfamily and contain immunoglobulin-V-like and immunoglobulin-C-like domains. B7-1 and B7-2 are expressed on APCs, and function primarily to regulate the initial T cell priming by engaging CD28 or CTLA-4 receptors found on naïve and activated T cells, respectively. The expression of other B7 ligands is not limited to APCs, and is also found in non-lymphoid organs. ICOSL, PD-L1, PD-L2, B7-H3 and B7-H4 signaling mediate peripheral tolerance, and their expression in cancer have been predictive of patient prognosis. CD28 family receptors are also part of the immunoglobulin superfamily, but consist of a lone immunoglobulin-V-like domain. CD28 and CTLA-4 compete for B7-1 and B7-2 during early stages of T cell response, whereas the engagement of ICOS to ICOSL, and of PD-1 to PD-L1/PD-L2 mediate the function of pre-activated T cells. To date, the receptors for B7-H3 and B7-H4 remain unidentified, although several candidates have been proposed. In addition to the canonical B7:CD28 signaling pathways, B7-1 has also been demonstrated to reverse signal upon PD-L1 engagement, adding to the complexity of how B7-mediated signals function during tumor growth. Receptors with established T cell co-stimulatory functions are in red; those with co-inhibitory roles in blue.
In human tumors, the ability of cancer cells to evade immune destruction has been recently added to the list of cancer hallmarks, and represents a potential area of exploitation in the development of novel cancer therapeutics. The aberrant expression of numerous T cell co-inhibitory molecules belonging to the B7 family in the tumor microenvironment has been attributed to the suppression of anti-tumor immunity and immune evasion. Naturally, attempts to block the interactions mediated by inhibitory B7 ligands are currently being pursued in order to enhance T cell infiltration and effector functions, both of which have been demonstrated in a large majority of cancers to predict favorable outcome. Blocking antibodies targeting critical T cell co-inhibitory receptors, CTLA-4 and PD-1, have shown the most promise in reducing advanced melanoma and renal cell carcinoma burden, and has prompted the initiation of several clinical trials aimed at blocking other T cell co-inhibitory pathways. Additionally, the upregulation of multiple B7 ligands within the tumor milieu has also been shown to be useful for the prediction of patient outcome (Fig. 2) (1). In this review, we will summarize the mode of actions of the major T cell co-stimulation and co-inhibition pathways in the context of cancer immune evasion and immunotherapy.
Figure 2.
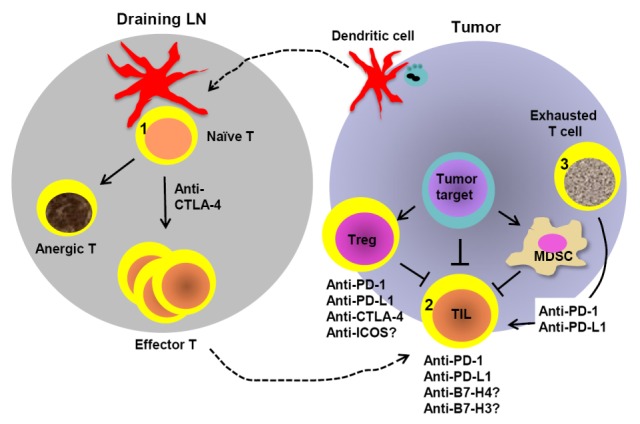
Stages of the anti-tumor T cell response in which B7 and CD28 blockade may function. In the initial T cell priming phase, DCs migrate from the tumor microenvironment to draining lymph nodes and present tumor antigens to naïve T cells. During this process, co-stimulatory signals provided by B7-1 and B7-2 to CD28 receptors prevent T cell anergy. (1) CTLA-4 plays a significant role in diminishing CD28-mediated co-stimulation, and inhibition of CTLA-4 has been shown to enhance the expansion of T cells specific to tumor-associated antigens. (2) Following activation, effector T cells infiltrate into the tumor to exert their functions, yet a host of immunosuppressive factors (such as MDSCs and Tregs) can dampen the anti-tumor response. Tumor cells and Tregs routinely upregulate co-inhibitory molecules such as PD-L1, B7-H3, B7-H4 and CTLA-4, weakening T cell immunity. Abrogation of these pathways results in enhanced anti-tumor responses, whereas the engagement of ICOS on TILs may be required for the persistence of tumor-specific T cells. (3) Lastly, elevated PD-L1 on tumor and/or infiltrating immune cells leads to T cell exhaustion, which can be reversed by PD-1 or PD-L1 blockade. Combinatorial immunotherapies disrupting multiple immunosuppressive mechanisms are predicted to improve anti-tumor efficacy.
CTLA-4
Pre-clinical studies of CTLA-4 blockade in murine tumor models
Following T cell activation through the B7:CD28 signaling axis, CTLA-4 receptors are upregulated and bind to B7 molecules with higher affinity relative to CD28. By outcompeting CD28 for B7 ligands, CTLA-4 attenuates the T cell response, primarily through the inhibition of IL-2 and by blocking cell cycle progression (2). Similarly, constitutive CTLA-4 expression on T regulatory cells (Tregs) also reduces the level of B7 ligands on APCs, further inhibiting T cell immunity (3). Although the appearance of CTLA-4 on T cells during an acute antigen exposure is transient, chronic antigen exposure, as in the case of cancer, leads to a sustained expression of CTLA-4 (4). The inhibitive properties of CTLA-4 on T cell activation and its function in Treg suppression, then, are believed to contribute to the immunosuppressive phenotype observed in tumor-bearing hosts, and to cancer immune evasion.
Given the role of CTLA-4, substantial effort has been made to investigate CTLA-4 blockade in the hopes of rescuing T cell responses during cancer growth. Treatment with anti-CTLA-4 antibodies has shown to be effective in ameliorating disease in multiple murine tumor models (5,6). Collectively, these studies demonstrated improved tumor regression and survival, with concomitant increases in T cell activity. Notably, anti-CTLA-4 antibody therapy alone has been proven to be most effective with immunogenic tumor models, since low or non-immunogenic tumor models show no improvement upon CTLA-4 inhibition (7). Combinatorial therapy has been successful at circumventing this obstacle, as administration of anti-CTLA-4 antibodies alongside cancer vaccines, chemotherapeutic agents, radiation, and other therapies was shown to create a synergistic effect, allowing weakly immunogenic tumors to be targeted by activated T cells (8,9,10,11). In particular, the synergistic effects of CTLA-4 blockade and the engagement of another CD28 family T cell co-stimulatory molecule, ICOS, have shown promising results (see below).
Clinical findings of CTLA-4 blockade in human patients
Two human anti-CTLA-4 antibodies have been in clinical investigations for the past decade and have shown success as viable cancer immunotherapeutics particularly in melanoma. While both antibodies can neutralize CTLA-4 and enhance T cell responses, the efficacy appears to differ. Ipilimumab (IgG1 isotype) was demonstrated to enhance the expression of activation markers on circulating lymphocytes (12) and augment antigen-specific immune responses in patients with melanoma who were co-administered peptide vaccines (13). In 2010, a seminal phase III clinical trial of ipilimumab monotherapy provided encouraging results, as previously-treated metastatic melanoma patients saw increased overall survival (14). Another phase III study in 2011 observed similar results in untreated metastatic melanoma patients administered ipilimumab with dacarbazine (15). Based on these results, the U.S. FDA and European Medicines Agency have approved ipilimumab for metastatic melanoma therapy in 2011 and currently, studies examining ipilimumab as a monotherapy and in conjunction with other therapies are underway.
In contrast, studies involving tremelimumab (IgG2 isotype) have resulted in moderate success: in a phase I study, a cohort of melanoma patients saw long-term benefits upon tremelimumab treatment (16). These results were contrasted by a 2013 study, in which tremelimumab-treated melanoma patients had similar objective response rates to control patients (17). Possible explanations for the differences between ipilimumab and tremelimumab efficacy may stem from the properties of the immunoglobulin isotype, as murine studies have demonstrated that anti-CTLA-4 antibodies expressing the IgG2a isotype (equivalent to ipilimumab isotype) have enhanced antitumor activity relative to treatment with anti-CTLA-4 antibodies expressing other isotypes (18).
Clinical biomarkers for CTLA-4 blockade
In light of studies in mouse models demonstrating the importance of tumor immunogenicity on the impact of CTLA-4 blockade, emerging clinical evidence also suggests that patients with ongoing immune responses prior to and during immunotherapy respond better upon CTLA-4 treatment. Patients who exhibited NY-ESO-1-specific T cell activity were better responders to ipilimumab therapy compared to patients that were sero-negative (19). Further, in bladder cancer patients, the accumulation of tumor-specific, IFN-γ-producing CD4+ICOS+ T cells following treatment was associated with enhanced effector to Treg cell ratio, making this subset an attractive biomarker for predicting patient outcome (20).
PD-1: PD-L1/PD-L2
Mechanism of action and pattern of expression in cancer
PDL-1 and PDL-2 are additional members of the B7 family, and represent the two known ligands for the PD-1 receptor. PD-L1 is found on hematopoietic cells and parenchymal cells, and PD-L2 is restricted to macrophages and dendritic cells (DCs) (21). The PD-1 receptor is induced on activated T cells, but can also be found on B cells and NK cells (22). While CTLA-4 limits the amplitude of early T cell responses, PD-1 suppresses T cell function in peripheral tissues through the activation of phosphatases that inhibit kinases in the upstream of TCR signaling cascade. Additionally, PD-1 is crucial for the development and function of Tregs, representing an indirect mechanism leading to dampened T cell responses (23). Since PD-1 is selectively upregulated in conditions of persistent antigen exposure and chronic inflammation and has been shown to drive cellular exhaustion, the expression of PD-1 on T cells has become one of the hallmarks of exhausted T cells. Indeed, in multiple human tumors, a significant proportion of TILs have been shown to express PD-1, and often, this expression has been associated with impaired CD8+ T cell function (24,25). Rescue of T cell activity by PD-1/PD-L1 blockade has been demonstrated in models of chronic viral infections (26), providing evidence for the use of PD-1 blockade to restore T cell function amongst exhausted PD-1+ TILs.
While PD-1 is often associated with TILs in numerous cancers, PD-1 ligands are frequently observed on the surface of multiple human tumors and murine cancer cell lines, with PD-L1 as the ligand that is most commonly expressed (27,28,29). This is not surprising, as PD-L1 can be upregulated by inflammatory cytokines and via oncogenic signalling (23). PD-L2 expression has mainly been documented in B cell lymphomas, such as primary mediastinal B-cell lymphoma and Hodgkin's lymphoma (30,31). In hepatocellular carcinoma, melanoma and breast cancer, PD-L1 positivity was correlated with worse prognosis (32,33,34), consistent with its physiological role in negatively regulating T cell responses. Other studies have found that PD-L1 status on tumors has either a positive or no correlation with patient prognosis (23,35,36). These observations may be attributed to differences in cancer type, stage of cancer, and/or patient treatment history, indicating the requirement for further studies examining PD-1 ligand positivity and patient outcome.
Pre-clinical evidence for PD1, PD-L1 and PD-L2 blockade in murine tumor models
In numerous murine models of cancer, inhibition or absence of PD-1 has been demonstrated to rescue anti-tumor T cell responses, resulting in diminished tumor burden (37,38). Likewise, blockade of PD-L1 could also rescue T cell function and enhance tumor regression in a variety of murine cancer models (27,39). Studies on PD-L2 blockade, however, have provided contradicting results regarding the inhibitory or stimulatory role of PD-L2 (39,40), and may indicate the involvement of an undiscovered co-stimulatory T cell receptor specific for PD-L2.
Clinical findings of PD1, PD-L1 and PD-L2 blockade
While the blockade of CTLA-4 has been characterised in human patients, clinical studies examining PD-1 blockade have only been initiated recently, and thus limited data is available. Nonetheless, initial clinical trials showed improved disease progression upon administration of anti-PD-1 antibodies. In the first clinical study involving a fully human IgG4 anti-PD-1 antibody (MDX-1106), PD-1 blockade elicited partial responses in patients with melanoma and renal cell carcinoma (41). An additional study with another anti-PD-1 antibody (BMS-936558) also demonstrated objective responses in patients with non-small cell lung cancer, melanoma and renal cell cancer, which did not occur in patients whose tumors were negative for PD-L1 (42). Treatment with lambrolizumab, another blocking PD-1 antibody, has yielded sustained tumor regression in patients with advanced melanoma (43).
Studies examining the effects of blocking PD-1 ligands in humans are scarce, although preliminary studies have been initiated and are presently ongoing. The first clinical trial with anti-PD-L1 antibodies (BMS-936559) demonstrated tumor regression and disease stabilization in non-small cell lung cancer, melanoma and renal cell cancer at 24-weeks (44). An ongoing clinical trial documenting the efficacy of another PD-L1 antibody (MPDL3280A) has also yielded objective responses in non-small cell lung cancer patients. This is particularly notable, as these patients had tumors that were difficult to treat, as evidenced by their treatment history (investigator update http://www.roche.com/investors/ir_update/inv-update-2013-09-29.htm). To date, only one reagent targeting PD-L2 has been tested in humans. A recombinant PD-L2-Fc fusion protein (AMP-224) has been developed, and in individuals with partial or mixed responses, AMP-224 treatment reduced PD-1hi exhausted cells and enhanced functional T cells in patients with advanced solid tumors (2013 ASCO Annual Meeting poster abstract, http://meetinglibrary.asco.org/content/117257-132).
Notably, combinatorial therapy involving PD-1 and CTLA-4 blockade has shown exceptionally promising results, as a phase I trial showed 53% of advanced melanoma patients had an objective response with tumor reduction of at least 80% (45). These observations have led to the initiation of phase III clinical studies, which is expected to yield encouraging data. These findings support the targeting of T cell co-inhibitory molecules involved in multiple phases of the T cell response, including those responsible for early T cell priming and those mediating late-stage effector functions.
Biomarkers for responsiveness to PD-1, PD-L1 blockade
In the majority of patients responding to PD-1 or PD-L1 blockade, the expression of PD-L1 in the tumor was correlated with treatment responsiveness. While it may appear counterintuitive that a T cell inhibitor may predict better prognosis, the upregulation of PD-L1 is often induced by cytokines produced by effector immune cells. Evidence for this in the context of tumor growth was provided in one study that demonstrated the localization of IFN-γ to the same area where PD-L1+ tumors associated with TILs (35), and studies in mice have shown that the induction of PD-L1 in the tumor relied upon IFN-γ-producing tumor-associated T cells (46). This suggests a negative feedback loop, as blockade of PD-1 signaling would enhance the production of inflammatory cytokines by immune cells, which would then promote the upregulation of PD-L1 in the tumor. Expression of PD-L1 would presumably suppress PD-1+ TILs, and this mechanism of tumor-mediated immunosuppression following T cell attack has been termed "adaptive immune resistance". This is in contrast to the constitutive expression of PD-L1 on tumor cells which is driven by oncogenic signalling, and suggests that the blockade of PD-1 and PD-L1, along with the careful timing of treatments, would produce the most effective anti-tumor response. These observations also imply that the expression of PD-L1 in the tumor milieu is indicative of pre-existing immune responses, and may be useful in predicting the responsiveness of immunotherapies that rely on the presence of immunosurveillance in the growing tumor.
ICOS AND ICOSL
Mechanism of action and physiological expression
The inducible co-stimulatory receptor (ICOS) shares much homology with CD28, yet key differences in signaling mechanisms and unique expression patterns of ICOS ligand suggest non-redundant functions. Similar to CTLA-4, ICOS is induced following T cell activation (2). During the initial priming of naïve T cells, the contribution of ICOS appear minimal relative to that of CD28; T cells lacking ICOS show minor differences in proliferation (1,2,47,48). However, while both CD28 and ICOS can bind phosphoinositide 3-kinase (PI3K), engagement of ICOS has been shown to induce greater PI3K signaling than CD28 co-stimulation (49). The significance of this finding was demonstrated by our group and others in being crucial for the production IL-21 and IL-4, key cytokines involved in the differentiation and function of T follicular helper (Tfh) cells (50,51,52). ICOS has also been detected on Tregs, where its expression was shown to influence the homeostasis and function of this subset (1).
The ICOS receptor is engaged by ICOSL, another member of the B7 family. ICOSL is expressed in APCs (B cells, macrophages, dendritic cells) and can be induced by inflammatory cytokines in non-hematopoietic cells including endothelial cells and epithelial cells (53).
Expression of ICOS and ICOSL in human cancers
ICOS and ICOSL have been observed in several human cancers, yet how this signaling pathway contributes to the anti-tumor response remains unclear. In the peripheral blood of colon cancer patients, co-stimulatory genes such as ICOS were significantly diminished and this reduction was linked to lymph node metastasis and aggressive tumor invasion (54). Moreover, high ICOS expression on TILs in metastatic melanoma lesions was associated with post-recurrence survival (55). While these findings support the co-stimulatory role of ICOS:ICOSL in facilitating the anti-tumor T cell response, others have revealed an inhibitory, pro-tumor role for ICOS signaling related to its function in Treg homeostasis. In one study, acute myeloid leukemia patients exhibiting ICOSL positivity had significantly decreased survival (56). In freshly isolated human melanomas, 25% of samples expressed ICOSL and 50% of metastatic samples demonstrated high ICOSL expression (57). The ICOSL present in these melanoma samples were able to engage ICOS on activated Tregs and stimulate suppressive functions, thus providing a means of tumor immune evasion. Notably, in melanoma patients, the specific expansion of ICOS+ Tregs following the first cycle of high-dose IL-2 therapy is correlated with worse clinical outcome (58).
ICOS as biomarker of CTLA-4 blockade efficacy
Despite contradictory observations regarding the role of ICOS signaling in cancer progression, recent findings support the role of ICOS as a biomarker for host responsiveness to CTLA-4 blockade. Ipilimumab treatment in patients with urothelial carcinoma of the bladder led to an increased frequency of CD4+ICOShi T cells (59), and administration of both tremelimumab and exemestane in hormone-responsive advanced breast cancer patients resulted in enhanced circulating ICOS+ T cells (60). CD4+ICOShi T cells obtained from bladder cancer patients treated with anti-CTLA-4 antibodies also showed IFN-γ production, the ability to recognize the NY-ESO-1 tumor antigen, and an increase in the ratio of T effector cells to Tregs (20). Correlations between ICOS positivity following CTLA-4 blockade and clinical benefit has also been examined, albeit to a lesser extent. In a small cohort of metastatic melanoma patients treated with ipilimumab, retrospective analysis showed that a sustained increase in CD4+ ICOShi T cells was associated with increased likelihood of clinical benefit (59). Findings from murine studies further proposes that ICOS engagement may be required for the optimal effects of CTLA-4 inhibition, as ICOS-deficient mice bearing B16 melanomas exhibited drastically diminished anti-tumor T cell responses upon CTLA-4 blockade (61). Recently, modified ICOS-based chimeric antigen receptors generated tumor-specific IL-17+ T cells with greater persistence (62). Collectively, these findings support the requirement of ICOS for maximal benefits of CTLA-4 inhibition, and prompt further studies examining the synergy between CTLA-4 blockade and ICOS stimulation in generating optimal anti-tumor T cell immunity.
B7-H3
Function and expression pattern
B7-H3 is a B7 family whose receptor remains unidentified. Despite this, one candidate, the triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2), has been proposed. While the engagement of B7-H3 with TLT-2 resulted in enhanced proliferation and IFN-γ production (63), a subsequent report disputed these findings in mouse and human systems (64). Consequently, contradictory findings regarding the co-stimulatory or co-inhibitory function of B7-H3 persists, as evidence demonstrating both co-stimulatory and co-inhibitory functions have been reported by numerous groups (65,66,67,68). In terms of expression, B7-H3 is constitutively found on murine APCs, but must be induced on human immune cells (69). B7-H3 is not restricted to immune cells and is found on osteoblasts, fibroblasts, epithelial cells and other cells of non-lymphoid lineage (70).
Pre-clinical evidence for B7-H3 in tumor immunity
While in vitro studies and murine disease models have provided support for both the co-stimulatory and co-inhibitory capacities of B7-H3, experiments with tumor models appear to largely support a co-stimulatory role for B7-H3 in the regulation of anti-tumor immunity. For instance, P815 tumors transfected with B7-H3 showed enhanced immunogenicity and tumor regression (71), and intratumoral administration of a B7-H3 expression plasmid led to the significant reduction of EL-4 tumors (72).
Expression of B7-H3 in human cancers and implications
Despite the evidence from murine studies implicating B7-H3 as a positive regulator of anti-tumor immunity, expression of B7-H3 in human cancers tends to favor poor prognosis. To date, only a handful of clinical studies have reported a co-stimulatory function and good prognosis associated with B7-H3 (73,74). The majority of reports demonstrate an inverse correlation between B7-H3 staining and patient outcome (75,76). In one instance, strong B7-H3 positivity was observed in colorectal carcinomas and correlated with tumor grade and decreased TILs; notably, TNF-α, an inflammatory protumorigenic molecule, could induce the shedding of soluble B7-H3 by colon cancer cell lines, suggesting another method of cancer immune evasion via B7-H3 (77).
To date, a monoclonal antibody against a human isoform of B7-H3 (8H9) has been tested in early-phase clinical trials. High-risk patients with solid tumors and central nervous system metastasis exhibited prolonged survival upon 8H9 administration (78), no doubt prompting further inquiries into the efficacy of targeting B7-H3 in other cancer types.
B7-H4
Function and expression pattern
As a more recent addition to the B7 family, B7-H4 represents a T cell co-inhibitory molecule whose expression pattern in the tumor microenvironment has garnered significant attention. B7-H4 transcripts are ubiquitous in healthy individuals, and B7-H4 mRNA has been found in both lymphoid and non-lymphoid organs such as the lung, liver, spleen, thymus, kidney, pancreas, and other tissues. B7-H4 protein, however, remains highly restricted in both mice and humans, indicating a tightly regulated translational mechanism (79,80). On hematopoietic cells, B7-H4 protein can be induced following in vitro stimulation of human T cells, B cells, monocytes, and DCs (79). Similarly, tumor-associated macrophages were observed to express B7-H4, and this upregulation was attributed to the presence of IL-6 and IL-10 in the tumor microenvironment (81).
Functionally, B7-H4 has been well-documented to inhibit T cell responses. In vitro studies on murine and human T cells revealed an inhibitory role for B7-H4 in regulating cell cycle progression, proliferation and cytokine secretion (82,83). Accordingly, mice deficient in B7-H4 displayed upregulated Th1 response upon Leishmania major infection, but did not show enhanced hypersensitive inflammatory responses or increased CTL activity during viral infections, suggesting that the role of B7-H4 may be one of a fine tuner (84). B7-H4 has also been found to regulate the activity of myeloid cells, as B7-H4 knockout mice showed enhanced CD11b+Gr-1+ neutrophils and lower Listeria monocytogenes burden, concordant with the observations that in vitro administration of B7-H4 fusion protein diminishes the expansion of neutrophil progenitors (85). We have also observed enhanced suppressive capacities of CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) in the absence of B7-H4 (86). Notably, despite the restricted profile of B7-H4 in healthy individuals, an array of human cancers overexpress B7-H4 molecules. These findings have prompted the study of B7-H4 on cell growth outside of their capacity to modulate immunity. For instance, overexpression of B7-H4 on cancer cells appear to enhance proliferation, adhesion, migration and protect from apoptosis in vitro, and accordingly, tumor cells overexpressing B7-H4 showed enhanced growth in SCID mice (87,88).
To date, the receptor for B7-H4 has not been identified, and although initial studies pointed to BTLA as the B7-H4 binding partner, a subsequent study showed a lack of direct binding between the two (89). Recent studies attributing a function for B7-H4 in influencing myeloid cell activity further suggest the presence of a B7-H4 receptor on this subset. Indeed, neutrophils and tumor-associated neutrophils have been shown to bind B7-H4 protein (85,90).
Expression of B7-H4 in cancer and implications from murine tumor models
While B7-H4 protein has limited expression in healthy individuals, numerous human cancers exhibit aberrant B7-H4 expression (91). In the majority of instances, B7-H4 positivity is linked with poor prognosis and is thus believed to have potential as a biomarker (88,92,93). These findings provide a basis for targeting B7-H4 in cancer, and several studies performed in mice have further validated this notion.
In an experimental model of lung metastasis, host B7-H4 was demonstrated to reduce the level of tumor-specific T cell responses and augment the infiltration of immunosuppressive subsets into the lung. As a result, B7-H4 WT mice exhibited more lung metastases relative to B7-H4 knockout mice (90). We have also shown that in the 4T1 tumor transplantation model, B7-H4-deficiency leads to enhanced IFN-γ in the tumor microenvironment, but no difference in tumor burden was observed between WT and B7-H4 knockout mice (86). This may be explained by our finding that in the absence of B7-H4, MDSCs showed enhanced immunosuppressive activity, in line with previous data suggesting a negative role for B7-H4 in the regulation of neutrophils which share similar markers. Notably, B7-H4 deficiency and treatment with gemcitabine, a current chemotherapeutic agent which was shown to decrease the level of MDSCs without affecting other immune subsets (94), was seen to drastically reduce 4T1 tumor burden relative to gemcitabine treatment alone, and thus provides support for combinatorial therapy. In a humanized murine model of ovarian cancer, administration of anti-B7-H4 single-chain fragments variable (scFv) was able to delay tumor growth; the same reagent was also able to rescue tumor-specific T cell activity from inhibition mediated by B7-H4+ APCs in vitro (95). To date, no anti-human B7-H4 blocking antibody has been tested in the clinic, yet based on the findings in murine tumor models and the high degree of B7-H4 staining in cancers relative to non-cancerous tissue, it is of great interest to see if blockade of B7-H4 can be used in combination with other immunotherapeutics or other chemotherapies.
NEW B7 MEMBERS IN ANTI-TUMOR IMMUNITY
In addition to the T cell co-inhibitory and co-stimulatory ligands discussed above, several recently discovered B7 ligands have also shown possibilities of being exploited for cancer therapeutics. Murine studies targeting another T cell co-inhibitor B7-H5, or VISTA, showed enhanced anti-tumor T cell immunity and reduced melanoma tumor burden (96). In the same vein, B7-H7, or HHLA2, has been demonstrated to inhibit CD4 and CD8 proliferation and cytokine production in vitro (97). Evidently, blockade of VISTA and B7-H7 may help enhance T cell responses in cancer patients, and further studies regarding these pathways will offer new avenues to explore in ameliorating current immunotherapeutics. B7-H6, another B7 molecule, has been reported to bind the NK receptor NKp30 and activate anti-tumor cytotoxicity and cytokine production. Intriguingly, B7-H6 was expressed on human tumors despite its absence in non-cancerous tissues, prompting further interest to examine B7-H6 during cancer growth (98).
CONCLUSION
B7 ligands play a significant role in the regulation of T cell immunity, whereby co-inhibitory B7 molecules restrain responses to minimize tissue damage and maintain self-tolerance. Tumor cells have been shown to evade immune surveillance by exploiting these immune checkpoints, and it is this notion that has given rise to the development of reagents designed to overcome these B7-mediated immunosuppressive signals. Evidence to support the success of immunotherapy stem from clinical trials with advanced melanoma patients that saw overall survival benefit upon CTLA-4 blockade, as well as from encouraging results obtained from preliminary studies targeting the PD-1:PD-L1 pathway. Moreover, other members of the B7 family have also been identified as biomarkers, and have been useful in predicting patient responsiveness and treatment management. Certainly, the discovery of the receptors for several orphan B7 ligands will also prove beneficial in elucidating their potential as drug targets.
As we expand our knowledge of how cancer cells can manipulate the regulation of the immune response, greater possibilities to target these mechanisms will become available. Indeed, accumulating evidence suggests that while monotherapy may aid patient prognosis, combinatorial treatment appears to enhance the effects of single therapy alone. Key examples have been discussed regarding the possible synergy between CTLA-4 blockade and ICOS stimulation, as well as between B7-H4 and gemcitabine. As more options become available to patients through fundamental research, and as additional biomarkers are being discovered, the means of providing the most efficient and effective therapies will be greatly enhanced.
ACKNOWLEDGEMENTS
The authors' research is funded by the Canadian Cancer Society (grant #702047).
Abbreviations
- PD1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PD-L2
programmed death-ligand 2
- ICOSL
inducible T cell co-stimulator ligand
- Treg
T regulatory cell
- DC
dendritic cell
- TIL
tumor-infiltrating lymphocyte
- CTL
cytotoxic lymphocyte
- MDSC
myeloid-derived suppressor cells
- WT
wild-type
- KO
knockout
Footnotes
The authors have no financial conflict of interest.
References
- 1.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 4.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 6.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van EA, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/ macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 9.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 11.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, Allison JP. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–439. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, Yellin M, Weber J. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 16.Ribas A. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with tremelimumab (CP-675,206) Oncologist. 2008;13(Suppl 4):10–15. doi: 10.1634/theoncologist.13-S4-10. [DOI] [PubMed] [Google Scholar]
- 17.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, Lorigan P, Kendra KL, Maio M, Trefzer U, Smylie M, McArthur GA, Dreno B, Nathan PD, Mackiewicz J, Kirkwood JM, Gomez-Navarro J, Huang B, Pavlov D, Hauschild A. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson M, Shankar EM, Che KF, Saeidi A, Ellegard R, Barathan M, Velu V, Kamarulzaman A. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology. 2013;10:31. doi: 10.1186/1742-4690-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 27.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 28.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin AS, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, Johnson NA, Zhao Y, Telenius A, Neriah SB, McPherson A, Meissner B, Okoye UC, Diepstra A, van den BA, Sun M, Leung G, Jones SJ, Connors JM, Huntsman DG, Savage KJ, Rimsza LM, Horsman DE, Staudt LM, Steidl U, Marra MA, Gascoyne RD. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 34.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H, Wang X, Hu Y, Ji J. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 38.Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer. Clin Pharmacol Ther. 2014;96:214–223. doi: 10.1038/clpt.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kurihara C, Kawaguchi A, Nagao S, Azuma M, Yagita H, Miura S. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–749. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 48.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 49.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 50.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gigoux M, Lovato A, Leconte J, Leung J, Sonenberg N, Suh WK. Inducible costimulator facilitates T-dependent B cell activation by augmenting IL-4 translation. Mol Immunol. 2014;59:46–54. doi: 10.1016/j.molimm.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Rolf J, Fairfax K, Turner M. Signaling pathways in T follicular helper cells. J Immunol. 2010;184:6563–6568. doi: 10.4049/jimmunol.1000202. [DOI] [PubMed] [Google Scholar]
- 53.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Kim JH, Yang SY, Kong J, Oh M, Jeong DH, Chung JI, Bae KB, Shin JY, Hong KH, Choi I. Peripheral blood gene expression of B7 and CD28 family members associated with tumor progression and microscopic lymphovascular invasion in colon cancer patients. J Cancer Res Clin Oncol. 2010;136:1445–1452. doi: 10.1007/s00432-010-0800-4. [DOI] [PubMed] [Google Scholar]
- 55.Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, Lonardi S, Zavadil J, Osman I, Bhardwaj N. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, Hyodo H, Wang SD, Dong H, Chen L, Ogata K. Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11:5708–5717. doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- 57.Martin-Orozco N, Li Y, Wang Y, Liu S, Hwu P, Liu YJ, Dong C, Radvanyi L. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer Res. 2010;70:9581–9590. doi: 10.1158/0008-5472.CAN-10-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P, Radvanyi L. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP, Sharma P. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Ruter J, Mariani GL, Usari T, Domchek SM. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 61.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71:5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 62.Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, Lee J, Posey AD, Jr, Scholler J, Scholler N, Bonneau R, June CH. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 66.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da CJ, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 67.Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, Chen L, Hancock WW. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35:428–438. doi: 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 68.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 69.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loos M, Hedderich DM, Friess H, Kleeff J. B7-h3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:683875. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, Liu Y, Chen L. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 72.Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10:1728–1734. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 73.Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 77.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 80.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 81.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ou D, Wang X, Metzger DL, Ao Z, Pozzilli P, James RF, Chen L, Warnock GL. Suppression of human T-cell responses to beta-cells by activation of B7-H4 pathway. Cell Transplant. 2006;15:399–410. [PubMed] [Google Scholar]
- 84.Suh WK, Wang S, Duncan GS, Miyazaki Y, Cates E, Walker T, Gajewska BU, Deenick E, Dawicki W, Okada H, Wakeham A, Itie A, Watts TH, Ohashi PS, Jordana M, Yoshida H, Mak TW. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol. 2006;26:6403–6411. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, Rietz AC, Huang J, Xu H, Flies AS, Flies SJ, Tamada K, Colonna M, van Deursen JM, Chen L. B7-H4-deficient mice display augmented neutrophilmediated innate immunity. Blood. 2009;113:1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leung J, Suh WK. Host B7-H4 regulates antitumor T cell responses through inhibition of myeloid-derived suppressor cells in a 4T1 tumor transplantation model. J Immunol. 2013;190:6651–6661. doi: 10.4049/jimmunol.1201242. [DOI] [PubMed] [Google Scholar]
- 87.Cheng L, Jiang J, Gao R, Wei S, Nan F, Li S, Kong B. B7-H4 expression promotes tumorigenesis in ovarian cancer. Int J Gynecol Cancer. 2009;19:1481–1486. doi: 10.1111/IGC.0b013e3181ad0fa2. [DOI] [PubMed] [Google Scholar]
- 88.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 90.Abadi YM, Jeon H, Ohaegbulam KC, Scandiuzzi L, Ghosh K, Hofmeyer KA, Lee JS, Ray A, Gravekamp C, Zang X. Host b7x promotes pulmonary metastasis of breast cancer. J Immunol. 2013;190:3806–3814. doi: 10.4049/jimmunol.1202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He C, Qiao H, Jiang H, Sun X. The inhibitory role of b7-h4 in antitumor immunity: association with cancer progression and survival. Clin Dev Immunol. 2011;2011:695834. doi: 10.1155/2011/695834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 93.Bignotti E, Tassi RA, Calza S, Ravaggi A, Romani C, Rossi E, Falchetti M, Odicino FE, Pecorelli S, Santin AD. Differential gene expression profiles between tumor biopsies and short-term primary cultures of ovarian serous carcinomas: identification of novel molecular biomarkers for early diagnosis and therapy. Gynecol Oncol. 2006;103:405–416. doi: 10.1016/j.ygyno.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 94.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 95.Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, net-Desnoyers G, Powell DJ, Jr, Scholler N. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le M, Chen IW, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, Ohaegbulam KC, Ghosh K, Zhao A, Scharff MD, Zang X. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110:9879–9884. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]


