Abstract
Tauopathies are devastating and ultimately fatal neurodegenerative diseases, which are histopathologically defined by insoluble filamentous deposits of abnormally phosphorylated tau protein within neurons and glia. Identifying the causes of abnormal tau phosphorylation and subsequent aggregation has been the focus of much research, and is currently a major target for the development of therapeutic interventions for tauopathies, including Alzheimer’s disease. Recently much has been learned about the sequence of events that lead from tau dysfunction to neuronal death. This review focuses on the cascade of events that are catalyzed by pathological tau, and highlights current and potential therapeutic strategies to target this pathway.
Keywords: tauopathy, Alzheimer, actin, chromatin, cell cycle, therapeutics
Involvement of tau in neurodegenerative diseases
In 1907, Alois Alzheimer first described neurofibrillary tangles [1], which are now known to be a key pathological feature of a number of neurodegenerative diseases. Eight decades later, the major component of neurofibrillary tangles was identified, a hyperphosphorylated, filamentous form of the tau protein [2]. The subsequent discovery of a group of inherited tauopathies termed frontotemporal dementia with parkinsonism associated with chromosome 17 (FTDP-17) caused by dominant mutations in the tau gene [3–5] unequivocally demonstrated that tau dysfunction can drive neurodegeneration.
Current treatments for Alzheimer’s disease, the most common tauopathy, are solely symptomatic. Given the close association between tau pathology and severity of disease, along with evidence that tau works downstream of amyloid beta (Aβ) to induce neuronal death [6, 7], it has become increasingly recognized that tau-based therapies may be effective in treating Alzheimer’s disease with the added benefit of applying to other comparatively rarer tauopathies. Current strategies include decreasing tau aggregation, blocking abnormal tau phosphorylation, or stopping the spread of tau pathology through the brain (Table 1). These targets have been reviewed elsewhere [8, 9]. We instead highlight recent work that provides significant insight into the mechanisms downstream of tau dysfunction that promote neuronal death. The genetic players in this pathway represent potentially untapped targets for therapeutic intervention.
Table 1.
Mechanisms of tau-induced toxicity that are current and potential targets for therapeutic intervention.
| Mechanism | Potential Targets | Drug Effort |
|---|---|---|
| Tau Hyperphosphorylation/Misfolding | PAR-1* GSK-3β* HSPs* |
Lithium
 Methylene Blue  Tideglusib** Nicotinomide** Valproic Acid** LMT-X** Sodium Selenate** Compound A* Thiamet-G* SNR-003-556* |
| Tau Spread | HSPGs | MC1 Antibody* PHF1 Antibody* |
| Synapse Loss | STEP NMDAR Fyn |
MEM 1003
 Neramexane  Sabeluzole 
|
| Microtubule Destabilization | AL-108
 Epothilone D** TPI-287** AL-208** Paclitaxel* |
|
| Impaired Axonal Transport | JIP1 KLC1/2 DIC |
|
| Actin Stabilization | CFL2* GSN* ACTB* |
|
| Mitochondrial Dysfunction/Oxidative Stress | DRP1* MFN2* OPA1* Complex V PRDX2* SOD2* REST |
Lithium
 ALCAR  Idebenone  Propentofylline  AC-1204** α-tocopherol** Resveratrol** Circumin** NSAIDs* |
| DNA Damage | p53* MDM4 ART |
|
| Heterochromatin Relaxation/Aberrant Gene Expression | BPTF* PPA2* PIWIL1* ASH1L* SFPQ |
|
| Cell Cycle Activation | CDK1* TSC2* RBL2* Rb |
Olomoucine* Rapamycin* |
 Discontinued clinical trial,
Discontinued clinical trial,
Clinical trial,
Drug or protein whose genetic manipulation suppresses aspects of tau toxicity in animal models. Proteins selected as potential therapeutic targets are those discussed in the text and/or whose genetic manipulation suppresses aspects of tau toxicity, and do not represent the entirety of proteins implicated in tauopathy. For proteins that were identified as dysfunctional in animal models of tauopathy, the human homolog is listed in the table. Information about clinical trials was obtained from ClinicalTrials.gov.
Mechanisms of tau neurotoxicity
The aggregation of misfolded tau protein, the autosomal dominant inheritance pattern in familial tauopathies [3–5], and the lack of an obvious neurodegenerative phenotype in tau knockout animal models [10], suggests a dominant gain of function pathogenic mechanism. Accordingly, transgenic expression of human wild-type or mutant tau causes progressive neuronal death in various animal models of tauopathy [11, 12]. These models have identified and characterized key cellular processes that promote apoptosis in tauopathy, including synapse loss, impaired axonal transport, overstabilization of filamentous actin, mitochondrial dysfunction, oxidative stress, DNA damage, epigenetic changes, and aberrant cell cycle activation in postmitotic neurons (Figure 1). We describe the evidence that supports a role for each of these processes in tauopathy in further detail.
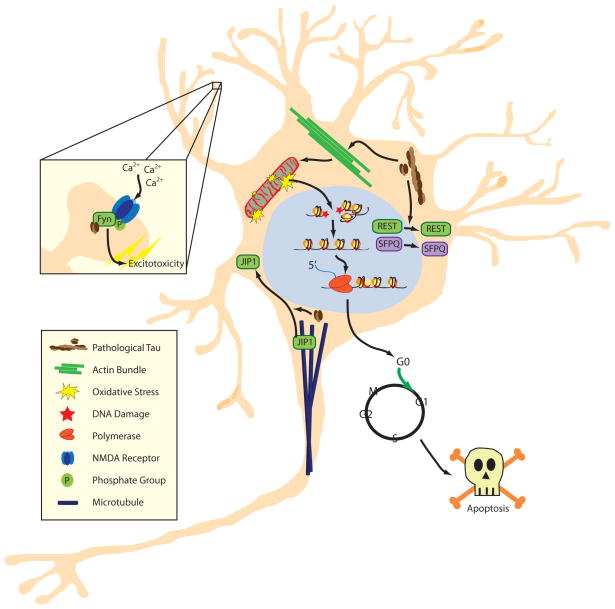
Figure 1.
Model of tau-induced neurodegeneration. Soluble tau becomes abnormally phosphorylated and forms oligomers and larger filamentous aggregates. Misfolded, hyperphosphorylated tau causes the bundling and stabilization of filamentous actin, which gives rise to elongated, dysfunctional mitochondria and oxidative stress. Oxidative stress induced by dysfunctional mitochondria or lack of nuclear REST causes DNA damage, which stimulates loss of heterochromatin. Genes that are normally silenced by heterochromatin are aberrantly transcribed, leading to cell cycle activation in postmitotic neurons and subsequent apoptosis. In parallel, pathological tau causes nuclear depletion of REST and SFPQ, along with defects in axonal transport due in part to the relocalization of JIP1 from microtubules to the neuronal soma. Furthermore, tau localizes Fyn to the NMDA receptor in dendritic spines, facilitating an Aβ-mediated influx of calcium and subsequent excitotoxicity.
Unplugged - Synapse loss in tauopathy
Synapse loss can be elicited either by the failure of neurons to maintain functional axons and dendrites or by neuronal death [13]. In Alzheimer’s disease with its slow progression, many studies indicate that synapse loss precedes neuronal loss by several decades. Not only does the initial decrease in synapse number and density seem to be disproportionate to the loss of neuronal cell bodies, suggesting that pruning of synaptic endings precedes neuronal loss [14], but synapse loss also appears to be an early event in pathogenesis as shown in patients with mild cognitive impairment and early Alzheimer’s disease [15–17].
Soluble, extracellular species of Aβ are capable of triggering both acute neuronal death and synaptic dysfunction [18]. Whereas in a pathocascade Aβ has been placed upstream of tau [7], the toxic effects of Aβ depend at least in part on soluble, cytoplasmic tau, as shown by crossing Aβ plaque-forming mice onto a tau knock-out background [6, 19]. Here, reducing tau levels was sufficient to improve or even fully rescue the clinical features that characterize mice with Aβ deposition, such as reduced lifespan, memory impairment and susceptibility to experimentally induced excitotoxic seizures. Importantly, these improvements occurred in the absence of any changes to Aβ levels or plaque load. A recent study in transgenic mice overexpressing mutant forms of human amyloid precursor protein (APP) and presenilin-1 even suggests a feedback mechanism with tau also regulating Aβ because, in addition to protecting from neuronal and synaptic loss, removing tau resulted in a lower plaque load [20]. With evidence increasing that spontaneous seizures have a role in the pathogenesis of Alzheimer’s disease, interestingly, tau reduction is also capable of preventing spontaneous epileptiform activity in multiple lines of Aβ plaque-forming mice, as shown by EEG recordings [21].
How do Aβ and tau interact in the spine? In mediating Aβ toxicity via tau, the Src kinase Fyn has a crucial role. Tau is required to target Fyn to the spine where Fyn mediates the downstream toxicity of Aβ by over-activating cellular receptors such as the NMDA receptor either directly or indirectly [19] (Figure 1). Aβ further causes a missorting of tau into dendrites as well as a loss of spines [22]. A time- resolved model for how Aβ, via Fyn, ultimately causes neuronal demise, posits that Aβ in a first step activates Fyn causing downstream excitotoxicity, and that Aβ later in disease activates STEP, a Fyn-phosphatase that eventually inactivates Fyn, leading to the loss of synapses [23]. Whether this inactivation of Fyn then results in the reduction of tau in the spine remains to be determined.
While tau is traditionally perceived as an axonal protein, with a somatodendritic relocalization characterizing Alzheimer’s disease and related tauopathies, already under physiological conditions, tau is localized - albeit at lower levels as known for the axon - to the dendritic compartment including spines [19]. This localization is tightly regulated. Both depolarization and LTP induction target tau to the spine, as does exposure to Aβ, however depending on the type of trigger tau seems to be specifically phosphorylated, and manipulating these phosphorylation sites abrogates the localization of tau to spines [24]. Together these studies present tau as a scaffolding protein with diverse functions in a physiological and pathological context, many of which await elucidation. More research needs to go into the cellular role of distinct phospho-species of tau, and the role of tau’s many isoforms.
Derailed - Impaired axonal transport in tauopathy
Concerning the axonal transport of tau, there are two important questions in the field, one relates to how tau is actually transported, and the second how pathologically elevated tau (which is inevitably hyperphosphorylated) impairs axonal transport. Both processes are highly interrelated.
Several models have been presented to understand tau transport: motor protein-dependent cotransport with microtubule fragments, diffusion, and kinesin-driven transport that is tau phosphorylation-dependent [25]. Recent studies suggest that tau diffuses along the microtubule lattice, a behavior also adopted by non-microtubule-associated proteins such as antibodies [26]. It was further found that about half of the tau molecules on microtubules are in fact not stationary but rather move bidirectionally along these microtubules. By using a range of tau concentrations it was suggested that tau molecules that diffuse along the length of a microtubule do not block each other but rather pass each other, either because they are bound to neighboring protofilaments or by switching onto another protofilament [26]. Interestingly, tau lacking a microtubule-binding domain moves into the axon, suggesting a mechanism that is independent of the binding to microtubules [19].
What is neglected in these studies is that at least in the experimental species investigated, there is an additional layer of complexity, namely with the tau-encoding MAPT mRNA being targeted to the axonal shaft [27]. The detailed process of mRNA transport and local translation of tau is still poorly understood. In Alzheimer’s disease, tau is missorted from the axonal to the somatodendritic compartment. What causes this missorting is partly explained by the existence of the axon initial segment (AIS) that acts as a retrograde barrier, and breaks down when tau is hyperphosphorylated [28].
Several studies addressed the role of kinesin and its interaction with tau. Protein levels of both the kinesin motor-mediated axonal transport machinery and of the dynein-mediated retrograde transport machinery are reduced in Alzheimer’s disease [29]. Kinesin motors enable long-range transport. Traditionally this molecule has been investigated in single-motor experiments although kinesin motors are often linked together to transport the same cargo in vivo [30]. Tau inhibits kinesin-mediated transport not only by limiting cargo travel distance, but the tau-mediated reduction in single-kinesin travel distance also leads to a modest reduction in multiple-kinesin velocity [31]. This is important because reductions in single-kinesin velocity increase the probability that at least one kinesin motor will remain bound to the microtubule per unit time, thereby increasing the travel distance of each cargo [32]. Pathological tau impairs the axonal transport of distinct kinesin cargoes by trapping the kinesin adapter-molecule JIP1 in the soma [33, 34] (Figure 1), but the reverse is also true because kinesin-deficiency leads to tau hyperphosphorylation, aggregate formation and neurodegeneration [35].
Tau’s role is evident in many cellular compartments. In recent years a new role emerged in the dendritic compartment: tau also causes the depletion of nuclear factors and their accumulation in the soma as shown for SFPQ (also known as PSF) (Figure 1), a nuclear splicing factor and transcriptional regulator. Strikingly, in affected brain areas in Alzheimer’s disease, SFPQ was massively depleted from nuclei of neurons and astrocytes [36]. Together this demonstrates a crucial role for pathological tau in axonal transport and possibly also, nucleo-cytoplasmic transport.
Actin’ up - Cytoskeletal dysfunction in tauopathy
In addition to its role as a microtubule-associated protein, tau partners with the actin cytoskeleton [37], and actin dynamics goes significantly awry in tauopathies. The finding that Hirano bodies, eosinophilic inclusions that are frequently observed in postmortem Alzheimer’s disease brains, are composed of bundles of filamentous actin [38] provided the first hint of an association between actin dysfunction and neurodegeneration. Neuronal Hirano bodies also form in mouse and Drosophila models of tauopathy, and can colocalize with tau [39–41].
Early evidence suggested that tau induces the formation of actin bundles by directly crosslinking filamentous actin based on the ability of a synthetic 18 amino acid fragment of tau’s microtubule binding domain to induce bundling of actin filaments in vitro [42]. Subsequent work supports and extends these findings. Full-length purified bovine tau induces bundling of actin filaments in vitro, and immunodepletion of tau prior to incubation with actin blocks this process [41]. The proline-rich domain of tau protein directly precedes the microtubule-binding domain, and can promote actin bundling in vitro in the absence of the microtubule-binding domain, indicating that multiple domains within the tau protein can facilitate actin bundling [43]. Unlike conventional actin filaments, actin bundles are resistant to the actin-depolymerizing drug Swinholide-A, suggesting that bundling confers stabilization of filamentous actin [41]. In flies, filamentous actin stabilization correlates with the degree of toxicity induced by transgenic expression of either human wild-type or disease-associated mutant tau, and occurs downstream of tau phosphorylation [41]. The interaction between tau and filamentous and/or bundled actin at the post-synaptic density [24] is increased upon synaptic activation, supporting a role for tau as a regulator of synaptic plasticity [24].
Excess stabilized actin reduces actin turnover and dynamics, which has significant consequences for cellular function. In cultured cells, jasplakinolide- or phalloidin-based actin stabilization significantly inhibit myosin-mediated organelle transport [44], which may underlie the reduced organelle motility that has been described in tauopathy. In yeast, genetically reducing actin dynamics causes oxidative stress and apoptosis [45] via hyperactivation of the Ras signaling pathway [46]. Similarly, genetically promoting stabilization of filamentous actin causes oxidative stress and significantly enhances tau neurotoxicity in Drosophila [47]. Collectively, these studies indicate that excess stabilized actin, a concomitant reduction in actin dynamics, and subsequent oxidative stress are significant contributors to neurotoxicity in tauopathies (Figure 1).
Power plant shutdown - Mitochondrial dysfunction in tauopathies
Early on, the presence of abnormally shaped mitochondria in dystrophic neurites was reported in brains affected by Alzheimer’s disease [48]. Comprehensive morphological and morphometric studies on neuronal mitochondria in various regions of postmortem human Alzheimer’s disease brain followed, and also demonstrated that mitochondria are morphologically distorted in Alzheimer’s disease [49]. Evidence suggests that abnormal mitochondrial morphology correlates with mitochondrial dysfunction, as mitochondrial complex V activity is significantly reduced in postmortem Alzheimer’s disease and FTDP-17 brains [50, 51]. Consistent with these findings, tau transgenic flies and mice have significantly elongated mitochondria [47], and tau transgenic mice have reduced mitochondrial complex I and V activity and other mitochondrial respiratory defects [51]. Studies in cell culture further support a role for structural and functional disturbances in mitochondria, as tau expression in neuroblastoma cells causes impaired mitochondrial fission and fusion, reduced mitochondrial complex I activity and reduced ATP levels [52].
Additional genetic and biochemical experiments in tau transgenic Drosophila provide mechanistic insight into how tau promotes mitochondrial dysfunction. In tauopathy model flies, a physical interaction between excess filamentous actin and the mitochondrial fission protein DRP1 blocks the myosin-based translocation of DRP1 to mitochondria, blocking mitochondrial fission and promoting the formation of elongated mitochondria [47]. This result, combined with the observation that DRP1 levels are depleted in pyramidal neurons from postmortem Alzheimer’s disease brain [53], supports a role for stabilized actin as a key disruptor of mitochondrial dynamics in tauopathy. While the role of tau truncation in disease is still under investigation, expression of disease-associated truncated forms of tau in cultured neurons causes mitochondrial fragmentation, whereas expression of full-length tau causes mitochondrial elongation [54], suggesting that full length tau versus tau cleavage products may affect mitochondria in distinct ways. Whether mitochondria tend toward elongation or fragmentation, these studies demonstrate that mitochondrial dynamics are impaired in tauopathy. When Aβ and tau pathologies are combined in transgenic mouse models, synergistic effects, as evident by a reduced mitochondrial membrane potential, reduced ATP synthesis, increased levels of reactive oxygen species, and defective respiration are observed [55].
The brain is a highly metabolic organ and, as such, heavily relies on proper mitochondrial function. Mitochondria buffer calcium ion levels and provide energy to cells in the form of ATP. A byproduct of this reaction is the formation of reactive oxygen species, that, when improperly balanced, give rise to oxidative stress. We will discuss the presence and repercussions of oxidative stress in tauopathies in the following section.
Zapped - Oxidative stress in tauopathy
With its relatively low levels of antioxidants, high demand for oxygen, and high concentration of polyunsaturated fatty acids, the nervous system is particularly susceptible to oxidative stress. The Alzheimer’s disease brain has ample evidence of oxidative stress, including protein carbonyls, 3-nitrotyrosine, markers of oxidative damage to DNA and RNA, products of lipid peroxidation, and alterations in the activity or expression of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione [56]. Similarly, evidence of oxidative stress is abundant in animal models of tauopathy. Tau transgenic mice have free radical damage [51] and, based on elevated levels of the superoxide-dependent fluorescent probe dihydroethidium, high levels of reactive oxygen species, as is also seen in tauopathy model flies [47]. In addition, neuronal cultures derived from tauopathy model rats have an excess of ascorbyl free radicals and are more sensitive to oxidative insults than controls [57].
Adding insult to injury, neurons affected by tau pathology are not well equipped to handle high levels of oxidative stress. In cultured neurons, tau blocks the transport of antioxidant-filled peroxisomes into neurites, and the lack of neuritic peroxisomes renders cells more vulnerable to oxidative damage [58]. In addition, a recent study identified the nuclear loss of repressor element 1-silencing transcription factor (REST), a stress response protein, as a mechanism that increases the sensitivity of neurons to oxidative stress in tauopathy (Figure 1). REST is depleted in neuronal nuclei of postmortem tauopathy brains compared to controls, and instead appears in autophagosomes alongside abnormally phosphorylated tau. Loss of nuclear REST correlates with decreased expression of stress response genes and increased DNA damage [59].
A critical cellular consequence of oxidative stress in tauopathies is DNA damage (Figure 1). DNA repair proteins are most active during DNA replication associated with cell division, thus postmitotic neurons have relatively limited DNA damage defense mechanisms [60]. Oxidative stress causes nucleic acid lesions including DNA double and single strand breaks, base modifications, and deletions [61]. Previous studies have found evidence of DNA damage in postmortem brains of patients with Alzheimer’s disease [62], and the recent detection of DNA adducts in pre-clinical Alzheimer’s disease suggests that DNA damage is an early event in the course of disease [63]. Tau transgenic mice and flies have increased levels of pH2Ax or pH2Av, respectively, which are specific markers of DNA double strand breaks, and increased levels of p53, an effector of the DNA damage checkpoint [64].
Recently, activation of the DNA damage checkpoint in response to DNA damage has been shown to alleviate tau neurotoxicity. Reducing the function of ATM, Chk2, or p53, effectors of the DNA damage checkpoint, exacerbates tau neurotoxicity in Drosophila [64]. ATM levels are increased in postmortem Alzheimer’s disease brain, along with DNA damage response genes MDM4 and ART [65], demonstrating that activation of the DNA damage checkpoint occurs in both animal models of tauopathy and in human disease. An additional neuroprotective role for tau in response to oxidative stress is based on recent evidence from cell culture experiments in which oxidative stress causes increased levels of nuclear tau, which renders DNA more resilient to heat-shock induced damage [66], potentially by a direct interaction between tau and DNA [67]. Oxidative stress is a driver of heterochromatin loss in tauopathy, which has been recently identified as a novel mechanism of tau neurotoxicity as discussed below [68].
Unraveled – Epigenetic changes in tauopathy
DNA is packaged into chromatin domains termed euchromatin and heterochromatin, and the maintenance of these domains is critical for cellular viability. While there are exceptions, heterochromatin is typically associated with gene silencing, and loss of heterochromatic silencing is known to cause an increase in gene expression. Histone methylation and acetylation, as well as DNA methylation regulate the balance between euchromatin and heterochromatin [69]. Studies in nonneuronal cells gave the first clue that chromatin structure may be altered in Alzheimer’s disease, as heterochromatin is undercondensed in lymphocytes derived from Alzheimer’s disease patients based on the sensitivity of chromosomes to 5-azacytidine [70]. Recently, a global relaxation of heterochromatin in neurons from postmortem human Alzheimer’s disease brain, along with mouse and fly models of tauopathy have been shown [68]. In support of an overall opening of chromatin in Alzheimer’s disease, a second recent study reports increased levels of the transcription-activating histone modification acetylated H3K9 (H3K9Ac), in neurons isolated from postmortem human Alzheimer’s disease brain [59]. The H3K9Ac histone modification is normally reduced by REST, and may increase in Alzheimer’s disease as a result of decreased nuclear REST levels [59]. In contrast, a reduction of H3K18 and H3K23 acetylation has been reported in postmortem temporal lobe from patients with Alzheimer’s disease [71], however this study did not measure acetylation specifically in neurons. In a pair of monozygotic twins discordant for Alzheimer’s disease, neuronal nuclei from the temporal neocortex of the affected twin contained reduced levels of DNA methylation [72], and total DNA and RNA methylation is reduced in neurons from entorhinal cortex layer II of postmortem Alzheimer’s disease brains [73]. Collectively, studies that have measured global histone modifications and DNA methylation specifically in neurons indicate a widespread opening of chromatin in Alzheimer’s disease that is conducive to transcriptional activation (Figure 1).
In flies, ectopic oxidative stress caused by mutations in thioredoxin reductase 1 or superoxide dismutase gives rise to heterochromatin relaxation, possibly via DNA damage, providing one mechanism whereby tau causes heterochromatin relaxation [68]. While the presence of tau in the nucleus has been the subject of some debate, recent work supports the idea that nuclear tau exists and demonstrates that visualization of nuclear tau is dependent upon several factors, including antibody, experimental system, and the staining protocol [74]. Disease associated TG-3 and Alz-50 positive tau colocalize with heterochromatin in human Alzheimer’s disease brain based on electron microscopy [75], and tau colocalizes with pericentromeric heterochromatic DNA in cultured cells based on immunofluorescence [76]. While some evidence suggests that oxidative DNA damage causes a loss of heterochromatin, it is also possible that heterochromatin loss may result from a direct interaction between pathological tau and DNA or an as yet uncharacterized mechanism. These hypotheses are not mutually exclusive.
Genes that are aberrantly expressed in tau transgenic Drosophila as a result of heterochromatin relaxation have also been identified [68]. The aberrant expression of genes that are normally silenced by heterochromatin is conserved in human Alzheimer’s disease, as large-scale gene expression analyses from laser- captured neurons reveal a transcriptional increase in over 30% of genes in Alzheimer’s disease that are silenced by heterochromatin in controls. Of the genes that are aberrantly expressed in human Alzheimer’s disease as a result of heterochromatin loss, PIWIL1 is of particular interest. The fly homolog of PIWIL1, Ago3, is expressed at higher levels in tau transgenic flies, and reduction of Ago3 levels suppresses tau neurotoxicity [68]. PIWIL1 promotes the biogenesis of piwi-interacting RNAs (piRNAs), which bind to RNA transcripts of transposons and facilitate their degradation [77, 78]. A potential imbalance between piRNAs and transposable element transcripts in the tauopathy brain is an intriguing topic for future experimentation.
Double trouble - Cell cycle activation in postmitotic neurons in tauopathy
The ectopic expression of cell cycle markers in post-mitotic neurons and their coincidence with tau pathology is a well-described feature of tauopathies, and has been reviewed elsewhere [79]. In tau transgenic Drosophila, activation of the cell cycle in postmitotic neurons results from tau-induced actin stabilization [41], mitochondrial dysfunction [47], oxidative stress [80], DNA damage [64], and heterochromatin relaxation [68], and is known to be mediated by target of rapamycin kinase (TOR) [81], suggesting that these events participate in a toxic cascade toward aberrant cell cycle activation in vivo. Additional players that promote cell cycle activation in tauopathy have recently been identified. MicroRNA 26b (miR-26b) is elevated in postmortem brain tissue from human Alzheimer’s disease [82]. MiR-26b is a known regulator of the cell cycle in dividing cells [83], and its overexpression in cultured postmitotic rat primary neurons causes expression of multiple cell cycle markers alongside a decrease in proteins that inhibit the cell cycle. The authors go on to show that miR-26b induces cell cycle activation through the targeted degradation of retinoblastoma protein (Rb) [82].
In dividing cells, elevated levels of proteins that promote the cell cycle can cause tumorigenesis through unrestrained cell division. In post-mitotic, fully differentiated neurons, however, ectopic expression of cell cycle related proteins causes cell death rather than cell division [81, 84]. Taken together, these studies provide key evidence that pathological tau sparks a series of cellular events that ultimately sentences neurons to death by aberrant cell cycle activation [79] (Figure 1).
Concluding Remarks
There is a significant unmet need for therapies that slow or prevent the progression of tauopathies. As the most common tauopathy, Alzheimer’s disease has been the focus of multiple past and recent clinical trials, with disappointing results (Table 1). Current treatments for Alzheimer’s disease, cholinesterase inhibitors and NMDA antagonists, modestly and temporarily reduce symptoms associated with the disease but do not stop disease progression [85]. Table 1 summarizes human clinical trials, drugs that have been promising in animal models of tauopathy, and proteins that are potential targets for drug development. A number of points should be considered when designing and implementing therapies for Alzheimer’s disease. First, the disorder is multifactorial. Similar to the treatment of cancers, a combinatorial therapeutic approach that targets multiple aspects of the disease may be more effective than strategies targeting a single aspect of the disease. Second, Alzheimer’s disease patient populations are heterogeneous and distinct therapies may be more or less effective in particular patient populations. Third, tauopathy patients often have a significant tau load by the time they begin to show symptoms, and cellular events that occur downstream of tau pathology may thus be more practical targets than tau aggregation or phosphorylation itself. Fourth, the identification of prognostic and predictive biomarkers will greatly aid disease treatment in sporadic tauopathies. To effectively address these issues, we must understand the basic cellular processes that connect tau dysfunction to neuronal death.
Much progress has been made in identifying the underlying causes of cell death that occur downstream of tau dysfunction (Figure 1). Genetic or pharmacological reversal of many events in the pathway between tau dysfunction and apoptosis: actin stabilization, mitochondrial dysfunction, oxidative stress, DNA damage, epigenetic changes, and reentry of postmitotic neurons into the cell cycle, significantly rescue tau neurotoxicity in animal models of the disease (Table 1, potential targets). Each node in the cascade of cellular dysfunction sparked by pathological tau is a valid candidate for a disease-modifying therapy in its own right, and may be beneficial in the treatment of tauopathies, including Alzheimer’s disease, either alone or in combination with a strategy targeting Aβ or tau protein. For example, in fly models of tauopathy, the CDK1 inhibitor olomoucine alleviates tau-induced neurotoxicity, as does genetic reduction of CDK1 function, supporting the idea that blocking aberrant neuronal cell cycle reentry is an excellent candidate for therapeutic intervention. Similarly, genetic reduction of Ago3, the fly homolog of mammalian PIWIL1, rescues neuropathological and locomotor deficits in tau transgenic flies, suggesting that silencing the aberrant expression of this gene may relieve tau neurotoxicity in humans. Stabilization of microtubules with Epothilone D alleviates neuropathological changes as well as cognitive defects in tau transgenic mice, and human clinical trials are underway. Similarly, drugs that reduce oxidative stress are efficacious in tau transgenic mice, and are currently in clinical trials. These studies are encouraging, however it is unknown if reversal of events downstream of pathological tau will slow disease progression in humans. Importantly, pathological tau is now known to spread between neurons in a manner similar to prions [86–90], suggesting that clearance of extracellular tau may halt the spread of tau pathology through the brain. As our understanding of the intricate pathway connecting tau dysfunction to cell death grows, so too does the likelihood that we will develop effective diagnostic and therapeutic tools for the treatment of tauopathies.
Highlights.
Downstream mechanisms of tau neurotoxicity are discussed.
Tau drives cytoskeletal, mitochondrial, chromatin, and cell cycle dysfunction.
Processes connecting tau dysfunction to apoptosis are potential therapeutic targets.
Acknowledgments
B.F. is supported by NINDS (R01NS083391 awarded to M.B.F.). J.G. is supported by the Australian Research Council (DP13300101932) and the National Health and Medical Research Council of Australia (APP1037746, APP1003150). M.B.F. is supported by NIA (R21AG040796 and R01AG044113) and NINDS (R01NS083391 and R01NS083391).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer A, et al. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clinical anatomy. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. The Journal of biological chemistry. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 3.Poorkaj P, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Annals of neurology. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 4.Hutton M, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, et al. Tau pathology in two Dutch families with mutations in the microtubule-binding region of tau. The American journal of pathology. 1998;153:1359–1363. doi: 10.1016/S0002-9440(10)65721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 7.Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nature reviews Neuroscience. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 8.Gotz J, et al. Tau-targeted treatment strategies in Alzheimer’s disease. British journal of pharmacology. 2012;165:1246–1259. doi: 10.1111/j.1476-5381.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina M, Avila J. New perspectives on the role of tau in Alzheimer’s disease. Implications for therapy. Biochemical pharmacology. 2014;88:540–547. doi: 10.1016/j.bcp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Ke YD, et al. Lessons from tau-deficient mice. International journal of Alzheimer’s disease. 2012;2012:873270. doi: 10.1155/2012/873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mhatre SD, et al. Invertebrate models of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2013;33:3–16. doi: 10.3233/JAD-2012-121204. [DOI] [PubMed] [Google Scholar]
- 12.LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012:2. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom GS. Amyloid-beta and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA neurology. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 14.Davies CA, et al. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. Journal of the neurological sciences. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 15.Masliah E, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 16.Scheff SW, et al. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiology of aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Scheff SW, et al. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 18.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature neuroscience. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ittner LM, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Leroy K, et al. Lack of tau proteins rescues neuronal cell death and decreases amyloidogenic processing of APP in APP/PS1 mice. The American journal of pathology. 2012;181:1928–1940. doi: 10.1016/j.ajpath.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Roberson ED, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zempel H, et al. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehm J. A ‘danse macabre’: tau and Fyn in STEP with amyloid beta to facilitate induction of synaptic depression and excitotoxicity. The European journal of neuroscience. 2013;37:1925–1930. doi: 10.1111/ejn.12251. [DOI] [PubMed] [Google Scholar]
- 24.Frandemiche ML, et al. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-Beta oligomers. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:6084–6097. doi: 10.1523/JNEUROSCI.4261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuchillo-Ibanez I, et al. Phosphorylation of tau regulates its axonal transport by controlling its binding to kinesin. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:3186–3195. doi: 10.1096/fj.08-109181. [DOI] [PubMed] [Google Scholar]
- 26.Hinrichs MH, et al. Tau protein diffuses along the microtubule lattice. The Journal of biological chemistry. 2012;287:38559–38568. doi: 10.1074/jbc.M112.369785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronov S, et al. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:6577–6587. doi: 10.1523/JNEUROSCI.21-17-06577.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, et al. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. The EMBO journal. 2011;30:4825–4837. doi: 10.1038/emboj.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel M, et al. Levels of kinesin light chain and dynein intermediate chain are reduced in the frontal cortex in Alzheimer’s disease: implications for axoplasmic transport. Acta neuropathologica. 2012;123:71–84. doi: 10.1007/s00401-011-0901-4. [DOI] [PubMed] [Google Scholar]
- 30.Hendricks AG, et al. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Current biology: CB. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, et al. Interplay between velocity and travel distance of Kinesin-based transport in the presence of Tau. Biophysical journal. 2013;105:L23–25. doi: 10.1016/j.bpj.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, et al. Tuning multiple motor travel via single motor velocity. Traffic (Copenhagen, Denmark) 2012;13:1198–1205. doi: 10.1111/j.1600-0854.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ittner LM, et al. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15997–16002. doi: 10.1073/pnas.0808084105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ittner LM, et al. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. The Journal of biological chemistry. 2009;284:20909–20916. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falzone TL, et al. Kinesin-1 transport reductions enhance human tau hyperphosphorylation, aggregation and neurodegeneration in animal models of tauopathies. Human molecular genetics. 2010;19:4399–4408. doi: 10.1093/hmg/ddq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke YD, et al. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer’s and Pick’s disease. PloS one. 2012;7:e35678. doi: 10.1371/journal.pone.0035678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriquez JP, et al. Subpopulations of tau interact with microtubules and actin filaments in various cell types. Cell biochemistry and function. 1995;13:239–250. doi: 10.1002/cbf.290130404. [DOI] [PubMed] [Google Scholar]
- 38.Galloway PG, et al. Hirano body filaments contain actin and actin-associated proteins. Journal of neuropathology and experimental neurology. 1987;46:185–199. doi: 10.1097/00005072-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Galloway PG, et al. Hirano bodies contain tau protein. Brain research. 1987;403:337–340. doi: 10.1016/0006-8993(87)90071-0. [DOI] [PubMed] [Google Scholar]
- 40.Davis RC, et al. A cell culture model for investigation of Hirano bodies. Acta neuropathologica. 2008;115:205–217. doi: 10.1007/s00401-007-0275-9. [DOI] [PubMed] [Google Scholar]
- 41.Fulga TA, et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nature cell biology. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 42.Moraga DM, et al. A tau fragment containing a repetitive sequence induces bundling of actin filaments. Journal of neurochemistry. 1993;61:979–986. doi: 10.1111/j.1471-4159.1993.tb03611.x. [DOI] [PubMed] [Google Scholar]
- 43.He HJ, et al. The proline-rich domain of tau plays a role in interactions with actin. BMC cell biology. 2009;10:81. doi: 10.1186/1471-2121-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenova I, et al. Actin dynamics is essential for myosin-based transport of membrane organelles. Current biology: CB. 2008;18:1581–1586. doi: 10.1016/j.cub.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gourlay CW, et al. A role for the actin cytoskeleton in cell death and aging in yeast. The Journal of cell biology. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gourlay CW, Ayscough KR. Actin-induced hyperactivation of the Ras signaling pathway leads to apoptosis in Saccharomyces cerevisiae. Molecular and cellular biology. 2006;26:6487–6501. doi: 10.1128/MCB.00117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuBoff B, et al. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kidd M. ALZHEIMER’S DISEASE--AN ELECTRON MICROSCOPICAL STUDY. Brain: a journal of neurology. 1964;87:307–320. doi: 10.1093/brain/87.2.307. [DOI] [PubMed] [Google Scholar]
- 49.Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2006;9:119–126. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- 50.Schagger H, Ohm TG. Human diseases with defects in oxidative phosphorylation. 2. F1F0 ATP-synthase defects in Alzheimer disease revealed by blue native polyacrylamide gel electrophoresis. European journal of biochemistry/FEBS. 1995;227:916–921. doi: 10.1111/j.1432-1033.1995.tb20219.x. [DOI] [PubMed] [Google Scholar]
- 51.David DC, et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. The Journal of biological chemistry. 2005;280:23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 52.Schulz KL, et al. A new link to mitochondrial impairment in tauopathies. Molecular neurobiology. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintanilla RA, et al. Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiology of aging. 2012;33:619, e625–635. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhein V, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt K, et al. Insights into mitochondrial dysfunction: aging, amyloid-beta, and tau-A deleterious trio. Antioxidants & redox signaling. 2012;16:1456–1466. doi: 10.1089/ars.2011.4400. [DOI] [PubMed] [Google Scholar]
- 57.Cente M, et al. Expression of a truncated human tau protein induces aqueous-phase free radicals in a rat model of tauopathy: implications for targeted antioxidative therapy. Journal of Alzheimer’s disease: JAD. 2009;17:913–920. doi: 10.3233/JAD-2009-1107. [DOI] [PubMed] [Google Scholar]
- 58.Stamer K, et al. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. The Journal of cell biology. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu T, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507:448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanawalt PC, et al. Genomic heterogeneity of DNA repair. Role in aging? Annals of the New York Academy of Sciences. 1992;663:17–25. doi: 10.1111/j.1749-6632.1992.tb38644.x. [DOI] [PubMed] [Google Scholar]
- 61.McKinnon PJ. Maintaining genome stability in the nervous system. Nature neuroscience. 2013;16:1523–1529. doi: 10.1038/nn.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullaart E, et al. Increased levels of DNA breaks in cerebral cortex of Alzheimer’s disease patients. Neurobiology of aging. 1990;11:169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- 63.Bradley-Whitman MA, et al. Nucleic acid oxidation: an early feature of Alzheimer’s disease. Journal of neurochemistry. 2014;128:294–304. doi: 10.1111/jnc.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khurana V, et al. A neuroprotective role for the DNA damage checkpoint in tauopathy. Aging cell. 2012;11:360–362. doi: 10.1111/j.1474-9726.2011.00778.x. [DOI] [PubMed] [Google Scholar]
- 65.Katsel P, et al. Cycle checkpoint abnormalities during dementia: a plausible association with the loss of protection against oxidative stress in Alzheimer’s disease. PloS one. 2013;8:e68361. doi: 10.1371/journal.pone.0068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Violet M, et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Frontiers in cellular neuroscience. 2014;8:84. doi: 10.3389/fncel.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sultan A, et al. Nuclear tau, a key player in neuronal DNA protection. The Journal of biological chemistry. 2011;286:4566–4575. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frost B, et al. Tau promotes neurodegeneration through global chromatin relaxation. Nature neuroscience. 2014;17:357–366. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dillon N. Heterochromatin structure and function. Biology of the cell/under the auspices of the European Cell Biology Organization. 2004;96:631–637. doi: 10.1016/j.biolcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Payao SL, et al. Differential chromosome sensitivity to 5-azacytidine in Alzheimer’s disease. Gerontology. 1998;44:267–271. doi: 10.1159/000022023. [DOI] [PubMed] [Google Scholar]
- 71.Zhang K, et al. Targeted proteomics for quantification of histone acetylation in Alzheimer’s disease. Proteomics. 2012;12:1261–1268. doi: 10.1002/pmic.201200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mastroeni D, et al. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer’s disease. PloS one. 2009;4:e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mastroeni D, et al. Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation. Neurobiology of aging. 2010;31:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu J, et al. Visualizing the microtubule-associated protein tau in the nucleus. Science China Life sciences. 2014;57:422–431. doi: 10.1007/s11427-014-4635-0. [DOI] [PubMed] [Google Scholar]
- 75.Luna-Munoz J, et al. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2005;8:29–41. doi: 10.3233/jad-2005-8104. [DOI] [PubMed] [Google Scholar]
- 76.Sjoberg MK, et al. Tau protein binds to pericentromeric DNA: a putative role for nuclear tau in nucleolar organization. Journal of cell science. 2006;119:2025–2034. doi: 10.1242/jcs.02907. [DOI] [PubMed] [Google Scholar]
- 77.Brennecke J, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 78.Aravin A, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 79.Arendt T. Cell cycle activation and aneuploid neurons in Alzheimer’s disease. Molecular neurobiology. 2012;46:125–135. doi: 10.1007/s12035-012-8262-0. [DOI] [PubMed] [Google Scholar]
- 80.Dias-Santagata D, et al. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. The Journal of clinical investigation. 2007;117:236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khurana V, et al. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Current biology: CB. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 82.Absalon S, et al. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:14645–14659. doi: 10.1523/JNEUROSCI.1327-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huse JT, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes & development. 2009;23:1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herrup K, Busser JC. The induction of multiple cell cycle events precedes target-related neuronal death. Development. 1995;121:2385–2395. doi: 10.1242/dev.121.8.2385. [DOI] [PubMed] [Google Scholar]
- 85.Ballard C, et al. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 86.Frost B, et al. Propagation of tau misfolding from the outside to the inside of a cell. The Journal of biological chemistry. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature cell biology. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim W, et al. Interneuronal transfer of human tau between Lamprey central neurons in situ. Journal of Alzheimer’s disease: JAD. 2010;19:647–664. doi: 10.3233/JAD-2010-1273. [DOI] [PubMed] [Google Scholar]
- 89.Holmes BB, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3138–3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker LC, et al. Mechanisms of protein seeding in neurodegenerative diseases. JAMA neurology. 2013;70:304–310. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]