Abstract
Purpose
Data on the association between body mass index (BMI) and risk of knee osteoarthritis (KOA) are sparse for Asian populations who are leaner than Western populations. We evaluated the association between BMI and risk of total knee replacement (TKR) due to severe KOA among Chinese in Singapore.
Methods
We used data from the Singapore Chinese Health Study, a population based prospective cohort of 63,257 Chinese men and women, aged 45–74 years at enrollment from 1993 to 1998. Information on height, weight, diet and lifestyle factors were obtained via in-person interviews. TKR cases for severe KOA were identified via linkage with the nationwide hospital discharge database through 2011. Cox regression and weighted least squares regression were used in the analysis.
Results
The mean BMI among cohort participants was 23.1 kg/m2, and more than two-thirds had BMI below 25 kg/m2. A total of 1,649 had TKR attributable to severe KOA. Risk of TKR increased in a strong dose-dependent manner with increasing BMI throughout the 15–32 kg/m2 range and became less clear at BMI > 32 kg/m2. In the BMI range 16–27 kg/m2, there was a 27% increase in TKR risk for each unit increase in BMI (p for trend < 0.001). Compared to BMI 19–20 kg/m2, the risk estimates of TKR were all statistically significant with increasing unit of BMI ≥ 21 kg/m2. Results were similar for men and women.
Conclusion
Our results provided evidence for a constant mechanical mechanism underlying BMI and KOA initiation and/or progression.
Keywords: Knee osteoarthritis, total knee replacement, body mass index, Chinese, cohort study
INTRODUCTION
Knee osteoarthritis (KOA) is a leading cause of disability among the aged population worldwide [1]. A positive association between obesity or being overweight and increased risk of KOA has been shown in numerous studies [2–4]. Hence, the global prevalence of KOA will continue to rise in tandem with the increasing prevalence of obesity worldwide. Guidelines for managing KOA recommend weight loss for body mass index (BMI) ≥ 25 kg/m2 [5, 6]. However, there is a paucity of data on risk of initiation and progression of KOA among people with BMI lower than 25 kg/m2. It is unknown whether there is a threshold effect of BMI on risk of KOA or a critical level of BMI above which risk of KOA rises dramatically. The identification of these BMI levels may serve as a weight reduction target in managing patients with KOA.
In this study we characterize the association between BMI and risk of total knee replacement (TKR) due to severe KOA using prospective data from the Singapore Chinese Health Study (SCHS), a population with a relatively lower BMI than western populations. This population-based cohort of middle-aged to elderly Chinese in Singapore provided ample data to investigate BMI as a risk factor for TKR attributable to severe KOA over a wide range of BMI values and in particular for BMI < 25kg/m2.
RESEARCH METHODS
Study population
The SCHS recruited 27,959 Chinese men and 35,298 women (n = 63,257) of ages 45–74 years between 1993 and 1998 in Singapore [7]. Subjects were recruited from public housing estates, where 86% of Singapore’s population lived at the time of recruitment. Study subjects were restricted to the two major dialect groups in Singapore: Hokkien and Cantonese, originating from the Fujian and Guangdong Provinces in Southern China, respectively. This study was approved by the Institutional Review Boards at the National University of Singapore and the University of Pittsburgh.
Baseline exposure assessment
The baseline assessment was conducted via in-person interview at recruitment using a structured questionnaire. Information was obtained on educational level, height, weight, cigarette smoking, habitual physical activity, sleep hours, self-reported medical history (e.g., physician-diagnosed hypertension, diabetes, coronary artery disease and stroke), alcohol consumption and habitual dietary intake (using a validated 165-item food frequency questionnaire). Body weight and height at baseline were self-reported during the interview. There were 10,054 cohort participants with unknown weight, 99 with unknown height, and 196 with both unknown weight and height. Their BMIs were calculated using imputed weight and/or height derived from the linear regression equation: Weight = y-intercept + gradient × height, where values for the y-intercept and gradient were obtained from gender-specific weight-height regression lines drawn from all cohort participants with known heights and weights. If only weight or height was missing, the linear regression equation was used to estimate the missing value. If both weight and height were both missing, the missing height was assigned the sex-specific median value and missing weight value calculated from the linear regression equation. We analysed data for participants with known body weight and height in the main models, and for the whole cohort that included imputed values for those with missing BMI in secondary models.
For cigarette smoking, current smokers were asked about the number of cigarettes smoked per day and the number of years of smoking, and former smokers were asked about the number of years since quitting. In assessing physical activity, subjects were asked to estimate the number of hours per week spent on moderate activities (such as brisk walking, bowling, bicycling on level ground, tai chi or chi kung), vigorous work (such as moving heavy furniture, loading and unloading trucks, shovelling or equivalent manual labor) and strenuous sports (such as jogging, bicycling on hills, tennis, squash, swimming laps or aerobics). This section of the questionnaire was adapted from the physical activity questionnaire in the European Prospective Investigation in Cancer (EPIC) Study [8]. Participants were also asked for the number of hours per day spent watching TV and sleeping.
Identification of incident cases of TKR for severe KOA
Identification of TKR for severe KOA was accomplished via record linkage analysis using the MediClaim System hospital discharge database through 31 December 2011. The system has been in use in Singapore since 1990 and records surgical procedures and up to 3 diagnoses per patient for inpatient discharges from public and private hospitals based on the ninth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-9) [9]. Since TKR may be bilateral or repeated, only first-time TKR cases were included. All TKR cases were verified by checking diagnosis code, and only subjects who underwent TKR for severe KOA (ICD-9 code 715) were counted as cases. A total of 128 prevalent cases of TKR which occurred prior to subject enrolment into the cohort were excluded from analysis. We also excluded those who underwent TKR for diagnoses such as septic arthritis, osteomyelitis, villonodular synovitis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and other inflammatory arthritis, or secondary causes of KOA such as avascular or aseptic necrosis of joint, meniscus or ligament injuries, and other congenital or acquired deformities of the knee (n=89) [10]. Deaths were identified through record linkage with the Singapore Registry of Births and Deaths. As of December 2011, only 47 subjects were known to be lost to follow-up due to migration out of Singapore or for other reasons, suggesting the ascertainment of vital status for cohort participants is virtually complete.
Statistical analysis
For each study subject, person-years were counted from the baseline interview to the date of TKR operation, death, lost to follow-up, or 31 December 2011, whichever occurred first. Association between BMI and risk of TKR was investigated using multivariable Cox proportional hazards models. The hazards ratio (HR) of TKR and its 95% confidence interval (CI) were estimated using the Cox model with BMI (kg/m2) per unit change. We set BMI of 19–<20 kg/m2 as the reference category to have a relatively large sample size and thus relatively stable estimates of HR. In all analyses, HRs were adjusted for the following variables: age at recruitment (years); year of recruitment (1993–1995, 1995–1998); dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher); self-reported histories of physician-diagnosed diabetes mellitus, ischemic heart disease, and stroke; number of hours per day of sleeping, sitting at work, and watching TV; numbers of hours per week spent in moderate physical activity, vigorous work, and strenuous sports; and smoking status (never, former, or current), number of cigarettes per day, and number of years of smoking for current smokers; and for former smokers, number of years since quitting smoking. The association between BMI and risk of TKR was investigated separately for men and women. The proportional hazards assumption was found to be tenable. The potential for interaction effects between BMI and age, gender, and physical activities or smoking on TKR risk was also examined.
In a graphical approach to investigate the relationship between BMI and risk of TKR, BMI was partitioned into one-unit intervals, and the natural logarithm of the hazards ratio (ln HR) was plotted against the median value of each interval. It was immediately apparent that in the BMI range 16–27 kg/m2, the relationship between BMI and ln HR was represented very well by a straight line. A line was fitted using weighted least squares regression with interval sample size as the weight. Exponentiating the slope of the fitted line (b) gives the multiplicative factor for change in risk (i.e., HR) per unit change in BMI. In addition, we calculated the change in HR per unit change in BMI within the range 16–27 kg/m2 in a separate Cox regression model with BMI as a continuous variable.
All hypothesis tests performed were two-sided and p < 0.05 was considered statistically significant. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Over a mean [± standard deviation (SD)] follow-up time of 14.5 ± 4.1 years, 1,649 (3.1%) incident cases of TKR for severe KOA were observed among the 52,780 subjects (23,936 men, 28,844 women) who reported weight and height at baseline in this study. Women accounted for 82.5% of cases. Mean age at TKR was 67.7 ± 6.6 years. The mean BMI of all cohort participants was 23.1 ± 3.6 kg/m2, and 71.5% of the cohort had BMI below 25 kg/m2. Table 1 shows the baseline characteristics stratified according to four BMI categories (< 21.0, 21.0–<23, 23.0–<25, ≥ 25.0 kg/m2). Across the different BMI categories, there were no large differences in distributions of gender, level of education, number of hours of sleep per day and number of hours of physical activities per week. There were higher proportions of current smokers in lower than higher BMI categories, whereas there were higher proportions of subjects with self-reported physician-diagnosed history of heart disease and diabetes in higher than lower BMI categories.
Table 1.
Baseline demographic and lifestyle characteristics by body mass index category, The Singapore Chinese Health Study 1993–2011
| Total | Body Mass Index Category (kg/m2) | ||||
|---|---|---|---|---|---|
| < 21 | 21– <23 | 23– <25 | ≥ 25 | ||
| No. of subjects | 52,780 | 15,057 | 12,411 | 11,588 | 13,724 |
| Gender, n (%) | |||||
| Men | 23,936 (45.35) | 6,883 (45.71) | 5,631 (45.37) | 5,383 (46.45) | 6,039 (44.00) |
| Women | 28,844 (54.65) | 8,174 (54.29) | 6,780 (54.63) | 6,205 (53.55) | 7,685 (56.00) |
| Age at recruitment ± SD | 56.02 ± 7.91 | 56.4 ± 8.1 | 55.8 ± 7.9 | 55.9 ± 7.8 | 55.9 ± 7.8 |
| Level of education, n (%) | |||||
| No formal education | 12,800 (24.25) | 3,365 (22.35) | 2,870 (23.12) | 2,741 (23.65) | 3,824 (27.86) |
| Primary | 23,380 (44.3) | 6,785 (45.06) | 5,442 (43.85) | 5,084 (43.87) | 6,069 (44.22) |
| Secondary or above | 16,600 (31.45) | 4,907 (32.59) | 4,099 (33.03) | 3,763 (32.47) | 3,831 (27.91) |
| Smoking status | |||||
| Never | 36,799 (69.72) | 9,761 (64.83) | 8,761 (70.59) | 8,320 (71.80) | 9,957 (72.55) |
| Former | 5,979 (11.33) | 1,537 (10.20) | 1,340 (10.80) | 1,389 (11.98) | 1,713 (12.49) |
| Current | 10,002 (18.95) | 3,759 (24.97) | 2,310 (18.61) | 1,879 (16.22) | 2,054 (14.97) |
| Hours of sleep/day ± SD | 7.03 ± 1.13 | 7.04 ± 1.15 | 7.04 ± 1.10 | 7.02 ± 1.11 | 7.02 ± 1.15 |
| Weekly vigorous work, n (%) | |||||
| No. | 48,626 (92.13) | 13,911 (92.39) | 11,396 (91.82) | 10,649 (91.90) | 12,670 (92.32) |
| 0.5–4 hours/week | 2,248 (4.26) | 616 (4.09) | 550 (4.43) | 516 (4.46) | 566 (4.12) |
| 4–7 hours/week | 605 (11.46) | 165 (1.10) | 150 (1.21) | 134 (1.16) | 156 (1.14) |
| 7+ hours/week | 1,301 (24.65) | 365 (2.42) | 315 (2.53) | 289 (2.49) | 332 (2.41) |
| Weekly strenuous sports, n (%) | |||||
| No. | 48,613 (92.10) | 13,972 (92.79) | 11,352 (91.47) | 10,552 (91.06) | 12,737 (92.81) |
| 0.5–2 hours/week | 1,889 (35.79) | 535 (3.55) | 451 (3.63) | 455 (3.93) | 448 (3.26) |
| 2–4 hours/week | 1,394 (2.64) | 346 (2.30) | 367 (2.96) | 365 (3.15) | 316 (2.30) |
| 4+ hours/week | 884 (1.67) | 204 (1.37) | 241 (1.94) | 216 (1.87) | 223 (1.62) |
| Weekly moderate activity, n (%) | |||||
| No. | 40,401 (76.55) | 11,794 (78.33) | 9,377 (75.55) | 8,772 (75.70) | 10,458 (76.20) |
| 0.5–4 hours/week | 7,721 (14.63) | 2,059 (13.67) | 1,901 (15.32) | 1,745 (15.06) | 2,016 (14.69) |
| 4+ hours/week | 4,658 (8.84) | 1,204 (8.00) | 1,133 (9.13) | 1,071 (9.24) | 1,250 (9.11) |
| Heart Disease (%) | 2,162 (4.10) | 456 (3.03) | 476 (3.84) | 480 (4.14) | 750 (5.46) |
| Diabetes (%) | 4,708 (8.92) | 830 (5.51) | 1,010 (8.14) | 1,162 (10.03) | 1,706 (12.43) |
| Stroke (%) | 748 (1.42) | 217 (1.44) | 157 (1.27) | 178 (1.54) | 196 (1.43) |
Compared to BMI 19–20 kg/m2, the risk estimates of TKR were all statistically significant with increasing unit of BMI ≥ 21 kg/m2 (Table 2). The risk of TKR increased at a constant rate of 27% with increasing levels of BMI in the range 16–27 kg/m2 after adjustment for potential confounders in both men and women (Table 2). Compared to men, women experienced significantly higher risk of TKR [HR = 2.66, 95% CI (2.28, 3.11)]. However, the association between risk of TKR and BMI was similar for both women and men (HR=1.27 for per unit increase of BMI in women vs 1.28 in men). The results for the whole cohort including imputed values for participants with missing BMI was essentially the same (Supplementary Table 1).
Table 2.
Body mass index in relation to hazard ratio (HR) of total knee replacement (TKR) in total subjects in men and women separately, the Singapore Chinese Health Study, 1993–2011
| Body mass index (kg/m2) |
Total subjects | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cases | HR (95% CI) |
N | Cases | HR (95% CI) |
N | Cases | HR (95% CI) |
|
| <16 | 505 | 1 | 0.20 (0.00, 1.39) | 217 | 0 | 0.00 (0.00, --) | 288 | 1 | 0.23 (0.03,1.65) |
| 16–<17 | 803 | 4 | 0.51 (0.19, 1.43) | 389 | 0 | 0.00 (0.00, --) | 414 | 4 | 0.64 (0.23,1.80) |
| 17–<18 | 1,618 | 11 | 0.66 (0.34, 1.29) | 759 | 1 | 0.29 (0.04, 2.26) | 859 | 10 | 0.76 (0.38,1.53) |
| 18–<19 | 2,551 | 23 | 0.91 (0.55, 1.50) | 1,228 | 3 | 0.53 (0.14, 1.96) | 1,323 | 20 | 1.01 (0.58,1.74) |
| 19–<20† | 4,263 | 45 | 1.00 (ref.) | 1,900 | 9 | 1.00 (ref.) | 2,363 | 36 | 1.00 (ref.) |
| 20–<21 | 5,317 | 74 | 1.30 (0.90, 1.89) | 2,390 | 14 | 1.16 (0.50, 2.67) | 2,927 | 60 | 1.34 (0.88,2.02) |
| 21–<22 | 6,255 | 108 | 1.61 (1.13, 2.27) ** | 2,771 | 20 | 1.43 (0.65, 3.14) | 3,484 | 88 | 1.64 (1.12,2.42)* |
| 22–<23 | 6,156 | 147 | 2.26 (1.62, 3.16) ‡ | 2,860 | 21 | 1.45 (0.67, 3.18) | 3,296 | 126 | 2.48 (1.71,3.59)‡ |
| 23–<24 | 6,022 | 175 | 2.73 (1.97, 3.79) ‡ | 2,789 | 33 | 2.26 (1.08, 4.73)* | 3,233 | 142 | 2.84 (1.97,4.09)‡ |
| 24–<25 | 5,566 | 214 | 3.58 (2.60, 4.94) ‡ | 2,594 | 41 | 3.03 (1.47, 6.25)** | 2,972 | 173 | 3.72 (2.59,5.32)‡ |
| 25–<26 | 4,165 | 194 | 4.36 (3.15, 6.03) ‡ | 1,920 | 38 | 4.01 (1.93, 8.30)** | 2,245 | 156 | 4.44 (3.09,6.38)‡ |
| 26–<27 | 2,974 | 162 | 5.27 (3.78, 7.33) ‡ | 1,416 | 31 | 4.25 (2.02, 8.94)‡ | 1,558 | 131 | 5.54 (3.83,8.01)‡ |
| ≥27 | 6,585 | 491 | 6.95 (5.12, 9.43) ‡ | 2,703 | 77 | 5.70 (2.85, 11.4)‡ | 3,882 | 414 | 7.24 (5.15,10.2)‡ |
| 52,780 | 1,649 |
b = 1.27 (p < 0.001) |
23,936 | 288 |
b = 1.28 (p < 0.001) |
28,844 | 1,361 |
b = 1.27 (p < 0.001) |
|
CI = confidence interval. BMI = body mass index. HR = adjusted hazard ratio.
= reference group.
b = multiplier for increase in HR per unit increase in BMI (e.g., 27% for cohort) applicable over shaded range; estimated by weighted linear regression of ln(HR) on BMI group median.
Significantly different from reference at * p < 0.05, ** p < 0.01, ‡ p < 0.0001
HR adjusted for age at recruitment (years), year of recruitment (1993–1995, 1995–1998), dialect group (Hokkien, Cantonese), dose of smoking (none, number of cigarettes per day), duration of smoking (years), duration of smoking cessation (years), level of education in categories (no formal education, primary school, secondary school or higher), self-reported diabetes mellitus, ischemic heart disease, stroke, hours per day sleeping, hours per week in moderate activity, hours per week in vigorous work, hours per week in strenuous sports, hours per day sitting at work, hours per day watching TV.
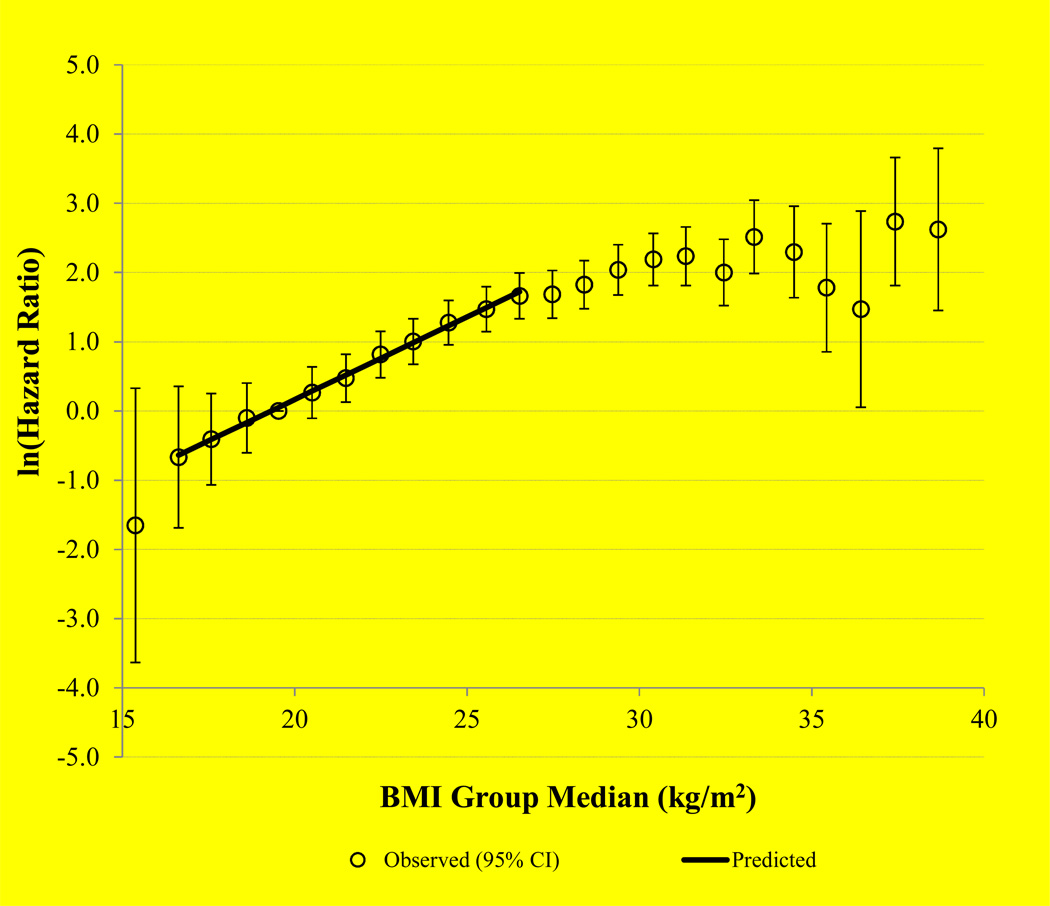
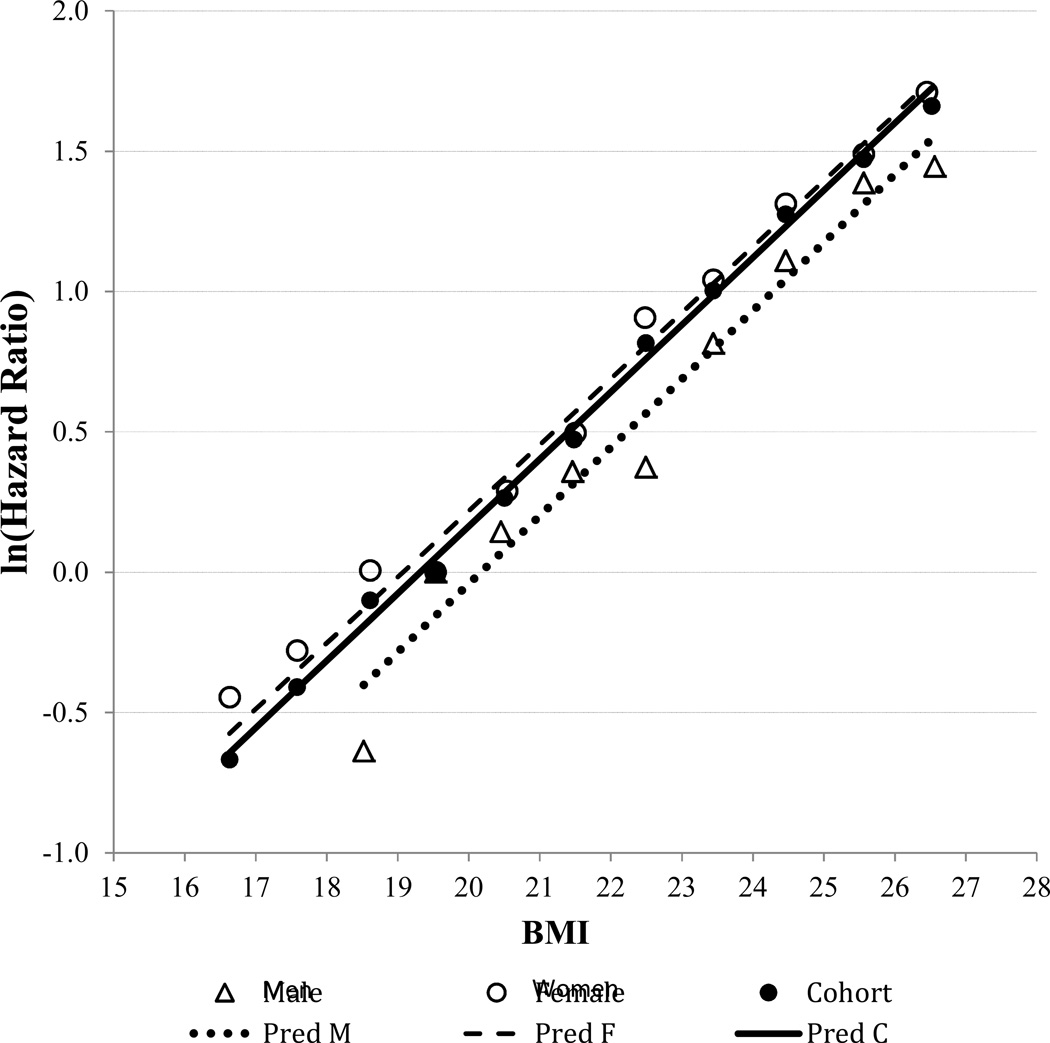
A semi-log plot of HR estimates with 95% CIs according to BMI value in the range of 15–39 kg/m2 is shown in Figure 1. A straight line was seen for BMI 16–27 kg/m2. In the BMI range of 27–32 kg/m2, HRs increased at a reduced rate, and beyond 32 kg/m2, HR estimates became less stable as sample size diminished. A straight line of ln (HR) with BMI on the semi-log plot indicated a constant rate of change in the log HR per unit change in BMI in the range of 16–27 kg/m2 (Figure 2). Adequacy of a linear fit and absence of significant curvilinearity over the range of BMI 16–27 kg/m2 was confirmed by fitting both linear (p < 0.013) and quadratic (p = 0.524) terms—the quadratic term was not statistically significant.
Figure 1.
Natural logarithm of hazard ratio against body mass index (BMI) group median with a straight line fitted for subjects with BMI range 16–27 kg/m2
Figure 2.
Natural logarithm of hazard ratio, by gender, regressed on BMI group median for subjects in BMI range 16–27 kg/m2 using weight least square regression
Natural logarithms of the HRs were obtained from the Cox model for each unit interval in the BMI range 16–27 kg/m2 and regressed on median BMI values using weighted least squares. The equation of the fitted line for the full cohort was ln(HR) = −4.6265 + 0.2395 × BMI, with adjusted R2 = 0.994 (Figure 2). The resulting estimate (95% CI) of the TKR risk multiplier was b = exp(0.2395) = 1.27 (1.25, 1.29), corresponding to a 27% increase in the HR (risk) of TKR per unit (kg/m2) increase in BMI. The TKR risk multiplier was also obtained using multivariable Cox regression analysis with BMI modeled as a continuous variable. After adjustment for covariates and potential confounders, the estimated HR (i.e., risk multiplier) was 1.26 (95% CI = 1.23, 1.30) per unit change in BMI.
For the purpose of comparison with other published studies [11, 12], we analyzed the data in predefined BMI categories in men [11] and women [12] separately. Among men, using a BMI reference of 20–<25 kg/m2, the HRs (95% CIs) were 0.38 (0.22, 0.68) for 17–<20 kg/m2, 2.35 (1.83, 3.01) for 25–<30 kg/m2, and 4.68 (3.04, 7.20) for 30–35 kg/m2. Among women, using a reference category of <22.5 kg/m2, the HRs (95% CIs) were 2.34 (2.01, 2.73) for 22.5–24.9 kg/m2, 3.69 (3.15, 4.33) for 25.0–27.4 kg/m2, 4.90 (4.08, 5.89) for 27.5–29.9 kg/m2, and 6.61 (5.43, 80.4) for ≥30 kg/m2.
We also examined the potential interactive effect of BMI and other selected variables on TKR risk, and did not find any statistically significant effect between BMI and age group (below or above 60 years) (p for interaction = 0.508), gender (p for interaction = 0.93), physical activity measures (all p for interaction > 0.70), or cigarette smoking (p for interaction = 0.99) (results not shown).
DISCUSSION
The present study represents a comprehensive evaluation of the risk of TKR across a wide range of BMI, especially in the lower end of the spectrum, using prospective data from a population-based cohort in Asia. The results showed that BMI was a strong risk factor for TKR. Over the BMI range of 16–27 kg/m2, the HR for TKR increased exponentially by greater than tenfold in both the entire cohort and in men and women separately. The linear relationship between ln(HR) and BMI in the range 16–27 kg/m2 suggests that the underlying anatomical and physiological mechanisms due to increasing BMI on risk of severe KOA are constant over this range. For BMI 27–32 kg/m2 the risk of TKR continued to increase but at a reduced rate, and for BMI > 32 kg/m2, the relationship became less well defined, suggesting more complex biological mechanisms underlying BMI and etiology of severe KOA at higher BMI levels.
From previous cohort studies, obesity has been implicated as causal risk factor for the onset [13–19] and progression [16, 19–20] of KOA; and risk of knee replacement surgeries [11, 12, 21–23]. Since the vast majority of these studies were conducted among Western populations with relatively higher BMI levels, the risk of KOA in overweight (BMI 25–29.9kg/m2) and obese (BMI > 30kg/m2) subjects were often compared to subjects with BMI <25 kg/m2. In a meta-analysis of 47 studies with 34 studies conducted in US and Europe, relative to BMI of < 25 kg/m2, the pooled odd ratios for overweight (BMI 25–29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2) were 2.02 (95% CI: 1.84, 2.22) and 3.91 (95% CI: 3.32, 4.56), respectively [4]. In another meta-analysis of 21 studies conducted primarily among Caucasian populations in Europe, US and Australia, with the exception of two studies in Japan, a five-unit increase in BMI was associated with a 35% increased risk of KOA (RR: 1.35; 95% CI: 1.21, 1.51) [2]. The majority of these previous studies did not investigate the relationship between BMI below 25 kg/m2 and risk of KOA. The present study, based on a population with an overall low BMI, demonstrated that for BMI below 25 kg/m2 the risk of TKR decreased monotonically with decreasing BMI without a threshold effect. The effect of BMI on risk of TKR in our study (330% per 5-unit increase in BMI) was much higher than that reported in the meta-analysis by Jiang et al (35% per 5-unit increase in BMI) [2].
Few studies have accumulated sufficient data to investigate increased risk of KOA in lean subjects. In a cohort study among male construction site workers in Sweden compared to lean subjects with BMI 20–24 kg/m2, the relative risk (95% CI) of TKR for BMI 17–19, 25–29, 30–35 were 0.50 (0.19, 1.36), 2.39 (1.93, 2.94), and 4.82 (3.65, 6.38), respectively [11]. In another study among women in the United Kingdom, compared to subjects with BMI <22.5 kg/m2, the relative risk (95% CIs) of TKR for BMI 22.5–24.9 kg/m2; 25.0–27.4 kg/m2; 27.5–29.9 kg/m2; and ≥30 kg/m2 were 1.65 (1.37, 1.98); 3.19 (2.75, 3.69); 5.63 (4.88, 6.48); and 10.51 (9.52, 11.62), respectively [12]. These risk estimates were comparable to our results. The present study, together with the previous two studies, showed that the risk of TKR began to increase at relatively low levels of BMI, and that the risk increase per BMI unit was greater in the low BMI spectrum (e.g., below 27 kg/m2) than in the high BMI range (e.g., >27 kg/m2). These findings suggest that the current recommendation for weight reduction management for individuals with BMI greater than 25 kg/m2 may be not optimal, as weight reduction may be beneficial to relatively lean subjects for reducing their risk of severe KOA. In fact, our data alluded that among lean populations such those in Asian countries, the effect of increasing weight on the risk of severe KOA could possibly be more prominent than that in the more obese Western populations. In the range beyond 27 kg/m2, the effect of BMI on risk of TKR seemed attenuated, notwithstanding that the small number of obese subjects in our study was a limitation.
Possible causal mechanisms linking overweight to the initiation and progression of KOA include biomechanical loading onto joints and the metabolic factors associated with adipose tissue and related adipokines [24–26]. Two studies evaluated different measures of body mass, including waist-hip ratio, fat mass and percentage body fat in relation to risk of primary hip and knee replacement [22, 23], and found that BMI had the strongest association with joint replacements. The strict linearity of the semi-log plot of HR against BMI in the range 16–27 kg/m2 in this study suggests that the overall effect from the anatomical and physiological mechanisms underlying increasing BMI and progression of KOA remains constant over this BMI range, and there is no evidence of altered or increased effect that could be due to metabolic factors at higher BMI levels operating in this BMI range. Hence, our study supports biomechanical stress as the constant mechanism linking body mass and KOA. Studies have shown that KOA is more common among Chinese as compared to Caucasian population despite their relative leanness [27], and a possible factor was thought to be related to frequent squatting habit among Chinese [28]. Other factors that could account for a greater influence of BMI increase on KOA among Chinese could be differences in joint alignment, thickness of cartilage and joint morphology that may render the knee joints of Chinese more vulnerable to OA from weight loading [27, 29].
The unique strength of this study is the relative leanness (mean BMI of 23.1 kg/m2) of subjects and the high prevalence of lean subjects (71.5% of subjects with BMI < 25 kg/m2) compared to studies in more obese Western populations. The exposure data can be assumed to be free of recall bias since they were obtained prior to TKR. An additional strength of this study is the large number of TKR cases for severe KOA identified from a population-based prospective cohort with a long follow-up time. Furthermore, our case ascertainment of TKR through linkage with the comprehensive, nationwide hospital database can be considered complete. In our study, we included only the first incident TKR for each case and verified that the surgical indication was primary KOA by checking the diagnosis code and including only subjects who underwent TKR for severe KOA as cases. Nevertheless, there is a possibility that KOA secondary to knee injury could have been included as primary KOA, although it could be clinically difficult to differentiate between the two since injury is a major risk factor of KOA. In our cohort, we excluded 89 cases of TKR done for other diagnoses, which accounted for 5% of all incident TKR in this cohort. This concurs with a previous report from the orthopaedic department of one of the public acute-care hospitals in Singapore, in which case notes were reviewed for more than 1,600 TKR cases over a 6-year period from January 2000 to December 2005, and it was reported that 96% of all TKR had been performed for osteoarthritis [30]. Finally, we included other established risk factors for severe KOA in our analyses, as well as other possible risk factors as covariates in our regression-based models to minimize the likelihood of spurious associations resulting from inadequate control of confounding.
One limitation of the study was the use of TKR as a surrogate outcome for severe KOA, which may exclude subjects who had severe KOA but did not undergo surgery due to medical, financial or other reasons. However, Singapore is a small city-state with a system for easy access to specialized medical care, and the comparatively high incidence of TKR in this cohort suggested adequate accessibility to TKR for severe KOA. In addition, using TKR as a surrogate outcome, we were unable to discern whether BMI was associated with the initiation or progression of KOA. Moreover, we could have missed out a few cases of TKR performed before 1990 that were not captured by the MediClaim System, and included these participants as non-cases in our analysis. However, we do not think this small number of participants will impact the results of the study materially. Another limitation of the study is the use of self-reported body weight and height in calculating BMI. However, self-reported body weight and height have been shown to be valid for epidemiologic studies across many populations [31], and specifically in lean Asian [32]. Moreover, we did not examine the effects of change in BMI in relation to risk of TKR.
In conclusion, in this large cohort of Chinese Singaporeans, the log-linear relationship between risk of TKR for severe KOA and BMI even in the relative low range emphasizes BMI as one of the most important predictors of KOA, and supports a constant biomechanical mechanism underlying BMI and KOA initiation and/or progression.
Supplementary Material
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study. We thank the Ministry of Health in Singapore for assistance with the identification of TKR cases and mortality via database linkages. We also thank Professor Ngai-Nung Lo of Department of Orthopedics, Singapore General Hospital for assistance in case notes review. Finally, we acknowledge the founding, long-standing Principal Investigator of the Singapore Chinese Health Study – Mimi C Yu.
Funding
This study was supported by the National Institutes of Health, USA (NCI RO1 CA55069, R35 CA53890, R01 CA80205, and R01 CA144034).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
The authors declare the following contributions to the preparation of the manuscript study: conception and design (YYL and WPK), acquisition of data (YYL, LWA and WPK), data analysis (JCA, RW, and WPK), interpretation of data (all authors), drafting of intellectual content (all authors) and final approval of manuscript (all authors). YYL (katyccc@hotmail.com) and WPK (woonpuay.koh@duke-nus.edu.sg) take responsibility for the integrity of the work as a whole.
Conflict of Interest
All authors (YYL, JCA, MN, LWA, RW, JMY and WPK) have no financial disclosures or notable competing interests.
References
- 1.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79:291–297. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Muthuri SG, Hui M, Doherty M, Zhang W. What if we prevent obesity? Risk reduction in knee osteoarthritis estimated through a meta-analysis of observational studies. Arthritis care & research. 2011;63:982–990. doi: 10.1002/acr.20464. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes L, Hagen KB, Bijlsma JWJ, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 7.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 8.Cust AE, Smith BJ, Chau J, van der Ploeg HP, Friedenreich CM, Armstrong BK, et al. Validity and repeatability of the EPIC physical activity questionnaire: a validation study using accelerometers as an objective measure. The international journal of behavioral nutrition and physical activity. 2008;5:33. doi: 10.1186/1479-5868-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heng DM, Lee J, Chew SK, Tan BY, Hughes K, Chia KS. Incidence of ischaemic heart disease and stroke in Chinese, Malays and Indians in Singapore: Singapore Cardiovascular Cohort Study. Ann Acad Med Singapore. 2000;29:231–236. [PubMed] [Google Scholar]
- 10.Leung YY, Ang LW, Thumboo J, Wang R, Yuan YM, Koh WP. Cigarette smoking and risk of total knee replacement for severe osteoarthritis among Chinese in Singapore - the Singapore Chinese health study. Osteoarthritis Cartilage. 2014;22:764–770. doi: 10.1016/j.joca.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Järvholm B, Lewold S, Malchau H, Vingård E. Age, bodyweight, smoking habits and the risk of severe osteoarthritis in the hip and knee in men. Eur J Epidemiol. 2005;20:537–542. doi: 10.1007/s10654-005-4263-x. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Balkwill A, Banks E, et al. Relationship of height, weight and body mass index to the risk of hip and knee replacements in middle-aged women. Rheumatology (Oxford) 2007;46:861–867. doi: 10.1093/rheumatology/kel434. [DOI] [PubMed] [Google Scholar]
- 13.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 14.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42:17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Shiozaki H, Koga Y, Omori G, Tamaki M. Obesity and osteoarthritis of the knee in women: results from the Matsudai Knee Osteoarthritis Survey. Knee. 1999;6:189–192. [Google Scholar]
- 16.Reijman M, Pols HAP, Bergink AP, Hazes JMW, Belo JN, Lievense AM, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66:158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toivanen AT, Heliövaara M, Impivaara O, Arokoski JPA, Knekt P, Lauren H, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis--a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49:308–314. doi: 10.1093/rheumatology/kep388. [DOI] [PubMed] [Google Scholar]
- 18.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years followup. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mnatzaganian G, Ryan P, Norman PE, Davidson DC, Hiller JE. Smoking, body weight, physical exercise, and risk of lower limb total joint replacement in a population-based cohort of men. Arthritis Rheum. 2011;63:2523–2530. doi: 10.1002/art.30400. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Simpson JA, Wluka AE, Teichtahl AJ, English DR, Giles GG, et al. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res Ther. 2009;11:R31. doi: 10.1186/ar2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engström G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68:490–496. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 24.Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65:1403–1405. doi: 10.1136/ard.2006.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women: The Framingham Study. Ann Intern Med. 1992;116:535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 26.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight Loss Reduces Knee-Joint Loads in Overweight and Obese Older Adults With Knee Osteoarthritis. Arthritis Rheum. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu W, Qin M, et al. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: The Beijing Osteoarthritis Study. Arthritis Rheum. 2001;44:2065–2071. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Hunter DJ, Nevitt MC, Xu L, Niu J, Yu W, et al. Association of squatting with increased prevalence of radiographic tibiofemoral knee osteoarthritis: the Beijing Osteoarthritis Study. Arthritis Rheum. 2004;50:1187–1192. doi: 10.1002/art.20127. [DOI] [PubMed] [Google Scholar]
- 29.Felson DT. Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthop Relat Res. 2004;(427 Suppl):S16–S21. doi: 10.1097/01.blo.0000144971.12731.a2. [DOI] [PubMed] [Google Scholar]
- 30.Xu GG, Sathappan SS, Jaipaul J, Chan SP, Lai CH. A review of clinical pathway data of 1,663 total knee arthroplasties in a tertiary institution in Singapore. Ann Acad Med Singapore. 2008;37:924–928. [PubMed] [Google Scholar]
- 31.Hu FB. Obesity Epidemiology. Oxford University Press; 2008. [Google Scholar]
- 32.Wada K, Tamakoshi K, Tsunekawa T, Otsuka R, Zhang H, Murata C, et al. Validity of self-reported height and weight in a Japanese workplace population. Int J Obes (Lond) 2005;29:1093–1099. doi: 10.1038/sj.ijo.0803012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.