Abstract
Objectives
To determine if labor-associated inflammatory markers differ between low-risk, nulliparous women in pre-active vs. active labor at hospital admission and over time.
Study Design
Prospective comparative study of low-risk, nulliparous women with spontaneous labor onset at term (N=118) sampled from two large Midwestern hospitals. Circulating concentrations of inflammatory markers were measured at admission and again 2 and 4 hours later: namely, neutrophil and monocyte counts; and serum inflammatory cytokines (interleukin [IL]-1β, IL-6, tumor necrosis factor [TNF]-α, IL-10) and chemokines (IL-8). Biomarker concentrations and their patterns of change over time were compared between pre-active (n=63) and active (n=55) labor admission groups using Mann-Whitney U tests.
Results
Concentrations of IL-6 and IL-10 in the active labor admission group were significantly higher than concentrations in the pre-active labor admission group at all three time points. Neutrophil levels were significantly higher in the active group at 2 and 4 hours after admission. The rate of increase in neutrophils and IL-10 between admission and 2 hours later was faster in the active group (p<0.001 and p=0.003, respectively).
Conclusions
Circulating concentrations of several inflammatory biomarkers are higher and their rate of change over time since admission is faster among low-risk, nulliparous women admitted to hospitals in active labor, as compared to those admitted in pre-active labor. More research is needed to determine if progressive changes in inflammatory biomarkers might be a useful adjunct to improving the assessment of labor progression and determining the optimal timing of labor admission.
Keywords: Cytokines, Inflammation, Interleukins, Labor Onset, Nulliparity
Introduction
Inflammatory events not seen before labor onset can be observed during parturition in the cervix, myometrium, and fetal membranes.1–5 Coincident with these events, maternal peripheral leukocytes (primarily neutrophils and monocytes) infiltrate the reproductive tissues, even in the absence of infection.6–10 These leukocytes are a major source of pro-inflammatory peptides in uterine and cervical tissues during labor, while the reproductive tissues also synthesize cytokines/chemokines (e.g., interleukin [IL]-8) that may attract additional leukocytes through chemotaxis.8,11 The pro-inflammatory peptides most implicated in labor progression are IL-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α, which contribute to recruitment and activation of additional leukocytes, augmentation of prostaglandin production, cervical ripening and dilation, membrane rupture, and uterine contractions.2,6,12–16 Thus, a positive feedback loop of cytokine production by activated leukocytes in maternal and fetal tissues is at least permissive, and perhaps essential, to labor onset and progression.
Activation of the inflammatory response likely explains the marked leukocytosis commonly found in the maternal blood during physiologic labor. Serum concentrations of IL-1β,17–19 IL-6,17,18,20–24 IL-8,17,20,23 and TNF-α25,26 are also significantly higher during labor than levels found before labor onset. Hebisch and colleagues23 reported that IL-6 concentrations during latent labor were significantly lower than concentrations associated with •established• and •advanced• labor. Moreover, serum IL-6 and IL-8 levels were positively related to cervical dilatation,23 and IL-6 was significantly higher with stronger and more frequent contractions,27,28 which are more likely to occur during active labor. Production of anti-inflammatory cytokines such as IL-10 (which is produced by almost every immune cell29 and within reproductive tissues30–33) is enhanced by pro-inflammatory stimuli; thus, increases in serum concentrations of IL-10 are also expected with advancing labor. These findings suggest that women in earlier versus more advanced labor may be at distinctly different points in the inflammatory pathway. A better understanding of the physiological differences between women in pre-active versus active labor is important to improving birth outcomes in light of the higher rates of oxytocin augmentation and cesarean delivery rates seen in nulliparous women admitted to hospitals before active labor begins.34–39 Knowledge of the progression of inflammatory processes known to be associated with efficient labor progress will advance our understanding of labor physiology and may eventually inform admission decisions and evaluation of labor progress.
In this study, we examined neutrophil and monocyte counts and serum cytokine/chemokine (IL-1β, IL-6, IL-8, TNF-α, and IL-10) concentrations in low-risk, nulliparous women at term admitted to the hospital following the onset of spontaneous contractions. Our primary aim was to evaluate differences in these biomarkers at admission and at two and four hours after admission between women later determined to be admitted in pre-active or active labor. We hypothesized that women admitted in active labor would have greater concentrations of inflammatory biomarkers than women admitted in pre-active labor, indicating a more advanced stage of the inflammatory pathway driving labor progress. Our secondary aim was to evaluate patterns of biomarker changes over time between the pre-active and active labor admission groups.
Materials and Methods
We performed a prospective comparative study at two large Midwestern hospitals in the United States. Institutional Review Board approval was granted, and written informed consents were obtained from all participants. Recruitment took place from March 2011 to December 2012 and was conducted by research team members in the labor and delivery triage unit or in the labor room soon after admission. All eligible women were approached for participation when a research team member was present on the unit. Approximately 70% of approached women accepted participation; we confirmed that study acceptance rates did not differ between those admitted in pre-active versus active labor. The predominant rationale for declining participation was to avoid blood draws required by the study protocol.
Participants (N=118) were nulliparous women carrying a single, cephalic presenting fetus at term (37–42 weeks gestation) admitted by their providers for spontaneous labor onset and an anticipated vaginal delivery. Eligible women were experiencing two or more uterine contractions every 10 minutes as objectively determined by external monitoring or palpation at admission, were dilated no more than 6 cm at admission, and had fetal membranes that were either intact or ruptured for not more than 4 hours prior to admission. Additional eligibility criteria included maternal age of 18–39 years, no significant medical history, absence of major pregnancy complications (e.g., preeclampsia, diabetes, oligohydramnios), absence of identified fetal complications (e.g., anomalies, non-reassuring status, intrauterine growth restriction), afebrile at study entry, lack of antibiotic or anti-inflammatory medication use in the past 6 weeks, and ability to read and speak English. Women with pre-existing conditions known to be associated with chronic, low-grade inflammation were excluded (e.g., asthma, autoimmune diseases, cardiovascular disease, metabolic syndrome, type 2 diabetes, atherosclerosis, acid reflux, COPD, chronic pain). Women undergoing inductions of labor were not eligible. Care during labor was at the discretion of the providers.
All digital cervical exams by labor care providers during the course of labor were retrieved from the labor record, and the average dilation slope for the first 4 hours post-admission was determined. Because cervical exams are rarely performed at exactly 4 hours after the admission exam, slope calculations based on the exams immediately prior to and after the 4-hour time-point were used to approximate dilatation at the 4-hour post-admission time point. The average dilation slope (cm/hour) for the first 4 hours post-admission was then calculated. Finally, each participant’s labor admission was retrospectively classified as either pre-active labor or active labor based on the rate of cervical change during the first 4 hours after admission using a priori criteria: a labor admission was classified as pre-active when average dilation was <0.5 cm/hour for the first 4 hours post-admission or as active when average dilation was ≥0.5 cm/hour. This differentiation cut point was based on contemporary labor progression research40,41 which is now formally supported by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine in their joint obstetric care consensus on the safe prevention of the primary cesarean delivery.42 Demographic data were collected from each participant via interview; labor process and outcome data were extracted from electronic health care records following birth.
Maternal blood was drawn at admission and 2 and 4 hours later. Blood at admission was sampled within 90 minutes of the cervical exam on which the labor admission was based; the median time to initial blood sampling was 33 minutes. Blood for neutrophil and monocyte counts was collected into ethylenediaminetetraacetic acid (EDTA)-containing tubes and quantified using a Sysmex XE-2100 within 30 minutes of blood collection (Sysmex America, Inc., Lincolnshire, IL). Blood for serum cytokine/chemokine determinations was collected into serum separator tubes. These samples were allowed to clot for up to 30 minutes followed by centrifugation at 4°C for 10 minutes at 3000 rpm. Serum was then stored as 1.5 mL aliquots at −70°C. All serum samples from a single participant were analyzed simultaneously in duplicate. Cytokines/chemokines were assayed using Human Pro-Inflammatory 7-Plex II Ultra-Sensitive kits measuring IL-1β, IL-6, IL-8, TNF-α, and IL-10 (Meso Scale Discovery, Rockville, MD) according to manufacturer’s instructions. Assay sensitivity varies by cytokine: IL-1β=0.58 pg/mL; IL-6=0.18 pg/mL; IL-8=0.10 pg/mL; TNF-α=0.28 pg/mL; and IL-10=0.57 pg/mL.
Statistical analyses were performed using SPSS Statistics 21 and SAS version 9.3. Maternal demographic characteristics and labor outcomes were compared by Mann-Whitney U tests for continuous variables and Fisher’s exact tests for categorical variables. Median neutrophil, monocyte, and cytokine/chemokine concentrations and their patterns of change over time (slope) were compared between the pre-active and active labor admission groups using Mann-Whitney U tests with Holm’s sequential Bonferroni correction.43 Alpha level was set at 0.05; with Holm’s approach, p-values considered significant were sequentially determined to account for multiple testing.
Results
Maternal demographic characteristics and labor outcomes are summarized in Table 1. Of the 118 low-risk nulliparous women, 63 (53.4%) were admitted in pre-active labor and 55 (46.6%) in active labor. Women in the pre-active group were more racially diverse. Groups had similar dilatations at admission, although women in the pre-active group had less cervical effacement. Women admitted in pre-active labor received oxytocin more often than the active labor admission group (88.9% vs. 43.6%, p<0.001) and had a higher cesarean rate (17.5% vs. 5.5%, p=0.040). In-hospital labor duration was longer in the pre-active admission group (12.3 vs. 8.0 hours, p<0.001).
Table 1.
Demographic Characteristics and Labor Outcomes of Low-Risk Nulliparous Women Admitted in Pre-Active or Active Labor at Term (N = 118)a
| Pre-Active Labor (n = 63) |
Active Labor (n = 55) |
p | |
|---|---|---|---|
| Maternal age (y) | 26.0 (20.4, 32.6) | 28.0 (21.0, 33.4) | 0.243 |
| Gestational age at admission (wk) | 39.6 (37.9, 40.6) | 39.6 (38.2, 40.6) | 0.413 |
| Race | |||
| White | 47 (74.6%) | 49 (89.1%) | <0.05 |
| Black | 13 (20.6%) | 2 (3.6%) | |
| Other | 6 (4.8%) | 4 (7.3%) | |
| Body mass index at admission (kg/m2) | 30.7 (25.0, 38.2) | 28.9 (24.1, 36.8) | 0.109 |
| Cervical dilatation at admission (cm) | 3.0 (1.0, 4.5) | 3.0 (1.5, 4.7) | 0.123 |
| Cervical effacement at admissionc | |||
| 50–75% | 19 (30.2%) | 1 (1.8%) | <0.001 |
| ≥ 80% | 44 (69.8%) | 54 (98.2%) | |
| Fetal station at admission | −2 (−2, −1) | −2 (−2, −0.6) | 0.227 |
| Membrane status at admission | |||
| Intact | 36 (57.1%) | 39 (70.9%) | 0.130 |
| Ruptured | 27 (42.9%) | 16 (29.1%) | |
| Number of cervical exams during labor | 8 (5, 11) | 6 (3.6, 9) | <0.001 |
| Rupture of membranes | |||
| Spontaneous | 30 (47.6%) | 25 (45.5%) | 0.480b |
| Amniotomy | 33 (52.4%) | 30 (54.5%) | |
| Oxytocin augmentation | |||
| No | 7 (11.1%) | 31 (56.4%) | <0.001b |
| Yes | 56 (88.9%) | 24 (43.6%) | |
| Narcotic analgesia used | 13 (20.6%) | 5 (9.1%) | 0.123 |
| Epidural analgesia used | 62 (98.4%) | 51 (92.7%) | 0.183 |
| Mode of birth | |||
| Vaginald | 52 (82.5%) | 52 (94.5%) | 0.040b |
| Cesarean | 11 (17.5%) | 3 (5.5%) | |
| Indication for cesarean (n) | |||
| Dystocia (1st stage) | 6 | 0 | <0.05 |
| Arrest of fetal descent (2nd stage) | 1 | 1 | 1.000 |
| Non-reassuring fetal well-being | 4 | 2 | 0.684 |
| Time from admission to complete dilation (h) | 10.9 (7.3, 17.2) | 6.0 (3.7, 10.8) | <0.001 |
| Second stage duration (min) | 79 (30, 167) | 83 (30, 198) | 0.859 |
| In-hospital labor duration (h) | 12.3 (8.3, 19.3) | 8.0 (4.6, 12.1) | <0.001 |
| Maximum temperature during labor >100.4°F | 5 (7.9%) | 3 (5.5%) | 0.722 |
| Infant sex | |||
| Female | 31 (49.2%) | 33 (60.0%) | 0.270 |
| Male | 32 (50.8%) | 22 (40.0%) | |
| Weight (infant) (g) | 3404 (2749, 3909) | 3386 (2807, 3812) | 0.285 |
| Apgar Scores | |||
| <8 at 1 min | 9 (14.3%) | 3 (5.5%) | 0.134 |
| <8 at 5 min | 1 (1.6%) | 2 (3.6%) | 0.600 |
| Neonatal admission to NICU | 3 (4.8%) | 1 (1.8%) | 0.622 |
ROM = rupture of membranes.
Data are n (%) and median (10th, 90th percentile). Mann-Whitney U tests performed for continuous level data comparisons due to violations of normality. Fisher’s exact tests (two-tailed) performed for categorical level data comparisons, unless otherwise specified.
Fisher’s exact test (one-tailed) performed as test of directional hypothesis that women admitted in pre-active labor are more prone to the intervention, as compared to women admitted in active labor.
Although percent effacement was not an inclusion/exclusion criterion, no woman was <50% effaced at admission.
Includes assisted vaginal births (i.e., vacuum or forceps), of which there were 6 and 3, respectively, in the pre-active and active labor admission groups.
Median concentrations of IL-6 and IL-10 were significantly higher among women admitted in active labor at all three sampling points while neutrophil concentrations were higher at 2 and 4 hours after admission with a trend toward significance at the admission time point (see Table 2). There were no between group differences in monocyte, IL-1β, IL-8, or TNF-α concentrations at any time point.
Table 2.
Comparisons of Inflammatory Markers in the Maternal Circulation at Admission, Admission+2hrs, and Admission+4hrs between Low-Risk Nulliparous Women Admitted in Pre-Active or Active Labor (N = 118)
| Pre-Active Labor (n = 63) | Active Labor (n = 55) | |||||
|---|---|---|---|---|---|---|
| n | n | p | ||||
| Neutrophils | admission | 61 | 9.28 (4.07–19.51) | 55 | 10.76 (6.02–20.04) | 0.030 |
| +2 hours | 54 | 9.63 (3.83–22.73) | 51 | 12.00 (7.23–23.11) | < 0.001 | |
| +4 hours | 49 | 10.54 (4.69–23.15) | 46 | 12.91 (7.82–23.31) | < 0.001 | |
| Monocytes | admission | 61 | 0.75 (0.30–2.31) | 55 | 0.72 (0.38–1.82) | 0.866 |
| +2 hours | 54 | 0.71 (0.12–1.35) | 51 | 0.69 (0.22–1.63) | 0.850 | |
| +4 hours | 49 | 0.70 (0.34–1.26) | 46 | 0.64 (0.34–1.38) | 0.826 | |
| IL-1β | admission | 63 | 0.51 (0.00–10.61) | 55 | 0.58 (0.00–3.32) | 0.352 |
| +2 hours | 58 | 0.49 (0.00–4.01) | 53 | 0.50 (0.00–3.10) | 0.906 | |
| +4 hours | 56 | 0.48 (0.00–4.50) | 49 | 0.50 (0.00–2.87) | 0.916 | |
| IL-6 | admission | 63 | 2.9 (0.8–63.9) | 55 | 5.1 (1.4–30.8) | 0.002 |
| +2 hours | 58 | 3.6 (1.3–26.7) | 53 | 6.9 (1.9–39.4) | < 0.001 | |
| +4 hours | 56 | 5.2 (1.7–86.2) | 49 | 9.9 (2.3–46.8) | < 0.001 | |
| IL-8 | admission | 63 | 5.7 (1.2–16.6) | 55 | 5.5 (1.8–96.5) | 0.728 |
| +2 hours | 58 | 5.8 (2.1–17.2) | 53 | 5.7 (2.1–27.7) | 0.468 | |
| +4 hours | 56 | 6.3 (1.9–14.6) | 49 | 5.3 (1.9–16.3) | 0.318 | |
| TNF-α | admission | 63 | 6.8 (1.9–34.7) | 55 | 6.8 (1.9–25.8) | 0.861 |
| +2 hours | 58 | 7.2 (2.4–33.3) | 53 | 6.5 (1.8–25.2) | 0.189 | |
| +4 hours | 56 | 7.7 (2.6–33.4) | 49 | 6.1 (1.6–27.1) | 0.191 | |
| IL-10 | admission | 63 | 3.6 (0.4–78.5) | 55 | 5.2 (0.6–70.5) | 0.003 |
| +2 hours | 57 | 3.6 (0.7–27.9) | 53 | 7.3 (1.6–132.8) | < 0.001 | |
| +4 hours | 55 | 3.4 (0.7–28.6) | 49 | 6.8 (0.7–70.5) | 0.001 | |
Median (Range). Mann-Whitney U tests. Leukocytes (absolute) x 1000/µL. Cytokines in pg/mL. The number of research participants sampled for blood at each biomarker collection time point varies because blood was collected only if the woman was still in labor at the sampling time point and because a few sampling time points were inadvertently missed by research team members. Holm’s sequential rejective multiple test procedure was applied to sequentially determine significant p-values, i.e., for 21 tests, the most significant p-value must be smaller than 0.05/21 = 0.0024, the second most significant p-value must be smaller than 0.05/20 = 0.0025, the third most significant p-value must be smaller than 0.05/19 = 0.0026, etc.
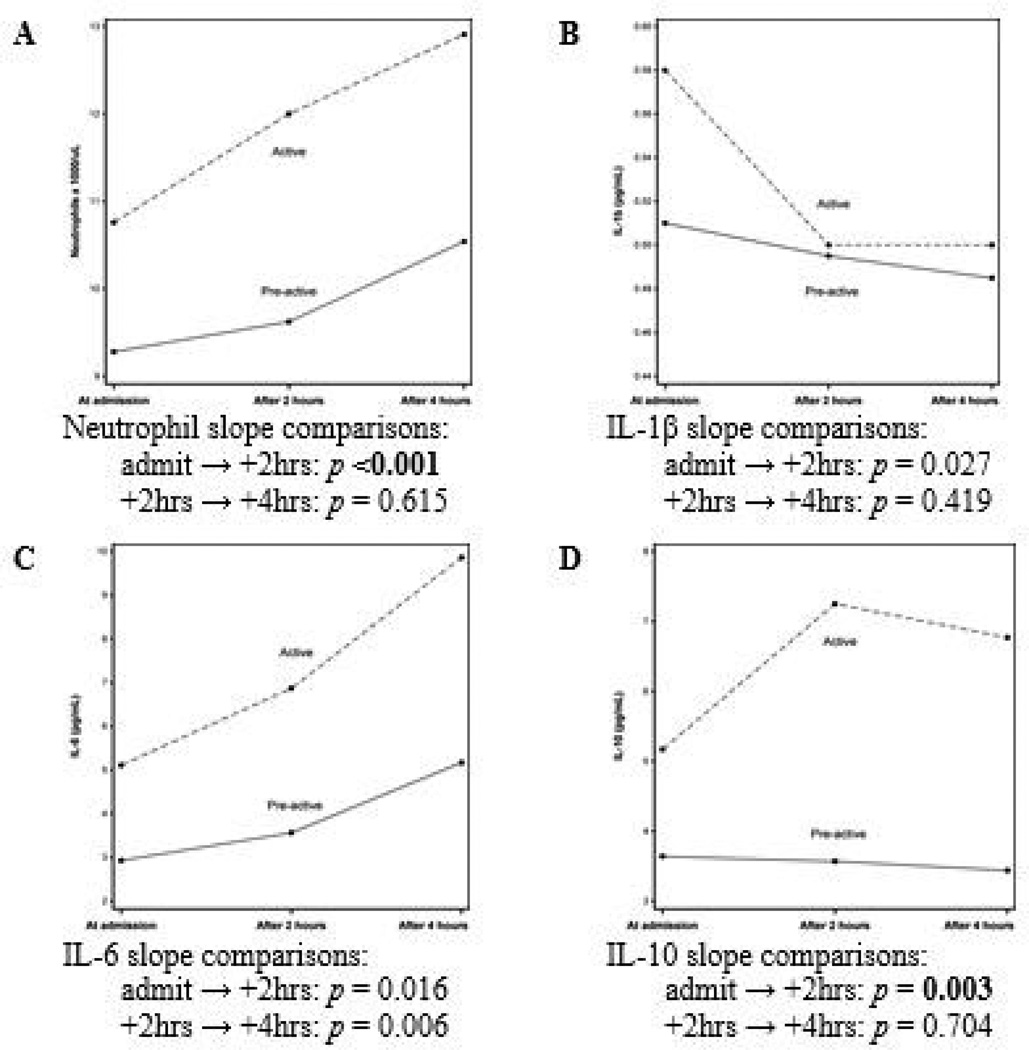
Inflammatory biomarker changes over time compared between the pre-active and active labor groups are shown in Figure 1. The magnitude of changes in neutrophil counts and IL-10 concentrations between admission and 2 hours were significantly different between the groups, i.e., slopes were more precipitous in the active group. IL-6 slope differences trended toward significance between the groups from admission to admission+2hrs and from admission+2hrs to admission+4hrs and IL-1β slopes trended toward significance from admission to admission+2hrs. There were no slope differences for monocytes, IL-8, or TNF-α (not shown).
Figure 1. Comparisons of inflammatory biomarkers in the maternal circulation over time between low-risk nulliparous women admitted in pre-active or active labor.
Mann-Whitney U tests. Analyses based on magnitude of biomarker change (slope) between admission → admission+2hrs (i.e., biomarkerAdmission+2hrs – biomarkerAdmission) and admission+2hrs → admission+4hrs (i.e., biomarkerAdmission+4hrs – biomarkerAdmission+2hrs).
Two comparisons (i.e., admit → +2hrs and +2hrs → +4hrs) were made between groups for each of the seven biomarkers measured (monocytes, IL-8, or TNF-α not shown). Holm’s sequential rejective multiple test procedure was applied to sequentially determine significant p-values, i.e., for 14 tests, the most significant p-value must be smaller than 0.05/14 = 0.0036, the second most significant p-value must be smaller than 0.05/13 = 0.0038, the third most significant p-value must be smaller than 0.05/12 = 0.0042, etc.
Comment
Our findings demonstrate physiological differences in inflammatory markers between women admitted to hospitals in pre-active and active labor. We found that neutrophils, IL-6, and IL-10 were in greater concentrations among low-risk, nulliparous women admitted in active labor as compared to women in pre-active labor, as measured at independent time points and/or by the magnitude of biomarker change over time during labor. This provides additional evidence that inflammation is involved in the initiation and propagation of term labor with a spontaneous onset, with actively laboring women perhaps being at a more advanced stage in the labor-related inflammatory pathway.
We also found that nulliparous women admitted in active labor received less intervention and were more likely to achieve vaginal birth than laboring women admitted in pre-active labor. This finding is supported by prior reports that women admitted earlier (e.g., <4 cm dilatation) are approximately twice as likely to be augmented with oxytocin34,35,38,39 and delivered via cesarean.34–39, when compared to women admitted later in labor. Unfortunately, true active labor can only be determined retrospectively based on an assessment of cervical dilation over time. The criteria traditionally taken as evidence of active labor onset—dilatation between 3 cm and 5 cm, in the presence of uterine contractions—have not proven to be reliable.44 Thus, a large percentage of nulliparous women may be admitted to hospitals prior to active labor onset, as suggested by the findings of our study. While it is possible that women who present earlier in labor may have an inherently higher risk of labor dystocia (i.e., “slow, abnormal progression of labor”47) at baseline,34 this explanation does not adequately explain why more than half of our sample was admitted prior to the onset of active labor. Our pre-active and active labor admission groups did not differ on the number of labor evaluation triage visits prior to admission or cervical dilatation at admission. Clearly, more reliable metrics for determination of active labor onset are needed.
Our finding that the pre-active labor admission group had less cervical effacement than the active group at admission, despite sharing a similar dilatation, warrants discussion. Ninety-eight percent of the women admitted in active labor had cervices that were ≥80% effaced compared to 70% among women in the pre-active group (p<0.001). This alone indicates that degree of cervical effacement must be carefully considered by clinicians making admission decisions since our group and others have found that women in active labor typically have advanced effacement.34,39,45 In light of the difference in cervical effacement at admission between our study groups, we performed post hoc analyses to determine if inflammatory biomarker differences between the groups persisted after all women with admission effacement <80% were excluded. For these analyses, the pre-active and active groups were comprised of 44 and 54 women, respectively; biomarker concentrations were compared between the groups using Mann-Whitney U tests and p-values <0.05 were considered significant. Interestingly, median concentrations of IL-10 remained significantly higher among women admitted in active labor at all three sampling points (p=0.005 at admission; p<0.001 at admission+2hrs; p=0.001 at admission+4hrs) while IL-6 and neutrophil concentrations remained higher at 2 and 4 hours after admission (for IL-6, p=0.006 and p=0.015 at admission+2hrs and admission+4hrs, respectively; for neutrophils, p=0.001 and p=0.003 at admission+2hrs and admission+4hrs, respectively). Indeed, of the inflammatory biomarkers that significantly differed between the pre-active and active groups prior to excluding women with effacement <80% at admission, only the difference in IL-6 concentrations at the admission time point was no longer significant after excluding the lessor effaced women (p=0.055). Thus, while it is reasonable to delay admission for presumed active labor until cervical effacement is complete or near complete, inflammatory biomarker differences between women in pre-active and active labor remain evident even when only women with advanced effacement are evaluated.
The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recently endorsed the idea that standards for active phase progress should not be applied before 6 cm dilatation,42 a consensus based primarily on labor progress work conducted by Zhang and colleagues using Consortium on Safe Labor data.40 A shortcoming of this approach is that a single dilatation point does not adequately discriminate pre-active from active labor for an individual39, i.e., some women may not be in active labor at 6 cm while many women may be in active labor before 6 cm, as shown in the present study. Moreover, because half of nulliparous women progress from 6 cm to complete dilatation in 3 hours or less40, it may not be reasonable to delay admission until 6 cm since doing so may result in a large percentage of women missing their window of opportunity for the care they desire (e.g., epidural analgesia and the possibility to acclimate to the birth environment) or undesired out-of-hospital birth. An even greater percentage of multiparous women would be affected by delaying admission until 6 cm since these women generally have more rapid active labors.40 Therefore, even before 6 cm, clinicians should carefully consider who they admit for labor based on an evaluation of cervical change over time rather than a single integer dilatation point.
Based on additional post hoc findings, consideration should be given to the possibility that many of the women in the pre-active labor admission group were only a few hours behind the active group in terms of the physiologic labor pathway. The majority of women admitted in pre-active labor in our study achieved dilation rates above 0.5 cm/hour once beyond the first 4 hours after admission (n=49 of 63). Although this could reflect the more frequent use of oxytocin augmentation in the pre-active admission group, an escalation in particular inflammatory biomarker concentrations was also observed in the pre-active admission group which, by 4 hours post-admission, reached levels similar to those observed in the active group at admission. Specifically, there were no significant differences between neutrophils or IL-6 when the pre-active labor group values at admission+4hrs were compared to the active labor group concentrations at admission (neutrophils 10.54 and 10.76 ×1000 cells/µL (p=1.000) and IL-6 5.2 and 5.1 pg/mL (p=0.333), respectively). Thus, delaying admissions for women in non-progressive labor may allow time for inflammatory changes important to efficient labor progress to more fully manifest. This may decrease the need for subsequent intervention aimed at accelerating labor progress and improve vaginal birth rates while also decreasing the woman’s time on the labor unit before progressive labor begins.
Our study included biologic samples collected during labor from a sample of low-risk, nulliparous women with spontaneous labor onset at term. The study had a few limitations that warrant mention. Firstly, common interventions that may affect rates of dilation (i.e., oxytocin, amniotomy, epidural analgesia) were received by many women within the first 4 hours after admission, before labor state was determined (i.e., pre-active or active). Secondly, although the percentage of women admitted with already ruptured membranes did not differ between the pre-active and active groups, eliminating these women would have yielded a cleaner, but less generalizable, sample. Finally, our measurement of cytokine concentrations in the maternal serum may not adequately reflect the cytokine-producing potential of immune cells because of the short half-lives of cytokines and the presence of various inhibitors in human sera. We recommend that this study be repeated in a more racially diverse sample that includes primiparous and multiparous women and, perhaps, with the addition of more frequent blood collection time points. Furthermore, because we speculate that differences and rate of change in biomarkers of inflammation may enhance our ability to accurately diagnose the onset of active labor in the future, we recommend that possible predictive models be developed and rigorously evaluated in subsequent research studies.
In the present study, we found that circulating biomarkers of inflammation differ between women admitted to hospitals in pre-active and active labor, suggesting that women in active labor have greater activation of the labor inflammatory pathway contributing to labor progress. Delaying admission of laboring women in pre-active labor may allow time for inflammatory events important to efficient labor progress to more fully develop.
Acknowledgments
Sources of Financial Support for the Research: This study was funded in part by R03 NR011493(JLN), the Midwest Nursing Research Society (JLN), and UL1RR025755 (JLN). The authors are solely responsible for the content which does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors report no conflict of interest.
Contributor Information
Jeremy L. Neal, Assistant Professor in the School of Nursing, Vanderbilt University, Nashville, TN.
Jane M. Lamp, Clinical Nurse Specialist in the Department of Women’s Health Services, Riverside Methodist Hospital, Columbus, OH.
Nancy K. Lowe, Professor in the College of Nursing, University of Colorado Denver, Aurora, CO.
Shannon L. Gillespie, Predoctoral Fellow in the College of Nursing, The Ohio State University, Columbus, OH.
Loraine T. Sinnott, Senior Statistician in the College of Nursing, The Ohio State University, Columbus, OH.
Donna O. Mccarthy, Professor in the College of Nursing, Marquette University, Milwaukee, WI.
References
- 1.Shynlova O, Lee Y, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 2.Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC pregnancy childbirth. 2007;7(Suppl 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol. 2011;335(1):52–59. doi: 10.1016/j.mce.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(1):104.e1–104.11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 7.Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 8.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: Evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86(2):39–39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 10.Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE. Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol Hum Reprod. 2009;15(11):713–724. doi: 10.1093/molehr/gap054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86(2):223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell MD, Goodwin V, Mesnage S, Keelan JA. Cytokine-induced coordinate expression of enzymes of prostaglandin biosynthesis and metabolism: 15-hydroxyprostaglandin dehydrogenase. Prostaglandins Leukot Essent Fatty Acids. 2000;62(1):1–5. doi: 10.1054/plef.1999.0117. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Fung W, Moore RM, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod. 2006;74(1):29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 14.Tribe RM, Moriarty P, Dalrymple A, Hassoni AA, Poston L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod. 2003;68(5):1842–1849. doi: 10.1095/biolreprod.102.011403. [DOI] [PubMed] [Google Scholar]
- 15.Roh CR, Oh WJ, Yoon BK, Lee JH. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: A cytokine-mediated process in uterine smooth muscle cells. Mol Hum Reprod. 2000;6(1):96–102. doi: 10.1093/molehr/6.1.96. [DOI] [PubMed] [Google Scholar]
- 16.Sennstrom MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6(4):375–381. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- 17.Jarocki S, Redzko S, Przepiesc J, Urban J. [Maternal serum Th1 and Th2 cytokines in preterm and term delivery] Ginekol Pol. 2007;78(4):284–287. [PubMed] [Google Scholar]
- 18.Torbe A, Czajka R, Kordek A, Rzepka R, Kwiatkowski S, Rudnicki J. Maternal serum proinflammatory cytokines in preterm labor with intact membranes: Neonatal outcome and histological associations. Eur Cytokine Netw. 2007;18(2):102–107. doi: 10.1684/ecn.2007.0092. [DOI] [PubMed] [Google Scholar]
- 19.Vit6oratos N, Mastorakos G, Kountouris A, Papadias K, Creatsas G. Positive association of serum interleukin-1beta and CRH levels in women with pre-term labor. J Endocrinol Invest. 2007;30(1):35–40. doi: 10.1007/BF03347393. [DOI] [PubMed] [Google Scholar]
- 20.Nowak M, Oszukowski P, Jaczewski B, et al. [Maternal serum cytokines in labor, pregnancy and chorioamnionitis] Ginekol Pol. 2001;72(12):1158–1162. [PubMed] [Google Scholar]
- 21.Nowak M, Oszukowski P, Szpakowski M, et al. [Maternal serum cytokines concentrations during normal pregnancy and labor] Ginekol Pol. 1998;69(12):1283–1287. [PubMed] [Google Scholar]
- 22.Greig PC, Murtha AP, Jimmerson CJ, Herbert WN, Roitman-Johnson B, Allen J. Maternal serum interleukin-6 during pregnancy and during term and preterm labor. Obstet Gynecol. 1997;90(3):465–469. doi: 10.1016/s0029-7844(97)00294-9. [DOI] [PubMed] [Google Scholar]
- 23.Hebisch G, Grauaug AA, Neumaier-Wagner PM, Stallmach T, Huch A, Huch R. The relationship between cervical dilatation, interleukin-6 and interleukin-8 during term labor. Acta Obstet Gynecol Scand. 2001;80(9):840–848. doi: 10.1034/j.1600-0412.2001.080009840.x. [DOI] [PubMed] [Google Scholar]
- 24.Opsjln SL, Wathen NC, Tingulstad S, et al. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169(2):397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 25.Vitoratos N, Papadias K, Makrakis E, Christodoulakos G, Panoulis K, Creatsas G. Association between serum tumor necrosis factor-alpha and corticotropin-releasing hormone levels in women with preterm labor. J Obstet Gynaecol Res. 2006;32(5):497–501. doi: 10.1111/j.1447-0756.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 26.Gucer F, Balkanli-Kaplan P, Yuksel M, Yuce MA, Ture M, Yardim T. Maternal serum tumor necrosis factor-alpha in patients with preterm labor. J Reprod Med. 2001;46(3):232–236. [PubMed] [Google Scholar]
- 27.Arntzen KJ, Lien E, Austgulen R. Maternal serum levels of interleukin-6 and clinical characteristics of normal delivery at term. Acta Obstet Gynecol Scand. 1997;76(1):55–60. doi: 10.3109/00016349709047785. [DOI] [PubMed] [Google Scholar]
- 28.Papatheodorou DC, Karagiannidis LK, Paltoglou G, et al. Pulsatile interleukin-6 leads CRH secretion and is associated with myometrial contractility during the active phase of term human labor. J Clin Endocrinol Metab. 2013;98(10):4105–4112. doi: 10.1210/jc.2012-4023. [DOI] [PubMed] [Google Scholar]
- 29.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: The expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 31.Bennett WA, Lagoo-Deenadayalan S, Whitworth NS, et al. First-trimester human chorionic villi express both immunoregulatory and inflammatory cytokines: A role for interleukin-10 in regulating the cytokine network of pregnancy. Am J Reprod Immunol. 1999;41(1):70–78. doi: 10.1111/j.1600-0897.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 32.Bennett WA, Lagoo-Deenadayalan S, Whitworth NS, Brackin MN, Hale E, Cowan BD. Expression and production of interleukin-10 by human trophoblast: Relationship to pregnancy immunotolerance. Early Pregnancy. 1997;3(3):190–198. [PubMed] [Google Scholar]
- 33.Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: A role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21(8):529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailit JL, Dierker L, Blanchard MH, Mercer BM. Outcomes of women presenting in active versus latent phase of spontaneous labor. Obstet Gynecol. 2005;105(1):77–79. doi: 10.1097/01.AOG.0000147843.12196.00. [DOI] [PubMed] [Google Scholar]
- 35.Holmes P, Oppenheimer LW, Wen SW. The relationship between cervical dilatation at initial presentation in labour and subsequent intervention. BJOG. 2001;108(11):1120–1124. doi: 10.1111/j.1471-0528.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- 36.Impey L, Hobson J, O’Herlihy C. Graphic analysis of actively managed labor: Prospective computation of labor progress in 500 consecutive nulliparous women in spontaneous labor at term. Am J Obstet Gynecol. 2000;183(2):438–443. doi: 10.1067/mob.2000.105899. [DOI] [PubMed] [Google Scholar]
- 37.Rahnama P, Ziaei S, Faghihzadeh S. Impact of early admission in labor on method of delivery. Int J Gynaecol Obstet. 2006;92(3):217–220. doi: 10.1016/j.ijgo.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Mikolajczyk R, Zhang J, Chan L, Grewal J. Early versus late admission to labor/delivery, labor progress and risk of caesarean section in nulliparous women. Am J Obstet Gynecol. 2008;199(6 Suppl A):S49. [Google Scholar]
- 39.Neal JL, Lamp JM, Buck JS, Lowe NK, Gillespie SL, Ryan SL. Outcomes of nulliparous women with spontaneous labor onset admitted to hospitals in preactive versus active labor. J Midwifery Womens Health. 2014;59(1):28–34. doi: 10.1111/jmwh.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. Am J Obstet Gynecol. 2002;187(4):824–828. doi: 10.1067/mob.2002.127142. [DOI] [PubMed] [Google Scholar]
- 42.Caughey AB, Cahill AG, Guise J, Rouse DJ. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193. doi: 10.1016/j.ajog.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6(2):65–70. [Google Scholar]
- 44.Neal JL, Lowe NK, Ahijevych KL, Patrick TE, Cabbage LA, Corwin EJ. “Active labor” duration and dilation rates among low-risk, nulliparous women with spontaneous labor onset: A systematic review. J Midwifery Womens Health. 2010;55(4):308–318. doi: 10.1016/j.jmwh.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragusa A, Mansur M, Zanini A, Musicco M, Maccario L, Borsellino G. Diagnosis of labor: A prospective study. MedGenMed. 2005;7(3):61–61. [PMC free article] [PubMed] [Google Scholar]