Abstract
Background
Previous studies have shown that genetic risk for externalizing (EXT) disorders is greater in the context of adverse family environments during adolescence, but it is unclear whether these effects are long-lasting. The current study evaluated developmental changes in gene-environment interplay in the concurrent and prospective associations between parent-child relationship problems and EXT at ages 18 and 25.
Method
The sample included 1,382 twin pairs (48% male) from the Minnesota Twin Family Study, participating in assessments at ages 18 (M = 17.8 years, SD = 0.69) and 25 (M = 25.0 years, SD = 0.90). Perceptions of parent-child relationship problems were assessed using questionnaires. Structured interviews were used to assess symptoms of adult antisocial behavior and nicotine, alcohol, and illicit drug dependence.
Results
We detected a gene-environment interaction at age 18, such that the genetic influence on EXT was greater in the context of more parent-child relationship problems. This moderation effect was not present at age 25, nor did parent-relationship problems at age 18 moderate genetic influence on EXT at age 25. Rather, common genetic influences accounted for this longitudinal association.
Conclusions
Gene-environment interaction evident in the relationship between adolescent parent-child relationship problems and EXT is both proximal and developmentally limited. Common genetic influence, rather than a gene-environment interaction, accounts for the long-term association between parent-child relationship problems at age 18 and EXT at age 25. These results are consistent with a relatively pervasive importance of gene-environmental correlation in the transition from late adolescence to young adulthood.
Keywords: Adolescence, Externalizing behaviors, Gene-environment correlation, Gene-environment interaction, Parent-child relationship quality
Introduction
Twin and adoption studies have shown that externalizing (EXT) disorders (substance use disorders and antisocial behavior) are influenced by common genetic factors (Haberstick et al., 2011, Hicks et al., 2011, Krueger et al., 2002, McGue et al., 2006, Prescott et al., 2006). The heritability of a general EXT factor that indexes the common genetic influences among multiple EXT disorders has been estimated to fall between 40%–60%% (Hicks et al., 2013; Rhee & Waldman, 2005). Heritability estimates, however, are influenced by environmental context. Gene-environment interplay refers to the notion that genes influence exposure to specific environments (gene-environment correlation, rGE) and that genetic influence varies as a function of the environmental context (gene-environment interaction, GxE) (Dick, 2011, Moffitt, 2005). Several studies have reported that genetic risk for EXT is greater under conditions of poorer parent-child and family relationships relative to more positive relationships (Button et al., 2008, Feinberg et al., 2007, Hicks et al., 2009b). More generally, Hicks et al. (2009b) found the same pattern of a greater genetic influence on EXT in the context of greater adversity across family, peer, and academic environments, suggesting a general and robust mechanism of gene-environment interaction in the development of adolescent EXT.
Additionally, genetic influences have been shown to contribute to the association between parent-child relationship quality and EXT in adolescence (Narusyte et al., 2011, Neiderhiser et al., 1999, Shelton et al., 2008). This is often interpreted as evidence of rGE, which refers to the notion that exposure to environments is not random, but rather is influenced by an individual’s genetic predispositions (for a review, see Dick, 2011). For example, adolescents may evoke a negative response from their parents as a result of their genetically influenced EXT traits (i.e., evocative rGE), or parenting behaviors such as monitoring, discipline, and interpersonal warmth may be influenced by parents’ genetically influenced EXT traits (i.e., passive rGE). Supporting the notion of child-driven effects, or evocative rGE, Burt et al. (2005) demonstrated that both parent-child conflict and EXT at age 11 predicted the other at age 14. Such results have been reported elsewhere (Larsson et al., 2008), suggesting evocative rGE is evident in the relationship between parenting and child EXT.
Much of the research on gene-environment interplay for EXT, however, has been limited to cross-sectional findings in childhood and adolescence (Burt et al., 2005, Button et al., 2008, Cadoret et al., 1995, Feinberg et al., 2007, Hicks et al., 2009b, Larsson et al., 2008, Legrand et al., 2008, Tuvblad et al., 2006). Thus, several important questions remain unanswered regarding the dynamics and limits of gene-environment interplay for EXT over time. For example, there is evidence for long-term associations between adolescent parent-child relationship quality and adult EXT (Bailey et al., 2011, Epstein et al., 2013), but it is unclear if adolescent parent-child relationship quality continues to moderate genetic influence on adult EXT. Relatedly, there is evidence of associations between adults’ reports of relationship quality with their parents and their own psychosocial development (Amato, 1994, Crook et al., 1981, Riggio, 2004, Seiffge-Krenke, 2006), but it is unclear if the quality of parent-child relationships in adulthood moderates genetic influence on adult EXT, or whether such effects are limited to childhood and adolescence. Questions such as these are important to address to better understand the role of development in the etiology of EXT.
The few studies that have taken development into account in evaluations of GxE interaction are somewhat contradictory. Two studies suggest at least some long-lasting effects of GxE mechanisms on EXT phenotypes. Dick et al. (2007) found that less parental monitoring at age 12 was associated with greater genetic influence on smoking at ages 14 and 17. Using a sample of female twins, Agrawal et al. (2010) found that a greater number of substance using peers in adolescence was associated with a greater genetic influence on substance use in adulthood. In contrast, Kendler et al. (2011) showed that while the genetic influence on EXT was greater as a function of a greater degree of alcohol availability, deviant peers, and less parental monitoring in early adolescence (ages 12–14), these GxE effects decreased slightly in magnitude in mid-adolescence (ages 15–17), and were essentially non-existent past age 18, suggesting GxE effects are more influential earlier in development.
To extend this research, we evaluated the concurrent and prospective relationships between parent-child relationship problems in EXT in adolescence and young adulthood. First, we expected to demonstrate the cross-sectional GxE effect in adolescence. Specifically, we hypothesized that the genetic influence on EXT would be greater in the context of a greater degree of parent-child relationship problems at age 18. We also evaluated the cross-sectional relationship between parent-child relationship problems and EXT at age 25 and the prospective association between parent-child relationship problems at age 18 and EXT at age 25, controlling for EXT at age 18. We anticipated that GxE interaction influences would be temporarily limited and dependent on the developmental context (Moffitt et al., 2005). Rather than a GxE interaction, we predicted that gene-environment correlation (as evidenced by common genetic influences) would account for the prospective association between adolescent parent-child relationship problems and EXT at age 25.
Method
Participants
Participants were twins from the Minnesota Twin Family Study, a twin study designed to investigate the development and etiology of substance use and related behaviors (Iacono et al., 1999). The study included two age cohorts: the younger cohort first assessed at age 11, and the older cohort, first assessed at age 17. Follow-up assessments were conducted every 3–5 years in both cohorts. Families were identified based on birth certificates that are public in the state of Minnesota (birth years 1972 to 1984) and were located using public databases. Approximately 90% of twin families were located. Study eligibility included living within a day’s drive to the university laboratories, and neither twin having a mental or physical handicap that would impair participation in the assessments. All participating families included twins who were the biological offspring of their parents. Among eligible families, 17% declined to participate. This project was approved by the University of Minnesota’s Institutional Review Board.
The sample included 1,382 same-sex (665 male, 717 female) twin pairs (902 monozygotic [MZ], 480 dizygotic [DZ]). Consistent with the Minnesota demographics in the relevant birth years, almost all participants (96%) were of European ancestry. All twins provided informed consent or assent (depending on their age of assessment, parents consented for children to participate). For this study, we used data from the intake and the second follow-up assessments for the older cohort and the second and fourth follow-up assessments for the younger cohort. We refer to these as the ages 18 and 25 assessments, which are the average ages of participants at these assessments across cohorts (age 18 assessment M age = 17.8 years, SD = 0.69, Range = 16.55 to 20.34, 80% of the sample was between ages 17.0–18.7; age 25 assessment M age = 25.0 years, SD = 0.90, Range = 22.6 to 29.3, 80% of the sample was between ages 23.9–26.1). Parent-child relationship problems and EXT measures were obtained at the same within-age assessment. Zygosity was determined using a questionnaire administered to parents concerning the resemblance of twin pairs, and with an algorithm comparing twins on anthropometric characteristics and fingerprint ridge counts; if results were not in agreement in these two measures, DNA was analyzed to resolve zygosity.
Measures
Parent-child relationship problems at ages 18 and 25
At the age 18 assessment, parent-child relationship problems was assessed using the adolescent report of the Parent Environment Questionnaire (Elkins et al., 1997), a 50-item self-report questionnaire that assesses multiple dimensions of the parent-child relationship. Due to high intercorrelations (r’s range .52 to .70, p’s < .001), ratings of the mother-child and father-child relationship were averaged at the scale level. Scales included parent-child conflict (e.g., “My [Mother/Father] and I often get into arguments,” α’s = .85 – .93), involvement (e.g., “My [Mother/Father] tries to keep up with how well I do in school and/or in my the job,” α’s = .80 – .92), parent’s positive regard for child (e.g., “My [Mother/Father] loves me no matter what I do,” α’s = .86 – .94) and child’s positive regard for parent (e.g., “I am really proud of my [Mother/Father]” α’s = .77 – .90); each item was answered on a scale of 1 = Definitely True to 4 = Definitely False. Prior to computing the composite score, all scales were coded in the same direction with higher scores indicative of a greater degree of parent-child relationship problems. The mean z-score across the scales was then used for the measure of parent-child relationship problems at age 18 (the mean r between all pairs of the z-scored scales = .67).
At the age 25 assessment, parent-child relationship problems was assessed using a 6-item scale from the Social Adjustment Interview (“I have what I consider to be a close relationship with my mother/father,” “I confide in/talk about personal things with my mother/father,” “I have problems getting along with my mother/father.”). Prior to computing the composite score, items were coded in the same direction (higher score indicated a greater degree of parent-child relationship problems). Items were standardized and averaged for the measure of parent-child relationship problems at age 25 (α = .77).
EXT at ages 18 and 25
EXT was measured using the same assessment tools at both ages 18 and 25. Structured diagnostic interviews were conducted by trained staff to assess symptoms of adult antisocial behavior (AAB; Criterion A symptoms of Antisocial Personality Disorder), nicotine dependence (NCD), alcohol dependence (ALD), and illicit drug abuse and dependence (DRUG). Interviews were reviewed by at least 2 individuals with advanced clinical training, who reached consensus prior to assigning symptoms. AAB symptoms were assessed using an interview comparable to the Structured Clinical Interview for DSM-III-R Axis II (Spitzer et al., 1987). Substance use disorder symptoms were assessed using the Substance Abuse Module (Robins et al., 1987) of the Composite International Diagnostic Interview. Kappa coefficients indexing diagnostic reliability were > .90 for all substance use disorders and .79 for AAB. The mean z-score across the symptom count measures was used to calculate an EXT composite score (the mean r between all pairs of the z-scored scales = .54 and .40 at ages 18 and 25).
Analysis Plan
Structural equation modeling was used to examine gene-environment interplay between parent-child relationship problems and EXT using Mx software (Neale, 2006). Mx software handles missing data using full information maximum likelihood, shown to be superior to other ways of handling missing data (Enders and Bandalos, 2001, Johnson and Young, 2011). Consistent with previous research, gender, age, age2, and age*gender were covaried out of all phenotypes prior to modeling. Also, EXT at ages 18 and 25 were log-transformed to better approximate normality. Univariate models were fit to estimate the additive genetic (A), shared environmental (C), and nonshared environmental (E) influences on each variable. ACE parameters were estimated by comparing the relative similarity of MZ and DZ twin pairs. Additive genetic effects are inferred when rMZ > rDZ. Shared environmental effects are inferred when rDZ > ½ rMZ). Nonshared environmental effects are inferred when rMZ < 1.0).
Figure 1 illustrates a bivariate decomposition, including moderation effects on the ACE parameters. This analysis, based on Purcell’s (2002) model of GxE in the presence of rGE, decomposes the ACE contributions to the covariance between the parent-child relationship problems and EXT (e.g., a11*a21) and the variance unique to EXT (e.g., a22). Standardizing the genetic and environmental covariance gives the genetic and environmental correlations (rA, rC, rE), which index the degree to which the two phenotypes share the same latent genetic and environmental influences. ACE parameters are derived from the bivariate model and are adjusted for direction and the size of the moderation (β) and the level of the moderator (M). Moderation can occur on ACE effects in common between parent-child relationship problems and EXT (e.g., a21 + βa21*M) or ACE effects unique to EXT (e.g, a22 + βa22*M). We extended the bivariate GxE model into a trivariate model that accounted for moderation in the relationship between parent-child relationship problems at age 18 and EXT at age 25, adjusting for moderation in the relationship between parent-child relationship problems at age 18 and EXT at age 18. Due to figure/table constraints, we have provided a figure describing this model in more detail as well as accompanying Mx script in supplementary material. We describe this model in more detail after reporting the bivariate GxE results (from which the trivariate model was based) in the results section.
Figure 1. Model of Gene-Environment Interplay.
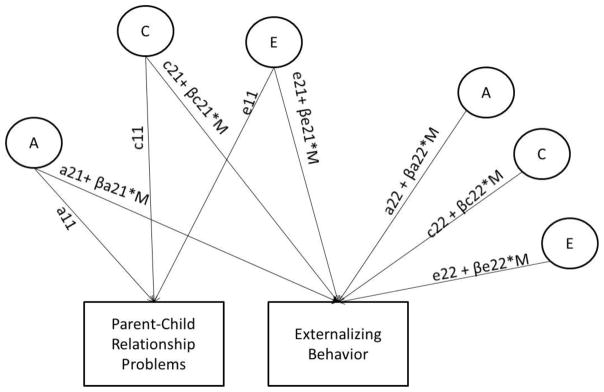
Separate models were evaluated for externalizing behaviors (EXT) at age 18 and age 25. A refers to genetic influences, C refers to shared environmental influences, and E refers to nonshared environmental influences. Parameters a11, c11, e11 refer to the genetic and environmental influences on the moderator (parent-child relationship problems). Parameters a21, c21, e21 refer to the genetic and environmental influences on the moderator (parent-child relationship problems) in common with the dependent variable (EXT). Parameters a22, c22, e22 refer to the unique genetic and environmental influence on the dependent variable (EXT). β describes the magnitude and direction of moderation effect, M indicates the level of the moderator. Moderation can influence both/either the common or unique variance with externalizing.
Model fit was evaluated by using the -2 x loglikelihood (-2lnL) and calculating the likelihood ratio test between comparison models. Model fit was also evaluated using several information theoretic fit indices that balance overall fit with parsimony by incorporating a penalty for the number of parameters including the Akaike Information Criterion, the sample-size adjusted Bayesian information criterion, and the Deviance Information Criterion. Lower values for each information theoretic index indicate better fit (Kenny, 2012, Spiegelhalter et al., 2002). For model comparisons, we first compared the full ACE moderation model to the no ACE moderation model. Additionally, fit of the full ACE moderation model was compared to a scalar moderation model, wherein moderator terms were constrained to be equal across the three ACE variance components. If there was evidence for full ACE moderation, follow up comparisons were made by dropping unnecessary moderation parameters (i.e., 95% confidence intervals included zero in the full ACE moderation model) to identify the most parsimonious, best-fitting model.
Results
Preliminary Analyses
As expected for a community sample, the sample overall reported relatively low levels of clinical EXT symptoms (prevalence of diagnoses at age 18: AAB = 4.5%, NCD = 15.1%, ALD = 7.8%, DRUG = 5.2%; age 25: AAB = 4.8%, NCD = 22.2%, ALD = 14.2%, DRUG = 6.8%). Following this, an evaluation of the descriptive statistics of the scales used to assess parent-child relationship problems indicated the scales were generally positively skewed (after recoding so the higher the score, the more the relationship problems); standardized skewness ranged from .44 to 1.52 for scales at age 18 and .63 for the scale at age 25. Due to table/figure limitations, additional descriptive statistics on the raw variables is available in the supplementary materials.
Phenotypic correlations among the variables are presented in Table 1 (all p’s < .01). There was substantial stability in EXT from age 18 to 25 (r = .58) and moderate stability in parent-child relationship problems over time (r = .35). Parent-child relationship problems at age 18 was significantly correlated with EXT at ages 18 (r = .26) and 25 (r = .24). Parent-child relationship problems at age 25 was weakly (but significantly) correlated with EXT at age 25 (r = .15).
Table 1.
Phenotypic Correlations (95% Confidence Intervals)
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
|
|
||||
| 1. Parent-Child Relationship Problems at age 18 | 1.0 | |||
| 2. Externalizing at age 18 | .26 (.20, .31) | 1.0 | ||
| 3. Parent-Child Relationship Problems at age 25 | .35 (.30, .39) | .07 (.03, .12) | 1.0 | |
| 4. Externalizing at age 25 | .24 (.19, .29) | .58 (.55, .62) | .15 (.10, .19) | 1.0 |
NOTE: All correlations were significant at p < .01.
Twin correlations and univariate ACE estimates are provided in Table 2. EXT at ages 18 and 25 exhibited large genetic and moderate nonshared environmental influence, but little to no shared environmental influence. At both ages, Parent-child relationship problems had moderate genetic and nonshared environmental influences, and small and nonsignificant shared environmental influences.
Table 2.
Intraclass Twin Correlations and Univariate ACE Estimates (95% Confidence Intervals)
| MZ twin pairs (n = 902) | DZ twin pairs (n = 480) | A | C | E | |
|---|---|---|---|---|---|
|
|
|||||
| Parent-Child Relationship Problems at age 18 | .57 (.52, .62) | .36 (.27, .45) | .43 (.25, .61) | .15 (.00, .31) | .42 (.38, .47) |
| Externalizing at age 18 | .70 (.66, .73) | .42 (.34, .50) | .66 (.52, .75) | .07 (.00, .20) | .27 (.24, .31) |
| Parent-Child Relationship Problems at age 25 | .46 (.40, .52) | .26 (.16, .35) | .38 (.17, .51) | .08 (.00, .27) | .54 (.49, .60) |
| Externalizing at age 25 | .60 (.55, .64) | .31 (.23, .40) | .62 (.46, .66) | .00 (.00, .14) | .38 (.34, .42) |
NOTE: MZ = Monozygotic, DZ = dizygotic. A = Additive genetic variance, C = Shared environmental variance, E = Nonshared environmental variance. Ninety-five confidence intervals are shown in parentheses; the estimate is significantly different from zero if the interval does not cross zero.
Common Genetic and Environmental Influences on Parent-Child Relationship Problems and EXT in Late Adolescence and Young Adulthood
Before testing for GxE, we evaluated the zero-order genetic and environmental influences on the associations between parent-child relationship problems and EXT across time. The genetic correlation between parent-child relationship problems at age 18 and EXT at age 18 was significant and moderate in effect (rA = .32, 95% CI: .09 .54), much like the genetic correlation for the longitudinal association between parent-child relationship problems at age 18 and EXT at age 25 (rA = .27, 95% CI: .03, .52). The genetic correlation for the cross-sectional association at age 25 was weaker in magnitude and not significantly different from zero (rA = .15, 95% CI: −.11, .41). The nonshared environmental correlations were small in effect for the cross-sectional relationship between EXT and parent-child relationship at age 18 (rE = .12; 95% CI: .05, .20) and the longitudinal relationship (rE = .13, 95% CI: .05, .21), and not significantly different from zero for the cross-sectional relationship at age 25 (rE = .07, 95% CI: −.00, .14). The shared environmental correlations were not significantly different from zero for all bivariate associations.
GxE Interaction between Parent-Child Relationship Problems and EXT in Late Adolescence and Young Adulthood
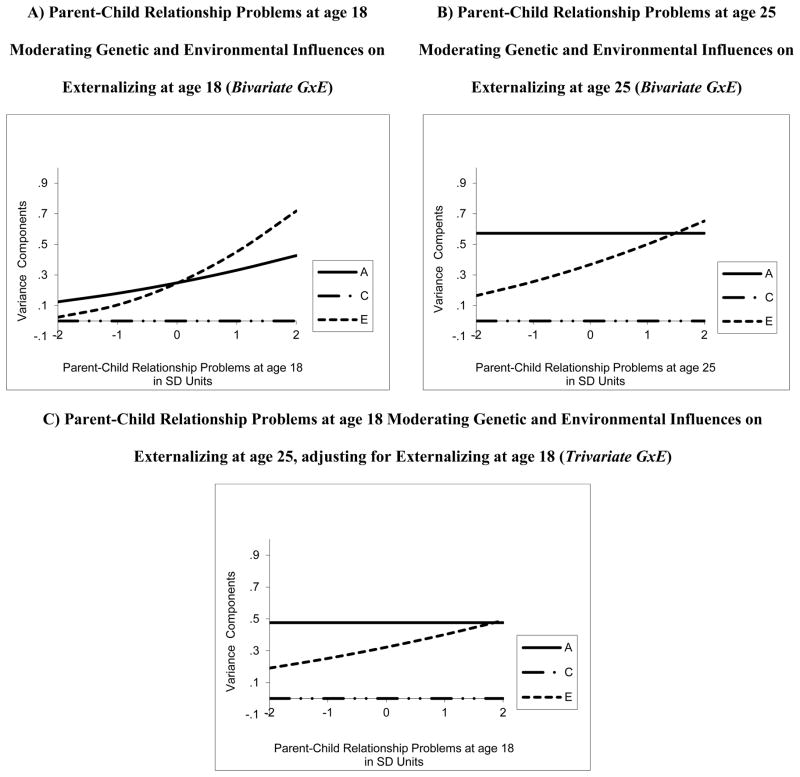
Fit statistics for both cross-sectional and longitudinal GxE interaction models are presented in Table 3. Figure 2 provides visual illustrations of the moderation effects for the best fitting models. For the cross-sectional model at age 18, fit statistics indicated that the no ACE moderation model (dropping all ACE moderation parameters common to parent-child relationship quality and EXT as well as those unique to EXT) fit significantly worse than the full ACE moderation model (keeping all ACE moderation parameters common to parent-child relationship quality and EXT as well as those unique to EXT) as evidenced by a significant likelihood ratio test and greater values for each information theoretic fit index. An evaluation of the 95% CIs of all ACE moderation parameters from the full ACE moderation model showed evidence of moderation on the A and E paths that are unique to EXT only; all other moderation parameters were not significantly different from zero. Additionally the c11 and c21 parameters could be dropped without worsening model fit, thus, this was considered the best fitting model (see Table 3). This model allowed moderation on unique AE variance only, after accounting for AE correlations between parent-child relationship problems and EXT. As illustrated in panel A of Figure 2, the genetic variance of EXT at age 18 was greater under conditions of a greater degree of parent-child relationship problems. The same pattern of effects was found for the nonshared environmental influence that was unique to EXT.
Table 3.
Fit Statistics for Gene-Environment Interplay Models of Parent-Child Relationship Problems and EXT
| -2LnL | df | Δχ2 | Δdf | p | AIC | Adjusted BIC | DIC | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Parent-Child Relationship Problems at Age 18 and EXT at Age 18 (Bivariate GxE) | ||||||||
| Full ACE Moderation | 10305.16 | 4250 | 1805.16 | −2914.82 | −5758.72 | |||
| No ACE Moderation | 10515.68 | 4256 | 210.52 | 6 | <.001 | 2003.68 | −2820.95 | −5668.86 |
| Scalar Model | 10316.51 | 4254 | 11.35 | 4 | 0.02 | 1808.51 | −2916.74 | −5763.31 |
| Unique AE Moderation | 10308.03 | 4254 | 2.87 | 4 | 0.58 | 1800.03 | −2920.98 | −5767.55 |
| a Unique AE Moderation, paths c11, c21 dropped | 10311.41 | 4256 | 6.25 | 6 | 0.40 | 1799.41 | −2923.09 | −5770.99 |
| Parent-Child Relationship Problems at Age 25 and EXT at Age 25 (Bivariate GxE) | ||||||||
| Full ACE Moderation | 11497.54 | 4295 | 2907.54 | −2426.04 | −5300.09 | |||
| No ACE Moderation | 11540.41 | 4301 | 42.87 | 6 | <.001 | 2938.41 | −2416.02 | −5294.09 |
| Scalar Model | 11508.48 | 4299 | 10.94 | 4 | 0.03 | 2910.48 | −2428.18 | −5304.91 |
| Unique E Moderation | 11500.81 | 4300 | 3.27 | 5 | 0.77 | 2900.81 | −2433.92 | −5311.31 |
| a Unique E Moderation, paths c11, c21, c22 and e21 dropped | 11504.52 | 4304 | 6.98 | 9 | 0.64 | 2896.52 | −2439.68 | −5319.75 |
| Parent-Child Relationship Problems at Age 18 and EXT at Age 25, adjusting for EXT at age 18 (Trivariate GxE) | ||||||||
| Unique AE moderation on EXT (at both ages 18 and 25) | 14630.13 | 6235 | 2160.132 | −4520.29 | −8692.45 | |||
| No unique AE moderation on EXT (at both ages 18 and 25) | 14849.67 | 6239 | 219.535 | 4 | <.001 | 2371.67 | −4418.12 | −8592.96 |
| Scalar Model | 14637.55 | 6237 | 7.421 | 2 | 0.02 | 2163.551 | −4520.38 | −8693.88 |
| Unique A moderation on EXT at age 18 only, unique E moderation on EXT at both ages 18 and 25 (i.e., unique A moderation on EXT at age 25 dropped) | 14630.13 | 6236 | 0.00 | 1 | 1.0 | 2158.132 | −4522.19 | −8695.02 |
| a Unique A moderation on EXT at age 18 only, unique E moderation on EXT at both ages 18 and 25; paths c11, c33, c31, c21, c32 dropped | 14638.1 | 6241 | 7.969 | 6 | 0.24 | 2156.1 | −4527.7 | −8703.87 |
NOTE: -2LnL = -2 x Loglikelihood, df = degrees of freedom, Δχ2 = chi-square change, Δdf = degree of freedom change, AIC = Akaike Information Criterion, BIC = Bayesian Information Criterion, DIC = Deviance Information Criterion. A = additive genetic effects, C = shared environmental effects, E = nonshared environmental effects. The baseline model of comparison used in chi-square difference tests is the full ACE moderation model, which allows for ACE moderation on all common and unique parameters. Scalar models refer to models that held moderators constant across genetic and environmental variance, rather than allowing moderators specific to genetic and environmental variance. Lower values across fit statistics indicate better fit. The change in χ2 is the difference between the -2 x log likelihood (-2LnL) in the baseline model (full ACE moderation) compared to the other modes tested.
Considered the best fitting model.
Figure 2. Estimates of Genetic and Environmental Variance Components at Different Levels of Parent-Child Relationship Problems Across Time.
Changes in the unstandardized ACE variance components (in standard deviation [SD] units) of EXT are given as a function of parent-child relationship problems for the best-fitting models. A = additive genetic influence, C = shared environmental influences, E = nonshared environmental influences. Parent-child relationship problems is measured such that the higher the score, the greater degree of parent-child relationship problems. Cross-sectional results at age 18 (parent-child relationship problems at age 18 moderating EXT at age 18) are shown in panel A. Cross-sectional results at age 25 (parent-child relationship problems at age 25 moderating EXT at age 25) are shown in panel B. Longitudinal results (parent-child relationship problems at age 18 moderating EXT at age 25, adjusting for EXT at age 18) are shown in panel C.
Table 4 provides both the unstandardized and standardized variance components of EXT at age 18 as a function of parent-child relationship problems at age 18. While a GxE interaction was only detected in the cross-sectional results at age 18 (such that genetic variance was greater in the context of a greater degree of parent-child relationship problems), all analyses showed that the heritability (i.e., proportion of total variance accounted for by genetic variance) of EXT was greater under conditions of less parent-child relationship problems. This “flip” in findings arises because the total EXT variance increases with greater parent-child relationship problems and although both the nonshared and genetic contributions to variance increase with increasing environmental adversity, the former increases more rapidly than the latter leading to declines in heritability. In other words, the standardized results concealed absolute changes in genetic and nonshared environmental variance.
Table 4.
Unstandardized and Standardized Variance Components for the Best-Fitting Models
| Unstandardized Variance Components | Standardized Variance Components | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| A | C | E | Total Variance | A | C | E | |
|
|
|||||||
| EXT at age 18 as a function of Parent-Child Relationship Problems at age 18 | |||||||
| −2 | 0.12 | 0.00 | 0.02 | 0.15 | 0.84 | 0.00 | 0.16 |
| −1 | 0.18 | 0.00 | 0.10 | 0.28 | 0.63 | 0.00 | 0.37 |
| 0 | 0.25 | 0.00 | 0.25 | 0.50 | 0.50 | 0.00 | 0.50 |
| 1 | 0.33 | 0.00 | 0.45 | 0.78 | 0.42 | 0.00 | 0.58 |
| 2 | 0.43 | 0.00 | 0.72 | 1.14 | 0.37 | 0.00 | 0.63 |
| EXT at age 25 as a function of Parent-Child Relationship Problems at age 25 | |||||||
| −2 | 0.57 | 0.00 | 0.17 | 0.74 | 0.78 | 0.00 | 0.22 |
| −1 | 0.57 | 0.00 | 0.26 | 0.83 | 0.69 | 0.00 | 0.31 |
| 0 | 0.57 | 0.00 | 0.37 | 0.94 | 0.61 | 0.00 | 0.39 |
| 1 | 0.57 | 0.00 | 0.50 | 1.07 | 0.53 | 0.00 | 0.47 |
| 2 | 0.57 | 0.00 | 0.65 | 1.22 | 0.47 | 0.00 | 0.53 |
| EXT at age 25 as a function of Parent-Child Relationship Problems at age 18, after adjusting for EXT at age 18 | |||||||
| −2 | 0.48 | 0.00 | 0.19 | 0.67 | 0.71 | 0.00 | 0.29 |
| −1 | 0.48 | 0.00 | 0.25 | 0.73 | 0.65 | 0.00 | 0.35 |
| 0 | 0.48 | 0.00 | 0.32 | 0.80 | 0.60 | 0.00 | 0.40 |
| 1 | 0.48 | 0.00 | 0.40 | 0.88 | 0.54 | 0.00 | 0.46 |
| 2 | 0.48 | 0.00 | 0.49 | 0.97 | 0.49 | 0.00 | 0.51 |
NOTE: A = additive genetic influence, C = shared environmental influence, E = nonshared environmental influence. Unlike the unstandardized variance components, the standardized variance components add up to 1.0.
In contrast to the cross-sectional analyses at age 18, dropping the genetic moderation parameters for the cross-sectional analysis at age 25 did not worsen model fit. Thus, parent-child relationship problems at age 25 did not moderate the genetic influence on EXT at age 25 (see Table 3). However, there was significant moderation of the unique E on EXT at age 25. Additionally, paths c11, c21, c22, and e21 could be dropped without worsening model fit; thus this parsimonious model was considered the best fitting model (see Table 3). As illustrated in panel B of Figure 2, unique nonshared environmental influences on EXT were greater under conditions of a greater degree of parent-child relationship problems at age 25.
Does Parent-Child Relationship Problems at Age 18 Moderate the Genetic and Environmental Influences on EXT at Age 25?
Next, we evaluated whether parent-child relationship problems at age 18 moderated genetic and environmental influences on EXT at age 25, after adjusting for the moderating influence of parent-child relationship problems at age 18 on EXT at age 18. Based on the best fitting bivariate results which documented unique AE moderation at age 18 and unique E moderation at age 25, we evaluated a trivariate model that tested for unique AE moderation on EXT at ages 18 and 25 (Figure describing this model and Mx script available in the supplementary materials). As described in the fit statistics in Table 3, unique A moderation on EXT at age 18 and unique E moderation on EXT at ages 18 and 25 was evident, but unique A moderation on EXT at age 25 could be dropped without reducing model fit; thus there was no evidence of genetic moderation on adult EXT by adolescent parent-child relationship problems. Paths c11, c33, c31, c21, and c32 could additionally be dropped without reducing model fit (see Table 3 for details); thus this parsimonious model was considered the best fitting. As illustrated in Figure 2, the unique E moderation effect on EXT at age 25 by parent-child relationship problems at age 18 was consistent with the pattern of nonshared environmental moderation of the cross-sectional relationships at age 18 and 25.1
Discussion
The goal of this study was to evaluate developmental changes in gene-environment interplay between parent-child relationship problems and EXT. As in our previous analysis and consistent with other studies, we detected a GxE interaction in adolescence (Button et al., 2008, Feinberg et al., 2007, Hicks et al., 2009b), such that genetic influence on adolescent EXT was greater in the context of a greater degree of parent-child relationship problems. There was no evidence for this GxE interaction between parent-child relationship problems and EXT at age 25, or for the longitudinal relationship between parent-child relationship problems at age 18 and EXT at age 25. Moreover, while the cross-sectional association was weak in adulthood, the longitudinal association between parent-child relationship problems at age 18 and EXT at age 25 was moderate and predominately accounted for by common genetic influences, which is consistent with a gene-environment correlation hypothesis. In summary, results indicate that the moderating influence of adolescent parent-child relationship quality on the genetic influence of EXT is specific to the developmental context of adolescence (Moffitt et al., 2005).
Johnson (2007) suggested models of GxE interaction in the presence of rGE can be interpreted to support a primarily social causation versus social selection explanation for the relationship between parent-child relationship problems and EXT. Social causation refers to the hypothesis that unfavorable environments (e.g., poor parenting) trigger genetic predisposition for mental illness and behavior problems (e.g., EXT). Social selection refers to the hypothesis that biological predispositions result in drift towards or creation of unfavorable environments (e.g., children have a greater degree of parent-child relationship problems as a result of their EXT). Our pattern of results appears to be consistent with both explanations as both GxE and rGE were detected at age 18. However, the direction of effects remains unclear as both EXT and parent-child relationship problems were measured at the same time point at the age 18 assessment (i.e., rather than parent-child relationship problems triggering genetic risk for EXT, it could be that greater EXT triggers genetic risk for greater parent-child relationship problems).
Unlike the cross-sectional results in adolescence, we failed to detect a longitudinal moderating influence of adolescent parent-child relationship problems on EXT in adulthood. Rather, results supported gene-environment correlation between adult EXT and prior parent-child relationship problems. Together, findings from this study and others (Burt et al., 2005; Dick et al., 2007; Larsson et al., 2008) generally support a developmental cascade theory of EXT, such that a genetic predisposition towards EXT is associated with greater environmental risk through processes of evocative and active gene-environment correlation (demonstrated by common genetic influences; e.g., Burt et al., 2005; Neiderhiser et al.,, 1999). Above and beyond these effects, environmental context may additionally buffer or amplify genetic influence on EXT (Button et al., 2008, Dick et al., 2007; Feinberg et al., 2007); however, our results suggest such GxE interaction effects may be temporally and developmentally limited, at least for parent-child relationship problems. Additional longitudinal GxE analyses are needed to evaluate the impact of other key environmental influences in adolescence and young adulthood.
We presented moderation results for both the unstandardized and standardized variance components. Unstandardized variance components are necessary to evaluate for GxE because standardized variance components can concealed absolute changes in genetic and nonshared environmental variance. Nonetheless, standardized results can be useful to compare to prior cross-sectional research documenting EXT heritability estimates in childhood and adolescence. The pattern of results in Table 4 suggest that studies documenting substantial heritability estimates of EXT may have used samples that had less environmental stress (on average) than studies documenting markedly low heritability estimates (on average). It is useful to note Rhee and Waldman’s (2005 meta-analysis confirmed moderate heritability estimates (h2) for EXT for children (h2 = .46), adolescents (h2 = .43), and adults (h2 = .41), but decreasing shared environmental estimates (c2), and increasing nonshared environmental estimates (e2) with age (children: c2 = .20, e2 = .34; adolescents: c2 = .16, e2 = .41; c2 = .09, e2 = .50).
Some study limitations should be noted. Although representative of the state from which they were sampled, study participants were almost all of European ancestry; results may or may not generalize to other populations. Second, the measurement of parent-child relationship problems at age 25 was not as extensive as the measurement at age 18. Nonetheless, the correlation of our measures for parent-child relationship problems from ages 18 to 25 was moderate in magnitude (r =.35), supporting the notion that these measures tap into a related construct at both ages. Notably, parent-child relationship problems at age 18 does not likely represent the beginning of a causal model, as it was likely affected by earlier child externalizing problems and parent-child dynamics throughout childhood and adolescence (Burt et al., 2005; Larsson et al., 2008). Finally, we do not know how results might apply to other forms of psychopathology (such as internalizing disorders, Hicks et al., 2009a), nor do we know how results might apply to clinical populations. Study strengths include the use of a genetically-informed design, a large community-based sample, the use of prospective data, and the inclusion of both males and females.
This study demonstrated notable changes in the influence of gene-environment interplay in the development of EXT over time. First, we showed that while GxE interaction effects on EXT may be evident during childhood and adolescence, such effects may be temporarily limited and specific to developmental context. Second, we demonstrated that the influence of environmental variables changes over the course of development (i.e., parent-child relationship problems in adulthood is a less salient predictor of adult EXT than parent-child relationship problems in adolescence). Finally, results are consistent with the notion that gene-environment correlation is a pervasive mechanism that accounts for person-environment associations during this developmental period spanning late adolescence to young adulthood. Altogether, this study is one of few (Agrawal et al., 2010, Kendler et al., 2011) that has evaluated long-term influences of gene-environment interplay. It remains important for future research to continue to identify what may potentially exacerbate or ameliorate genetic risk of EXT and related psychopathology in adulthood, particularly using longitudinal models that account for childhood and adolescent risk factors relevant for EXT over the life-course.
Supplementary Material
Acknowledgments
Financial Support
This research was supported by Grant DA05147 from the National Institute on Drug Abuse and Grant AA09367 from the National Institute of Alcohol Abuse and Alcoholism. Diana R. Samek was also supported by the National Institute of Mental Health postdoctoral training grant MH017069. Brian M. Hicks was supported by K01 DA025868.
Footnotes
While Mx accounts for missing data using full information maximum likelihood (FIML), shown superior to other methods for handling of missing data (Enders and Bandalos, 2001, Johnson and Young, 2011), we also analyzed results for those with valid data at all time points and for all phenotypes for comparison (972 pairs of twins, 639 MZ, 333 DZ). We found the same pattern of results for this sub-sample.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest:
All authors declare that they have no conflict of interest.
Ethical Standards:
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Agrawal A, Balasubramanian S, Smith EK, Madden PA, Bucholz KK, Heath AC, Lynskey MT. Peer substance involvement modifies genetic influences on regular substance involvement in young women. Addiction. 2010;105:1844–53. doi: 10.1111/j.1360-0443.2010.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato PR. Father-Child Relations, Mother-Child Relations, and Offspring Psychological Well-Being in Early Adulthood. Journal of Marriage and the Family. 1994;56:1031–1042. [Google Scholar]
- Bailey JA, Hill KG, Meacham MC, Young SE, Hawkins JD. Strategies for characterizing complex phenotypes and environments: general and specific family environmental predictors of young adult tobacco dependence, alcohol use disorder, and co-occurring problems. Drug and Alcohol Dependence. 2011;118:444–51. doi: 10.1016/j.drugalcdep.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results, from a genetically informative cross-lagged study. Development and Psychopathology. 2005;17:145–165. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TM, Lau JY, Maughan B, Eley TC. Parental punitive discipline, negative life events and gene-environment interplay in the development of externalizing behavior. Psychological Medicine. 2008;38:29–39. doi: 10.1017/S0033291707001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. Archives of General Psychiatry. 1995;52:916–24. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- Crook T, Raskin A, Eliot J. Parent-Child Relationships and Adult Depression. Child Development. 1981;52:950–957. [PubMed] [Google Scholar]
- Dick DM. Gene-environment interaction in psychological traits and disorders. Annalu Review of Clinical Psychology. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007;116:213–8. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–63. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Structural Equation Modeling- A Multidisciplinary Journal. 2001;8:430–457. [Google Scholar]
- Epstein M, Hill KG, Bailey JA, Hawkins JD. The effect of general and drug- specific family environments on comorbid and drug-specific problem behavior: A longitudinal examination. Developmental Psychology. 2013;49:1151–64. doi: 10.1037/a0029309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg ME, Button TM, Neiderhiser JM, Reiss D, Hetherington EM. Parenting and adolescent antisocial behavior and depression: evidence of genotype x parenting environment interaction. Archives of General Psychiatry. 2007;64:457–65. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, Hewitt JK. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011;106:215–24. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, McGue M. Gene-environment interplay in internalizing disorders: consistent findings across six environmental risk factors. Journal of Child Psychology and Psychiatry. 2009a;50:1309–17. doi: 10.1111/j.1469-7610.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Foster KT, Iacono WG, McGue M. Genetic and environmental influences on the familial transmission of externalizing disorders in adoptive and twin offspring. Journal of the American Medication Association: Psychiatry. 2013;70:1076–1083. doi: 10.1001/jamapsychiatry.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, South SC, Dirago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009b;66:640–8. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Developmental Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Young R. Toward best practices in analyzing datasets with missing data: Comparisons and recommendations. Journal of Marriage and the Family. 2011;73:926–945. [Google Scholar]
- Johnson W. Genetic and Environmental Influences on Behavior: Capturing All the Interplay. Psychological Review. 2007;114:423–440. doi: 10.1037/0033-295X.114.2.423. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine. 2011;41:1507–16. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA. Measuring model fit. Jul 5, 2012. [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–24. [PubMed] [Google Scholar]
- Larsson H, Viding E, Rijsdijk FV, Plomin R. Relationships between parental negativity and childhood antisocial behavior over time: A bidirectional effects model in a longitudinal genetically informative design. Journal of Abnormal Child Psychology. 2008;36:633–645. doi: 10.1007/s10802-007-9151-2. [DOI] [PubMed] [Google Scholar]
- Legrand LN, Keyes M, McGue M, Iacono WG, Krueger RF. Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior. Psychological Medicine. 2008;38:1341–50. doi: 10.1017/S0033291707001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: a multivariate behavioral genetic perspective. Behavorial Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131:533–54. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–81. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Narusyte J, Neiderhiser JM, Andershed AK, D’Onofrio BM, Reiss D, Spotts E, Ganiban J, Lichtenstein P. Parental criticism and externalizing behavior problems in adolescents: the role of environment and genotype-environment correlation. Journal of Abnormal Psychology. 2011;120:365–76. doi: 10.1037/a0021815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical modeling. 7. Department of Psychiatry; Richmond, VA: 2006. [Google Scholar]
- Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Relationships between parenting and adolescent adjustment over time: genetic and environmental contributions. Developmental Psychology. 1999;35:680–92. doi: 10.1037//0012-1649.35.3.680. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Madden PA, Stallings MC. Challenges in genetic studies of the etiology of substance use and substance use disorders: introduction to the special issue. Behavior Genetics. 2006;36:473–82. doi: 10.1007/s10519-006-9072-9. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- Riggio HR. Parental marital conflict and divorce, parent-child relationships, social support, and relationship anxiety in young adulthood. Personal Relationships. 2004;11:99–114. [Google Scholar]
- Robins LM, Babor T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. Authors; St. Louis: 1987. [Google Scholar]
- Seiffge-Krenke I. Leaving home or still in the nest? Parent-child relationships and psychological health as predictors of different leaving home patterns. Developmental Psychology. 2006;42:864–876. doi: 10.1037/0012-1649.42.5.864. [DOI] [PubMed] [Google Scholar]
- Shelton KH, Harold GT, Fowler TA, Rice FJ, Neale MC, Thapar A, van den Bree M. Parent-child relations, conduct problems and cigarette use in adolescence: Examining the role of genetic and environmental factors on patterns of behavior. Journal of Youth and Adolescence. 2008 Nov;37:1216–1228. [Google Scholar]
- Spiegelhalter DJ, Best N, Carlin B, AvdL Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002 Oct;64:583–640. [Google Scholar]
- Spitzer RL, Williams JBW, MG . Structured Clinical Interview for DSM-III-R (SCID) New York State Psychiatric Institute, Biometrics Research; New York: 1987. [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. Journal of Child Psychology and Psychiatry. 2006;47:734–43. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.