Abstract
Glioblastomas are aggressive adult brain tumors, characterized by inadequately organized vasculature and consequent nutrient and oxygen (O2)-depleted areas. Adaptation to low nutrients and hypoxia supports glioblastoma cell survival, progression, and therapeutic resistance. However, specific mechanisms promoting cellular survival under nutrient and O2 deprivation remain incompletely understood. Here, we show that miR-124 expression is negatively correlated with a hypoxic gene signature in glioblastoma patient samples, suggesting that low miR-124 levels contribute to pro-survival adaptive pathways in this disease. Since miR-124 expression is repressed in various cancers (including glioblastoma), we quantified miR-124 abundance in normoxic and hypoxic regions in glioblastoma patient tissue, and investigated whether ectopic miR-124 expression compromises cell survival, during tumor ischemia. Our results indicate that miR-124 levels are further diminished in hypoxic/ischemic regions within individual glioblastoma patient samples, compared to regions replete in O2 and nutrients. Importantly, we also show that increased miR-124 expression affects the ability of tumor cells to survive under O2 and/or nutrient deprivation. Moreover, miR-124 re-expression increases cell death in vivo, and enhances the survival of mice bearing intracranial xenograft tumors. miR-124 exerts this phenotype in part by directly regulating TEAD1, MAPK14/p38α and SERP1, factors involved in cell proliferation and survival under stress. Simultaneous suppression of these miR-124 targets results in similar levels of cell death as caused by miR-124 restoration. Importantly, we further demonstrate that SERP1 re-introduction reverses the hypoxic cell death elicited by miR-124, indicating the importance of SERP1 in promoting tumor cell survival. In support of our experimental data, we observed a significant correlation between high SERP1 levels and poor patient outcome in glioblastoma patients. Collectively, among the many pro-tumorigeneic properties of miR-124 repression in glioblastoma, we delineated a novel role in promoting tumor cell survival under stressful microenvironments, thereby supporting tumor progression.
Keywords: miR-124, glioblastoma, hypoxia, ischemia, nutrient deprivation
Introduction
Glioblastoma Multiforme (GBM) is the most aggressive adult brain tumor. At only 12 months, median GBM patient survival is one of the lowest for all cancers (1). The standard of care for GBM treatment is surgery, followed by radiation and chemotherapy (2). However, glioblastomas rapidly become resistant to these therapies, thereby making them ineffective at significantly improving prognosis. GBM tumors are considered Grade IV (the most aggressive gliomas) and occur either de novo or as a progression from lower grade lesions (3). In both instances, the key feature of GBM, as compared with lower grade gliomas, is the presence of severely hypoxic/ischemic regions (4). Low oxygen tension (hypoxia) is defined as less than 2% O2 and occurs in most solid tumors due to rapid proliferation, or aberrant angiogenesis, resulting in poor perfusion. The presence of hypoxic/ischemic areas is detrimental to GBM patients, as it positively correlates with recurrence and negatively correlates with patient survival (5, 6). Therefore, identifying factors mediating cellular adaptation to nutrient deprivation and hypoxia is crucial for improving therapeutic approaches to GBM.
Recent studies, such as the Cancer Genome Atlas (TCGA), have elucidated genetic aberrations associated with glioblastomas. In addition to deregulated oncoproteins and tumor suppressors (such as EGFR, PDGFR, PI3K, PTEN, NF1, etc.) (7, 8), numerous micro-RNAs (miRNAs) are differentially expressed in GBMs relative to adjacent non-neoplastic tissue (9, 10). miRNAs are ~22 nucleotide small RNAs that function as post-transcriptional negative regulators of ~30% of all mammalian genes (11, 12). While the inhibition of any single miRNA target is relatively modest, each miRNA impacts the expression of numerous genes. Thus, by targeting genes involved in multiple pathways, a single miRNA can significantly influence systems involved in cell cycle progression, differentiation, and cell death, as well as broad responses to stress (13).
Previous studies have measured miRNA levels in glioblastoma and compared them to adjacent non-neoplastic tissues, or to lower-grade gliomas. In particular, miR-124 levels were shown to be significantly reduced in glioblastomas as compared to both adjacent non-neoplastic tissues (10) and lower-grade tumors (14, 15). miR-124 is a brain-enriched miRNA critical for regulating neuronal differentiation (16-20). As miR-124 levels are differentially expressed in distinct brain cell types, low miR-124 levels in glioblastoma may be a result of the cellular heterogeneity between glioma and adjacent tissue (21, 22). Alternatively, it is possible that miR-124 functions as a tumor suppressor in GBM. This has been suggested in the context of other tumors (9, 23, 24) and the abundance of known miR-124 targets negatively correlates with miR-124 levels in brain tumor patient samples (15, 21), leaving open the possibility that glioblastoma cells expressing low miR-124 levels exhibit a selective growth or survival advantage.
Hypoxic glioblastoma cells are often found in perinecrotic areas, where surviving cells experience low levels of O2 in addition to diminished nutrient and growth factor availability (25). Such cells must therefore adapt to steep O2 and nutrient gradients, and these adaptive responses are partly mediated by the Hypoxia Inducible Factors (HIFs) (26). Recent studies have shown that miRNAs also play a key role in modulating cellular survival or death under limiting O2 and nutrient availability. For example, miR-210 is elevated in hypoxic regions and promotes survival under low O2 (27). Additionally, we have shown that restoring miR-218 levels in glioblastoma opposes a Receptor-Tyrosine Kinase/HIF signaling pathway necessary for glioblastoma progression, particularly in the Mesenchymal subtype (28).
Here, we show that miR-124 levels inversely correlate with a hypoxic signature in TCGA patient samples. Moreover, miR-124 levels are diminished in pseudopalisading regions within individual glioblastoma patient tissues, when compared to relatively well-perfused regions. We demonstrate that increased miR-124 expression in glioblastoma cells experiencing nutrient and O2 deprivation promotes cell death, suggesting that miR-124 targets factors important for glioblastoma survival under stressful microenvironments. Moreover, miR-124 expression leads to increased cell death in vivo, in a doxycycline-induced tumor xenograft model that faithfully recapitulates hypoxic/ischemic regions in GBM. We identify three factors, TEAD1, p38α (MAPK14) and SERP1, as direct miR-124 targets, and show that they are overexpressed in hypoxic/ischemic conditions. Combined inhibition of these targets recapitulates the increased cell death observed under low nutrient/O2 stress, upon miR-124 expression. In addition, SERP1 re-expression reverses the miR-124 cell death phenotype in glioblastoma cells grown under hypoxia. Finally, we show that miR-124 restoration confers increased mouse overall survival in an intracranial orthotopic xenograft model. These data suggest further investigation of miR-124 and its targets, in particular SERP1, is strongly warranted.
Results
miR-124 levels inversely correlate with hypoxia signatures in glioblastoma patients and are further decreased in pseudopalisading necrotic regions within patient tissues
While several studies have shown that miR-124 levels are low in glioblastomas as compared to adjacent non-neoplastic tissues, little is known about how miR-124 levels correlate with low O2 microenvironments. We took advantage of the large TCGA glioblastoma dataset (7), and compared miR-124 levels to a “hypoxia” signature identified in the GBM patients’ expression profile. We defined a Hypoxia Inducible Factor (HIF) metagene by averaging the expression of known HIF-regulated genes (15) from each TCGA patient into a single expression value. A high HIF metagene score positively correlates with high levels of hypoxia in each patient sample. Interestingly, miR-124 levels negatively correlated with the HIF metagene, suggesting that GBM tissues experiencing highly hypoxic microenvironments have even lower miR-124 levels (Figure 1a, S1). Furthermore, we stratified TCGA patients based on miR-124 levels (high vs. low) and observed that patients expressing the lowest levels of miR-124 exhibited a higher correlation with the HIF metagene. To validate our analysis, we also queried the levels of miR-210, a known miRNA stimulated by hypoxia, which positively correlated with the HIF metagene (Figure 1b).
Figure 1. miR-124 levels inversely correlate with hypoxia signatures in glioblastoma patients and are further decreased in pseudopalisading necrotic regions within patient tissues.
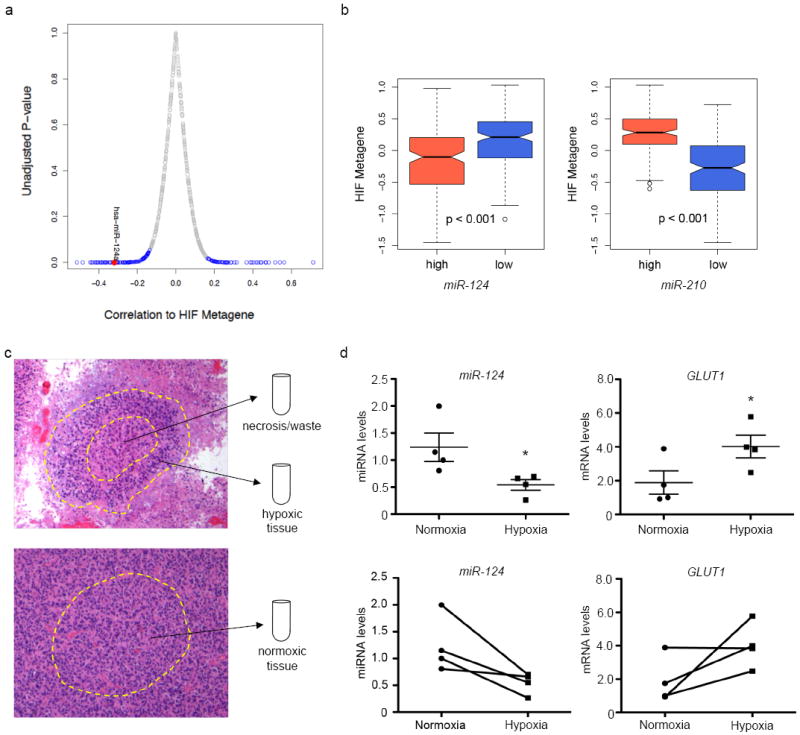
(a) Hypoxia Inducible Factor (HIF) metagene correlation to miRNA levels. MiRNAs that are positively and negatively correlated with the metagene are indicated blue = p - value < 0.05. Red dot = miR-124. (b) HIF metagene in TCGA GBM patient samples divided into high and low miR-124 / miR-210 levels. (c) Schematic of Laser-Capture Microdissection. Top: region of pseudopalisading necrosis. The inner necrotic region is discarded, while the pseudopalisade is collected as “hypoxic tissue”. Bottom: Non-necrotic region, collected as the perfused (“normoxic”) tissue counterpart. (d) miR-124 and GLUT1 mRNA levels in perfused or “normoxic” and hypoxic regions in patient samples. Top: averages; bottom: paired representation (each pair is from an individual patient). * p - value = 0.047 (miR-124 changes); p - value = 0.035 (GLUT1 changes). n = 4 patients.
While the TCGA contains a large repository of patient samples, it does not compare normoxic and hypoxic regions of glioblastoma within the same tumor. To determine whether miR-124 levels were changing in hypoxic/ischemic domains within an individual glioblastoma patient sample, we performed laser-capture microdissection, collected regions of pseudopalisading necrosis, and compared them to non-necrotic, better-perfused areas. (Figure 1c, Supplemental Figure 1b). We compared miR-124 and GLUT1 levels between the perfused (“normoxic”) and hypoxic regions of each individual patient, and showed that, while miR-124 levels are already low in glioblastoma tissue, they are further diminished in domains experiencing hypoxia/ischemia (Figure 1d, left panel). Conversely, GLUT1, a hypoxically induced HIF target gene, was elevated in the hypoxic regions (Figure 1d, right panel). Taken together, these findings suggest the possibility that miR-124 levels may be further decreased in hypoxic/ischemic domains, potentially to confer a survival advantage within a stressful microenvironment.
miR-124 expression increases cell death in glioblastoma cells grown under limiting nutrients and oxygen
To determine whether miR-124 levels affect glioblastoma cells during stress, we increased miR-124 expression in U87MG and LN18 glioblastoma cells and incubated them for 48 hours under nutrient deprivation (no glucose, no serum) or hypoxia (0.5% O2). Interestingly, we found that miR-124 re-expression resulted in a significant increase in cell death in glioblastoma cells cultured under nutrient or O2 deprivation, as measured by Annexin/PI staining and flow cytometry (Figure 2a, c). Consequently, a slight increase in cleaved PARP accumulation was observed (Figure 2b). As shown previously, no significant change in cell viability was observed in glioblastoma cells grown under replete O2 and nutrients upon miR-124 expression (Figure 2a), suggesting that cell death is due to miR-124 negatively regulating factors responsible for pro-survival, adaptive responses specifically during stress. We confirmed these results by modulating serum and O2 levels in U373-MG cells and imaging miR-124-dependent cell death (Figure S2a). Of note, miR-124 enhanced cell death was also observed when cells were treated with tunicamycin, a small molecule that induces ER stress (Figures 2d-e, S2b). Based on these results, we hypothesized that miR-124 may be targeting factors that are active under stress conditions, and subsequently their pro-survival effects.
Figure 2. miR-124 expression increases cell death in glioblastoma cells grown under limiting nutrients and oxygen.
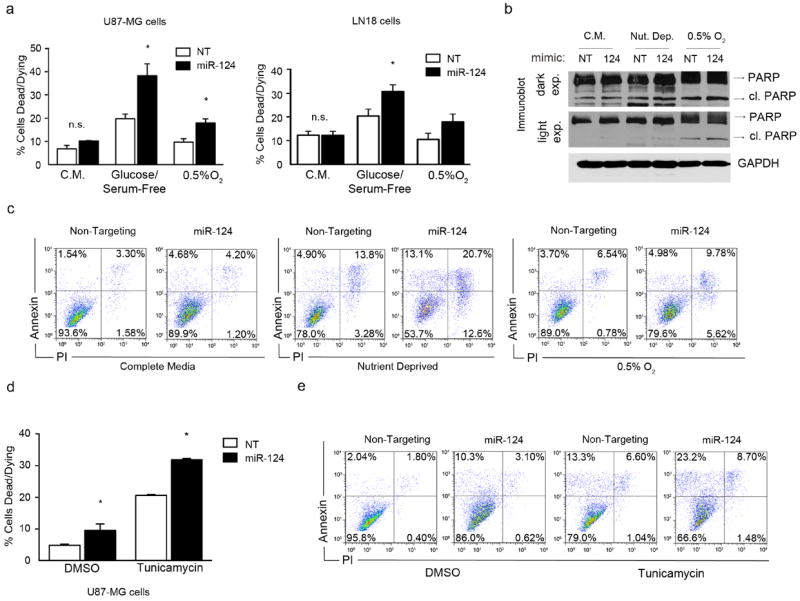
(a) U87-MG and LN18 cells were grown under the following conditions: Complete Media (C.M.), Glucose/Serum-Free, and Hypoxia (0.5% O2). Cells were transfected with either a Non-Targeting (NT) or miR-124 mimic (miR-124) for 48 hours, followed by Annexin/PI staining and flow cytometry for cell death analysis. (b) Immunoblots of cleaved PARP in cells treated as described in Figure 1a. (c) Representative Annexin – PI flow cytometry plots of U87MG cells corresponding to Figure1a. (d) U87-MG cells were treated with tunicamycin or DMSO control, and transfected with either a NT or miR-124 mimic for 24 hours, followed by Annexin/PI staining and cell death analysis by flow cytometry. (e) Representative Annexin – PI flow cytometry plots from Figure 1d. * p - value < 0.05.
TEAD1, MAPK14/p38α, and SERP1 are miR-124 direct targets
To identify potential miR-124 targets promoting survival during stress, we queried various miRNA target prediction bioinformatic databases, including TargetScan™, miRBase™ and DIANA-LAB, as well as previously published microarrays of genes modulated by miR-124 overexpression (16, 20), and identified three putative miR-124 targets that may play a role in pro-survival stress responses: MAPK14/p38α, TEAD1 and SERP1.
TEAD1 (TEA Domain 1) is a transcription factor regulated by the Hippo pathway, which controls the expression of many pro-proliferation genes (29). Interestingly, a recent study has shown that TEAD1 overexpression leads to an anti-apoptotic phenotype in HeLa cells (30). p38α (also known as MAPK14, MAP kinase 14) is a kinase typically activated as a response to upstream stress signaling. Although p38α is thought to be tumor suppressive in some settings (31), it may have a pro-tumorigenic role in GBM: an activated p38-MAPK pathway signature correlates with poor survival in glioblastoma (14), and increased glioblastoma invasion (32). Interestingly, a recent study demonstrated that miR-124 can decrease MAPK14 levels, to counteract p38α signaling in neurons (33). TEAD1 and p38α are upregulated in several cancers, including colon, prostate, lung and pancreatic cancer (30, 34), suggesting a pro-tumorigenic role for these proteins. SERP1 (stress-associated endoplasmic reticulum protein, also known as RAMP4) is a 7 kD peptide located within the sec61 ER translocon protein complex. While very little is known about SERP1 biological functions, it has been proposed that this peptide is critical for the folding of newly synthesized proteins under stress conditions: SERP1 was originally identified as a peptide exhibiting increased expression during hypoxia and hypoglycemia (35), and Serp1-/- mice are more susceptible to ER stress (36).
According to TargetScan™, MAPK14 harbors a putative conserved miR-124 binding site in its 3’ Untranslated Region (UTR), while TEAD1 and SERP1 have two putative conserved binding sites each (Figure 3a). We ectopically increased Non-Targeting (NT) and miR-124 levels in U87MG and LN18 cell lines, and assessed target mRNA levels by quantitative RT-PCR (Figure 3b) and protein levels in U87MG, U373 and LN18 cell lines by immunoblot (Figure 3c). In all cases, miR-124 expression led to decreased levels of each of the three mRNA and protein targets, respectively. We then transduced lentiviruses expressing either a Non-Targeting or a pre-miR-124 vector into human glioblastoma “stem like cells” grown as tumor spheres and again showed that miR-124 overexpression leads to decreased levels of target mRNA (Figure 3d). To investigate whether MAPK14, TEAD1 and SERP1 are direct miR-124 targets, we employed a 3’ UTR luciferase assay comparing luciferase levels in cells transfected with plasmids expressing wild type 3’ UTRs or those harboring mutations in the miR-124 seed binding sites. We determined that all three targets are directly regulated by miR-124, as luciferase levels decreased upon miR-124 expression in cells expressing the wild type 3’UTR regions, but not in cells expressing 3’UTR regions with mutated miR-124 seed sequences (Figures 3e-g). (Note: only one SERP1 seed sequence binding [S2] site appears to be a bona fide miR-124 binding site [Figure 3g]).
Figure 3. TEAD1, MAPK14/p38α, and SERP1 are miR-124 direct targets.
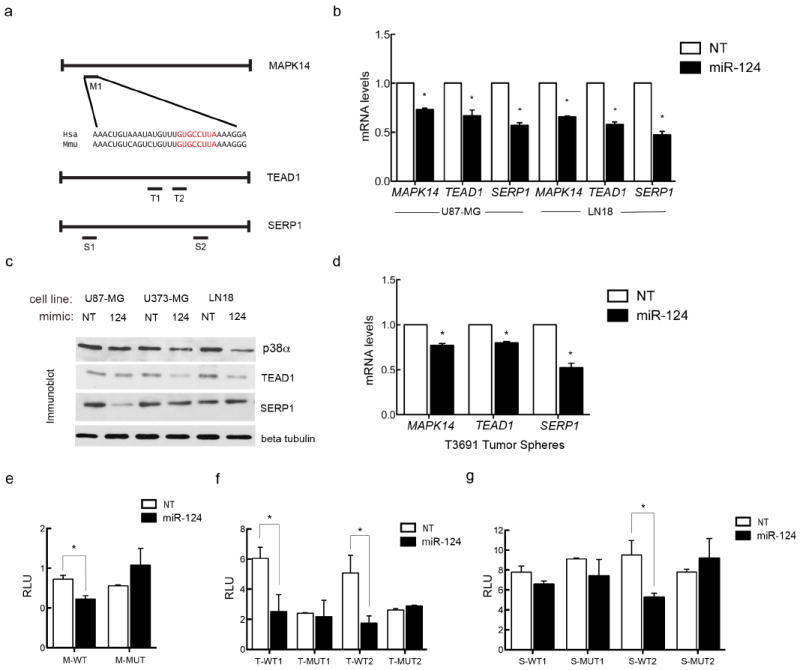
(a) Schematic of miR-124 seed sequence binding sites in the 3’ untranslated regions of the three putative targets. (b) MAPK14/p38, TEAD1 and SERP1 mRNA levels in U87-MG and LN18 cells treated either with a Non-Targeting (NT) or a miR-124 mimic after 12 hours of transfection. (c) p38, TEAD1 and SERP1 protein levels in U87-MG, U373-MG, and LN18 cells treated either with a Non-Targeting (NT) or miR-124 mimic after 72 hours of transfection. (d) mRNA levels of glioblastoma stem like cells grown as tumor spheres transduced with either an empty control or pre-miR-124 expressing virus. (e-g) T98G cells transfected with pMIR-REPORT with intact or mutated seed sequences were tested for luciferase activity in the presence of stable miR-124 expression. Luciferase levels for (e) MAPK14, (f) TEAD1 and (g) SERP1 3’UTR constructs.
TEAD1, MAPK14 and SERP1 promote glioblastoma progression
To assess whether MAPK14, TEAD1, and SERP1 play a role in glioblastoma tumorigenesis, we obtained RNA from formalin-fixed, paraffin embedded (FFPE) glioblastoma patient samples, procured from the University of Pennsylvania Department of Pathology. A comparison of average miR-124 levels between glioblastoma RNA versus normal brain tissue RNA showed that miR-124 levels are greatly diminished in GBM, relative to normal brain, as previously shown (10) (Figure 4a). Of note, MAPK14, TEAD1 and SERP1 mRNA levels exhibited the opposite result: mRNA levels of these targets were higher in GBM samples compared to normal brain tissue (Figures 4b-d, S3a-d). These data suggest an important role for all three genes in glioblastoma. In order to verify whether they promote glioblastoma cell survival, we employed short hairpin RNAs (shRNAs) to inhibit TEAD1, MAPK14, and SERP1 individually (Figure 4e; Figure 4f shows resulting mRNA levels) and as part of a combined triple depletion (Figure 4g; Figure 4h depicts corresponding protein levels). Decreased expression of each miR-124 target partially enhanced cell death under nutrient or O2 deprivation (Figure 4e); in contrast, combined loss of TEAD1, MAPK14 and SERP1 fully recapitulated the increased apoptosis (Figure 4g) observed in miR-124 expressing cells (see Figure 2).
Figure 4. TEAD1, MAPK14 and SERP1 promote glioblastoma progression.
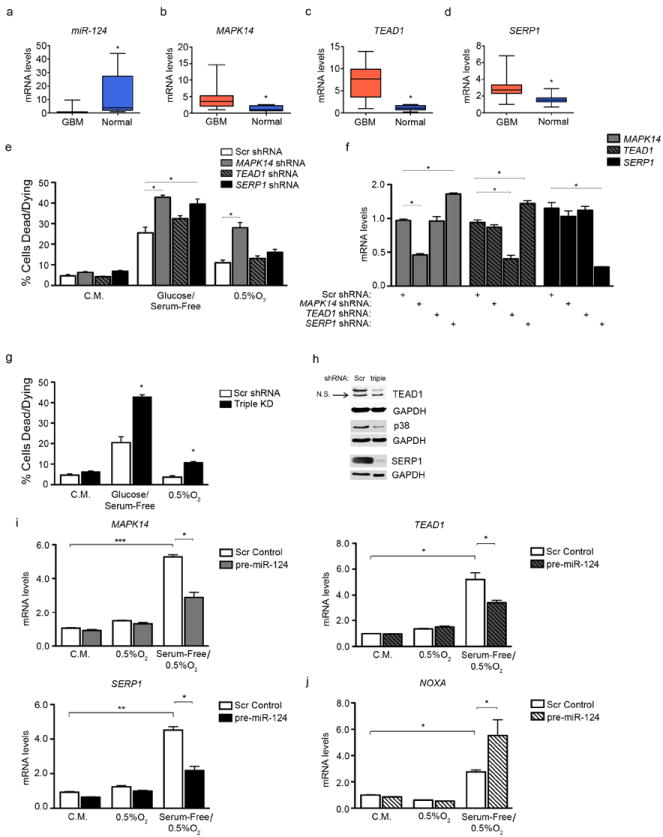
(a) miR-124, (b) MAPK14, (c) TEAD1, and (d) SERP1 levels in GBM (n=30) and normal brain samples (n=10). * p value < 0.05. (e) shRNA-mediated inhibition of individual targets partially recapitulates the cell death phenotype observed in Figure 2a. * p value < 0.05. (f) Transcript levels of all three targets, upon shRNA-mediated inhibition of any single target. (g) Cell death levels after shRNA-mediated depletion of all three targets combined (Triple KD). (h) Immunoblot showing target protein levels upon triple ablation. Scr = Scrambled control shRNA, triple = shRNAs for all three targets. (i) mRNA levels of MAPK14 (top left), TEAD1 (top right), and SERP1 (bottom left) under the following conditions: Complete Media (C.M.), Hypoxia (0.5% O2), and Serum Free + Hypoxia (0.5% O2). U87-MG cells were transduced with scrambled control (Scr) or pre-miR-124-expressing lentivirus and subjected to these conditions for 24 hours. (j) NOXA levels from experiment in (4i) * p - value < 0.05; ** p - value < 0.001, *** p - value < 0.0001.
Finally, we treated U87-MG cells with scrambled or pre-miR-124 lentivirus and subjected them to low O2 or serum-free/low O2 conditions for 24 hours. Upon stress, particularly the serum-free/low O2 conditions, a marked increase in all three targets was observed (6-8 fold) (Figure 4i). miR-124 treatment counteracted this increase, bringing target levels closer to baseline. At the same time, ectopic miR-124 expression under serum-free/low O2 conditions significantly increased NOXA levels, a marker of apoptotic cell death (Figure 4j).
miR-124 expression affects glioblastoma cell proliferation and survival in vivo
In order to establish a role for miR-124 re-expression/target gene downregulation in vivo, we transduced U87-MG cells with control or pre-miR-124 – GFP, and assessed growth both in vitro (cell counts) and in vivo (subcutaneous xenografts). Additionally, we performed the same experiments comparing non-targeting shRNA vs. TEAD1, SERP1, or MAPK14 shRNAs. As previously established (10), miR-124 re-expression leads to decreased cell proliferation in vitro (Figure S4a) and subcutaneous tumor growth in vivo (Figure 5a). TEAD1 and MAPK14/p38α inhibition also resulted in decreased in vitro proliferation when U87-MG cells were grown under replete media (Figures S4b, c). SERP1 knockdown did not result in significant differences in in vitro proliferation under replete media, but resulted in decreased proliferation when cells were grown under 0.5% O2. (Figure S4d). TEAD1 and SERP1 inhibition led to decreased tumor growth in vivo (Figure 5b, c; p38α modulation has been previously shown to lead to decreased tumor growth in glioblastoma growth in vivo [37], thus not shown here).
Figure 5. miR-124 expression affects glioblastoma cell proliferation and survival in vivo.
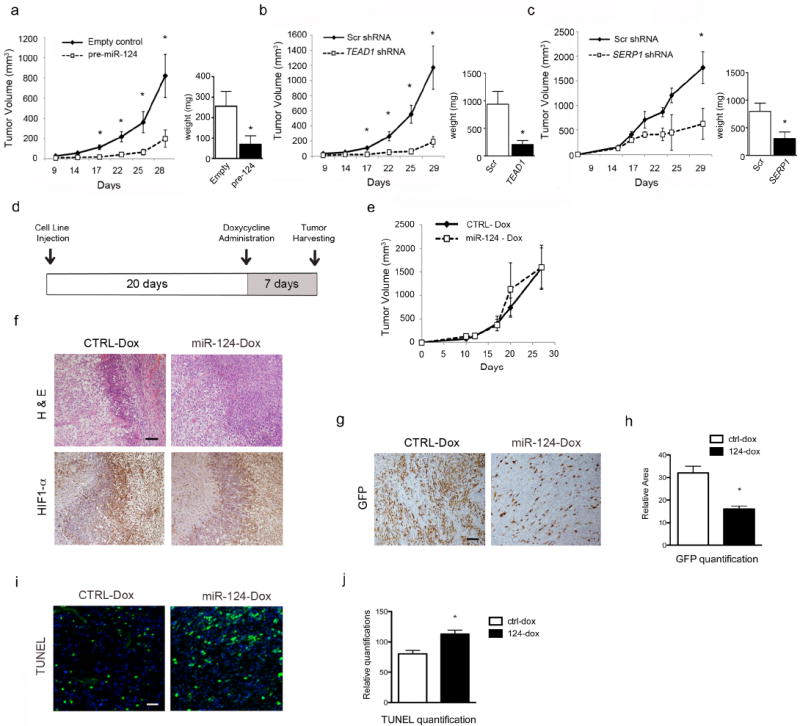
Growth curves and tumor weights of subcutaneous xenografts of U87-MG cells expressing miR-124 (a, n=9), TEAD1 shRNA (b, n=9), and SERP1 shRNA (c, n=6). (d) Schematic of doxycycline-inducible subcutaneous xenograft assay. (e) Growth curve of doxycycline inducible subcutaneous xenografts after expressing control or miR-124 for seven days. (f) H&E and HIF-1α IHC staining of doxycycline-inducible subcutaneous xenograft tumor sections. Scale bar is 200 μm. (g, h) GFP IHC staining and quantification, (i, j) TUNEL staining and quantification of doxycycline-inducible subcutaneous xenograft tumor sections. Scale bar is 100 μm. * p - value < 0.05.
Since increased miR-124 expression led to a significant difference in tumor size (Figure 5a), it was difficult to determine whether increased cell death occurred in areas experiencing limited nutrient and O2 availability, since smaller tumors exhibit fewer hypoxic/ischemic regions. In order to mitigate this scenario, we devised a doxycycline-inducible system where miR-124 expression is regulated by doxycycline administration. Briefly, we created stable U87-MG cell lines expressing either a doxycycline-inducible control or miR-124, along with GFP, to assess the robustness of the system. As shown in the schematic in Figure 5d, subcutaneous tumors were allowed to grow for 20 days in nude mice, so that they achieved a size sufficient for the natural generation of O2/nutrient gradients. At day 20, doxycycline was administered, thereby activating expression of miR-124-GFP, or the control-GFP construct. The doxycycline-inducible system was validated in vitro as described in Figure S5a-b. Surprisingly, no statistically significant difference in tumor size was observed after doxycycline treatment. (Figure 5e). Subcutaneous tumor architecture was similar in both control and miR-124 expressing tumors, and both showed regions of hypoxia as demonstrated by HIF-1α staining (Figure 5f). However, when we stained for GFP (the proxy for expression of either the control or the miR-124 construct), a significant loss of GFP-expressing cells was detected in the tumor tissues expressing the miR-124 construct (Figures 5g, h), even though both constructs expressed GFP in a doxycycline-dependent manner in vitro (Figure S5a). We then quantified cell death using the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay, and observed increased apoptosis in tissues expressing miR-124, compared to those expressing the control construct (Figure 5i, j). While there are many possible explanations for these observations, increased TUNEL staining in miR-124 expressing tumors, coupled with decreased numbers of GFP-expressing cells, suggest that miR-124 expressing cells may be selected against in the seven days of doxycycline treatment, due to their inability to survive O2 and nutrient deprivation.
SERP1 is an important pro-survival target in glioblastoma
Since SERP1 has been shown to be elevated during brain ischemia (35), we focused on dissecting the role for SERP1 in survival under stress. We decreased SERP1 levels via an shRNA-mediated lentivirus in U87-MG cells and subjected them to low O2 or serum-free/low O2 conditions for 24 hours. Just as we observed in Figure 4i, SERP1 transcripts are elevated under stress (Figure 6a). Importantly, we demonstrate that shRNA treatment recapitulates the miR-124 phenotype by eliciting increased NOXA levels (Figure 6b). We therefore determined if re-expression of the SERP1 Open Reading Frame (lacking the 3’UTR) reverses miR-124-mediated cell death. Increased miR-124 expression led to enhanced cell death under hypoxia, while SERP1 re-expression almost completely abrogated miR-124-mediated cell death under hypoxia (Figure 6c). This underscores a previously unappreciated role for SERP1 in glioblastoma. As observed with our FFPE patient samples in Figure 4d, TCGA patient data also suggest that SERP1 mRNA levels are elevated in glioblastoma samples, as compared to normal brain tissue (p - value < 0.0001) (Figure 6d). Additionally, a query into the REMBRANDT database (https://caintegrator.nci.nih.gov/rembrandt/) showed that patients with increased SERP1 mRNA exhibit lower overall survival, compared to patients with low or intermediate levels (p - value = 0.006) (Figure 6e). Taken together, these data suggest that SERP1 may be an attractive target for further evaluation in glioblastoma survival.
Figure 6. SERP1 is an important factor in glioblastoma.
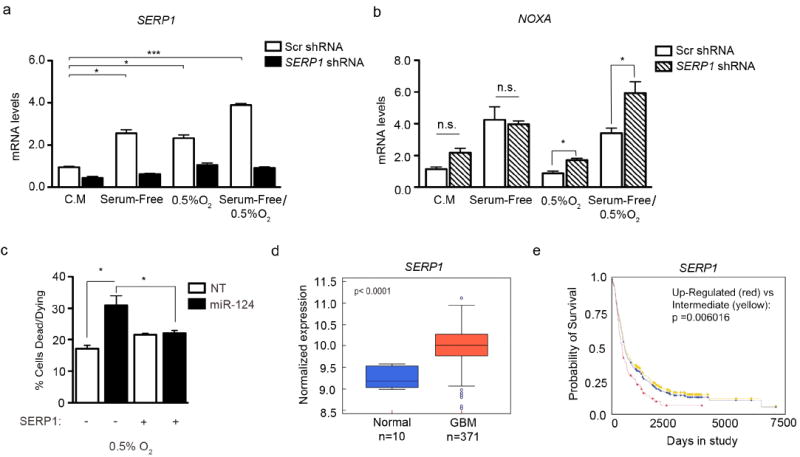
(a) SERP1 and (b) NOXA mRNA levels in U87-MG cells grown under the following conditions: Complete Media (C.M.), Serum-Free, Hypoxia (0.5% O2), and Serum Free + Hypoxia (0.5% O2). Cells were transduced with scrambled (Scr) or SERP1 shRNA and subjected to these conditions for 24 hours. * p - value < 0.05; ** p - value < 0.001, (c) SERP1 Open Reading Frame re-expression leads to rescue of cell death in U87-MG expressing miR-124 under hypoxia. (d) SERP1 levels in normal brain and GBM tissues from the TCGA dataset (p - value < 0.0001). (e) Kaplan – Meier survival curve of patients from the REMBRANDT database, stratified by SERP1 expression levels (p - value = 0.006).
miR-124 expression increases overall survival in an orthotopic intracranial mouse model
While several studies have investigated the role for miR-124 in glioblastoma (10, 38), the impact of miR-124 expression on glioblastoma cell survival in vivo is unclear. To test whether miR-124 expression led to a change in tumor cell survival, we performed an intracranial orthotopic experiment in Nu/Nu mice expressing either control or pre-miR-124 construct, as well as GFP. miR-124 levels were increased only ~50 fold (data not shown), similar to the difference in miR-124 levels between GBM and adjacent non-neoplastic tissue measured in patients (10, 39). GFP levels were similar between control and miR-124 constructs (Figure S5c). Mice were sacrificed upon showing neurological symptoms associated with tumor burden. We observed that miR-124 re-expression conferred mice with a higher survival rate (p - value < 0.0001) (Figure 7a, 7b shows brain sections upon sacrifice), suggesting the importance of silencing miR-124 during GBM tumor progression.
Figure 7. miR-124 expression increases overall survival in an orthotopic intracranial mouse model.
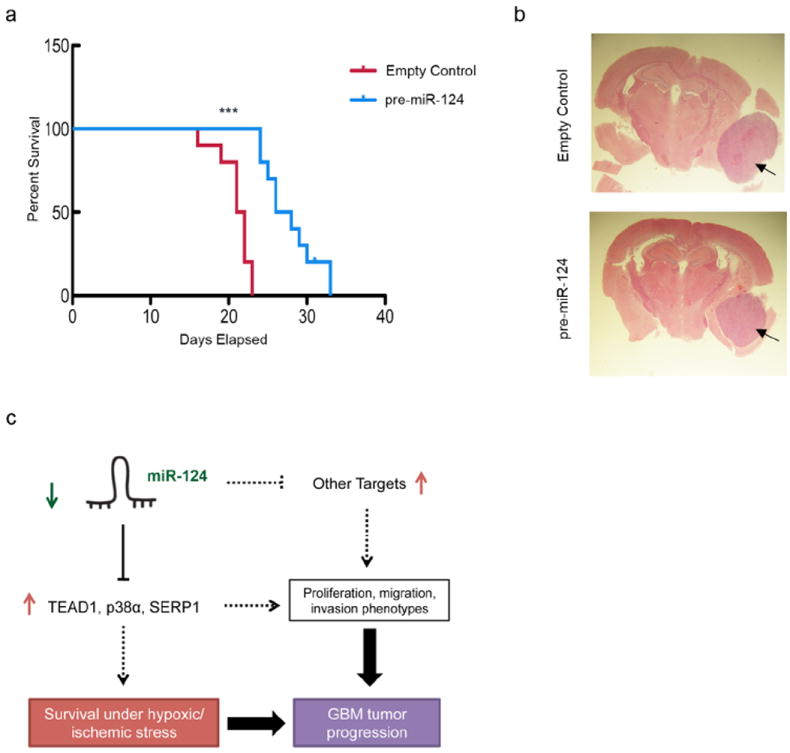
(a) U87-MG cells transduced with either an empty control or pre-miR-124 expressing virus were injected into nude mice, subsequently surveyed for survival. Kaplan-Meier curve, *** p - value < 0.0001. (b) Representative H&E images of xenografts in the brain showing tumor lesions (arrows) at the time of sacrifice. (c) Model for miR-124 role in glioblastoma. miR-124 loss contributes to glioblastoma tumorigenesis by promoting the elevated expression of cell proliferation factors, as well as pro-survival factors, especially in regions experiencing nutrient and oxygen deprivation.
Discussion
Glioblastomas are difficult to treat, primarily due to their resistance to standard of care therapy and eventual tumor recurrence. Both resistance to therapy and recurrence are closely associated with the frequent occurrence of hypoxic/ischemic regions in grade IV gliomas. The ability of glioblastoma cells to survive under nutrient and O2 deprivation ensures the opportunity for glioblastoma recurrence. Many factors are over - and underexpressed to promote cellular survival, and a majority have yet to be elucidated. Several micro-RNAs have been demonstrated to promote survival under stress. Here, we show that miR-124 loss in glioblastoma allows for survival during nutrient and O2 deprivation, as miR-124 re-expression in glioblastoma cells increased cell death under these conditions.
miRNAs function by targeting and modestly inhibiting a vast array of mRNAs and proteins. Given that miRNAs can inhibit up to 30% of the mammalian genome (13), differentially expressed miRNAs are a powerful discovery tool in cellular processes impacting development, physiology and disease. While a single miRNA inhibits any given mRNA or protein only ~10-20%, global pathway changes can be significant. Additionally, modulating miRNA levels often reveals new targets or pathways that have been under-investigated or not well understood. Previous studies have identified an anti-proliferative role for miR-124 in glioblastoma (10), but no consistent evidence has linked miR-124 to cell death under limiting O2 and nutrient conditions in the context of glioblastoma. During the preparation of this manuscript, a recently published study showed that miR-124 levels are downregulated in pulmonary artery smooth muscle cells (PASMC) subjected to hypoxia, implying that miR-124 expression could be utilized in the future to target effectors of pulmonary arterial hypertension (40). In this paper, we show that miR-124 levels (while decreased in glioblastoma compared to normal brain tissue) are further diminished in pseudopalisading necrotic/hypoxic regions as compared to better-perfused tissue. Based on TCGA data, the more patient samples express a hypoxic signature, the lower miR-124 levels become. While the observation that miR-124 levels anti-correlate with hypoxic regions and hypoxic signatures in patients (Figure 1) does not necessarily imply that miR-124 has been selectively inhibited to confer survival, endogenous miR-124 expression has a significant effect in inhibiting cell survival in hypoxic/ischemic glioblastoma tissue. We demonstrate for the first time how miR-124 controls cell death through inhibition of various pro-survival targets elevated under low nutrients, growth factors and O2, making miR-124 an attractive target for further investigation in glioblastoma. Our current model suggests that, due to lack of miR-124 expression, tumor cells acquire the ability to uncontrollably proliferate, as these cells express high levels of pro-proliferation factors including CDK-6 (10), and TEAD1 (Figure 3). During growth and expansion, glioblastoma tissues invariably experience gradients of nutrient and O2 availability, and in many cases, regions where nutrients and O2 are significantly depleted. Our study suggests that lack of miR-124 allows cells in these regions to upregulate pro-survival factors (such as SERP1, a novel miR-124 target). Taken together, lack of miR-124 expression allows glioblastoma cells to proliferate, but also survive under stress conditions (Figure 7c, model). While at the present time miRNA-based therapeutics await further development, investigating miRNA targets should lead to insights into the biological processes of glioblastoma, as well as potential new therapeutic modalities.
One interesting novel miR-124 target described here is SERP1. No previous connection has been made between SERP1 and glioblastoma progression, and very little is known about the biological roles for SERP1 in the endoplasmic reticulum. So far, SERP1 appears to have a role in modulating responses to ER stress (36) and protecting proteins from degradation while promoting consequent glycosylation (35). Additionally, it was shown that SERP1 levels are elevated in astrocytes during rat brain ischemia (35). Since miR-124 re-expression led to increased cell death under tumor ischemic and ER stress conditions, we hypothesized that miR-124 counteracts pro-survival stress responses in glioblastoma, in part by targeting SERP1. Here, we identify SERP1 as an important factor in glioblastoma patient survival. Additionally, we show that SERP1 overexpression rescues the cell death phenotype conferred by miR-124, suggesting that SERP1 may provide a pro-survival (hence, pro-tumorigenic) role in vivo.
Liu et al. have recently shown that miR-124 levels decrease in the sub-ventricular zone when mice are subjected to cerebral ischemia, in a model of stroke (41). The authors suggest that decreased miR-124 allows for increased proliferation in the ischemic region during stroke-induced neurogenesis. We demonstrate that, in addition to allowing for cellular proliferation, the decrease in miR-124 may also be permissive for cellular survival during stroke, potentially by allowing for SERP1 upregulation under low nutrients and O2, as described by Yamaguchi and colleagues (35). Thus, more research regarding the connection between miR-124, SERP1, and hypoxia/ischemia is warranted, in the settings of stroke and glioblastoma tumorigenesis.
In summary, we have shown glioblastoma cells undergo apoptosis under hypoxic/ischemic conditions, upon miR-124 reintroduction. This increase in cell death may be partially mediated by direct miR-124 inhibition of TEAD1, MAPK14/p38α and SERP1, in addition to numerous other factors. Further research on miR-124 targets involved in survival under nutrient and O2 deprivation may lead to novel glioblastoma therapeutics.
Materials and Methods
Cell Culture conditions, reagents, and lentiviral transduction
U87MG, U373, LN18 and HEK 293T cells were obtained from ATCC (Manassas, VA), and cultured in DMEM containing 10% FBS, glutamine, non-essential amino-acids, Penicillin/Streptomyicin antibiotics and HEPES buffer, and passaged on average every three days. Cells cultured in the nutrient deprived media were grown in glucose-free DMEM containing glutamine, non-essential amino-acids, antibiotics and HEPES buffer, without the addition of FBS. Cells were grown under hypoxic incubation in a Ruskinn invivO2 400 workstation. Patient-derived glioblastoma tumor spheres were a gift from Dr. Jeremy Rich (Cleveland Clinic, Cleveland, OH). These cells were maintained in Neurobasal medium, supplemented 1:50 with B27 (Invitrogen, Carlsbad, CA), Epidermal Growth Factor (Sigma) at 20 ng/mL, and basic Fibroblast Growth Factor at 20 ng/mL. Non-targeting and miR-124 mimics were obtained from Dharmacon (Lafayette, CO). microRNA mimic transfection was performed using HiPerFect transfection reagent (Qiagen). 100 mM miRNA mimics were used for all experiments. Tunicamycin (Sigma) was diluted in DMSO was administered at 1 μg/mL for 24 hours. All viruses were packaged using the third generation lentivector system (Invitrogen, USA) and expressed in HEK 293T cells. Supernatant containing virus was collected at 24 hr and 48 hr timepoints and concentrated using 10-kDa Amicon Ultra-15 centrifugal filter units (Millipore). Overexpression of SERP1 Open Reading Frame was achieved by cloning into the pCDH-CMV-MCS-EF1-Puro vector (Systems Biosciences) via EcoRI and NotI restriction sites.
Immunoblots
Lysates were collected using a whole cell elution buffer previously described. 20 or 40 μg protein was loaded in 12% or 15% SDS-PAGE gels, transferred to nitrocellulose and blotted with various antibodies for TEAD1 (Novus), p38α (Cell Signaling), SERP1 (Genetex), β-tubulin, PARP, cleaved PARP, (Cell Signaling). Primary antibodies were diluted 1:1000 in 5% non-fat milk TBST.
Cell death assays
Cell death was assessed using Annexin-PI staining on Flow Cytometry, using the FITC Annexin V Apoptosis Detection Kit I from BD Pharmingen (San Jose, CA). For nutrient deprivation/hypoxia experiments, cells were kept under stress conditions for 48 hours before cell death was assayed. For tunicamycin experiments, cells were kept under stress conditions for 24 hours.
In vivo xenograft assays
All experiments were performed in accordance with NIH guidelines and approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC). Sub-cutaneous xenografts were performed on Nu/Nu mice (Charles River) as previously described. 1,000,000 U87-MG cells expressing the empty pCDH-EF1-copGFP lentivector or the pCDH-EF1-copGFP-pre-miR-124 lentivector were injected on flanks of nude mice and the tumors were subsequently measured by caliper over the period of a month. Additionally, sub-cutaneous xenografts were performed with U87-MG cells expressing: Scr shRNA, TEAD1 shRNA, SERP1 shRNA, doxycycline-inducible miR-124 and doxycycline inducible control. Tumor volume was measured using the calculation: (X•Y2)π/6, where X is the longest caliper measurement, Y is the shortest, and π=3.14. For the doxycycline-inducible experiments, mice were fed 2 mg/mL doxycycline and 5% glucose in their drinking water for 7 days.
Orthotopic xenograft injections were performed by stereotactically injecting 500,000 U87-MG glioblastoma cells in 5 uL PBS, 5mm in the right cerebral hemisphere in 8-week-old Nu/Nu mice. As in the sub-cutaneous xenograft experiment, the U87-MG cells were expressing either the empty pCDH-EF1-copGFP, or the pCDH-EF1-copGFP-pre-miR-124 lentivector. Prior to injection, the mouse was prepared as previously described. The animals were sacrificed at the first sign of neurological symptoms.
Immunohistochemistry (IHC) and Immunofluorescence (IF) assays
IHC and IF were performed on 5 μm sections according to standard protocols. Antibody concentrations are the following: HIF1α: 1:100 (Abcam), GFP: 1:100 (Cell Signaling). Images were taken using a Leica 500 microscope (Leica) at 10X magnification. Paraffin embedded sections were stained for TUNEL using an ApopTag Plus Fluorescein In situ Apoptosis Detection Kit (Chemicon, CA, USA) according to the manufacturer’s instructions. Images were taken at 20X magnification using an Olympus IX81 microscope.
Laser Capture Microdissection
FFPE slides were lightly stained with Hematoxylin and Eosin and immediately subjected to laser capture under RNase-Free conditions. For pseudopalisading regions, the internal necrotic core was initially cut and discarded, before the pseudopalisading region was collected for testing. Microdissection was performed using a Leica microscope and the Leica LMD software. Upon collection, RNA was extracted using the Qiagen miRNeasy FFPE kit.
Patient samples
LCM tissue: At the Hospital of the University of Pennsylvania, we searched the departmental surgical pathology database for cases with the diagnosis of “Glioblastoma, WHO grade IV” over a two year period (2011-2013). Only primary glioblastomas that had not received prior radiation or chemotherapy were included. Subsequently, the cases were screened by a neuropathologist (P.P.G) for adequate areas of both pseudopalisading necrosis and solid tumor, and whether it was possible to obtain ten 10 um unstained tissue sections from the archived paraffin embedded formalin fixed tissue blocks.
mRNA extraction from whole patient sample: the formalin-fixed, paraffin-embedded (FFPE) patient samples were obtained from the University of Pennsylvania Department of Pathology and Laboratory Medicine (Philadelphia, PA). GBM blocks were screened by a neuropathologist (S.V) contained > 95% tumor cells. Controls (temporal lobectomy tissue obtained from intractable epilepsy patients) showed histopathologic evidence of mild to focally moderate gliosis, but no lesions. GBM patients age range: 24 to 89 years, (Median: 63 years). Control patients age range: 22 to 61 years, (Median: 38 years). RNA was obtained from FFPE samples using the RecoverAll™ Total Nucleic Acid Isolation kit (Ambion, CA, USA).
Bioinformatic analyses
mRNA expression data were downloaded from the TCGA Data Portal (https://tcga-data.nci.nih.gov/tcga/). Data generated on the Affymetrix microarray platform HT_HG-U133A for 385 tumor and 10 normal samples was subjected to GCRMA normalization (GCRMA background correction, quantile normalization, log2 transformation and Median polish probeset summarization) was used to determine mRNA expression. To obtain expression, previously normalized TCGA Level2 data from 426 tumor samples and 10 normal samples run on Agilent’s miRNA microarray was utilized. Data were analyzed using the Partek software (Partek Inc., St. Louis, MO, USA). To determine the association between miR-124 and expression of the HIF signature, we utilized the Gene Set Analysis (GSA) implementation (version 1.03) of Gene Set Enrichment Analysis (GSEA). We defined the HIF signature by using previously - established HIF regulated genes (15). To create a single value that represents the expression of the HIF signature (HIF metagene), the average expression of all the genes in the HIF signature was calculated for each tumor sample (using TCGA GBM data). This HIF metagene was then used as a continuous variable for various comparisons between groups. Analysis was performed using the R language and environment for statistical computing.
Statistics
All statistical analysis was performed using the GraphPad Prism™software (CA, USA). All data are represented as mean ± S.E.M. To establish whether a difference between two values is statistically significant, we performed Student’s t-test, where p < 0.05 defines statistical significance.
Supplementary Material
Acknowledgments
We thank Dr. Hongwei Yu for assistance with histological preparations, Dr. Jeremy Rich for providing the patient-derived tumor sphere cells, Dr. Stephen Prouty for assistance with Laser-Capture Microdissection, and the Simon laboratory for helpful discussions. This work was funded by the Howard Hughes Medical Institute and the NIH (T32 GM-07229; F31CA174211). M.C.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement
This manuscript presents original research not previously published and not considered for publication at any other journals. All the authors declare no conflict of interest.
References
- 1.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain : a journal of neurology. 2007;130(Pt 10):2596–606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Current cancer drug targets. 2009;9(3):381–90. doi: 10.2174/156800909788166637. [DOI] [PubMed] [Google Scholar]
- 5.Evans SM, Jenkins KW, Chen HI, Jenkins WT, Judy KD, Hwang W-T, et al. The Relationship among Hypoxia, Proliferation, and Outcome in Patients with De Novo Glioblastoma: A Pilot Study. Translational oncology. 2010;3(3):160–9. doi: 10.1593/tlo.09265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(9):2623–30. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. Epub 0906. (7216 SRC - GoogleScholar) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Sun L, Wang H, Yao J, Jiang C, Xu W, et al. MiR-328 expression is decreased in high-grade gliomas and is associated with worse survival in primary glioblastoma. PloS one. 2012;7(10):e47270. doi: 10.1371/journal.pone.0047270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC medicine. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nature reviews Cancer. 2006;6(4):259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Hamo R, Efroni S. Gene expression and network-based analysis reveals a novel role for hsa-miR-9 and drug control over the p38 network in glioblastoma multiforme progression. Genome medicine. 2011;3(11):77. doi: 10.1186/gm293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483(7390):484–8. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular cell. 2007;27(3):435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476(7359):228–31. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao F-B. Context-dependent functions of specific microRNAs in neuronal development. Neural development. 2010;5:25. doi: 10.1186/1749-8104-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visvanathan J, Lee S, Lee B, Lee JW, Lee S-K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes & development. 2007;21(7):744–9. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 21.Sonntag KC, Woo T-UW, Krichevsky AM. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Experimental neurology. 2012;235(2):427–35. doi: 10.1016/j.expneurol.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RHA, Mourelatos Z. RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain. RNA (New York, NY) 2006;12(2):187–91. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt S, Jones AV, Hinsley EE, Whawell SA, Lambert DW. MicroRNA-124 suppresses oral squamous cell carcinoma motility by targeting ITGB1. FEBS letters. 2011;585(1):187–92. doi: 10.1016/j.febslet.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, et al. An HNF4α-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147(6):1233–47. doi: 10.1016/j.cell.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong Y, Durden DL, Van Meir EG, Brat DJ. ’Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006 Jun;65(6):529–39. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Molecular cell. 2009;35(6):856–67. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathew LK, Skuli N, Mucaj V, Lee SS, Zinn PO, Sathyan P, et al. miR-218 opposes a critical RTK-HIF pathway in mesenchymal glioblastoma. Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):291–6. doi: 10.1073/pnas.1314341111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Lei Q-Y, Guan K-L. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Current opinion in cell biology. 2008;20(6):638–46. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malt A, Cagliero J, Legent K, Silber J, Zider A, Flagiello D, et al. Landin Alteration of TEAD1 expression levels confers apoptotic resistance through the transcriptional up-regulation of Livin. PLo. 2012;7:e45498. doi: 10.1371/journal.pone.0045498. Epub 1003. (9 SRC - GoogleScholar) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature reviews Cancer. 2009;9(8):537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 32.Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB, et al. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Molecular cancer therapeutics. 2007;6(4):1212–22. doi: 10.1158/1535-7163.MCT-06-0711. [DOI] [PubMed] [Google Scholar]
- 33.Lawson SK, Dobrikova EY, Shveygert M, Gromeier M. p38alpha mitogen-activated protein kinase depletion and repression of signal transduction to translation machinery by miR-124 and -128 in neurons. Mol Cell Biol. 2013 Jan;33(1):127–35. doi: 10.1128/MCB.00695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paillas S, Causse A, Marzi L, de Medina P, Poirot M, Denis V, et al. MAPK14/p38α confers irinotecan resistance to TP53-defective cells by inducing survival autophagy. Autophagy. 2012;8(7):1098–112. doi: 10.4161/auto.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi A, Hori O, Stern DM, Hartmann E, Ogawa S, Tohyama M. Stress-associated endoplasmic reticulum protein 1 (SERP1)/Ribosome-associated membrane protein 4 (RAMP4) stabilizes membrane proteins during stress and facilitates subsequent glycosylation. The Journal of cell biology. 1999;147(6):1195–204. doi: 10.1083/jcb.147.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hori O, Miyazaki M, Tamatani T, Ozawa K, Takano K, Okabe M, et al. Deletion of SERP1/RAMP4, a component of the endoplasmic reticulum (ER) translocation sites, leads to ER stress. Molecular and cellular biology. 2006;26(11):4257–67. doi: 10.1128/MCB.02055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cloninger C, Bernath A, Bashir T, Holmes B, Artinian N, Ruegg T, et al. Inhibition of SAPK2/p38 enhances sensitivity to mTORC1 inhibition by blocking IRES-mediated translation initiation in glioblastoma. Molecular cancer therapeutics. 2011;10(12):2244–56. doi: 10.1158/1535-7163.MCT-11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Chen P, Li X-Y, Zhang L-Y, Xiong W, Zhou M, et al. Grade-specific expression profiles of miRNAs/mRNAs and docking study in human grade I-III astrocytomas. Omics : a journal of integrative biology. 2011;15(10):673–82. doi: 10.1089/omi.2011.0064. [DOI] [PubMed] [Google Scholar]
- 39.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, et al. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer research. 2008;68(22):9125–30. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 40.Kang K, Peng X, Zhang X, Wang Y, Zhang L, Gao L, et al. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J Biol Chem. 2013 Aug 30;288(35):25414–27. doi: 10.1074/jbc.M113.460287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, et al. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PloS one. 2011;6(8):e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


