Abstract
The rd1 mouse is a model of retinitis pigmentosa, an inherited photoreceptor neurodegenerative disease. In rd1 retina, early onset rod degeneration is caused by a Pde6b mutation that leads to high levels of intracellular cyclic guanosine monophosphate (cGMP). Cyclic nucleotide gated ion channels (CNGCs), necessary for phototransduction, are regulated by cGMP. We previously demonstrated that inhibition of dopamine signaling blocks rd1 photoreceptor degeneration in retinal organ cultures. The mechanism underlying this protection remains unknown. The aim of this study was to determine whether inhibition of dopamine signaling alters cGMP accumulation or CNGC expression. Dopamine depletion from rd1 retinal organ cultures resulted in a significant decrease in cGMP compared to untreated rd1 organ cultures. However, cGMP levels in both treated and untreated rd1 organ cultures significantly exceeded cGMP levels in wild type (wt) retinal organ cultures. The CNGC expression profile was first determined in vivo. Both channel subunits, Cnga1 and Cngb1, are expressed at low levels by postnatal day 2 (P2), increasing sharply by P6 with modest increases after P12 in wt retina. A similar pattern is seen in rd1 retina until P12 when expression levels decrease, accompanying cell death. No significant difference was observed in the expression of either Cnga1 or Cngb1 in organ cultures from wt, rd1, or dopamine-depleted rd1 retinas. Our results demonstrate that dopamine depletion significantly decreases cGMP levels in rd1 retinal organ cultures, but that cGMP accumulation remains high, requiring additional mechanisms for photoreceptor protection. These mechanisms may include activation of PKG signaling pathways and/or crosstalk with dopamine signaling through cAMP pathways.
Keywords: retina, dopamine, retinal degeneration, rd1, cGMP, cyclic nucleotide gated channel, photoreceptor development
Introduction
The rd1 mouse retina was the first identified model of the human retinal degenerative disease retinitis pigmentosa [1]. It is characterized by a mutation in the Pde6b gene [2,3], which codes for an enzyme that plays a central role in phototransduction in adult rod photoreceptors. Specifically, in wild type (wt) photoreceptors, light-stimulated activation of Pde6 via rhodopsin results in breakdown of the second messenger cGMP, which in turn causes closure of cyclic nucleotide gated ion channels (CNGCs) [4]. In the rd1 mouse, the defect in Pde6 leads to an accumulation of cGMP. The rd1 retina contains approximately twice the normal level of cGMP by postnatal day 6 (P6), and differentiating rod photoreceptors begin to undergo cell death around P10 with complete degeneration by P21 [5,6]. High levels of Ca2+ influx through CNGCs are thought to initiate cell death signaling pathways [5], although recent reports are consistent with an additional role for cGMP-induced activation of protein kinase G (PKG) in retinal degeneration [7–9].
Dopamine is an important neuromodulator in the vertebrate retina that is released by amacrine and interplexiform cells and can diffuse in a paracrine fashion to act on every cell type in the retina. It signals through two families of G-protein coupled receptors, D1 and D2 receptors. Dopamine plays a variety of roles in retinal and cellular processes, including modulation of trophic effects on retinal development, regulation of circadian rhythms, mediation of light adaptation, and regulation of cAMP [10].
We have previously shown that inhibition of dopamine signaling through the addition of either D1 or D2 dopamine family receptor antagonists completely blocks rd1 photoreceptor degeneration in retinal organ culture for four weeks, whereas photoreceptors are reduced to a monolayer of cells in sham treated rd1 organ cultures, similar to in vivo animals at this age [11]. Protection is also achieved by dopamine depletion with the toxin 6-hydroxydopamine (6-OHDA), demonstrating a specific action through dopaminergic pathways. The mechanism underlying this protection is unknown [12]. Here we investigate whether dopamine depletion protects photoreceptors in rd1 retinal organ cultures by suppressing cGMP accumulation or by altering CNGC expression.
Methods
Animals
Retinas of rd1 and wt mice on a C57BL/6 background were used for all experiments. Animals were handled in accordance with the National Institutes of Health Guidelines on Laboratory Animal Welfare adhering to protocols approved by the Saint Louis University Institutional Animal Care and Use Committee.
Organ cultures
Retinas from wt and rd1 mice were grown in organ culture as previously described [11,13]. Eyes were enucleated from mice at P2 and incubated in Dulbecco’s Modified Eagle’s Media (DMEM, Sigma, St. Louis, MO) plus 1.25 µg/mL Fungizone with 0.5% Proteinase K (Invitrogen, Carlsbad, CA) at 37°C for 7 minutes followed by a brief wash in 10% fetal calf serum (FCS) to halt the enzymatic reaction. Retinas were removed and incubated in DMEM plus 1.25 µg/mL Fungizone and 10% FCS to detach retinal pigment epithelium. Retinas were positioned photoreceptor side down on Millipore Millicell-CM cell culture inserts (Millipore, Bedford, MA) with DMEM/ Fungizone/FCS and incubated at 37°C with 5% CO2. For dopamine-depleted rd1 retinal organ cultures, 100 µM each 6-OHDA (Sigma, St. Louis, MO) and pargyline (Sigma, St. Louis, MO) were added on the first two days in vitro (DIV), followed by 50 µM each on DIV 6 and 7 (P8 and 9). Media was changed in untreated wt and rd1 organ cultures on the same days that drugs were added to experimental cultures and every two-three days for all cultures during the remainder of the culture period.
Retinal organ cultures for cGMP analysis were dark-adapted overnight. They were harvested at P14 in total dark under infrared illumination and immediately frozen on dry ice. Prior to light exposure, hydrochloric acid was added to lyse cells and stop endogenous Pde activity. Organ cultures were harvested at P10 and immediately frozen on dry ice for quantitative (q)PCR analysis. For all assays, tissue samples were sonicated and stored at −20°C.
cGMP enzyme immunoassays
cGMP concentration was determined using the Direct Cyclic GMP Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI) following the manufacturer’s instructions. Sample concentrations were diluted 1:40 for rd1 retinal organ cultures with or without 6-OHDA-treatment and were undiluted for wt cultures. The acetylated format was used in all experiments for additional sensitivity of cGMP levels. Microplates were read with a scanning spectrophotometer (optical density 405 nm). Total protein content of each sample was determined using BCA assays (Pierce, Rockford, IL). Data were log-transformed to meet the assumptions of normality required for t-tests. Welch’s 1-tailed and 2-tailed t-tests were used to determine significance at p<0.05.
Real-time polymerase chain reaction
For in vivo analysis, retinas were harvested at ages between P2 and P21 and immediately frozen on dry ice. Tissue samples were sonicated and stored at −20°C. Total RNA was extracted with the Perfectpure RNA Tissue Kit (5 Prime, Gaithersburg, MD) according to manufacturer’s instructions. One microgram of total RNA was reverse transcribed into cDNA using the Quantitect Reverse Transcription Kit (Qiagen, Valencia, California, USA). qPCR was carried out with SYBR Green dye and specific primer pairs to determine the amplification curve of Cnga1, Cngb1, Hprt, and Actb on 96- well plates in a Chromo4 thermo-cycler. The following primers were used: Cnga1 forward: AATACGTGGCATTCCTTCGTAAA, reverse: GAGCCATTGTCATCGTCAGAAA; Cngb1 forward: CAGAGGAGGAACACTACTGCG, reverse: AAGTAATCCATGAGGAGCCAGA; Hprt forward: TCAGTCAACGGGGGACATAAA, reverse: GGGGCTGTACTGCTTAACCAG; Actb forward: GAAATCGTGCGTGACATCAAAG, reverse: AAGTAATCCATGAGGAGCCAGA. Three independent samples were assayed for each time point and genotype. Three replicates were performed for each sample. The ratio of Cnga1 and Cngb1 expression level between rd1 and wt mice was calculated by the 2-ΔΔCt method after normalization to Hprt or Actb.
Western blot
Retinas from wt and rd1 mice were harvested at ages between P2 and P21 and homogenized in cold RadioImmune Precipitation Assay buffer. Protein was normalized prior to separation by SDS-PAGE and transfer to nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in TBST for 2 hrs at room temperature, incubated with primary antibody to CNGβ1 (Gift from Robert Molday, UBC mouse monoclonal, dilution 1:15) or β-actin (Abcam, Ab 8226, mouse monoclonal, dilution 1:500) overnight at 4°C and incubated with HRP-conjugated anti-mouse IgG secondary antibody (Sigma-Aldrich, A9044, rabbit polyclonal, dilution 1:5000) for 1 hr at room temperature. After incubation with ECL solution (Sigma-Aldrich CPS160), the membrane was visualized with a GE LAS4000 imager.
Results and Discussion
Dopamine depletion reduces cGMP accumulation in rd1 retinal organ cultures
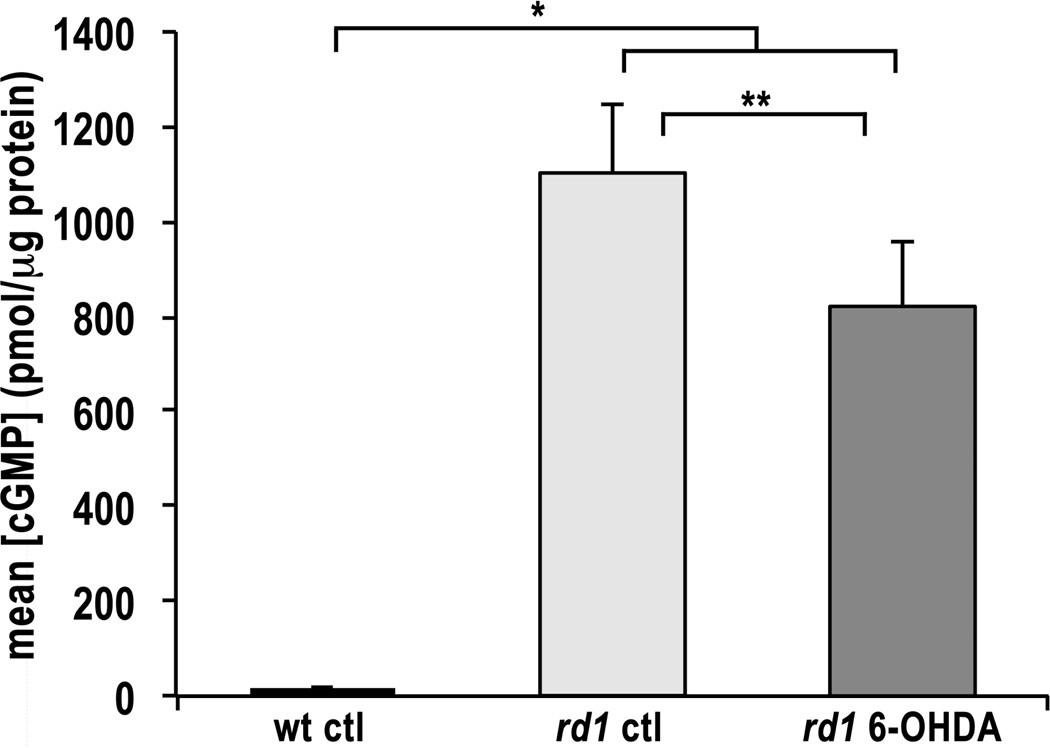
Since the concentration of cGMP in rd1 retinal organ cultures peaks at P14 (data not shown), this age was selected for analysis. The mean cGMP concentration in rd1 retinal organ cultures was approximately 55-fold higher than that in wt retinal organ cultures at P14 (Fig. 1). These results are consistent with previously reported experiments performed in vivo [5,6]. However in the latter case, cGMP concentration was about 10-fold higher in the rd1 retina compared to wt. The difference in magnitude is likely due to variance in the retinal organ culture model, including possible cGMP accumulation in the media and loss of some inner retinal neurons. For example, all ganglion cells degenerate when the optic nerve is cut to remove the retina from the organism, reducing the total protein.
Figure 1. cGMP Content in Retinal Organ Cultures.
Mean cGMP levels in wt, rd1, and 6-OHDA-treated rd1 retinal organ cultures at P14 as determined by enzyme immunoassay. cGMP levels are expressed as mean concentrations ± SEM in pmol/µg total protein. Sample numbers for each condition were as follows: wt control samples, n = 15; rd1 control samples, n = 20; rd1 6-OHDA-treated samples, n = 22. * p < 2.2e–16; ** p < 0.027.
Treatment of rd1 retinal organ cultures with 6-OHDA significantly reduced the cGMP concentration compared to untreated rd1 retinal organ cultures (Fig. 1). This reduction in cGMP is likely due to decreased cGMP synthesis. Dopamine has been demonstrated to regulate cGMP synthesis by acting through the neuromodulator nitric oxide (NO). Our result is consistent with experiments in which 6-OHDA treatment of rat brain resulted in 50% reduction in activity of the NO synthetic enzyme and a consistent, but not significant, decrease in cGMP levels [14]. Further studies in rat striatum have illuminated a complex downregulation of NO-cGMP signaling in response to 6-OHDA lesions [15]. The NO/cGMP signaling pathway is widely distributed in the mouse retina [16] and has been shown to play an important role in light-adaptation in lower vertebrates [17]. In teleost retina, exogenous dopamine increases NO production and inhibition of D1 receptors blocks light-evoked NO release [18]. Furthermore, activation of the NO pathway inhibits rod cell growth and neurite sprouting in salamander retina [19], which is consistent with synaptic defects seen in the rd1 retina [20]. Alternatively, decreased cGMP levels could be due to an increase in degradation by other members of the Pde family. However, we are unaware of any evidence to support this hypothesis. In either case, 6-OHDA-treated rd1 retinal organ cultures retained cGMP at levels approximately 45-fold higher than wt organ cultures. This result suggests that dopamine depletion may act, in part, to suppress cGMP and increase photoreceptor survival, but that additional effects downstream of cGMP are also required for photoreceptor protection in retinal organ cultures.
Expression of CNGC genes during development of wt and rd1 mouse retina
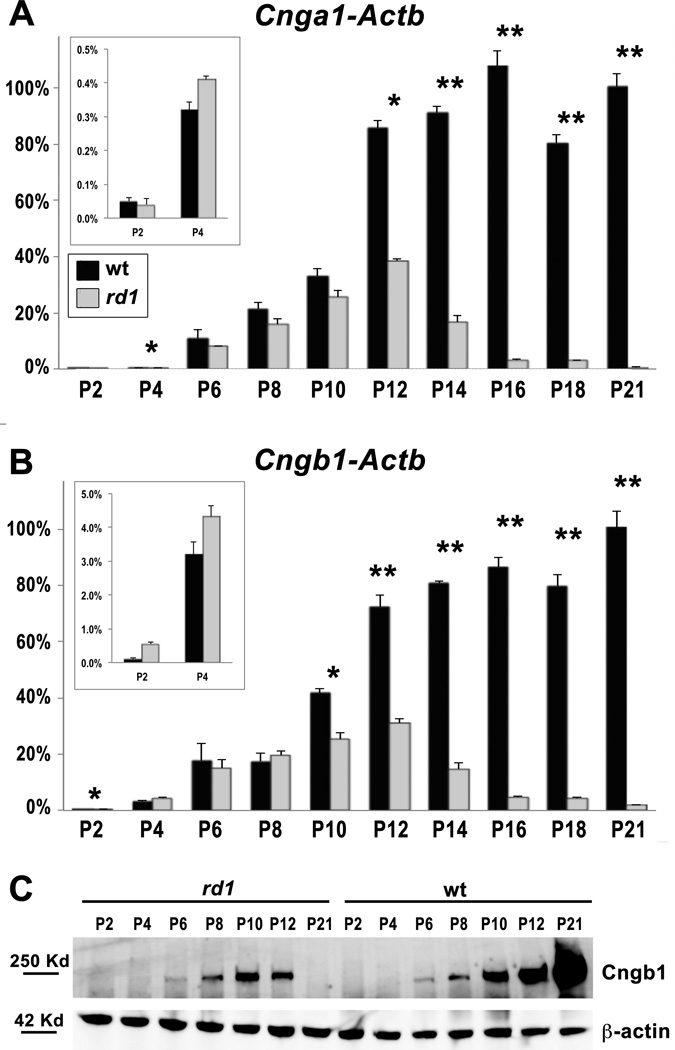
In the phototransduction cascade, cGMP acts directly on CNGCs, which are comprised of two subunits in rod photoreceptors, Cngα1 and Cngβ1 [4]. However, developmental defects have been identified in the rd1 retina well before initiation of phototransduction [5,7]. If high levels of Ca2+ influx induce cell death in rd1 photoreceptors, then CNGCs must be expressed early in photoreceptor cell differentiation. Since the developmental time course for Cnga1 and Cngb1 expression has not been previously reported, we first determined the relative expression of each gene in wt and rd1 retinas in vivo between P2 and P21 using qRT-PCR. Both Hprt and Actb (β-actin) were used to control for any potential variability in expression of housekeeping genes during development. We found similar expression patterns among Cnga1 and Cngb1 compared to either control gene (Fig. 2A, B, Fig. S1). In wt retinas, both CNGC genes were detected at P2 with a sharp increase in expression beginning at P6 and continuing through P12, coinciding with differentiation of rod photoreceptor outer segments. The overall expression pattern is similar to that seen for rhodopsin and other phototransduction genes, with the notable difference that CNGC expression is evident at P2, whereas rhodopsin expression is first detected at P5 in the mouse retina [21].
Figure 2. Expression of CNG channels in rd1 retinas compared to wt.
Relative expression of Cnga1 (A) and Cngb1 (B) in rd1 (grey) compared to wt (black) in postnatal retinas between P2 and P21. All time points were normalized to P21 wt. Early time points are shown on an amplified scale (insets). Actb (β-actin) was used as the reference gene. Significant differences between wt and rd1 are indicated (* p < 0.05; ** p < 0.001). The time course of Cngb1 protein expression as determined by Western blot (C) is consistent with mRNA expression. β-actin was used as a loading control.
In rd1 retinas, expression of Cnga1 and Cngb1 is comparable to wt at early time points but drops sharply beginning around P10-12 and is nearly absent by P21 (Fig. 2A, B, Fig. S1). This observation is consistent with the onset of photoreceptor degeneration at P10 and nearly complete loss of rods by P21 [5]. The developmental expression of Cngβ1 protein is consistent with the mRNA expression for both wt and rd1 retinas (Fig. 2C), although protein was below the level of detection prior to P6. The expression profile of CNGC subunits is consistent with the Ca2+ influx hypothesis.
Dopamine depletion does not alter CNG channel expression in rd1 retinal organ cultures
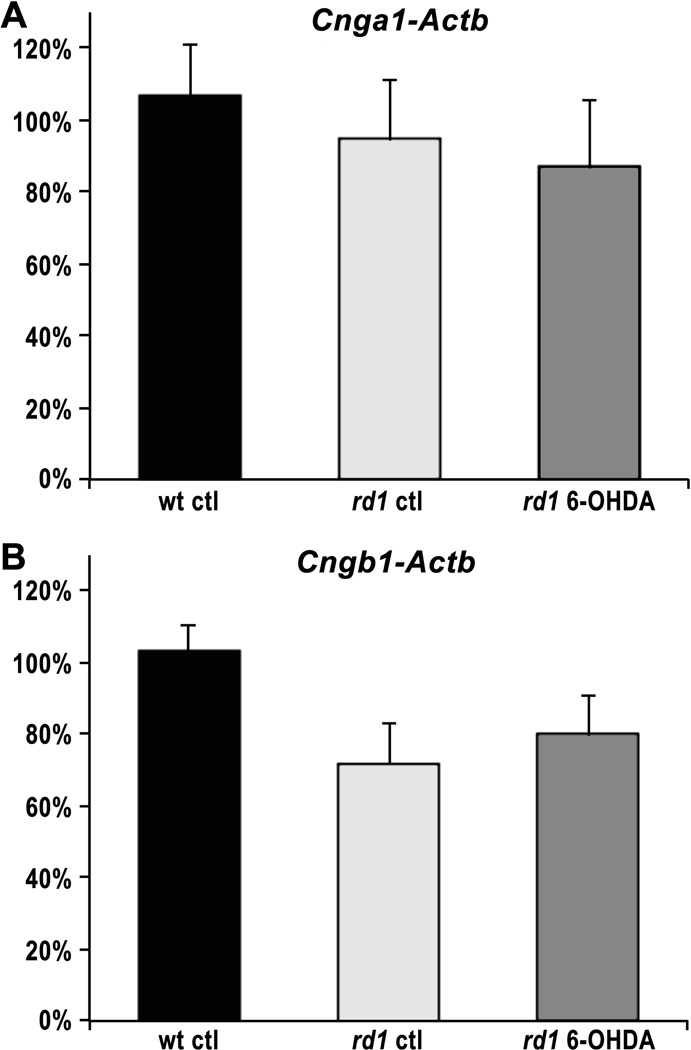
Dopamine signaling mediates changes in gene expression, in part through CREB-mediated activation of transcription factors [22]. Since loss of CNGCs has been shown to significantly protect rd1 photoreceptors in vivo [23], we next determined whether treatment with 6-OHDA alters expression of CNGC subunits in retinal organ cultures. At P10, the age at which rd1 rod cell death begins, we found no significant difference in expression of either Cnga1 or Cngb1 in organ cultures from wt, rd1, or 6-OHDA-treated rd1 retinas (Fig. 3). Actb was used as a control gene for normalization with 8–9 cultures for each condition (three-way ANOVA, p = 0.725 and 0.122 for Cnga1 and Cngb1, respectively). We conclude from these results that the neuroprotective effects of dopamine inhibition do not involve CNGC expression.
Figure 3. CNG channel expression in retinal organ cultures.
Cnga1 (A) and Cngb1 (B) expression in wt control, rd1 control, and rd1 6-OHDA-treated retinal organ cultures at P10. The ratio of mRNA expression level between wt and treated or untreated rd1 organ cultures was calculated by the 2−ΔΔCt method after normalization to Actb. For each condition, nine independent samples were tested. No significant difference in expression was observed among the three conditions.
Conclusion
Our results suggest that dopamine depletion may contribute to protection of rd1 photoreceptors in retinal organ culture via regulation of cGMP levels. Consistent with our results, dopamine inhibition has been established to act through NO to decrease cGMP synthesis in the central nervous system, including retina, of a variety of species [14,15,18], although the possibility that other members of the Pde family may increase cGMP degradation can not be eliminated. Nevertheless, additional mechanisms must be essential since the cGMP concentration is only reduced by ~18%, yet complete rd1 photoreceptor protection is observed [11]. Of the three downstream cGMP-specific signaling pathways that are known [24], two now seem unlikely to explain the protective effects of dopamine inhibition in rd1 retinal organ culture. Specifically, cGMP is known to act in a feedback loop onto Pde6, but this mechanism cannot occur in the rd1 mutant since Pde6 is absent. Secondly, cGMP can act through activation of CNGCs. Although dopamine signaling is an important transcriptional regulator, we found no evidence that dopamine regulates expression of CNGC subunits. We have also demonstrated that the time course of CNGC expression in vivo is consistent with the Ca2+ influx hypothesis in the rd1 retina, although it does not preclude involvement of other signaling pathways. Additional studies will investigate the possibility that dopamine depletion inhibits CNGC activity in rd1 organ cultures through a novel mechanism.
Two alternative cGMP signaling pathways warrant further consideration. The third known cGMP-specific signaling pathway is through activation of protein kinase G (PKG), although little is known about PKG or its downstream targets in the retina [24]. This is a particularly interesting possibility since a number of mutations causing retinal degenerative disease involve defects in cGMP pathways. A recent study has shown that PKG activity is both necessary and sufficient to cause cGMP-mediated photoreceptor degeneration and that PKG inhibitors are protective in rd1 retina [8]. Finally, high levels of cGMP may induce nonspecific crosstalk with cAMP signaling pathways [24,25]. Dopamine signaling acts directly through cAMP, modulating intracellular Ca2+ and mediating anti-apoptotic activity in developing rat retina [10,26,27]. In conclusion, inhibition of dopamine signaling significantly suppresses cGMP accumulation in rd1 retinal organ cultures, likely acting through NO to inhibit cGMP synthesis, but can not fully account for the protective effects. Further studies are required to determine whether signaling through PKG and/or crosstalk between cGMP and cAMP pathways contribute to the robust photoreceptor protection evident in rd1 retinal organ cultures.
Supplementary Material
Acknowledgements
We would like to thank Dr. Robert Molday for providing the CNGβ1 antibody, and Jon Fisher, Eric Westhus, Ameair Abu Irqeba, Sam Bhattacharya, Sean Hoge, and Betsy Boedeker for thoughtful comments and technical assistance.
Source of funding: This work was supported by grants from the National Eye Institute (R03EY15113, JMO), the National Institute of Childhood Health and Human Disease (R15HD064269, JMO), Sigma Xi Grants-in-Aid of Research (AMR), and the Beaumont Faculty Development Fund, Saint Louis University (JMO).
Footnotes
Conflicts of interest: None.
References
- 1.Keeler CE. The Inheritance of a Retinal Abnormality in White Mice. Proc Natl Acad Sci U S A. 1924;10:329–333. doi: 10.1073/pnas.10.7.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase ß-subunit gene of the rd mouse. Proceedings of the National Academy of Sciences, USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 4.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiological reviews. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 5.Farber DB, Flannery JG, Bowes-Rickman C. The rd Mouse Story: Seventy Years of Research on an Animal Model of Inherited Retinal Degeneration. Prog Retin Eye Res. 1994;13:31–64. [Google Scholar]
- 6.Lolley RN, Farber DB. Abnormal guanosine 3',5'-monophosphate during photoreceptor degeneration in the inherited retinal disorder of C3H/HeJ mice. Annals of Ophthalmology. 1976;8:469–473. [PubMed] [Google Scholar]
- 7.Dickison VM, Richmond AM, Abu Irqeba A, Martak JG, Hoge SC, Brooks MJ, et al. A role for prenylated rab acceptor 1 in vertebrate photoreceptor development. BMC Neurosci. 2012;13:152. doi: 10.1186/1471-2202-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paquet-Durand F, Hauck SM, van Veen T, Ueffing M, Ekstrom P. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J Neurochem. 2009;108:796–810. doi: 10.1111/j.1471-4159.2008.05822.x. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Morris L, Thapa A, Ma H, Michalakis S, Biel M, et al. cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J Neurosci. 2013;33:14939–14948. doi: 10.1523/JNEUROSCI.0909-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 11.Ogilvie JM, Speck JD. Dopamine Has a Critical Role in Photoreceptor Degeneration in the rd Mouse. Neurobiology of Disease. 2002;10:33–40. doi: 10.1006/nbdi.2002.0489. [DOI] [PubMed] [Google Scholar]
- 12.Ogilvie JM, Hakenewerth AM, Gardner RR, Martak JG, Maggio VM. Dopamine receptor loss of function is not protective of rd1 rod photoreceptors in vivo. Molecular vision. 2009;15:2868–2878. [PMC free article] [PubMed] [Google Scholar]
- 13.Ogilvie JM, Speck JD, Lett JM, Fleming TT. A reliable method for organ culture of neonatal mouse retina with long-term survival. J Neurosci Methods. 1999;87:57–65. doi: 10.1016/s0165-0270(98)00157-5. [DOI] [PubMed] [Google Scholar]
- 14.de Vente J, Markerink-van Ittersum M, van Abeelen J, Emson PC, Axer H, Steinbusch HW. NO-mediated cGMP synthesis in cholinergic neurons in the rat forebrain: effects of lesioning dopaminergic or serotonergic pathways on nNOS and cGMP synthesis. Eur J Neurosci. 2000;12:507–519. doi: 10.1046/j.1460-9568.2000.00927.x. [DOI] [PubMed] [Google Scholar]
- 15.Sancesario G, Giorgi M, D'Angelo V, Modica A, Martorana A, Morello M, et al. Down-regulation of nitrergic transmission in the rat striatum after chronic nigrostriatal deafferentation. Eur J Neurosci. 2004;20:989–1000. doi: 10.1111/j.1460-9568.2004.03566.x. [DOI] [PubMed] [Google Scholar]
- 16.Blom J, Giove T, Deshpande M, Eldred WD. Characterization of nitric oxide signaling pathways in the mouse retina. The Journal of comparative neurology. 2012;520:4204–4217. doi: 10.1002/cne.23148. [DOI] [PubMed] [Google Scholar]
- 17.Djamgoz MB, Sekaran S, Angotzi AR, Haamedi S, Vallerga S, Hirano J, et al. Light-adaptive role of nitric oxide in the outer retina of lower vertebrates: a brief review. Philosophical transactions of the Royal Society of London Series B. Biological sciences. 2000;355:1199–1203. doi: 10.1098/rstb.2000.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekaran S, Cunningham J, Neal MJ, Hartell NA, Djamgoz MB. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. Eur J Neurosci. 2005;21:2199–2208. doi: 10.1111/j.1460-9568.2005.04051.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, Beuve A, Townes-Anderson E. The nitric oxide-cGMP signaling pathway differentially regulates presynaptic structural plasticity in cone and rod cells. J Neurosci. 2005;25:2761–2770. doi: 10.1523/JNEUROSCI.3195-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanks JC, Adinolfi AM, Lolley RN. Photoreceptor Degeneration and Synaptogenesis in Retinal-degenerative (rd) Mice. Journal of Comparative Neurology. 1974;156:95–106. doi: 10.1002/cne.901560108. [DOI] [PubMed] [Google Scholar]
- 21.Bibb LC, Holt JK, Tarttelin EE, Hodges MD, Gregory-Evans K, Rutherford A, et al. Temporal and spatial expression patterns of the CRX transcription factor and its downstream targets. Critical differences during human and mouse eye development. Hum Mol Genet. 2001;10:1571–1579. doi: 10.1093/hmg/10.15.1571. [DOI] [PubMed] [Google Scholar]
- 22.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Journal of receptor and signal transduction research. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 23.Paquet-Durand F, Beck S, Michalakis S, Goldmann T, Huber G, Muhlfriedel R, et al. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum Mol Genet. 2011;20:941–947. doi: 10.1093/hmg/ddq539. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Cote RH. cGMP signaling in vertebrate retinal photoreceptor cells. Front Biosci. 2005;10:1191–1204. doi: 10.2741/1612. [DOI] [PubMed] [Google Scholar]
- 25.Francis SH, Blount MA, Zoraghi R, Corbin JD. Molecular properties of mammalian proteins that interact with cGMP: protein kinases, cation channels, phosphodiesterases, and multi-drug anion transporters. Front Biosci. 2005;10:2097–2117. doi: 10.2741/1684. [DOI] [PubMed] [Google Scholar]
- 26.Varella MH, de Mello FG, Linden R. Evidence for an antiapoptotic role of dopamine in developing retinal tissue. J Neurochem. 1999;73:485–492. doi: 10.1046/j.1471-4159.1999.0730485.x. [DOI] [PubMed] [Google Scholar]
- 27.Ivanova TN, Alonso-Gomez AL, Iuvone PM. Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain research. 2008;1207:111–119. doi: 10.1016/j.brainres.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.