Synopsis
This article reviews exogenous surfactant therapy and its use in mitigating acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) in infants, children, and adults. Biophysical and animal research documenting surfactant dysfunction in ALI/ARDS is described, and the scientific rationale for treatment with exogenous surfactant is discussed. Major emphasis is on reviewing clinical studies of surfactant therapy in pediatric and adult patients with ALI/ARDS. Particular advantages from surfactant therapy in direct pulmonary forms of these syndromes are described. Also discussed are additional factors affecting the efficacy of exogenous surfactants in ALI/ARDS, including the multifaceted pathology of inflammatory lung injury, the effectiveness of surfactant delivery in injured lungs, and composition-based activity differences among clinical exogenous surfactant preparations.
I. Introduction
Because of their extensive alveolar network and capillary vasculature, the lungs have significant exposure and susceptibility to injury from toxins and pathogens present either in the circulation or in the external environment. The medical consequences of acute pulmonary injury in pediatric and adult patients are frequently defined as the syndromes of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). The American-European Consensus Conference in 1994 defined ALI as respiratory failure of acute onset with a PaO2/FiO2 ratio ≤300 mmHg (regardless of the level of positive end expiratory pressure, PEEP), bilateral infiltrates on frontal chest radiograph, and a pulmonary capillary wedge pressure <18 mmHg (if measured) or no evidence of left atrial hypertension [1]. ARDS is defined identically except for a lower PaO2/FiO2 limit of <200 mmHg [1]. The Consensus Committee definitions of ALI/ARDS are widely-used clinically, supplemented by lung injury or critical care scores such as the Murray [2] or APACHE II [3] scores in adults, or the PRISM [4, 5], PIM [6], or Oxygenation Index [7] in children.
The incidence of ALI/ARDS has been variably reported to be 50,000–190,000 cases per year in the United States [1, 8–14]. The incidence of ALI in two recent studies has been estimated at 22–86 cases per 100,000 persons per year [13, 14], with 40–43 percent of these patients having ARDS [13]. These studies primarily considered adults; the incidence of ALI/ARDS has been reported to be significantly lower at 2–8 cases per 100,000 persons per year in the pediatric age group (e.g., [15–19]). Survival statistics for patients with ALI/ARDS vary depending on specific lung injury etiology and age, but overall mortality rates in both adult and pediatric patients remain very substantial despite sophisticated intensive care [1, 8–14, 16–19]. Mortality rates reported in a series of studies in pediatric patients with ALI/ARDS are given in Table 1 [15, 19–25]. The significance of distinguishing between the two clinical syndromes in a practical sense is uncertain, since a meta-analysis of 102 studies prior to 1996 suggested little or no difference in mortality rates between patients meeting criteria for ALI compared to ARDS [9]. This was also the conclusion in the recent NEJM article by Rubenfeld et al [13], which reported mortality rates of 38.5% for ALI and 41% for ARDS, with an estimated 74,500 deaths per year and an aggregate 3.6 million hospital days of care in the United States.
Table 1.
Mortality rates reported in a series of studies in pediatric ALI/ARDS.
| Author | Year | # Patients | Mortality (%) |
|---|---|---|---|
| Pfenninger [15] | 1982 | 20 | 40% |
| DeBruin [21]* | 1992 | 100 | 72% |
| Timmons [22] | 1995 | 470 | 43% |
| Dahlem [23] | 2003 | 44 | 27.3% |
| Curley [24] | 2005 | 102 | 8% |
| Willson [25] | 2005 | 152 | 28% |
| Flori [19] | 2005 | 328 | 22% |
| Pediatric Study Group (ANZICS) [20] | 2007 | 117 | 35% |
Patients consisted of mostly immune compromised children
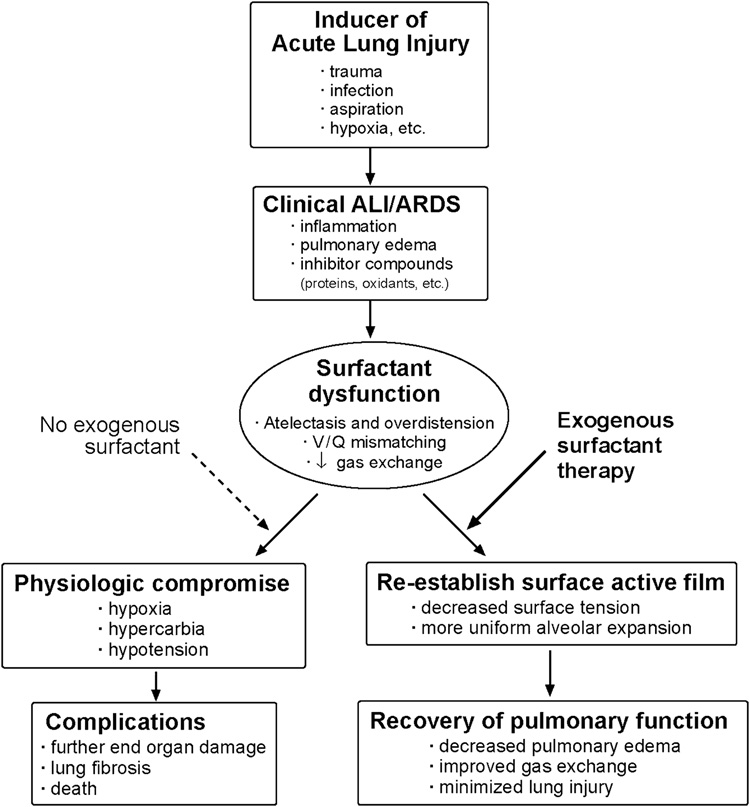
There is clearly a significant need for improved treatments for ALI/ARDS in all age groups, and this review focuses on the potential utility of exogenous surfactant replacement therapy. Although the clinical syndromes of ALI and ARDS encompass a broad spectrum of etiologies, it is important to consider specific etiologies in targeting surfactant therapy. In particular, it is important to distinguish between ALI/ARDS from direct pulmonary causes as opposed to systemic (indirect, non-pulmonary) causes. Direct pulmonary causes of ALI/ARDS include lung viral or bacterial infections, gastric aspiration, blunt thoracic trauma with lung contusion, meconium aspiration (infants), near-drowning, thoracic radiation, hyperoxia, and the inhalation of smoke or other toxicants. Indirect (systemic) causes of ALI/ARDS include sepsis, closed space burn injury, hypovolemic shock, non-thoracic trauma, multiple transfusions, and pancreatitis. The pathology of ALI/ARDS is particularly complex in indirect insults like sepsis, where multi-organ involvement is common and extra-pulmonary inflammation is severe and pervasive. The multifaceted systemic pathology of ALI/ARDS from non-pulmonary causes of lung injury likely renders therapy with exogenous surfactant less effective compared to direct pulmonary ALI/ARDS. As detailed later, post-hoc analyses in two recent clinical trials of surfactant therapy in ALI/ARDS suggest greater efficacy in direct as opposed to indirect pulmonary injury [25, 26]. In all forms of ALI/ARDS, the major rationale for surfactant therapy is the presence of surfactant dysfunction in the injured lungs (Figure 1). Although ALI/ARDS can include chronic lung injury in its later fibro-proliferative phase, surfactant dysfunction is most prominent in the acute phase of ALI/ARDS as described below.
Figure 1. Schematic of the rationale for exogenous surfactant therapy to mitigate surfactant dysfunction in ALI/ARDS.
The pathophysiology of acute inflammatory lung injury includes surfactant dysfunction, which contributes to respiratory failure in term infants, children, and adults with clinical acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS). Surfactant dysfunction reduces lung volumes and compliance, causes atelectasis and overdistension, increases ventilation/perfusion (V/Q) mismatching, and reduces gas exchange. In addition, surfactant dysfunction and lung injury can also be present in the clinical course of premature infants being treated with mechanical ventilation and supplemental oxygen for surfactant deficient lung disease in association with the neonatal respiratory distress syndrome (RDS). Scientific understanding indicates that surfactant dysfunction in lung injury can, at least in principle, be ameliorated by exogenous surfactant therapy as discussed in this article.
II. Surfactant dysfunction in acute pulmonary injury
Total lavaged phospholipid is either unchanged or increased in most animal models of ALI/ARDS, including models of direct pulmonary injury such as gastric aspiration or respiratory infection [27–32]. Although surfactant deficiency can potentially occur during the course of ALI/ARDS (e.g., from type II cell injury), surfactant dysfunction in association with inflammation and permeability injury is generally much more prominent. Impairments in lung surfactant activity, and reductions in the content or composition of active large surfactant aggregates, have been reported in BAL, edema fluid, or tracheal aspirates from patients with ALI/ARDS or other diseases involving lung injury [33–45]. Research over the last two decades has identified a number of pathways and mechanisms contributing to surfactant dysfunction in acute pulmonary injury as summarized below (for review see [27, 46, 47]):
One prevalent pathway of surfactant dysfunction in lung injury is through biophysical or chemical interactions with substances present in the alveoli as a result of permeability edema or inflammation. Biophysical studies in vitro have shown that the surface activity of lung surfactant can be impaired by multiple injury-related inhibitors including plasma and blood proteins [48–55], meconium [56], cell membrane lipids [50, 55, 57], fluid free fatty acids [55, 58–60], reactive oxidants [58, 61–63], and lytic enzymes including proteases [64] and phospholipases [65, 66]. Surfactant dysfunction associated with inhibitory substances in the lungs has also been widely-demonstrated in animal models of acute inflammatory lung injury [27–32, 46, 67–69]. In terms of physicochemical mechanisms of action, albumin and other blood proteins impair the surface activity of lung surfactant primarily by competitive adsorption that reduces the entry of active surfactant components into the air-water interface [52, 70]. In contrast, cell membrane lipids, lyso-phospholipids, or fatty acids act at least in part by mixing into the surface film and compromising its ability to reach low surface tension during dynamic compression [50, 55, 59, 70]. Also, phospholipases, proteases, and reactive oxygen-nitrogen species can act to chemically degrade or alter functionally-essential surfactant lipids or proteins [64, 66, 71]. It is well-documented that surfactant activity deficits from these various mechanisms can be overcome or at least mitigated in vitro by increasing the concentration of active surfactant even if inhibitor substances are still present [27, 46, 47].
Another pathway by which surfactant activity can be reduced during lung injury is by depletion or alteration of active large aggregates. Surfactant exists in the alveolar hypophase in a size-distributed microstructure of aggregates, the largest of which typically have the greatest surface activity and the highest apoprotein content [60, 72–78]. The percentage of large aggregates and their content of SP-A and SP-B are reduced in bronchoalveolar lavage from patients with ARDS [38–40]. Animal studies of ALI/ARDS indicate that large surfactant aggregates can be depleted or reduced in activity by molecular interactions with inhibitor substances as well as by changes in surfactant aggregate metabolism in the alveoli or involving altered reuptake or recycling in type II cells [30, 60, 79–81]. Although large aggregates are depleted or compromised in many forms of ALI/ARDS, information on total surfactant pools is inconsistent, with both decreased [35, 37] and unchanged amounts [34, 36] reported.
The ability of exogenous surfactant therapy to reverse or mitigate acute respiratory pathology in animal models of ALI/ARDS has also been extensively documented (Table 2). Examples of animal studies showing benefits to acute lung function or mechanics following surfactant therapy include acid aspiration [82–84], meconium aspiration [85–88], anti-lung serum [89], bacterial or endotoxin injury [90–95], bilateral vagotomy [96], hyperoxia [97–101], in vivo lavage [102–107], N-nitroso-N-methylurethane (NNNMU) injury [108–110], and viral pneumonia [111, 112]. These acute animal model studies typically did not address long-term outcomes. However, significant improvements in respiratory function would be expected to lead to improved long term outcomes in patients with ALI/ARDS, provided that untreated elements of pathology do not blunt this effect. As noted earlier, this is most likely to be the case in direct pulmonary forms of ALI/ARDS.
Table 2.
Animal models of ALI/ARDS lung injury that have been shown to respond acutely to exogenous surfactant therapy.
| Aspiration of acid [82–84]or meconium [85–88] |
| Anti-lung serum infusion [89] |
| Bacterial or endotoxin-induced injury [90–95] |
| Bilateral vagotomy [96] |
| Hyperoxic lung injury [97–101] |
| In vivo lung lavage with mechanical ventilation [102–107] |
| NNNMU-induced lung injury [108–110] |
| Viral pneumonia [111, 112] |
NNNMU is N-nitroso-N-methylurethane. See text for discussion.
III. Clinical exogenous surfactant drugs
Although endogenous surfactant contains similar chemical constituents across mammalian species, this is not true of exogenous surfactant drugs. The degree of resemblance of pharmaceutical surfactants to native surfactant is highly variable, and has direct consequences for surface and physiological activity. Pharmaceutical surfactants comprise three functionally-relevant classifications (Table 3): (I) organic solvent extracts of lavaged lung surfactant from animals; (II) organic solvent extracts of processed animal lung tissue with or without additional synthetic additives; and (III) synthetic preparations formulated in vitro without material from animal lungs. Organic solvent extracts of lavaged alveolar surfactant (Category I) contain all of the hydrophobic lipid and protein components of endogenous surfactant, although specific compositional details can vary depending on preparative methodology. Extracts of minced or homogenized lung tissue (Category II) necessarily contain some non-surfactant components, and require more extensive processing that can further alter composition compared to native surfactant. The synthetic surfactants in Category III that have been most widely studied are Exosurf® and ALEC® (artificial lung expanding compound), although neither of these two preparations is in active clinical use because they have been shown to have inferior activity compared to animal-derived surfactants (e.g., [46, 51, 113–117]). Two additional synthetic surfactants, KL4 (Surfaxin®) and recombinant SP-C surfactant (Venticute®), are presently undergoing clinical evaluation, and new synthetic lipid:peptide exogenous surfactants are currently under development.
Table 3.
Clinical exogenous surfactant drugs for treating diseases that involve lung surfactant deficiency or dysfunction.
| I. | Organic solvent extracts of lavaged animal lung surfactant |
| Infasurf® (CLSE) | |
| bLES® | |
| Alveofact® | |
| II. | Supplemented or unsupplemented organic solvent extracts of processed animal lung tissue |
| Survanta® | |
| Surfactant-TA® | |
| Curosurf® | |
| III. | Synthetic exogenous lung surfactants |
| Exosurf® | |
| ALEC® | |
| Surfaxin® (KL4) | |
| Venticute® (Recombinant SP-C surfactant) |
Curosurf ® (Chesi Farmaceutici and Dey Laboratories), Infasurf® (ONY, Inc and Forest Laboratories), and Survanta® (Abbott/Ross Laboratories) are currently FDA-approved in the USA, and Surfaxin® (KL4, Discovery Laboratories) is under clinical evaluation. Exosurf® (Glaxo-Wellcome) is also FDA-approved, but is no longer used clinically. Details on the composition, activity, and efficacy of these surfactants in treating or preventing RDS in premature infants are detailed elsewhere (e.g., [46]), and this article focuses on their use in ALI/ARDS. See text for details. Table adapted from Refs [46, 119].
Four exogenous surfactant preparations are currently licensed for clinical use in RDS in the United States: Infasurf®, Survanta®, Curosurf®, and Exosurf® (the latter is no longer used as noted above). As reviewed elsewhere [46, 118, 119], Infasurf® is a direct chloroform:methanol extract of large aggregate surfactant obtained by bronchoalveolar lavage from calf lungs. Survanta® is an extract of minced bovine lung tissue to which is added synthetic dipalmitoyl phosphatidylcholine (DPPC), tripalmitin, and palmitic acid. Curosurf® is prepared from minced porcine lung tissue by a combination of washing, chloroform-methanol extraction, and liquid-gel chromatography. In addition, Surfaxin® (KL4 surfactant), which is under consideration for FDA-approval, is made up of a 21 amino acid peptide that has repeating units of one leucine (K) and four lysine (L) residues and is combined at 3% by weight with a 3:1 mixture of DPPC and palmitoyl-oleoyl phosphatidylglycerol plus 15% palmitic acid. The synthetic surfactant Venticute®, which has also been studied in ARDS as noted later, contains synthetic lipids and palmitic acid plus a 34 AA modified human recombinant SP-C that has substitutions of phenylalanine for cysteine at two positions and isoleucine for methionine at another [46].
IV. Current studies on surfactant replacement therapy in patients with ALI/ARDS
A significant number of clinical studies have reported benefits following the instillation of exogenous surfactants to term infants, children, or adults with ALI/ARDS or related acute respiratory failure [25, 120–135] (Table 4). However, many of these are small case series or pilot studies, and found only improvements in acute lung function (oxygenation). Controlled trials of surfactant therapy in patients with ALI/ARDS have met with mixed success, particularly in adults with sepsis-associated ARDS [136, 137]. A summary of the clinical experience with exogenous surfactant therapy in term infants, children and adults is given below.
Table 4.
Clinical studies reporting benefits of exogenous surfactant therapy in acute respiratory failure (ALI/ARDS).
| Study | Patients (N) | Disease Or Syndrome | Surfactant | Outcomes |
|---|---|---|---|---|
| Güntheret al [120] | Adults (27) | ARDS | Alveofact® | Improved surfactant function |
| Walmrath et al. [155] | Adults (10) | ARDS from sepsis | Alveofact® | Improved oxygenation |
| Spragg et al [122] | Adults (6) | ARDS from multiple causes | Curosurf® | Improved oxygenation a and biophysical function |
| Wiswell et al [123] | Adults (12) | ARDS from multiple causes | Surfaxin® | Improved oxygenation |
| Willson et al [124, 125] | Children (29 & 42) | ARDS from multiple causes | Infasurf® | Improved oxygenation |
| Willson et al [25] | Children(152) | ARDS from multiple causes | Infasurf® | Improved survival, and improved ventilation |
| Lopez-Herce et al [126] | Children (20) | ARDS + post-op cardiac | Curosurf® | Improved oxygenation |
| Hermon et al [127] | Children (19) | ARDS + post-op cardiac | Curosurf® or Alveofact® | Improved oxygenation |
| Herting et al [128] | Children (8) | Pneumonia | Curosurf® | Improved oxygenation |
| Auten et al [129] | Infants (14) | Meconium aspiration or pneumonia | Infasurf®(CLSE) | Improved oxygenation |
| Lotze et al [130, 131] | Infants (28 & 328) | ECMO, multiple indications | Survanta® | Improved oxygenation, decreased ECMO |
| Khammash et al [132] | Infants (20) | Meconium aspiration | bLES® | Improved oxygenation in 75% of patients |
| Findlay et al [133] | Infants (40) | Meconium aspiration | Survanta® | Improved oxygenation, decreased pneumothorax and mechanical ventilation |
| Luchetti et al [134, 135] | Infants (20 & 40) | RSV bronchiolitis | Curosurf® | Improved oxygenation |
Tabulated clinical studies include both controlled and non-controlled trials as discussed in the text.
The best-studied application of surfactant therapy in term infants with acute pulmonary injury is in meconium aspiration syndrome [129–133]. Meconium obstructs and injures the lungs when aspirated and is known to cause surfactant dysfunction [56, 138]. Auten et al [129], Khammash et al [132], and Findlay et al [133] have all reported significant improvement from surfactant administration in infants with meconium aspiration. The randomized controlled study of Findlay et al [133] found reductions in the incidence of pneumothorax, duration of mechanical ventilation and oxygen therapy, time of hospitalization, and requirements for ECMO in 20 term infants treated with Survanta® compared to controls. Lotze et al [130, 131] also reported favorable results using Survanta® in a controlled trial in term infants referred for ECMO due to severe respiratory failure (meconium aspiration was a prevalent diagnosis in both studies). Twenty-eight infants treated with four doses of Survanta® (150 mg/kg) had improved pulmonary mechanics, decreased duration of ECMO treatment, and a lower incidence of complications after ECMO compared to control infants [130]. A subsequent multicenter controlled trial in 328 term infants also reported significant improvements in respiratory status and the need for ECMO following surfactant treatment [131]. Surfactant therapy has also been found to be beneficial to lung function or respiratory outcome in several studies in infants with viral respiratory (RSV) infection [134, 135, 139]. Luchetti et al [134] reported that 10 infants with RSV-bronchiolitis treated with tracheally-instilled porcine-derived surfactant (Curosurf®, 50 mg/kg body weight) had improved gas exchange and a reduced time on mechanical ventilation and in the pediatric intensive care unit compared to an equal number of control infants not treated with exogenous surfactant. A subsequent multicenter controlled trial in 40 patients also found that surfactant therapy with Curosurf® improved gas exchange and respiratory mechanics, and shortened the duration of mechanical ventilation and hospitalization, in infants with acute respiratory failure from RSV bronchiolitis [135]. Also, Tibby et al [139] have reported that 9 infants with severe RSV bronchiolitis who received two doses of Survanta® (100 mg/kg) had a more rapid improvement in oxygenation and ventilation indices in the first 60 hours compared to 10 control infants receiving air-placebo. Exogenous surfactant therapy is now used in neonatal intensive care units at many institutions to treat respiratory failure in term infants with meconium aspiration or pulmonary viral/bacterial infection. Surfactant therapy has also been studied in infants with congenital diaphragmatic hernia, but its use remains controversial in this context [140, 141].
Studies of surfactant therapy in children and adults with ALI/ARDS show positive results in a number of case reports or relatively small pilot trials [120, 121, 124–128]. However, particularly in adults, controlled clinical trials have been less successful. By far the largest prospective controlled study of surfactant therapy in adults with ARDS was definitively negative [136]. Anzueto et al [136] administered nebulized Exosurf® vs. placebo to 725 adults with ARDS secondary to sepsis and found no improvement in any measure of oxygenation and no effect on morbidity or mortality. However, interpretation of these negative results is confounded because both laboratory and clinical studies have documented that Exosurf® has low activity compared to animal-derived surfactants [51, 113–117, 142–145], and aerosolization has not been shown to be as effective as airway instillation in delivering surfactant. Gregory et al [137] reported small benefits in oxygenation in a controlled trial in adults with ARDS who received four 100 mg/kg doses of Survanta®, but with no overall advantage in survival in the 43 surfactant-treated patients studied. A more recent study by Spragg et al [26] using recombinant SP-C surfactant (Venticute®) in adults with ARDS showed immediate improvements in oxygenation, but no longer-term improvement in duration of mechanical ventilation, lengths of stay, or mortality. Post-hoc analysis did suggest, however, that the response in the subgroup of patients with ARDS due to “direct lung injury” was positive, and a follow-up prospective study in this group of patients is currently underway.
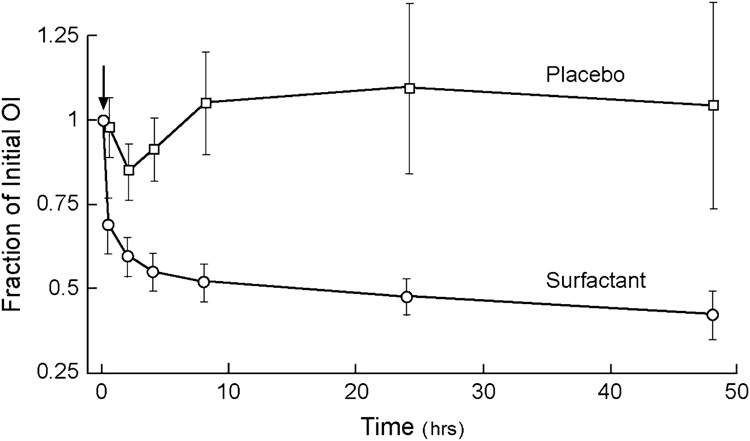
Controlled studies of surfactant therapy in children up to age 21 with ALI/ARDS have been more encouraging. A randomized but unblinded controlled trial by Willson et al [125] in 42 children at eight centers with ALI/ARDS showed that those receiving Infasurf® (70 mg/kg) had immediate improvement in oxygenation and fewer ventilator days and days in intensive care (Figure 2). This unblinded trial followed an initial open-label study by the same group demonstrating improved oxygenation in 29 children (0.1–16 yrs) treated with instilled Infasurf® [124]. In addition, a clinical trial by Moller et al [146] reported that children with ARDS showed an immediate improvement in oxygenation and less need for rescue therapy following treatment with Survanta®, although this latter study was underpowered for more definitive outcomes. Most recently, a large blinded, randomized, controlled study by Willson et al [25] in 152 pediatric patients with ALI/ARDS (77 surfactant treated and 75 placebo) yielded very positive results, showing both immediate improvements in oxygenation as well as a significant survival advantage for patients receiving calfactant (Infasurf®) relative to placebo (Table 5). Patients aged 1 wk through 21 years in this 21 center study were enrolled within 48 hr of endotracheal intubation with radiographic evidence of bilateral lung disease and an oxygenation index greater than 7. Patients with pre-existing lung, cardiac, or central nervous system were excluded. Ventilator-free days and mortality were primary outcomes. Surfactant treatment resulted in decreased oxygenation index, decreased mortality, and a higher percentage of response to conventional mechanical ventilation compared to air-placebo (Table 5). A post-hoc analysis indicated that the great majority of these beneficial effects were confined to patients with direct injury forms of ALI/ARDS (Table 6).
Figure 2. Improvements in oxygenation index (OI) after instillation of exogenous surfactant in children with ALI/ARDS.
Patients ranging in age from 1 day through 18 years in eight pediatric intensive care units were randomized to surfactant or control groups. Surfactant-treated patients received a dose of Infasurf® of 80 mL/m2 body surface (70 mg/kg body weight) by tracheal instillation during hand-ventilation with 100% oxygen (arrow). Control patients received hand-ventilation and 100% oxygen alone. Ten of 21 surfactant-treated patients received a second dose 12 or more hours after the first. Significant improvements were found in lung function in patients receiving exogenous surfactant therapy. OI is defined as: 100 × MAP × FiO2/PaO2, where MAP = mean airway pressure; FiO2 = fraction of inspired oxygen; PaO2 = arterial partial pressure of oxygen. Data from Willson et al [125].
Table 5.
Clinical outcomes from a recent controlled study using exogenous surfactant (Infasurf®; calfactant) in pediatric patients with ALI/ARDS [25].
| Calfactant (n=77) | Placebo (n=75) | P Value | |
|---|---|---|---|
| Mortality | |||
| Died (in hospital) | 15 (19%) | 27 (36%) | 0.03 |
| Died w/o extubation | 12 (16%) | 24 (32%) | 0.02 |
| Failed CMV* | 13 (21%) | 26 (42%) | 0.02 |
| ECMO | 3 | 3 | -- |
| Use of Nitric Oxide | 9 | 10 | 0.80 |
| HFOV after entry | 7 | 15 | 0.07 |
| Secondary Outcomes | |||
| PICU LOS | 15.2 ± 13.3 | 13.6 ± 11.6 | 0.85 |
| Hospital LOS | 26.8 ± 26 | 25.3 ± 32.2 | 0.91 |
| Days O2 therapy | 17.3 ± 16 | 18.5 ± 31 | 0.93 |
| Hospital Charges# | $205 ± 220 | $213 ± 226 | 0.83 |
| Hospital Charges/day# | $7.5 ± 7.6 | $7.9 ± 7.5 | 0.74 |
Some patients that failed CMV had more than one non-conventional therapy (ECMO, iNO, or HFOV);
Costs are given in thousands of dollars.
Abbreviations CMV = conventional mechanical ventilation; ECMO = extracorporeal membrane oxygenation; HFOV = high frequency oscillatory ventilation; iNO = inhaled nitric oxide. In addition to improving mortality and reducing the percentage of patients that failed CMV as reported in the table, instilled calfactant also significantly improved oxygenation index compared to placebo (P=0.01, data not shown). From Willson et al [25].
Table 6.
Efficacy of exogenous surfactant (Infasurf®, calfactant) in direct and indirect forms of lung injury in the controlled study of Willson et al [25] in children up to age 21 with ALI/ARDS.
| Placebo | Calfactant | P value | |
|---|---|---|---|
| Direct lung injury (n) | 48 | 50 | -- |
| OI ↓ 25% or more | 31% | 66% | 0.0006 |
| Ventilator days | 17 ± 10 | 13 ± 9 | 0.05 |
| Died | 38% | 8% | 0.0005 |
| Indirect lung injury (n) | 27 | 27 | -- |
| OI ↓ 25% or more | 41% | 37% | 0.79 |
| Ventilator days | 17 ± 10 | 18 ± 10 | 0.75 |
| Died | 33% | 41% | 0.65 |
Percentages of patients with an OI decrease of greater than 25%, days on mechanical ventilation, and percentage mortality were calculated in a post-hoc analysis. The efficacy of exogenous surfactant was confined to patients with direct pulmonary forms of ALI/ARDS. See text for details.
None of the above studies in infants, children, or adults showed any significant adverse long-term effects from surfactant administration, although transient hypoxia and some hemodynamic instability surrounding intratracheal or bronchoscopic instillation appear common. Transmission of infectious agents or allergic reactions has also not been reported with any of the exogenous surfactants currently licensed in the United States.
V. Considerations affecting the efficacy of exogenous surfactant therapy in ALI/ARDS
Several factors increase the difficulty of developing effective surfactant therapy for ALI/ARDS. As emphasized earlier, patients with ALI/ARDS share aspects of lung injury pathophysiology, but these diagnoses are clinical syndromes that comprise a diverse set of etiologies. The occurrence of ALI/ARDS in a heterogeneous population of patients with varying degrees of lung injury and systemic disease significantly reduces the resolving power of clinical trials of surfactant therapy. In addition, edema and inflammation in patients with acute pulmonary injury make it more difficult to deliver and distribute exogenous surfactant to the alveoli. Finally, meaningful evaluations of surfactant therapy in ALI/ARDS must account for differences in surfactant drug activity that can significantly impact therapeutic efficacy. Specific considerations for surfactant therapy in ALI/ARDS are discussed below.
Delivery methods and dosages for exogenous surfactant therapy in patients with lung injury
The primary method used to deliver exogenous surfactants to children and adults with ALI/ARDS is based on that shown to be most effective in premature infants with RDS, i.e., direct intratracheal instillation through an endotracheal tube or airway instillation through a bronchoscope. Surface active material instilled into the airways has the capacity to rapidly spread and distribute to the periphery of the lung [147–149]. Spreading from the central airways towards the alveoli is promoted by surface tension gradients that drive transport from regions of high surfactant concentration to regions of lower surfactant concentration. Typical doses of intratracheal surfactant in premature infants are 100 mg/kg body weight. This represents a significant excess over the amount of surfactant phospholipid needed to cover the surface of the alveolar network with a tightly packed surfactant film (only about 3 mg/kg of surfactant phospholipid at a molecular weight of 750 Daltons are needed to form a monomolecular film at a limiting area of 40 Å2/molecule over an alveolar surface of 1 m2/kg body weight [46, 150]). Excess instilled exogenous surfactant that reaches the alveoli provides a reservoir of material for the hypophase and interface, and is available for incorporation into endogenous surfactant pools via recycling pathways.
Although instillation is an effective method of delivering exogenous surfactants, total dosages required in older children and adults with ALI/ARDS are non-trivial. To achieve a comparable dose to that of premature infants based on body weight or body surface area, much larger total drug amounts and volumes are required. The prototypical “70 kg adult” requires 7 grams of exogenous surfactant at a dosage level of 100 mg/kg body weight. This necessitates an instilled volume of 87.5–280 ml at the phospholipid concentrations of current clinical surfactant drugs in saline (25–80 mg/ml). It is obviously important to minimize instilled surfactant volumes in patients with severe respiratory failure as in ALI/ARDS. At the same time, instilled surfactant volume impacts intrapulmonary drug distribution, which is already compromised by edema and inflammation as noted earlier. Studies in animal models of ALI/ARDS have indicated that the distribution of exogenous surfactant can be improved by instilling larger fluid volumes or utilizing associated bronchoalveolar lavage [151–154], but the feasibility and/or utility of these approaches in clinical practice is uncertain. Clinical studies on intratracheal or bronchoscopic instillation of exogenous surfactants in patients with ALI/ARDS have used a range of instilled volumes, with doses as high as 300 mg/kg [155] and as low as 25 mg/kg [26].
An alternative to administering exogenous surfactant drugs by instillation is to deliver them in aerosol form. In theory, aerosolization could significantly reduce required surfactant doses, since delivery can be targeted to the alveoli by controlling particle size. Phospholipid aerosols with stable particle sizes appropriate for alveolar deposition in normal lungs can be formed by ultrasonic or jet nebulization [150, 156, 157], and exogenous surfactants have been aerosolized to animals and patients with surfactant deficiency or dysfunction [91, 105, 110, 136, 158–160]. However, the theoretical potential of aerosols to improve alveolar deposition and reduce required surfactant doses has not been replicated in practice. Aerosol methodology to date has not been shown to deliver exogenous surfactants to the alveoli as effectively as instillation, although this may improve in the future as technology advances.
Another approach to facilitate the delivery and distribution of exogenous surfactants in injured lungs involves the use of specific modes and strategies of mechanical ventilation. For example, studies indicate that the distribution and/or efficacy of instilled exogenous surfactant can be improved by jet ventilation [161, 162] and partial liquid ventilation [163–165]. Additional mechanism-based research on the impact of specific ventilation methods and strategies on the delivery, distribution, and efficacy of exogenous surfactants may be important for optimizing this therapy in ALI/ARDS. The delivery and distribution of surfactant drugs in injured lungs could also potentially be improved by the use of low viscosity formulations to reduce transport resistance after tracheal or bronchoscopic instillation. Whole surfactant and animal-derived exogenous surfactants have complex non-Newtonian, concentration-dependent viscosities that vary significantly among preparations [166, 167]. For a given surfactant preparation at fixed shear rate, viscosity can be significantly reduced by altering the physical formulation through changes in dispersion methodology, ionic environment, or temperature [166, 167].
Activity and inhibition resistance of exogenous surfactant drugs
One of the most important considerations in the clinical efficacy of surfactant therapy is the relative activity of different exogenous surfactant drugs. Differences in efficacy between clinical exogenous surfactants have been demonstrated in a number of comparison trials in premature infants [114–117, 142–145, 168, 169]. Results of these clinical comparisons indicate that “natural” surfactants derived from animal lungs (Categories I and II in Table 3) have significantly greater efficacy than the protein-free synthetic surfactants Exosurf® and ALEC. This is also the conclusion of retrospective meta analyses combining clinical data from multiple surfactant trials [170–174]. As noted earlier, the largest study of surfactant therapy in adults with ALI/ARDS utilized the protein-free synthetic surfactant Exosurf® and found no therapeutic benefit [136]. The hydrophobic surfactant proteins are highly-active in endogenous surfactant, and substituting for them effectively in protein-free synthetic surfactants is extremely difficult. The addition of mixed bovine SP-B/SP-C to Exosurf® greatly improves its surface activity and its efficacy in reversing surfactant-deficient pressure-volume mechanics in excised rat lungs [113], documenting that the biochemical components of this surfactant are not adequate substitutes for the hydrophobic apoproteins in activity.
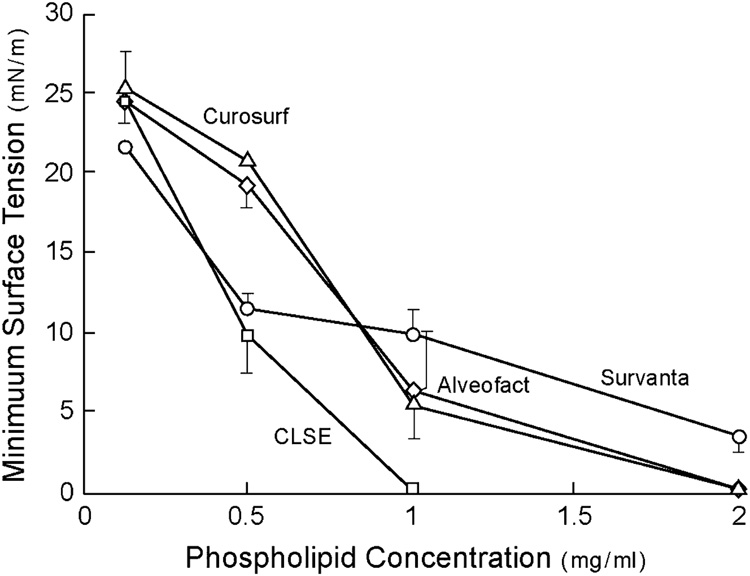
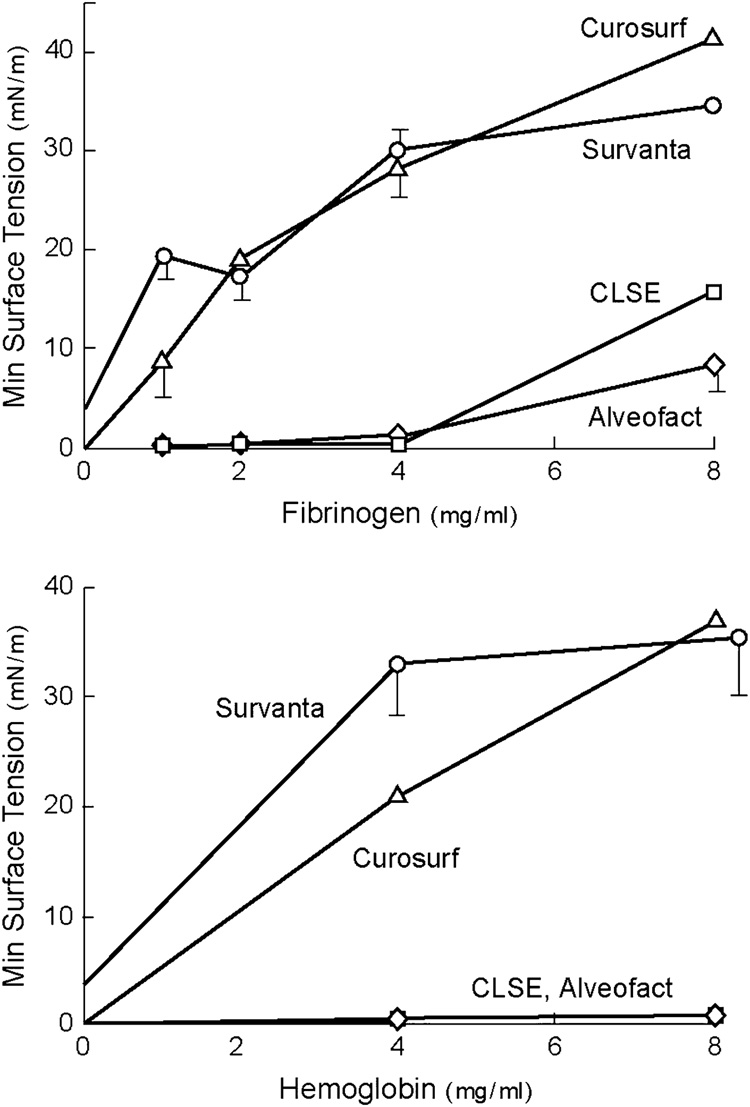
Animal-derived clinical exogenous surfactants themselves also differ substantially in their surface activity and ability to resist inhibitor-induced dysfunction. The consensus of available biophysical and animal research indicates that exogenous surfactants based on extracts of lavaged large aggregate endogenous surfactant in Category I of Table 3 have the greatest overall surface and physiological activity. This reflects the fact that these drugs have close compositional analogy to the mix of phospholipids and hydrophobic proteins in active alveolar surfactant. The surface tension lowering ability of several clinical surfactants during dynamic cycling on a pulsating bubble apparatus (37°C, 20 cycles/min, 50% area compression) is illustrated in Figure 3 from Seeger et al [51]. Suspensions of CLSE (Infasurf®) and Alveofact® were found to reach the lowest minimum surface tensions at the lowest surfactant concentrations among the preparations studied. Category I exogenous surfactants such as CLSE and Alveofact® also resist inhibition by blood proteins more effectively than exogenous surfactants like Survanta® and Curosurf® that are processed from lung tissue [51, 113] (Figure 4).
Figure 3. Overall surface tension lowering ability of clinical exogenous surfactants.
Minimum surface tension after 5 minutes of pulsation in a bubble surfactometer (37°C, 20 cycles/min, 50% area compression) is plotted as a function of surfactant phospholipid concentration for several clinical surfactants. The surfactants shown vary widely in overall surface tension lowering ability, with the most active being Category I surfactants from Table 3 (e.g., Infasurf® and Alveofact®). Data redrawn from Seeger et al [51].
Figure 4. Resistance of clinical surfactants to inhibition by blood proteins.
Minimum surface tension after 5 minutes of pulsation in a bubble surfactometer (37°C, 20 cycles/min, and 50% area compression) is plotted against the concentration of inhibitory blood proteins (fibrinogen and hemoglobin). The results indicate that animal-derived exogenous surfactants that closely mimic natural surfactant by being extracted from lung lavage (Category I surfactants from Table 3) have an improved ability to resist inhibition by plasma proteins compared to the other preparations shown. Surfactant phospholipid concentration was 2 mg/ml. Redrawn from [51].
The biophysical activity differences found between surfactants in general reflect differences in their functional biochemical compositions. For example, the activity differences between CLSE and Survanta® in Figure 3 and Figure 4 relate primarily to differences in the content of SP-B in the two preparations [106, 118, 175]. Survanta has an SP-B content by ELISA of only 0.044% by weight relative to phospholipid, while CLSE (Infasurf®) has an SP-B content of 0.9% by weight that approaches that of lavaged whole surfactant [118]. SP-B is known to be the most active of the hydrophobic surfactant proteins in enhancing the adsorption, dynamic surface activity, and inhibition resistance of phospholipids [118, 176–184]. The addition of bovine SP-B or synthetic SP-B peptides to Survanta® significantly improves its surface and physiological activity towards that of Infasurf and whole surfactant [106, 118, 175] (Figure 5)‥ Despite its lack of SP-B, other active components such as SP-C in Survanta® allow it to have significant efficacy in some forms of ALI/ARDS as noted earlier (e.g., [130, 131, 133]). Treatment with Survanta® has not been found to give substantial improvements in adult patients with ARDS [137], but Category I exogenous surfactants with the complete mix of surfactant lipids and near-endogenous amounts of both SP-B and SP-C may prove to have increased efficacy.
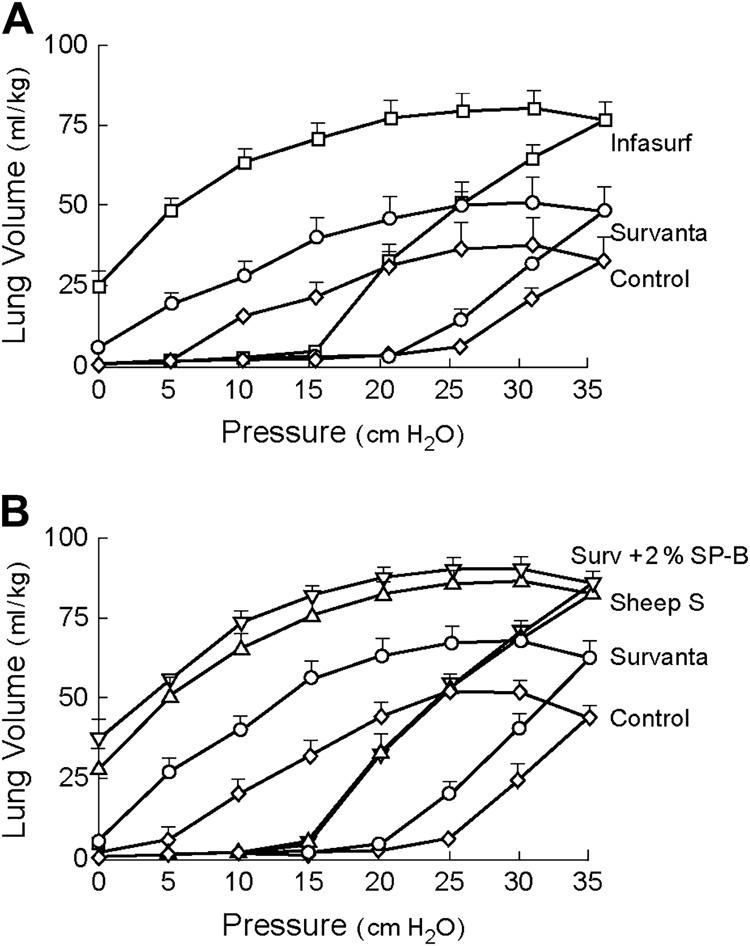
Figure 5. Effects on physiological activity from the addition of purified SP-B to Survanta®.
(Top): Premature rabbit fetuses (27 days gestation) treated with Survanta® or Infasurf®, and untreated controls; (Bottom): Premature rabbit fetuses treated with Survanta®, Survanta® + SP-B (2% by wt by ELISA), natural surfactant from adult sheep (Sheep S), or untreated controls. Infasurf® improved lung mechanics more than Survanta® (top panel), and the importance of SP-B in this behavior is shown by the increased activity of Survanta® + SP-B compared to Survanta® alone (bottom panel). Surfactants were instilled intratracheally at a dose of 100 mg/kg body weight, and quasistatic P-V curves were measured following 15 min of mechanical ventilation. Data are redrawn from Mizuno et al [175].
Activity differences between exogenous surfactants such as shown in Figure 3–Figure 5 are generally larger in magnitude than differences found in clinical comparison trials of surfactant drugs [114–117, 142–145, 168, 169]. This is because basic science studies are able to discriminate phenomena under controlled conditions and with more mechanistic detail than possible in clinical trials. Patient outcomes in clinical surfactant trials in patients with severe acute respiratory failure (whether they are premature infants or adults) are influenced by multiple variables unrelated to lung surfactant activity. Patient outcomes in surfactant trials can also be affected by secondary phenomena involving drug incorporation into type II cell recycling pathways or combination with small amounts of native surfactant apoproteins in the alveoli [46]. Even placebo-controlled studies of surfactant therapy in premature infants typically required substantial numbers of patients to demonstrate improvements in survival and other long term outcomes (as opposed to acute lung functional improvements) [46]. Mechanism-based differences in activity between exogenous surfactants in correlated biochemical, biophysical, and animal research are thus highly important in assessing and evaluating clinical findings.
VI. Examples of research on new synthetic exogenous surfactants for ALI/ARDS
In addition to investigating the activity and delivery of clinical exogenous surfactants, research is also attempting to develop new synthetic or semi-synthetic surfactants for treating lung injury ([46, 47, 185–188] for review). Synthetic lung surfactants manufactured under controlled conditions in the laboratory have significant potential advantages in purity, reproducibility, and quality control compared to animal-derived preparations. As biological products, animal surfactants have significant batch-to-batch variability that increases the cost and complexity of quality control testing during manufacture. Laboratory-synthesized drugs have the potential to be significantly less expensive over time once the costs of development are recovered, and they can be produced in unlimited amounts. Synthetic surfactants are also free from concerns about animal pathogens such as prions, and they are not subject to cultural or religious issues that can affect the use of bovine- or porcine-derived preparations in some countries. Considerations relating to drug dosage described in the preceding section illustrate the practical importance of synthetic preparations in treating ALI/ARDS, where amounts 50–100 times larger than those used in premature infants are required in adults and older children to achieve an equivalent dose on a body weight basis. A larger total number of surfactant doses per patient may also be necessary in patients with ALI/ARDS, further increasing the total amount of surfactant required. The cost of multiple doses of exogenous surfactant per adult patient would be prohibitive at a level even closely proportional to the expense of current 100–200 mg vials of animal-derived surfactants used in infants. The production of synthetic surfactants could theoretically be scaled up to large amounts with substantially reduced expense so as to allow more cost-effective therapy for ALI/ARDS.
Two important conceptual approaches being followed to develop synthetic exogenous surfactants are: (1) Combining human sequence recombinant proteins or synthetic amphipathic peptides with synthetic biological glycerophospholipids; and (2) combining synthetic amphipathic peptides with novel synthetic lipids designed to have favorable physicochemical properties such as high surface activity and structural resistance to inflammatory phospholipases during lung injury. Surfaxin® (KL4) [87, 107, 123, 189–195] and Venticute® (recombinant SPC surfactant) [26, 196–201] are two current examples of the first of these approaches (Table 3). However, the 21 amino acid KL4 peptide is only a very rough structural analog to native SP-B, and advances in peptide molecular modeling and synthesis technology support the feasibility of preparing new SP-B peptides with significantly greater sequence and molecular folding specificity, and correspondingly higher activity ([202–204] for review). The development of new synthetic SP-B peptides is particularly important for optimal synthetic exogenous surfactants given the greater activity of SP-B compared to SP-C in native surfactant noted earlier [118, 176–184]. In addition to peptide/protein components in new synthetic surfactants, it is also important to consider their lipid constituents. On a weight or molar basis, lipids are the major components of both endogenous and exogenous lung surfactants. Ester-linked glycerophospholipids of the kind found in native surfactant are the primary lipids used in current synthetic exogenous surfactants. However, synthetic ether-linked lipids are now available that have direct structural analogy to lung surfactant lipids plus designed molecular behavior that enhances adsorption and spreading while maintaining very high dynamic surface tension lowering in surface films (e.g., [71, 205–211]). Moreover, such lipids have structural features making them resistant to phospholipases such as phospholipase A2, which has been implicated in the pathology of ALI/ARDS [65, 212–218]. Phospholipase-induced degradation of lung surfactant glycerophospholipids not only reduces the concentration of active components, but also generates reaction products such as lysophosphatidylcholine and fluid free fatty acids that can further decrease surface activity by interacting biophysically with remaining surfactant at the alveolar interface [55, 59, 70]. Synthetic exogenous surfactants containing such phosphonolipids combined with purified surfactant proteins or synthetic peptides have very high overall surface activity plus an ability to resist phospholipases in ALI/ARDS [71, 208–211, 219]. On-going basic research is continuing to design and synthesize peptides related to SP-B and other surfactant proteins for use in highly-active fully-synthetic exogenous surfactants in combination with either normal glycerophospholipids or phospholipase-resistant analogs.
VI. Summary and future prospects for surfactant therapy in ALI/ARDS
Exogenous surfactant therapy is now standard in the prevention and treatment of RDS in premature infants, and basic science and clinical evidence support its use in at least some patients with lung injury-associated respiratory failure as described in this article. The efficacy of surfactant therapy in term infants with meconium aspiration is sufficiently documented that this is now a standard intervention in many neonatal intensive care units. Surfactant therapy is also frequently used in neonatal patients with ALI/ARDS (or related acute respiratory failure) from respiratory infections or pneumonia. Controlled trials of surfactant therapy in children with ALI/ARDS have shown significant benefits, with significant survival advantages in direct pulmonary lung injuries as reported in the recent trial of Willson et al [25]. The relative enthusiasm of pediatric intensive care specialists for surfactant therapy in respiratory failure to some extent probably reflects the fact that this intervention was originally defined for neonatal applications. The striking success of surfactant therapy in treating and preventing RDS in premature neonates, and the large body of accompanying basic research on the composition and activity of exogenous surfactants, have thus been particularly apparent to pediatric subspecialists.
Current clinical evidence supporting the use of surfactant in adults with ALI/ARDS is less extensive and compelling than in infants and children. However, the surfactants that have to date been most widely studied in adults with ARDS (Exosurf® and Survanta®) are known to have limitations in composition and activity compared to several other available preparations. Also, adult studies have not focused on direct pulmonary forms of ALI/ARDS, which is where surfactant therapy is most likely to be effective as a targeted intervention. In addition, neonatal data suggest that early surfactant administration generates improved responses compared to delayed administration (e.g., [220]), possibly as a result of better intrapulmonary drug distribution coupled with minimized ventilator-induced lung injury. It would make sense intuitively that similar advantages might accompany early surfactant administration in patients with ALI/ARDS. It is challenging and expensive to examine surfactant therapy in placebo-controlled clinical trials of substantial size in adults with ALI/ARDS, but further studies of direct pulmonary forms of these conditions treated with the most active available exogenous surfactant preparations are clearly warranted based both on pathophysiological understanding and on extensive biophysical and animal research.
Finally, a major issue with regard to surfactant therapy in ALI/ARDS involves its potential use in combination with agents or interventions that target additional aspects of the complex pathophysiology of acute pulmonary injury. This kind of combination therapy approach may be particularly important in adults with ALI/ARDS, where responses to exogenous surfactant have so far been disappointing. The use of multiple therapeutic agents or interventions based on specific rationales for potential synergy has the potential to significantly enhance patient outcomes in complex disease processes involving inflammatory lung injury. The potential use of exogenous surfactant therapy in the context of specific combined-modality interventions is described in detail elsewhere [119, 221, 222]. Examples of agents that might be synergistic with exogenous surfactant in ALI/ARDS include anti-inflammatory drugs such as steroids or receptor antagonists, antioxidants, and vasoactive drugs such as inhaled nitric oxide (iNO). In addition, specific ventilator modalities or ventilation strategies that reduce iatrogenic lung injury may be equally important to consider in conjunction with surfactant therapy. Given the known importance of surfactant dysfunction in inflammatory lung injury, it is likely that surfactant therapy alone or in combination with other agents will be applicable for adult as well as pediatric patients with ALI/ARDS associated with direct pulmonary injury.
Acknowledgments
This work was supported in part by grant HL-56176 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 3.American College of Chest Physicians Society of Critical Care Medicine Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies for sepsis; Crit Care Med; 1992. pp. 864–874. [PubMed] [Google Scholar]
- 4.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Slater A, Shann F. ANZICS Paediatric Study Group. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5:447–454. doi: 10.1097/01.PCC.0000138557.31831.65. [DOI] [PubMed] [Google Scholar]
- 6.Shann F, Pearson G, Slater A, et al. Paediatric index of mortality (PIM): A mortality prediction model for children in intensive care. Inten Care Med. 1997;23:201–207. doi: 10.1007/s001340050317. [DOI] [PubMed] [Google Scholar]
- 7.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 8.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 9.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, et al. The acute respiratory distress syndrome; definitions, severity, and clinical outcome. An analysis of 101 clinical investigations. Intensive Care Med. 1996;22:519–529. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 10.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury: Predictors of mortality. Am J Respir Crit Care Med. 1995;152:1818–1824. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000;342:1334–1348. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 12.Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003;31(suppl):S276–S284. doi: 10.1097/01.CCM.0000057904.62683.2B. [DOI] [PubMed] [Google Scholar]
- 13.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 14.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. ARDS Network. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 15.Pfenninger J, Gerber A, Tschappeler H, Zimmerman A. Adult respiratory distress syndrome in children. J Pediatr. 1982;101:352–357. doi: 10.1016/s0022-3476(82)80057-7. [DOI] [PubMed] [Google Scholar]
- 16.Bindl L, Dresbach K, Lentze M. Incidence of acute respiratory distress syndrome in German children and adolescents: A population based study. Crit Care Med. 2005;33:209–212. doi: 10.1097/01.ccm.0000151137.76768.08. [DOI] [PubMed] [Google Scholar]
- 17.Manzano F, Yuste E, Colmenero M, et al. Incidence of acute respiratory distress syndrome and its relation to age. J Crit Care. 2005;20:274–280. doi: 10.1016/j.jcrc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Randolph AG, Wypij D, Venkataraman ST, et al. Effect of mechanical ventilator weaning protocols on respiratory putcomes in infants and children. A randomized controlled trial. JAMA. 2002;288:2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 19.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury. Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 20.The Paediatric Study Group of the Australian and New Zealand Intensive Care Society (ANZICS) Acute lung injury in pediatric intensive care in australia and new zealand - A prospective, multicentre, observational study. Pediatr Crit Care Med. 2007;8:317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 21.DeBruin W, Notterman DA, Magid M, et al. Acute hypoxemic respiratory failure in infants and children: Clinical and pathologic characteristics. Crit Care Med. 1992;20:1223–1234. doi: 10.1097/00003246-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Timmons OD, Havens PL, Fackler JC. (Pediatric Critical Care Study Group and the Extracorporeal Life Support Organization). Predicting death in pediatric patients with acute respiratory failure. Chest. 1995;108:789–797. doi: 10.1378/chest.108.3.789. [DOI] [PubMed] [Google Scholar]
- 23.Dahlem P, van Aalderen WMC, Hamaker ME, et al. Incidence and short-term outcome of acute lung injury in mechanically ventilated children. Eur Respir J. 2003;22:980–983. doi: 10.1183/09031936.03.00003303. [DOI] [PubMed] [Google Scholar]
- 24.Curley MAQ, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury. A randomized controlled trial. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willson DF, Thomas NJ, Markovitz BP, DiCarlo JV, Pon S, Jacobs BR, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 26.Spragg RG, Lewis JF, Wurst W, Hafner D, Baughman RP, Wewers MD, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Holm BA, Matalon S, Notter RH. Surfactant activity and dysfunction in lung injury. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: Mechanisms, pathophysiology, and therapy. Boca Raton: Taylor Francis Group, Inc; 2005. pp. 297–352. [Google Scholar]
- 28.Russo TA, Bartholomew LA, Davidson BA, Helinski JD, Carlino UB, Knight PR, et al. Total extracellular surfactant is increased but abnormal in a rat model of Gram-negative bacterial pneumonitis. Am J Physiol. 2002;283:L655–L663. doi: 10.1152/ajplung.00071.2002. [DOI] [PubMed] [Google Scholar]
- 29.Russo TA, Wang Z, Davidson BA, Genagon SA, Beanan JM, Olsen R, et al. Surfactant dysfunction and lung injury due to the E. coli virulence factor hemolysin in a rat pneumonia model. Am J Physiol: Lung Cell Mol Physiol. 2007;292:L632–L643. doi: 10.1152/ajplung.00326.2006. [DOI] [PubMed] [Google Scholar]
- 30.Davidson BA, Knight PR, Wang Z, Chess PR, Holm BA, Russo TA, et al. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol: Lung Cell Mol Physiol. 2005;288:L699–L708. doi: 10.1152/ajplung.00229.2004. [DOI] [PubMed] [Google Scholar]
- 31.Wright TW, Notter RH, Wang Z, Harmsen AG, Gigliotti F. Pulmonary inflammation disrupts surfactant function during P. carinii pneumonia. Infect Immun. 2001;69:758–764. doi: 10.1128/IAI.69.2.758-764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruckner L, Gigliotti F, Wright TW, Harmsen A, Notter RH, Chess PR, et al. Pneumocystis carinii infection sensitizes the lung to radiation-induced injury after syngeneic marrow transplantation: Role of CD4+ T-cells. Am J Physiol: Lung Cell Mol Physiol. 2006;290:L1087–L1096. doi: 10.1152/ajplung.00441.2005. [DOI] [PubMed] [Google Scholar]
- 33.Petty T, Reiss O, Paul G, Silvers G, Elkins N. Characteristics of pulmonary surfactant in adult respiratory distress syndrome associated with trauma and shock. Am Rev Respir Dis. 1977;115:531–536. doi: 10.1164/arrd.1977.115.3.531. [DOI] [PubMed] [Google Scholar]
- 34.Hallman M, Spragg R, Harrell JH, Moser KM, Gluck L. Evidence of lung surfactant abnormality in respiratory failure. J Clin Invest. 1982;70:673–683. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeger W, Pison U, Buchhorn R, Obestacke U, Joka T. Surfactant abnormalities and adult respiratory failure. Lung. 1990;168(Suppl):891–902. doi: 10.1007/BF02718225. [DOI] [PubMed] [Google Scholar]
- 36.Pison U, Seeger W, Buchhorn R, Joka T, Brand M, Obertacke U, et al. Surfactant abnormalities in patients with respiratory failure after multiple trauma. Am Rev Respir Dis. 1989;140:1033–1039. doi: 10.1164/ajrccm/140.4.1033. [DOI] [PubMed] [Google Scholar]
- 37.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88:1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Günther A, Siebert C, Schmidt R, Ziegle S, Grimminger F, Yabut M, et al. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153:176–184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 39.Veldhuizen RAW, McCaig LA, Akino T, Lewis JF. Pulmonary surfactant subfractions in patients with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:1867–1871. doi: 10.1164/ajrccm.152.6.8520748. [DOI] [PubMed] [Google Scholar]
- 40.Griese M. Pulmonary surfactant in health and human lung diseases: State of the art. Eur Respir J. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- 41.Mander A, Langton-Hewer S, Bernhard W, Warner JO, Postle AD. Altered phospholipid composition and aggregate structure of lung surfactant is associated with impaired lung function in young children with respiratory infections. Am J Respir Cell Mol Biol. 2002;27:714–721. doi: 10.1165/rcmb.4746. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt R, Meier U, Markart P, Grimminger F, Velcovsky HG, Morr H, et al. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am J Physiol. 2002;283:L1079–L1085. doi: 10.1152/ajplung.00484.2001. [DOI] [PubMed] [Google Scholar]
- 43.Skelton R, Holland P, Darowski M, Chetcuti P, Morgan L, Harwood J. Abnormal surfactant composition and activity in severe bronchiolitis. Acta Paediatr. 1999;88:942–946. doi: 10.1080/08035259950168414. [DOI] [PubMed] [Google Scholar]
- 44.LeVine AM, Lotze A, Stanley S, Stroud C, O'Donnell R, Whitsett J, et al. Surfactant content in children with inflammatory lung disease. Crit Care Med. 1996;24:1062–1067. doi: 10.1097/00003246-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 45.Dargaville PA, South M, McDougall PN. Surfactant abnormalities in infants with severe viral bronchiolitis. Arch Dis Child. 1996;75:133–136. doi: 10.1136/adc.75.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notter RH. Lung surfactants: Basic science and clinical applications. New York: Marcel Dekker, Inc; 2000. [Google Scholar]
- 47.Notter RH, Wang Z. Pulmonary surfactant: Physical chemistry, physiology and replacement. Rev Chem Eng. 1997;13:1–118. [Google Scholar]
- 48.Holm BA, Notter RH, Finkelstein JH. Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem Phys Lipids. 1985;38:287–298. doi: 10.1016/0009-3084(85)90022-2. [DOI] [PubMed] [Google Scholar]
- 49.Seeger W, Stohr G, Wolf HRD, Neuhof H. Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J Appl Physiol. 1985;58:326–338. doi: 10.1152/jappl.1985.58.2.326. [DOI] [PubMed] [Google Scholar]
- 50.Holm BA, Notter RH. Effects of hemoglobin and cell membrane lipids on pulmonary surfactant activity. J Appl Physiol. 1987;63:1434–1442. doi: 10.1152/jappl.1987.63.4.1434. [DOI] [PubMed] [Google Scholar]
- 51.Seeger W, Grube C, Günther A, Schmidt R. Surfactant inhibition by plasma proteins: Differential sensitivity of various surfactant preparations. Eur Respir J. 1993;6:971–977. [PubMed] [Google Scholar]
- 52.Holm BA, Enhorning G, Notter RH. A biophysical mechanism by which plasma proteins inhibit lung surfactant activity. Chem Phys Lipids. 1988;49:49–55. doi: 10.1016/0009-3084(88)90063-1. [DOI] [PubMed] [Google Scholar]
- 53.Fuchimukai T, Fujiwara T, Takahashi A, Enhorning G. Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol. 1987;62:429–437. doi: 10.1152/jappl.1987.62.2.429. [DOI] [PubMed] [Google Scholar]
- 54.Keough KWM, Parsons CS, Tweeddale MG. Interactions between plasma proteins and pulmonary surfactant: Pulsating bubble studies. Can J Physiol Pharmacol. 1989;67:663–668. doi: 10.1139/y89-106. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Notter RH. Additivity of protein and non-protein inhibitors of lung surfactant activity. Am J Respir Crit Care Med. 1998;158:28–35. doi: 10.1164/ajrccm.158.1.9709041. [DOI] [PubMed] [Google Scholar]
- 56.Moses D, Holm BA, Spitale P, Liu M, Enhorning G. Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol. 1991;164:477–481. doi: 10.1016/s0002-9378(11)80003-7. [DOI] [PubMed] [Google Scholar]
- 57.Cockshutt A, Possmayer F. Lysophosphatidylcholine sensitizes lipid extracts of pulmonary surfactant to inhibition by plasma proteins. Biochim Biophys Acta. 1991;1086:63–71. doi: 10.1016/0005-2760(91)90155-b. [DOI] [PubMed] [Google Scholar]
- 58.Seeger W, Lepper H, Hellmut RD, Neuhof H. Alteration of alveolar surfactant function after exposure to oxidant stress and to oxygenated and native arachadonic acid in vitro. Biochim Biophys Acta. 1985;835:58–67. doi: 10.1016/0005-2760(85)90030-x. [DOI] [PubMed] [Google Scholar]
- 59.Hall SB, Lu ZR, Venkitaraman AR, Hyde RW, Notter RH. Inhibition of pulmonary surfactant by oleic acid: Mechanisms and characteristics. J Appl Physiol. 1992;72:1708–1716. doi: 10.1152/jappl.1992.72.5.1708. [DOI] [PubMed] [Google Scholar]
- 60.Hall SB, Hyde RW, Notter RH. Changes in subphase surfactant aggregates in rabbits injured by free fatty acid. Am J Respir Crit Care Med. 1994;149:1099–1106. doi: 10.1164/ajrccm.149.5.8173747. [DOI] [PubMed] [Google Scholar]
- 61.Hickman-Davis JM, Fang FC, Nathan C, Shepherd VL, Voelker DR, Wright JR. Lung surfactant and reactive oxygen-nitrogen species: antimicrobial activity and host-pathogen interactions. Am J Physiol. 2001;281:L517–L523. doi: 10.1152/ajplung.2001.281.3.L517. [DOI] [PubMed] [Google Scholar]
- 62.Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol. 1993;265:L555–L564. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- 63.Amirkhanian JD, Merritt TA. Inhibitory effects of oxyradicals on surfactant function: Utilizing in vitro Fenton reaction. Lung. 1998;176:63–72. doi: 10.1007/pl00007592. [DOI] [PubMed] [Google Scholar]
- 64.Pison U, Tam EK, Caughey GH, Hawgood S. Proteolytic inactivation of dog lung surfactant-associated proteins by neutrophil elastase. Biochim Biophys Acta. 1989;992:251–257. doi: 10.1016/0304-4165(89)90082-2. [DOI] [PubMed] [Google Scholar]
- 65.Holm BA, Kelcher L, Liu M, Sokolowski J, Enhorning G. Inhibition of pulmonary surfactant by phospholipases. J Appl Physiol. 1991;71:317–321. doi: 10.1152/jappl.1991.71.1.317. [DOI] [PubMed] [Google Scholar]
- 66.Enhorning G, Shumel B, Keicher L, Sokolowski J, Holm BA. Phospholipases introduced into the hypophase affect the surfactant film outlining a bubble. J Appl Physiol. 1992;73:941–945. doi: 10.1152/jappl.1992.73.3.941. [DOI] [PubMed] [Google Scholar]
- 67.Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147:218–233. doi: 10.1164/ajrccm/147.1.218. [DOI] [PubMed] [Google Scholar]
- 68.Seeger W, Günther A, Walmrath HD, Grimminger F, Lasch HG. Alveolar surfactant and adult respiratory distress syndrome. Pathogenic role and therapeutic prospects. Clin Invest. 1993;71:177–190. doi: 10.1007/BF00180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lachmann B, van Daal G-J. Adult respiratory distress syndrome: animal models. In: Robertson B, van Golde LMG, Batenburg JJ, editors. Pulmonary Surfactant: From Molecular Biology to Clinical Practice. Amsterdam: Elsevier Science Publishers; 1992. pp. 635–663. [Google Scholar]
- 70.Holm BA, Wang Z, Notter RH. Multiple mechanisms of lung surfactant inhibition. Pediatr Res. 1999;46:85–93. doi: 10.1203/00006450-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Schwan AL, Lairson LL, O'Donnell JS, Byrne GF, Foye A, et al. Surface activity of a synthetic lung surfactant containing a phospholipase-resistant phosphonolipid analog of dipalmitoyl phosphatidylcholine. Am J Physiol. 2003;285:L550–L559. doi: 10.1152/ajplung.00346.2002. [DOI] [PubMed] [Google Scholar]
- 72.Magoon MW, Wright JR, Baritussio A, Williams MC, Goerke J, Benson BJ, et al. Subfractionation of lung surfactant: Implications for metabolism and surface activity. Biochim Biophys Acta. 1983;750:18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- 73.Wright JR, Benson BJ, Williams MC, Goerke J, Clements JA. Protein composition of rabbit alveolar surfactant subfractions. Biochim Biophys Acta. 1984;791:320–332. doi: 10.1016/0167-4838(84)90343-1. [DOI] [PubMed] [Google Scholar]
- 74.Gross NJ, Narine KR. Surfactant subtypes in mice: Characterization and quantitation. J Appl Physiol. 1989;66:342–349. doi: 10.1152/jappl.1989.66.1.342. [DOI] [PubMed] [Google Scholar]
- 75.Putz G, Goerke J, Clements JA. Surface activity of rabbit pulmonary surfactant subfractions at different concentrations in a captive bubble. J Appl Physiol. 1994;77:597–605. doi: 10.1152/jappl.1994.77.2.597. [DOI] [PubMed] [Google Scholar]
- 76.Putman E, Creuwels LAJM, Van Golde LMG, Haagsman HP. Surface properties, morphology and protein composition of pulmonary surfactant subtypes. Biochem J. 1996;320:599–605. doi: 10.1042/bj3200599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Veldhuizen RAW, Hearn SA, Lewis JF, Possmayer F. Surface-area cycling of different surfactant preparations: SP-A and SP-B are essential for large aggregate integrity. Biochem J. 1994;300:519–524. doi: 10.1042/bj3000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gross NJ. Extracellular metabolism of pulmonary surfactant: the role of a new serine protease. Ann Rev Physiol. 1995;57:135–150. doi: 10.1146/annurev.ph.57.030195.001031. [DOI] [PubMed] [Google Scholar]
- 79.Lewis JF, Ikegami M, Jobe AH. Altered surfactant function and metabolism in rabbits with acute lung injury. J Appl Physiol. 1990;69:2303–2310. doi: 10.1152/jappl.1990.69.6.2303. [DOI] [PubMed] [Google Scholar]
- 80.Putman E, Boere AJ, van Bree L, van Golde LMG, Haagsman HP. Pulmonary surfactant subtype metabolism is altered after short-term ozone exposure. Toxicol Appl Pharmacol. 1995;134:132–138. doi: 10.1006/taap.1995.1176. [DOI] [PubMed] [Google Scholar]
- 81.Atochina EN, Beers MF, Scanlon ST, Preston AM, Beck JM. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am J Physiol. 2000;278:L599–L609. doi: 10.1152/ajplung.2000.278.3.L599. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi T, Ganzuka M, Taniguchi J, Nitta K, Murakami S. Lung lavage and surfactant replacement for hydrochloric acid aspiration in rabbits. Acta Anaesthesiol Scand. 1990;34:216–221. doi: 10.1111/j.1399-6576.1990.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 83.Zucker A, Holm BA, Wood LDH, Crawford G, Ridge K, Sznajder IA. Exogenous surfactant with PEEP reduces pulmonary edema and improves lung function in canine aspiration pneumonitis. J Appl Physiol. 1992;73:679–686. doi: 10.1152/jappl.1992.73.2.679. [DOI] [PubMed] [Google Scholar]
- 84.Schlag G, Strohmaier W. Experimental aspiration trauma: Comparison of steroid treatment versus exogenous natural surfactant. Exp Lung Res. 1993;19:397–405. doi: 10.3109/01902149309064354. [DOI] [PubMed] [Google Scholar]
- 85.Al-Mateen KB, Dailey K, Grimes MM, Gutcher GR. Improved oxygenation with exogenous surfactant administration in experimental meconium aspiration syndrome. Pediatr Pulmonol. 1994;17:75–80. doi: 10.1002/ppul.1950170202. [DOI] [PubMed] [Google Scholar]
- 86.Sun B, Curstedt T, Robertson B. Exogenous surfactant improves ventilation efficiency and alveolar expansion in rats with meconium aspiration. Am J Respir Crit Care Med. 1996;154:764–770. doi: 10.1164/ajrccm.154.3.8810617. [DOI] [PubMed] [Google Scholar]
- 87.Cochrane CG, Revak SD, Merritt TA, Schraufstatter U, Hoch RC, Henderson C, et al. Bronchoalveolar lavage with KL4-surfactant in models of meconium aspiration syndrome. Pediatr Res. 1998;44:705–715. doi: 10.1203/00006450-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Sun B, Curstedt T, Song GW, Robertson B. Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biol Neonate. 1993;63:96–104. doi: 10.1159/000243917. [DOI] [PubMed] [Google Scholar]
- 89.Lachmann B, Hallman M, Bergman K-C. Respiratory failure following anti-lung serum: Study on mechanisms associated with surfactant system damage. Exp Lung Res. 1987;12:163–180. doi: 10.3109/01902148709062839. [DOI] [PubMed] [Google Scholar]
- 90.Nieman G, Gatto L, Paskanik A, Yang B, Fluck R, Picone A. Surfactant replacement in the treatment of sepsis-induced adult respiratory distress syndrome in pigs. Crit Care Med. 1996;24:1025–1033. doi: 10.1097/00003246-199606000-00024. [DOI] [PubMed] [Google Scholar]
- 91.Lutz C, Carney D, Finck C, Picone A, Gatto L, Paskanik A, et al. Aerosolized surfactant improves pulmonary function in endotoxin-induced lung injury. Am J Respir Crit Care Med. 1998;158:840–845. doi: 10.1164/ajrccm.158.3.9801089. [DOI] [PubMed] [Google Scholar]
- 92.Lutz CJ, Picone A, Gatto LA, Paskanik A, Landas S, Nieman G. Exogenous surfactant and positive end-expiratory pressure in the treatment of endotoxin-induced lung injury. Crit Care Med. 1998;26:1379–1389. doi: 10.1097/00003246-199808000-00025. [DOI] [PubMed] [Google Scholar]
- 93.Tashiro K, Li W-Z, Yamada K, Matsumoto Y, Kobayashi T. Surfactant replacement reverses respiratory failure induced by intratracheal endotoxin in rats. Crit Care Med. 1995;23:149–156. doi: 10.1097/00003246-199501000-00024. [DOI] [PubMed] [Google Scholar]
- 94.Eijking EP, van Daal GJ, Tenbrinck R, Luyenduijk A, Sluiters JF, Hannappel E, et al. Effect of surfactant replacement on Pneumocystis carinii pneumonia in rats. Intensive Care Med. 1990;17:475–478. doi: 10.1007/BF01690770. [DOI] [PubMed] [Google Scholar]
- 95.Sherman MP, Campbell LA, Merritt TA, Long WA, Gunkel JH, Curstedt T, et al. Effect of different surfactants on pulmonary group B streptococcal infection in premature rabbits. J Pediatr. 1994;125:939–947. doi: 10.1016/s0022-3476(05)82013-x. [DOI] [PubMed] [Google Scholar]
- 96.Berry D, Ikegami M, Jobe A. Respiratory distress and surfactant inhibition following vagotomy in rabbits. J Appl Physiol. 1986;61:1741–1748. doi: 10.1152/jappl.1986.61.5.1741. [DOI] [PubMed] [Google Scholar]
- 97.Matalon S, Holm BA, Notter RH. Mitigation of pulmonary hyperoxic injury by administration of exogenous surfactant. J Appl Physiol. 1987;62:756–761. doi: 10.1152/jappl.1987.62.2.756. [DOI] [PubMed] [Google Scholar]
- 98.Loewen GM, Holm BA, Milanowski L, Wild LM, Notter RH, Matalon S. Alveolar hyperoxic injury in rabbits receiving exogenous surfactant. J Appl Physiol. 1989;66:1987–1992. doi: 10.1152/jappl.1989.66.3.1087. [DOI] [PubMed] [Google Scholar]
- 99.Engstrom PC, Holm BA, Matalon S. Surfactant replacement attenuates the increase in alveolar permeability in hyperoxia. J Appl Physiol. 1989;67:688–693. doi: 10.1152/jappl.1989.67.2.688. [DOI] [PubMed] [Google Scholar]
- 100.Matalon S, Holm BA, Loewen GM, Baker RR, Notter RH. Sublethal hyperoxic injury to the alveolar epithelium and the pulmonary surfactant system. Exp Lung Res. 1988;14:1021–1033. doi: 10.3109/01902148809064190. [DOI] [PubMed] [Google Scholar]
- 101.Novotny WE, Hudak BB, Matalon S, Holm BA. Hyperoxic lung injury reduces exogenous surfactant clearance in vitro. Am J Respir Crit Care Med. 1995;151:1843–1847. doi: 10.1164/ajrccm.151.6.7767528. [DOI] [PubMed] [Google Scholar]
- 102.Lachmann B, Fujiwara T, Chida S, Morita T, Konishi M, Nakamura K, et al. Surfactant replacement therapy in experimental adult respiratory distress syndrome (ARDS) In: Cosmi EV, Scarpelli EM, editors. Pulmonary Surfactant System. Amsterdam: Elsevier; 1983. pp. 221–235. [Google Scholar]
- 103.Kobayashi T, Kataoka H, Ueda T, Murakami S, Takada Y, Kobuko M. Effect of surfactant supplementation and end expiratory pressure in lung-lavaged rabbits. J Appl Physiol. 1984;57:995–1001. doi: 10.1152/jappl.1984.57.4.995. [DOI] [PubMed] [Google Scholar]
- 104.Berggren P, Lachmann B, Curstedt T, Grossmann G, Robertson B. Gas exchange and lung morphology after surfactant replacement in experimental adult respiratory distress induced by repeated lung lavage. Acta Anaesthesiol Scand. 1986;30:321–328. doi: 10.1111/j.1399-6576.1986.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 105.Lewis JF, Goffin J, Yue P, McCaig LA, Bjarneson D, Veldhuizen RAW. Evaluation of exogenous surfactant treatment strategies in an adult model of acute lung injury. J Appl Physiol. 1996;80:1156–1164. doi: 10.1152/jappl.1996.80.4.1156. [DOI] [PubMed] [Google Scholar]
- 106.Walther FJ, Hernandez-Juviel J, Bruni R, Waring A. Spiking Survanta with synthetic surfactant peptides improves oxygenation in surfactant-deficient rats. Am J Respir Crit Care Med. 1997;156:855–861. doi: 10.1164/ajrccm.156.3.9611053. [DOI] [PubMed] [Google Scholar]
- 107.Walther F, Hernandez-Juviel J, Bruni R, Waring AJ. Protein composition of synthetic surfactant affects gas exchange in surfactant-deficient rats. Pediatr Res. 1998;43:666–673. doi: 10.1203/00006450-199805000-00016. [DOI] [PubMed] [Google Scholar]
- 108.Harris JD, Jackson F, Moxley MA, Longmore WJ. Effect of exogenous surfactant instillation on experimental acute lung injury. J Appl Physiol. 1989;66:1846–1851. doi: 10.1152/jappl.1989.66.4.1846. [DOI] [PubMed] [Google Scholar]
- 109.Lewis JF, Ikegami M, Jobe AH. Metabolism of exogenously administered surfactant in the acutely injured lungs of adult rabbits. Am Rev Respir Dis. 1992;145:19–23. doi: 10.1164/ajrccm/145.1.19. [DOI] [PubMed] [Google Scholar]
- 110.Lewis J, Ikegami M, Higuchi R, Jobe A, Absolom D. Nebulized vs instilled exogenous surfactant in an adult lung injury model. J Appl Physiol. 1991;71:1270–1276. doi: 10.1152/jappl.1991.71.4.1270. [DOI] [PubMed] [Google Scholar]
- 111.van Daal GJ, So KL, Gommers D, Eijking EP, Fievez RB, Sprenger MJ, et al. Intratracheal surfactant administration restores gas exchange in experimental adult respiratory distress syndrome associated with viral pneumonia. Anesth Analg. 1991;72:589–595. [PubMed] [Google Scholar]
- 112.van Daal GJ, Bos JAH, Eijking EP, Gommers D, Hannappel E, Lachmann B. Surfactant replacement therapy improves pulmonary mechanics in end-stage influenza A pneumonia in mice. Am Rev Respir Dis. 1992;145:859–863. doi: 10.1164/ajrccm/145.4_Pt_1.859. [DOI] [PubMed] [Google Scholar]
- 113.Hall SB, Venkitaraman AR, Whitsett JA, Holm BA, Notter RH. Importance of hydrophobic apoproteins as constituents of clinical exogenous surfactants. Am Rev Respir Dis. 1992;145:24–30. doi: 10.1164/ajrccm/145.1.24. [DOI] [PubMed] [Google Scholar]
- 114.Hudak ML, Farrell EE, Rosenberg AA, Jung AL, Auten RL, Durand DJ, et al. A multicenter randomized masked comparison of natural vs synthetic surfactant for the treatment of respiratory distress syndrome. J Pediatr. 1996;128:396–406. doi: 10.1016/s0022-3476(96)70291-3. [DOI] [PubMed] [Google Scholar]
- 115.Hudak ML, Martin DJ, Egan EA, Matteson EJ, Cummings J, Jung AL, et al. A multicenter randomized masked comparison trial of synthetic surfactant versus calf lung surfactant extract in the prevention of neonatal respiratory distress syndrome. Pediatrics. 1997;100:39–50. doi: 10.1542/peds.100.1.39. [DOI] [PubMed] [Google Scholar]
- 116.Vermont-Oxford Neonatal Network. A multicenter randomized trial comparing synthetic surfactant with modified bovine surfactant extract in the treatment of neonatal respiratory distress syndrome. Pediatrics. 1996;97:1–6. [PubMed] [Google Scholar]
- 117.Horbar JD, Wright LL, Soll RF, Wright EC, Fanaroff AA, Korones SB, et al. A multicenter randomized trial comparing two surfactants for the treatment of neonatal respiratory distress syndrome. J Pediatr. 1993;123:757–766. doi: 10.1016/s0022-3476(05)80856-x. [DOI] [PubMed] [Google Scholar]
- 118.Notter RH, Wang Z, Egan EA, Holm BA. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids. 2002;114:21–34. doi: 10.1016/s0009-3084(01)00197-9. [DOI] [PubMed] [Google Scholar]
- 119.Chess P, Finkelstein JN, Holm BA, Notter RH. Surfactant replacement therapy in lung injury. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: Mechanisms, pathophysiology, and therapy. Boca Raton: Taylor Francis Group, Inc; 2005. pp. 617–663. [Google Scholar]
- 120.Gunther A, Schmidt R, Harodt J, Schmehl T, et al. Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: impact on biophysical and biochemical surfactant properties. Eur Respir J. 2002;10:797–804. doi: 10.1183/09031936.02.00243302. [DOI] [PubMed] [Google Scholar]
- 121.Walmrath D, Gunther A, Ghofrani HA, Schermuly R, Schnedier T, Grimminger F, et al. Bronchoscopic surfactant administration in patients with severe adult respiratory distress syndrome and sepsis. Am J Respir Crit Care Med. 1996;154:57–62. doi: 10.1164/ajrccm.154.1.8680699. [DOI] [PubMed] [Google Scholar]
- 122.Spragg R, Gilliard N, Richman P, Smith R, Hite R, Pappert D, et al. Acute effects of a single dose of porcine surfactant on patients with the adult respiratory distress syndrome. Chest. 1994;105:195–202. doi: 10.1378/chest.105.1.195. [DOI] [PubMed] [Google Scholar]
- 123.Wiswell TE, Smith RM, Katz LB, Mastroianni L, Wong DY, Willms D, et al. Bronchopulmonary segmental lavage with Surfaxin (KL(4) - surfactant) for acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:1188–1195. doi: 10.1164/ajrccm.160.4.9808118. [DOI] [PubMed] [Google Scholar]
- 124.Willson DF, Jiao JH, Bauman LA, Zaritsky A, Craft H, Dockery K, et al. Calf lung surfactant extract in acute hypoxemic respiratory failure in children. Crit Care Med. 1996;24:1316–1322. doi: 10.1097/00003246-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 125.Willson DF, Bauman LA, Zaritsky A, Dockery K, James RL, Stat M, et al. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Crit Care Med. 1999;27:188–195. doi: 10.1097/00003246-199901000-00050. [DOI] [PubMed] [Google Scholar]
- 126.Lopez-Herce J, de Lucas N, Carrillo A, Bustinza A, Moral R. Surfactant treatment for acute respiratory distress syndrome. Arch Dis Child. 1999;80:248–252. doi: 10.1136/adc.80.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hermon MM, Golej J, Burda H, et al. Surfactant therapy in infants and children: three years experience in a pediatric intensive care unit. Shock. 2002;17:247–251. doi: 10.1097/00024382-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 128.Herting E, Moller O, Schiffman JH, Robertson B. Surfactant improves oxygenation in infants and children with pneumonia and acute respiratory distress syndrome. Acta Paediatr. 2002;91:1174–1178. doi: 10.1080/080352502320777397. [DOI] [PubMed] [Google Scholar]
- 129.Auten RL, Notter RH, Kendig JW, Davis JM, Shapiro DL. Surfactant treatment of full-term newborns with respiratory failure. Pediatrics. 1991;87:101–107. [PubMed] [Google Scholar]
- 130.Lotze A, Knight GR, Martin GR, Bulas DI, Hull WM, O'Donnell RM, et al. Improved pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr. 1993;122:261–268. doi: 10.1016/s0022-3476(06)80131-9. [DOI] [PubMed] [Google Scholar]
- 131.Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. J Pediatr. 1998;132:40–47. doi: 10.1016/s0022-3476(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 132.Khammash H, Perlman M, Wojtulewicz J, Dunn M. Surfactant therapy in full-term neonates with severe respiratory failure. Pediatrics. 1993;92:135–139. [PubMed] [Google Scholar]
- 133.Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97:48–52. [PubMed] [Google Scholar]
- 134.Luchetti M, Casiraghi G, Valsecchi R, Galassini E, Marraro G. Porcine-derived surfactant treatment of severe bronchiolitis. Acta Anaesthesiol Scand. 1998;42:805–810. doi: 10.1111/j.1399-6576.1998.tb05326.x. [DOI] [PubMed] [Google Scholar]
- 135.Luchetti M, Ferrero F, Gallini C, Natale A, Pigna A, Tortorolo L, et al. Multicenter, randomized, controlled study of porcine surfactant in severe respiratory syncytial virus-induced respiratory failure. Pediatr Crit Care Med. 2002;3:261–268. doi: 10.1097/00130478-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 136.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. The New England journal of medicine. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 137.Gregory TJ, Steinberg KP, Spragg R, Gadek JE, Hyers TM, Longmere WJ, et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:109–131. doi: 10.1164/ajrccm.155.4.9105072. [DOI] [PubMed] [Google Scholar]
- 138.Clark DA, Nieman GF, Thompson JE, Paskanik AM, Rokhar JE, Bredenberg CE. Surfactant displacement by meconium free fatty acids: An alternative explanation for atelectasis in meconium aspiration syndrome. J Pediatr. 1987;110:765–770. doi: 10.1016/s0022-3476(87)80021-5. [DOI] [PubMed] [Google Scholar]
- 139.Tibby SM, Hatherill M, Wright SM, Wilson P, Postle AD, Murdoch IA. Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2000;162:1251–1256. doi: 10.1164/ajrccm.162.4.9909004. [DOI] [PubMed] [Google Scholar]
- 140.Ivascu FA, Hirschl RB. New approaches to managing congenital diaphragmatic hernia. Semin in Perinatology. 2004;28:185–198. doi: 10.1053/j.semperi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 141.Congenital Diaphragmatic Hernia Study Group. Van Meurs K. Is surfactant therapy beneficial in the treatment of the term newborn infants with congenital diaphragmatic hernia? J Pediatr. 2004;145:312–316. doi: 10.1016/j.jpeds.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 142.Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR, et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of RDS. Pediatrics. 1997;100:31–38. doi: 10.1542/peds.100.1.31. [DOI] [PubMed] [Google Scholar]
- 143.Rollins M, Jenkins J, Tubman R, Corkey C, Wilson D. Comparison of clinical responses to natural and synthetic surfactants. J Perinat Med. 1993;21:341–347. doi: 10.1515/jpme.1993.21.5.341. [DOI] [PubMed] [Google Scholar]
- 144.Sehgal SS, Ewing CK, Richards T, Taeusch HW. Modified bovine surfactant (Survanta) vs a protein-free surfactant (Exosurf) in the treatment of respiratory distress syndrome in preterm infants: A pilot study. J Natl Med Assoc. 1994;86:46–52. [PMC free article] [PubMed] [Google Scholar]
- 145.Choukroun ML, Llanas B, Apere H, Fayon M, Galperine RI, Guenard H, et al. Pulmonary mechanics in ventilated preterm infants with respiratory distress syndrome after exogenous surfactant administration: A comparison between two surfactant preparations. Pediatr Pulmonol. 1994;18:273–298. doi: 10.1002/ppul.1950180502. [DOI] [PubMed] [Google Scholar]
- 146.Surfactant ARDS Study Group. Moller JC, Schaible T, Roll C, et al. Treatment with bovine surfactant in severe acute respiratory distress syndrome in children: a randomized multicenter study. Inten Care Med. 2003;29:437–446. doi: 10.1007/s00134-003-1650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Davis JM, Russ GA, Metlay L, Dickerson B, Greenspan BS. Short-term distribution kinetics in intratracheally administered exogenous lung surfactant. Pediatr Res. 1992;31:445–450. doi: 10.1203/00006450-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 148.Espinosa FF, Shapiro AH, Fredberg JJ, Kamm RD. Spreading of exogenous surfactant in an airway. J Appl Physiol. 1993;75:2028–2039. doi: 10.1152/jappl.1993.75.5.2028. [DOI] [PubMed] [Google Scholar]
- 149.Grotberg JB, Halpern D, Jensen OE. Interaction of exogenous and endogenous surfactant: Spreading-rate effects. J Appl Physiol. 1995;78:750–756. doi: 10.1152/jappl.1995.78.2.750. [DOI] [PubMed] [Google Scholar]
- 150.Marks LB, Notter RH, Oberdoerster G, McBride JT. Ultrasonic and jet aerosolization of phospholipids and the effects on surface activity. Pediatr Res. 1984;17:742–747. doi: 10.1203/00006450-198309000-00012. [DOI] [PubMed] [Google Scholar]
- 151.Balaraman V, Meister J, Ku TL, Sood SL, Tam E, Killeen J, et al. Lavage administration of dilute surfactants after acute lung injury in neonatal piglets. Am J Respir Crit Care Med. 1998;158:12–17. doi: 10.1164/ajrccm.158.1.9704119. [DOI] [PubMed] [Google Scholar]
- 152.Balaraman V, Sood SL, Finn KC, Hashiro G, Uyehara CFT, Easa D. Physiologic response and lung distribution of lavage vs bolus Exosurf in piglets with acute lung injury. Am J Respir Crit Care Med. 1996;153:1838–1843. doi: 10.1164/ajrccm.153.6.8665043. [DOI] [PubMed] [Google Scholar]
- 153.Gommers D, Eijking EP, van't Veen A, Lachmann B. Bronchoalveolar lavage with a diluted surfactant suspension prior to surfactant instillation improves the effectiveness of surfactant therapy in experimental acute respiratory distress syndrome (ARDS) Intensive Care Med. 1998;24:494–500. doi: 10.1007/s001340050602. [DOI] [PubMed] [Google Scholar]
- 154.van Der Beek J, Plotz F, van Overbeek F, Heikamp A, Beekhuis H, Wildevuur C, et al. Distribution of exogenous surfactant in rabbits with severe respiratory failure: The effect of volume. Pediatr Res. 1993;34:154–158. doi: 10.1203/00006450-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 155.Walmrath D, Grimminger F, Pappert D, Knothe C, Obertacke U, Benzing A, et al. Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: Impact on gas exchange and haemodynamics. Eur Respir J. 2002;19:805–810. doi: 10.1183/09031936.02.00243402. [DOI] [PubMed] [Google Scholar]
- 156.Marks LB, Oberdoerster G, Notter RH. Generation and characterization of aerosols of dispersed surface active phospholipids by ultrasonic and jet nebulization. J Aerosol Sci. 1983;14:683–694. [Google Scholar]
- 157.Wojciak JF, Notter RH, Oberdoerster G. Size stability of phosphatidylcholine-phosphatidylglycerol aerosols and a dynamic film compression state from their interfacial impaction. J Colloid Interface Sci. 1985;106:547–557. [Google Scholar]
- 158.Dijk PH, Heikamp A, Bambang-Oetomo S. Surfactant nebulization versus instillation during high frequency ventilation in surfactant-deficient rabbits. Pediatr Res. 1998;44:699–704. doi: 10.1203/00006450-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 159.Ellyett KM, Broadbent RS, Fawcett ER, Campbell AJ. Surfactant aerosol treatment of respiratory distress syndrome in the spontaneously breathing rabbit. Pediatr Res. 1996;39:953–957. doi: 10.1203/00006450-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Zelter M, Escudier BJ, Hoeffel JM, Murray JF. Effects of aerosolized artificial surfactant on repeated oleic acid injury in sheep. Am Rev Respir Dis. 1990;141:1014–1019. doi: 10.1164/ajrccm/141.4_Pt_1.1014. [DOI] [PubMed] [Google Scholar]
- 161.Davis JM, Richter SE, Kendig JW, Notter RH. High frequency jet ventilation and surfactant treatment of newborns in servere respiratory failure. Pediatr Pulmonol. 1992;13:108–112. doi: 10.1002/ppul.1950130209. [DOI] [PubMed] [Google Scholar]
- 162.Davis JM, Notter RH. Lung surfactant replacement for neonatal pathology other than primary repiratory distress syndrome. In: Boynton B, Carlo W, Jobe A, editors. New Therapies for Neonatal Respiratory Failure: A Physiologic Approach. Cambridge: Cambridge University Press; 1994. pp. 81–92. [Google Scholar]
- 163.Leach CL, Greenspan JS, Rubenstein SD, Shaffer TH, Wolfson MR, Jackson JC, et al. Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The New England journal of medicine. 1996;335:761–767. doi: 10.1056/NEJM199609123351101. [DOI] [PubMed] [Google Scholar]
- 164.Leach CL, Holm BA, Morin FC, Fuhrman BP, Papo MC, Steinhorn D, et al. Partial liquid ventilation in premature lambs with respiratory distress syndrome: Efficacy and compatibility with exogenous surfactant. J Pediatr. 1995;126:412–420. doi: 10.1016/s0022-3476(95)70461-2. [DOI] [PubMed] [Google Scholar]
- 165.Chappell SE, Wolfson MR, Shaffer TH. A comparison of surfactant delivery with conventional mechanical ventilation and partial liquid ventilation in meconium aspiration injury. Respiratory Medicine. 2001;95:612–617. doi: 10.1053/rmed.2001.1114. [DOI] [PubMed] [Google Scholar]
- 166.King DM, Wang Z, Kendig JW, Palmer HJ, Holm BA, Notter RH. Concentration-dependent, temperature-dependent non-newtonian viscosity of lung surfactant dispersions. Chem Phys Lipids. 2001;112:11–19. doi: 10.1016/s0009-3084(01)00150-5. [DOI] [PubMed] [Google Scholar]
- 167.King DM, Wang Z, Palmer HJ, Holm BA, Notter RH. Bulk shear viscosities of endogenous and exogenous lung surfactants. Am J Physiol. 2002;282:L277–L284. doi: 10.1152/ajplung.00199.2001. [DOI] [PubMed] [Google Scholar]
- 168.Bloom BT, Delmore P, Rose T, Rawlins T. Human and calf lung surfactant: A comparison. Neonat Intensive Care. 1993 Mar-Apr;:31–35. [Google Scholar]
- 169.Speer CP, Gefeller O, Groneck P, Laufkotter E, Roll C, Hanssler L, et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child. 1995;72:F8–F13. doi: 10.1136/fn.72.1.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Soll RF, Merritt TA, Hallman M. Surfactant in the prevention and treatment of respiratory distress syndrome. In: Boynton BR, Carlo WA, Jobe AH, editors. New Therapies for Neonatal Respiratory Failure. New York: Cambridge University Press; 1994. pp. 49–80. [Google Scholar]
- 171.Kattwinkel J. Surfactant: Evolving issues. Clin Perinatol. 1998;25:17–32. [PubMed] [Google Scholar]
- 172.Halliday HL. Overview of clinical trials comparing natural and synthetic surfactants. Biol Neonate. 1995;67(Suppl):32–47. doi: 10.1159/000244205. [DOI] [PubMed] [Google Scholar]
- 173.Halliday HL. Controversies - synthetic or natural surfactant - the case for natural surfactant. J Perinat Med. 1996;24(5):417–426. doi: 10.1515/jpme.1996.24.5.417. [DOI] [PubMed] [Google Scholar]
- 174.Soll RF. Surfactant therapy in the USA: Trials and current routines. Biol Neonate. 1997;71:1–7. doi: 10.1159/000244444. [DOI] [PubMed] [Google Scholar]
- 175.Mizuno K, Ikegami M, Chen C-M, Ueda T, Jobe AH. Surfactant protein-B supplementation improves in vivo function of a modified natural surfactant. Pediatr Res. 1995;37:271–276. doi: 10.1203/00006450-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 176.Curstedt T, Jornvall H, Robertson B, Bergman T, Berggren P. Two hydrophobic low-molecular- mass protein fractions of pulmonary surfactant: Characterization and biophysical activity. Eur J Biochem. 1987;168:255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- 177.Oosterlaken-Dijksterhuis MA, Haagsman HP, van Golde LM, Demel RA. Characterization of lipid insertion into monomolecular layers mediated by lung surfactant proteins SP-B and SP-C. Biochemistry. 1991;30:10965–10971. doi: 10.1021/bi00109a022. [DOI] [PubMed] [Google Scholar]
- 178.Oosterlaken-Dijksterhuis MA, Haagsman HP, van Golde LM, Demel RA. Interaction of lipid vesicles with monomolecular layers containing lung surfactant proteins SP-B or SP-C. Biochemistry. 1991;30:8276–8281. doi: 10.1021/bi00247a024. [DOI] [PubMed] [Google Scholar]
- 179.Oosterlaken-Dijksterhuis MA, van Eijk M, van Golde LMG, Haagsman HP. Lipid mixing is mediated by the hydrophobic surfactant protein SP-B but not by SP-C. Biochim Biophys Acta. 1992;1110:45–50. doi: 10.1016/0005-2736(92)90292-t. [DOI] [PubMed] [Google Scholar]
- 180.Revak SD, Merritt TA, Degryse E, Stefani L, Courtney M, Hallman M, et al. The use of human low molecular weight (LMW) apoproteins in the reconstitution of surfactant biological activity. J Clin Invest. 1988;81:826–833. doi: 10.1172/JCI113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Seeger W, Günther A, Thede C. Differential sensitivity to fibrinogen inhibition of SP-C- vs. SP-B-based surfactants. Am J Physiol. 1992;261:L286–L291. doi: 10.1152/ajplung.1992.262.3.L286. [DOI] [PubMed] [Google Scholar]
- 182.Wang Z, Gurel O, Baatz JE, Notter RH. Differential activity and lack of synergy of lung surfactant proteins SP-B and SP-C in surface-active interactions with phospholipids. J Lipid Res. 1996;37:1749–1760. [PubMed] [Google Scholar]
- 183.Yu SH, Possmayer F. Comparative studies on the biophysical activities of the low-molecular-weight hydrophobic proteins purified from bovine pulmonary surfactant. Biochim Biophys Acta. 1988;961:337–350. doi: 10.1016/0005-2760(88)90081-1. [DOI] [PubMed] [Google Scholar]
- 184.Wang Z, Baatz JE, Holm BA, Notter RH. Content-dependent activity of lung surfactant protein B (SP-B) in mixtures with lipids. Am J Physiol. 2002;283:L897–L906. doi: 10.1152/ajplung.00431.2001. [DOI] [PubMed] [Google Scholar]
- 185.Johansson J, Curstedt T, Robertson B. Synthetic protein analogues in artificial surfactants. Acta Paediatr. 1996;85:642–646. doi: 10.1111/j.1651-2227.1996.tb14114.x. [DOI] [PubMed] [Google Scholar]
- 186.Johansson J, Gustafsson M, Zaltash S, Robertson B, Curstedt T. Synthetic surfactant protein analogs. Biol Neonate. 1998;74(Suppl):9–14. doi: 10.1159/000047029. [DOI] [PubMed] [Google Scholar]
- 187.Walther FJ, Gordon LM, Zasadzinski JM, Sherman MA, Waring AJ. Surfactant protein B and C analogues. Mol Gen Metab. 2000;71:342–351. doi: 10.1006/mgme.2000.3053. [DOI] [PubMed] [Google Scholar]
- 188.Robertson B, Johansson J, Curstedt T. Synthetic surfactants to treat neonatal lung disease. Molec Med Today. 2000;6:119–124. doi: 10.1016/s1357-4310(99)01656-1. [DOI] [PubMed] [Google Scholar]
- 189.Cochrane CG, Revak SD, Merritt TA, Heldt GP, Hallman M, Cunningham MD, et al. The efficacy and safety of KL4-surfactant in preterm infants with respiratory distress syndome. Am J espir Crit Care Med. 1996;153:404–410. doi: 10.1164/ajrccm.153.1.8542150. [DOI] [PubMed] [Google Scholar]
- 190.Cochrane CG, Revak SD. Surfactant lavage treatment in a model of respiratory distress syndrome. Chest. 1999;116(suppl):85S–86S. doi: 10.1378/chest.116.suppl_1.85s. [DOI] [PubMed] [Google Scholar]
- 191.Cochrane CG, Revak SD. Pulmonary surfactant protein B (SP-B): Structure-function relationships. Science. 1991;254:566–568. doi: 10.1126/science.1948032. [DOI] [PubMed] [Google Scholar]
- 192.Cochrane CG, Revak SD. Protein-phospholipid interactions in pulmonary surfactant. Chest. 1994;105:57S–62S. doi: 10.1378/chest.105.3_supplement.57s. [DOI] [PubMed] [Google Scholar]
- 193.Merritt TA, Kheiter A, Cochrane CG. Positive end-expiratory pressure during KL4 surfactant instillation enhances intrapulmonary distribution in a simian model of respiratory distress syndrome. Pediatr Res. 1995;38:211–217. doi: 10.1203/00006450-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 194.Revak S, Merritt T, Cochrane C, Heldt G, Alberts M, Anderson D, et al. Efficacy of synthetic peptide-containing surfactant in the treatment of respiratory distress syndrome in preterm infant rhesus monkeys. Pediatr Res. 1996;39:715–724. doi: 10.1203/00006450-199604000-00025. [DOI] [PubMed] [Google Scholar]
- 195.Revak SD, Merritt TA, Hallman M, Heldt G, La Polla RJ, Hoey K, et al. The use of synthetic peptides in the formation of biophysically and biologically active surfactants. Pediatr Res. 1991;29:460–465. doi: 10.1203/00006450-199105010-00010. [DOI] [PubMed] [Google Scholar]
- 196.Ikegami M, Jobe AH. Surfactant protein-C in ventilated premature lamb lung. Pediatr Res. 1998;44:860–864. doi: 10.1203/00006450-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 197.Davis AJ, Jobe AH, Hafner D, Ikegami M. Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant. Am J Respir Crit Care Med. 1998;157:553–559. doi: 10.1164/ajrccm.157.2.97-08019. [DOI] [PubMed] [Google Scholar]
- 198.Lewis J, McCaig L, Hafner D, Spragg R, Veldhuizen R, Kerr C. Dosing and delivery of a recombinant surfactant in lung-injured sheep. Am J Respir Crit Care Med. 1999;159:741–747. doi: 10.1164/ajrccm.159.3.9806069. [DOI] [PubMed] [Google Scholar]
- 199.Hafner D, Germann P-G, Hauschke D. Effects of rSP-C surfactant on oxygenation and histology in a rat-lung-lavage model of acute lung injury. Am J Respir Crit Care Med. 1998;158:270–278. doi: 10.1164/ajrccm.158.1.9712061. [DOI] [PubMed] [Google Scholar]
- 200.Spragg RG, Smith RM, Harris K, Lewis JF, Hafner D, Germann P. Effect of recombinant SP-C surfactant in a porcine lavage model of acute lung injury. J Appl Physiol. 1999;88:674–681. doi: 10.1152/jappl.2000.88.2.674. [DOI] [PubMed] [Google Scholar]
- 201.Spragg RG, Lewis JF, Rathgeb F, Hafner D, Seeger W. Intratracheal instillation of rSP-C surfactant improves oxygenation in patients with ARDS (Abstract) Am J Respir Crit Care Med. 2002;165:A22. [Google Scholar]
- 202.Notter RH, Schwan AL, Wang Z, Waring AJ. Novel phospholipase-resistant lipid/peptide synthetic lung surfactants. Mini-Rev Med Chem. 2007;7:932–934. doi: 10.2174/138955707781662627. [DOI] [PubMed] [Google Scholar]
- 203.Waring AJ, Walther FJ, Gordon LM, Hernandez-Juviel JM, Hong T, Sherman MA, et al. The role of charged amphipathic helices in the structure and function of surfactant protein B (SP-B) J Peptide Res. 2005;66:364–374. doi: 10.1111/j.1399-3011.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 204.Walther FJ, Waring AJ, Sherman MA, Zasadzinski J, Gordon LM. Hydrophobic surfactant proteins and their analogues. Neonatology. 2007;91:303–310. doi: 10.1159/000101346. [DOI] [PubMed] [Google Scholar]
- 205.Turcotte JG, Lin WH, Pivarnik PE, Sacco AM, Bermel MS, Lu Z, et al. Chemical synthesis and surface activity of lung surfactant phospholipid analogs. II. Racemic N-substituted diether phosphonolipids. Biochim Biophys Acta. 1991;1084:1–12. doi: 10.1016/0005-2760(91)90048-m. [DOI] [PubMed] [Google Scholar]
- 206.Turcotte JG, Sacco AM, Steim JM, Tabak SA, Notter RH. Chemical synthesis and surface properties of an analog of the pulmonary surfactant dipalmitoyl phosphatidylcholine analog. Biochim Biophys Acta. 1977;488:235–248. doi: 10.1016/0005-2760(77)90181-3. [DOI] [PubMed] [Google Scholar]
- 207.Chang Y, Wang Z, Notter RH, Wang Z, Long Q, Schwan AL. Synthesis and interfacial behavior of sulfur-containing analogs of lung surfactant dipalmitoyl phosphatidylcholine. Bioorg Med Chem Lett. 2004;14:5983–5986. doi: 10.1016/j.bmcl.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 208.Chang Y, Wang Z, Schwan AL, Wang Z, Holm BA, Baatz JE, et al. Surface properties of sulfur- and ether-linked phosphonolipids with and without purified hydrophobic lung surfactant proteins. Chem Phys Lipids. 2005;137:77–93. doi: 10.1016/j.chemphyslip.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 209.Notter RH, Wang Z, Wang Z, Davy J, Schwan AL. Synthesis and surface activity of diether-linked phosphoglycerols: Potential applications for exogenous lung surfactants. Bioorg Med Chem Lett. 2007;17:113–117. doi: 10.1016/j.bmcl.2006.09.083. [DOI] [PubMed] [Google Scholar]
- 210.Davy JA, Wang Z, Notter RH, Schwan AL. Synthesis of sulfur-containing glycerophospholipids. J Sulfur Chem. 2007;28:45–72. [Google Scholar]
- 211.Wang Z, Chang Y, Schwan AL, Notter RH. Activity and inhibition resistance of a phospholipase-resistant synthetic exogenous surfactant in excised rat lungs. Am J Respir Cell Mol Biol. 2007;37:387–394. doi: 10.1165/rcmb.2006-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim DK, Fukuda T, Thompson BT, Cockrill B, Hales C, Bonventre JV. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol. 1995;269:L109–L118. doi: 10.1152/ajplung.1995.269.1.L109. [DOI] [PubMed] [Google Scholar]
- 213.Touqui L, Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Molec Med Today. 1999;5:244–249. doi: 10.1016/s1357-4310(99)01470-7. [DOI] [PubMed] [Google Scholar]
- 214.Vadas P. Elevated plasma phospholipase A2 levels: correlation with the hemodynamic and pulmonary changes in Gram-negative septic shock. J Lab Clin Med. 1984;104:873–881. [PubMed] [Google Scholar]
- 215.Kostopanagiotou G, Routs C, Smyrniotis V, Lekka ME, Kitsiouli E, Arkadopoulos N, et al. Alterations in bronchoalveolar lavage fluid during ischemia-induced acute hepatic failure in the pig. Hepatology. 2003;37:1130–1138. doi: 10.1053/jhep.2003.50185. [DOI] [PubMed] [Google Scholar]
- 216.Attalah HL, Wu Y, Alaoui-El-Azher M, Thouron F, Koumanov K, Wolf C, et al. Induction of type-IIA secretory phospholipase A2 in animal models of acute lung injury. Eur Respir J. 2003;21:1040–1045. doi: 10.1183/09031936.03.00093002. [DOI] [PubMed] [Google Scholar]
- 217.Nakos G, Kitsiouli E, Hatzidaki E, Koulouras V, Touqui L, Lekka ME. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med. 2003;33:772–779. doi: 10.1097/01.ccm.0000158519.80090.74. [DOI] [PubMed] [Google Scholar]
- 218.Ackerman SJ, Kwatia MA, Doyle CB, Enhorning G. Hydrolysis of surfactant phospholipids catalyzed by phospholipase A2 and eosinophil lysophospholipases causes surfactant dysfunction: A mechanism for small airway closure in asthma. Chest. 2003;123:255S. doi: 10.1378/chest.123.3_suppl.355s. [DOI] [PubMed] [Google Scholar]
- 219.Walther FJ, Waring AJ, Hernandez-Juviel JM, Gordon LM, Schwan AL, Jung C-L, et al. Dynamic surface activity of a fully-synthetic phospholipase-resistant lipid/peptide lung surfactant. PLoS ONE. 2007 doi: 10.1371/journal.pone.0001039. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kendig JW, Notter RH, Cox C, Reubens LJ, Davis JM, Maniscalco WM, et al. A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks gestation. The New England journal of medicine. 1991;324:865–871. doi: 10.1056/NEJM199103283241301. [DOI] [PubMed] [Google Scholar]
- 221.Notter RH, Apostolakos M, Holm BA, Willson D, Wang Z, Finkelstein JN, et al. Surfactant therapy and its potential use with other agents in term infants, children and adults with acute lung injury. Perspectives in Neonatol. 2000;1(4):4–20. [Google Scholar]
- 222.Pryhuber GS, D'Angio CT, Finkelstein JN, Notter RH. Combined-modality therapies for lung injury. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: Mechanisms, pathophysiology, and therapy. Boca Raton: Taylor Francis Group, Inc; 2005. pp. 779–838. [Google Scholar]