Abstract
Glucocorticoid treatment given in late pregnancy in sheep resulted in altered placental development and function. An imbalance of placental survival and apoptotic factors resulting in an increased rate of apoptosis may be involved. We have now investigated the effects of dexamethasone (DEX) in early pregnancy on binucleate cells (BNCs), placental apoptosis, and fetal sex as a determinant of these responses. Pregnant ewes carrying singleton fetuses (n = 105) were randomized to control (n = 56, 2 mL saline/ewe) or DEX treatment (n = 49, intramuscular injections of 0.14 mg/kg ewe weight per 12 hours over 48 hours) at 40 to 41 days of gestation (dG). Placentomes were collected at 50, 100, 125, and 140 dG. At 100 dG, DEX in females reduced BNC numbers, placental antiapoptotic (proliferating cell nuclear antigen), and increased proapoptotic factors (Bax, p53), associated with a temporarily decrease in fetal growth. At 125 dG, BNC numbers and apoptotic markers were restored to normal. In males, ovine placental lactogen-protein levels after DEX were increased at 50 dG, but at 100 and 140 dG significantly decreased compared to controls. In contrast to females, these changes were independent of altered BNC numbers or apoptotic markers. Early DEX was associated with sex-specific, transient alterations in BNC numbers, which may contribute to changes in placental and fetal development. Furthermore, in females, altered placental apoptosis markers may be involved.
Keywords: binucleate cell, placental lactogen, apoptopic markers, glucocorticoid, placenta
Introduction
The administration of synthetic glucocorticoids (GCs) is an important clinical tool used both in late pregnancy, for the management of women at risk of early preterm birth,1–3 and in early pregnancy, in suspected cases of congenital adrenal hyperplasia (CAH) to prevent fetal virilization.4,5 The lifelong consequences of this treatment are not fully understood.6–10 Human fetuses with CAH, who had received dexamethasone (DEX), had normal pre- and postnatal growth11 but showed more shyness, greater emotionality, and less sociability than unexposed children.12
Previous studies in sheep have shown that maternal intravenously DEX treatment between 26 and 28 days of gestation (dG) did not result in fetal organ weight changes at 130 dG13 but were associated with enhanced coronary artery vascular reactivity with 4 months of age,14 altered brain renin–angiotensin function15 and hyperinsulinemia in response to glucose challenge at the age of 4 years.16 Female offspring demonstrated hypertension, which was still evident at the age of 7 years.17 Maternal intramuscular (im) DEX treatment between 40 and 41 dG in pregnant sheep resulted in changes in fetal and organ weights, some of which persisted into later life.18,19 Dexamethasone treatment resulted in the activation of the fetal adrenal near term, which was attributed, in part, to increased fetal adrenal steroidogenic activity.18 In addition, in females, early DEX treatment was accompanied by sex-specific fetal but not maternal changes in plasma insulin and glucose levels, suggesting that these animals were insulin resistant.20 A suppressed response of adrenocorticotropic hormone (ACTH) but an increased ratio of cortisol to ACTH after a neuroendocrine challenge in female offsprings indicated that early DEX treatment can produce enduring effects on the hypothalamic–pituitary–adrenal (HPA) axis and its responsiveness later in life.19,21 The HPA development and activity is associated with increased levels of ACTH and adrenal corticosteroids in the circulation of sheep and human fetuses that may be implicated in determining gestation length and producing pathophysiologic adjustments in later life.22,23 Since the placenta is the conduit between the maternal and fetal environment, early DEX treatment at the starting point of rapid placental growth in sheep24 may influence placental development and function and may play a role in mediating fetal GC exposure.23,25–33
The sheep has a synepitheliochorial, noninvasive placenta,34 compared to hemochorial placentation in primates. The ovine cotyledonary placenta consists of placentomes that have been classified previously according to gross morphological appearance into 4 types (A, B, C, and D), reflecting the degree of eversion of the hemophagous zone.35 Placental lactogen (PL), a member of the growth hormone (GH) family, is associated with the regulation of maternal carbohydrate, lipid, and protein metabolism.36 In the fetus, PL may influence fetal growth indirectly through alterations in the maternal metabolic environment, maternal placental nutrient transfer to the fetus, or through stimulation of insulin-like growth factor release.37,38 Placental lactogen is found in humans and sheep but produced by different trophoblast cell types. In sheep, ovine PL (oPL) is produced by binucleate cells (BNCs).39 Antenatal betamethasone exposure late in gestation reduced the mean number of BNCs, reduced placental oPL-protein and maternal and fetal oPL-plasma levels as well as lowered birth weight.26 Binucleate cells are formed from 2 uninucleate cells which, after a period of maturation, migrate through the fetal–maternal placental interface to fuse with the maternal epithelium.40,41 The observed decrease in BNC number after GC treatment or after the rise in endogenous cortisol near term may result from an increased rate of BNC migration across the fetal–maternal interface,42 prevention of the usual increase in BNC numbers during pregnancy,26 or from inhibition of BNC formation and/or an imbalance of survival and apoptotic factors resulting in an increased rate of BNC apoptosis.26,42 Glucocorticoid-induced apoptosis has been implicated in the generation of the immune response repertoire and clinically in the therapy of lymphoid malignancies.43,44 Two pathways, the extrinsic pathway, dependent on the ligand binding to a “death signal” receptor (FAS), and the intrinsic pathway, regulated by the members of the B-cell lymphoma 2 (BCL2) family, consisting of pro- (Bax) and antiapoptotic (BCL2) proteins and mitochondria-derived proteins, trigger apoptosis (Figure S1).45 Glucocorticoid might directly induce apoptosis by regulating components of either the extrinsic or intrinsic pathway or both. The fine balance between survival factors (proliferating cell nuclear antigen [PCNA]) and apoptosis with the activation of caspases (Caspase-3), as central initiators and executioners of apoptosis,46,47 may determine placental function. It is not known whether DEX treatment in early pregnancy is associated with changes in BNC numbers and function and whether placental apoptosis is involved.
We hypothesized that DEX treatment in early pregnancy (early DEX) would alter placental development and function and therefore contribute to the previously reported immediate and long-term changes in HPA development observed after DEX treatment. Further, these effects could be sex specific.18–21,48 To address this, we investigated changes in BNC localization and distribution, placental oPL-protein, maternal and fetal oPL-plasma levels, and placental apoptotic markers at 4 different gestational ages to determine the effect and interaction of early DEX treatment, fetal sex, and placentome subtype.
Materials and Methods
All experimental procedures were approved by the Animal Experimentation Ethics Committee of the University of Western Australia and/or the Western Australian Department of Agriculture. Briefly, pregnant Merino ewes (Ovis aries) with singleton pregnancies (total n = 105) of known gestational age were randomized to control (2 mL saline/ewe) or DEX-treated groups (im injections of 0.14 mg/kg ewe weight per 12 hours over 48 hours) at 40 and 41 dG as described previously.18 Hysterectomy was performed at 49 to 51 (50), 101 to 103 (100), 125 to 127 (125), and 140 to 142 (140) dG, and placentomes were dissected from the uterus.26 The number and weights of all placentomes per animal were recorded, and 1 representative placentome of each available subtype was randomly collected from each pregnancy. Changes in organ weights have been reported previously.18,19
Immunohistochemical Localization and Quantification of BNCs
Saggital cross-sections (6 μm) were taken in the middle of the placentomes. A monoclonal rabbit antibody against oPL (1:20.000 dilution, rabbit antihuman) was used as described previously.26 Semi-quantitative analyses were performed using computerized image analysis (ImagePro Plus 4.5; Media Cybernetics, Silver Spring, Maryland).26 A total of 12 random fields of view within 3 levels, L1-3, referring to previously described zones in a placentome,26 were counted in each section of immunostained tissue at a magnification of 20×. A counting frame was used and BNCs were counted in an area of 0.75 μm2. Binucleate cell with less than 30% cytoplasm visible were excluded. At least 4 sections per placentome (placentomes n = 204 from 105 sheep) were counted (average coefficient of variation [CV] = 7.2%). The mean number of BNCs was expressed per 0.750 mm2 ± standard error of the mean (SEM).
Quantification of oPL-Protein Levels: Western Blotting
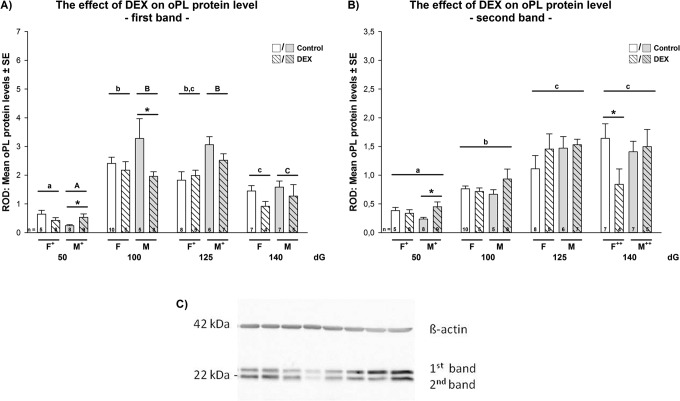
Quantification of oPL-protein levels with Western blotting was performed as described previously.26 Briefly, samples from different treatment groups, sex, placentome subtypes, and days of gestation were run together on 1 gel to facilitate comparison between all factors. In total, n = 106 control and n = 111 DEX-treated placentomes were analyzed. Each blot was repeated at least 3 times with the same samples, each run 3 times. Membranes were incubated overnight with blocking solution (7.5% skim milk powder in phosphate-buffered saline-Tween) and then overnight with the same primary antibody as used for immunohistochemistry, but at a dilution of 1:80.000 for oPL. All blots were reincubated with anti-β-actin (ACTB) 1:20.000 (107K4800; Sigma, Sigma- Aldrich Chemie Gmbh, Eschenstrasse 5, Munich, Germany) as an internal control to allow correction for gel loading and transfer. The oPL-protein was identified as a doublet at 22 kDa (upper band = first band and lower band = second band; Figure 1C). The intensity of both oPL bands and ACTB was quantified by densitometry using Quantity One 4.6.2 (Bio-Rad, Bio-Rad Laboratories GmbH, Heidemannstrasse, Munich, Germany). Results were expressed as the ratio of protein to ACTB as relative optical density (ROD) ± SEM (average CV = 6.4%). As described previously, placental oPL-protein was identified as 2 close bands at 22 and 23 kDa with no other background signal.26 Both bands were analyzed together (mean) as well as each band separately (upper band = first and lower band = second band; Figure 1).
Figure 1.
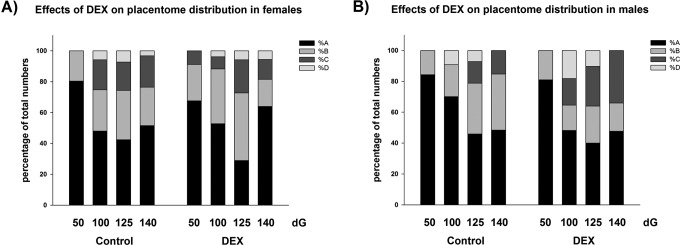
(A-B) The effect of early DEX treatment on placentome distribution as percentage of total numbers of placentomes in (A) females and (B) males. Data were analyzed by a full factorial model (MANOVA) with treatment, gender, and dG as type as factors, followed by a pairwise comparison (Holm Sidak) when main effects were P < .05. Data are presented as mean ± standard error of the mean (SEM). In females, the proportion of A subtypes placentomes in controls was lowest at 125 dG, whereas the proportion of B and C subtypes was highest at 125 dG (P < .05). This was similar in the DEX groups (MANOVA main effects: dG P < .001, type P < .05; interaction: dG × type P < .05). In males, the highest proportion of A subtypes was found at 50 dG, whereas the proportion of B subtypes was highest at 125 dG (P < .05). At 125 dG, DEX increased significantly the proportion of C subtypes compared to controls (P < .05). DEX indicates dexamethasone; dG, days of gestation.
Quantification of oPL-Plasma Levels: Radioimmunoassay
Concentrations of oPL-plasma were measured using equilibrium radioimmunoassay as described previously and validated in sheep.37 There was no significant cross-reaction with ovine PRL, GH, follicle-stimulating hormone, lutenizing hormone, or thyroid-stimulating hormone.49 The minimal detectable dose was 0.1 ng/mL, the intra-assay CV was 9.8%, and the interassay CV was 16%. Values are expressed in terms of recombinant oPL (M3RD86; Gentech, Arcade, JY).
Quantification of Placental Proliferation-, Pro-, and Antiapoptotic Markers: Quantitative Polymerase Chain Reaction
Total placenta RNA was extracted using the RNeasy Midi kit (QIAGEN, Australia) and stored at −80°C until further use. For quantitative polymerase chain reaction (q-PCR), primer pairs for sheep (Table 1) were either designed (ACTB, Caspase-3, PCNA, p53, and FAS) using Primer 6.0 (PRIMER-E Ltd, United Kingdom) according to the manufacture’s manual or have been reported previously (ribosomal protein, large P0 [RPLP0],50 hypoxanthine phosphoribosyltransferase 1 [HPRT1],51 Bax,52 and BCL-2 53). The q-PCR assays were run on an ABI 7500 Real Time PCR System (Applied Biosystems, Life Technologies GmbH, Darmstadt, Germany). Primer sequences have been tested and verified with fluorescent color band sequencing (Seqlab; Sequence Laboratories, Germany). All samples for each gene were run in triplicate. To determine the reliability of internal control genes, the average expression stability values (M) of 4 ICGs (HPRT1, ACTB, RPLP0, 18S ribosomal RNA) were analyzed in all samples with geNorm Visual basic application (V 3.5; Biogazelle NV, Belgium) according to the manufacture’s manual and the procedures described by Vandesompele et al.54 Stepwise elimination of successive genes showed that HPRT1, ACTB, and RPLP0 were the 3 most stable housekeeping genes to be used (pairwise variation in V3/4 = 0.135). Rescaled normalized expression levels of target genes were calculated according to the manufacture’s manual and as described previously.54
Table 1.
Primer Used for Quantitative RT-PCR in Sheep Placenta and Cycling Conditions.a
| Gene | Primer Sequences (5′ → 3′) | Product Size, bp | Efficiency, % | Annealing Temp, °C | Denaturation and Extension, °C | Cycles | Accession Number |
|---|---|---|---|---|---|---|---|
| RPLP0 | F: CAA CCC TGA AGT GCT TGA CAT R: AGG CAG ATG GAT CAG CCA | 227 | 95.9 | 60 | 95, 72 | 40 | NM_001012682 |
| ACTB | F: CAT CGG CAA TGA GCG GTT CC R: CCG TGT TGG CGT AGA GGT | 146 | 97.75 | 60 | 95, 72 | 40 | NM_001009784 |
| HPRT1 | F: GCT GAG GAT TTG GAG AAG GTG T R: GGC CAC CCA TCT CCT TCA T | 94 | 97.6 | 60 | 95, 72 | 40 | NM_001034035.1 |
| PCNA | F: GCTGTTACCATAGAGATGAATG R: ATACTGAGTGTTACTGTAGGAG | 107 | 99.9 | 57.2 | 95, 72 | 40 | AF416380 |
| Caspase-3 | F: TCTTCAGAGGGGACTGTTGC R: ACTTTGAGTTTCGCCAGGAA | 206 | 99.9 | 60 | 95, 72 | 40 | AF068837.1 |
| BCL-2 | F: TTCGCCGAGATGTCCAGcC R: TTGACGCTCTCCACACACATG | 155 | 96.5 | 63 | 95, 72 | 40 | DQ152929.1 |
| Bax | F: CAG GAT GCA TCC ACC AAG AAG C R: TTG AAG TTG CCG TCG GAA AAC ATT | 164 | 95.4 | 60 | 95, 72 | 40 | AF163774 |
| p53 | F: GAAGAATCGCAGGCAGAA R: CTCGGAGGACAGAAGGTT | 102 | 96.93 | 61.8 | 95, 72 | 35 | FJ855223.1 |
| FAS | F: CGTGGCTGGTATCAACTC R: ACACATTCTGGCATATCTCC | 168 | 95.5 | 59 | 95, 72 | 37 | NM-001123003 |
Abbreviations: RPLP0, ribosomal protein, large, P0; ACTB, β-actin; HPRT, hypoxanthine phosphoribosyltransferase; PCNA, proliferating nuclear cell antigen; BCL-2, B-cell lymphoma 2; Bax, proapoptotic Bcl-2-family protein; p53, tumor protein 53; FASR, FAS receptor; PCR, polymerase chain reaction; temp, temperature.
aEfficiencies of PCR were determined using the formula (10− 1/slope − 1) × 100% according to the manufacture’s manual.
Statistical Analyses
Analyses were performed by using SPSS 20 statistical software (SPSS Inc, Chicago, Illinois). Data were analyzed first for normality and equal variance (Levene test). Data that were not normally distributed were log transformed to achieve normality. Total placentome numbers and the mean numbers of each subtype were calculated for each gestational age for each treatment group. To determine treatment, dG, gender, and placentome subtype (where applicable) effects as well as an interaction between them, data sets were analyzed using a full factorial model (multivariate analysis of variance [MANOVA]) with treatment, gender, dG, and placentome subtype as factors, followed by a pairwise comparison (Holm Sidak) when main effects were P < .05. Main effects and interactions are indicated in Result section as well as in the figure legend when significant (P < .05), post hoc P values (Holm-Sidak) are indicated in figures. Data are presented as mean ± SEM. The relationship between the number of BNCs, placental oPL-protein, or oPL-plasma levels with fetal and placental weights and placental gene expression as combined data of placentome subtypes across gestation was assessed by correlation analysis (Pearson).
Results
The Effect of DEX on Placental Weight, Placentome Numbers, and Fetal Anthropometrics
In females, DEX did not affect total placenta weight or total placentome numbers (Table 2). In control animals, the proportion A subtypes was lowest at 125 dG, whereas the proportion of B and C subtypes was highest at 125 dG (P < .05; Figure 2A). Dexamethasone did no significantly change this distribution (MANOVA main effects: dG P < .05, type P < .05; interactions: dG × type P < .05). Mean placentome weight in females after DEX treatment was only different from controls in C subtypes at 100 and 140 dG (MANOVA main effects: dG P < .001, type P < .001; interactions: gender × type P < .05, dG × gender × type × treatment P < .05; Table 2). As reported previously,18,19 DEX significantly reduced fetal weight in females at 100 dG (control 888 g ± 23.4 vs DEX = 853 g ± 52.3; MANOVA main effects: dG P < .05, gender P < .05; interaction: dG × treatment P < .05; Holm-Sidak post hoc analysis P = .035), but weight was restored to normal at 125 dG (control 2787 g ± 116.8 vs DEX 2694 g ± 143.6; P > .05).18,19 Crown–rump length was significantly reduced at 100 dG in females after DEX compared to controls (control 36.4 cm ± 0.8 vs DEX = 33.5 cm ± 0.8; MANOVA main effects: dG P < .05, treatment P < .05; interaction: P > .05; Holm-Sidak Post hoc analysis P = .009; Table 3).
Table 2.
Total Placental Weight, Mean Placentome Weight, and Placentome Numbers.a
| Days of Gestation | Total | A | B | C | D | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight, Mean ± SEM | Numbers, Mean ± SEM | Weight, Mean ± SEM | Numbers, Mean ± SEM | Weight, Mean ± SEM | Numbers, Mean ± SEM | Weight, Mean ± SEM | Numbers, Mean ± SEM | Weight, Mean ± SEM | Numbers, Mean ± SEM | |
| (A) Placentome type in females | ||||||||||
| 50 | ||||||||||
| Control | 61 ± 9.3a | 59 ± 4.6a, c | 1.0 ± 0.14a | 56.2 ± 4.3a | 1.2b | 16b | NA | NA | NA | NA |
| DEX | 40 ± 10.3A | 47 ± 5.1A | 0.9 ± 0.29A | 44.7 ± 5.7A | 1.2b | 16b | NA | NA | NA | NA |
| 100 | ||||||||||
| Contro | 473 ± 35.8b | 73 ± 2.2b | 5.8 ± 0.68b | 56.9 ± 7.4a | 8.2 ± 1.52a | 19.4 ± 5.8a | 8.8 ± 4.03a | 13.3 ± 9.9a | 8.8 ± 4.04a | 6.7 ± 4.0 |
| DEX | 431 ± 32.0B | 60 ± 8.1A | 5.0 ± 1.03A, B | 38.6 ± 11.1A | 8.6 ± 1.55A, B | 17.6 ± 6.9A | 22.4 ± 3.25A | 3.2 ± 1.6A | 10.3 ± 1.95 | 2.0 ± 1.0 |
| 125 | ||||||||||
| Control | 418 ± 40.9b | 57 ± 4.6c | 4.5 ± 0.65a, b | 29.2 ± 12.8b | 7.5 ± 0.67a | 25.3 ± 4.5a | 8.9 ± 1.9a | 15.0 ± 2.8a | 8.6 ± 1.52a | 4.7b |
| DEX | 423 ± 46.2B | 60 ± 5.1A | 5.4 ± 0.85A, B | 20.0 ± 9.0A | 4.8 ± 1.20A | 33.7 ± 8.9A | 12.0 ± 5.4B | 12.0 ± 5.4A | 7.3b | 5.0b |
| 140 | ||||||||||
| Control | 499 ± 58.2b | 70 ± 6.0a, b, c | 6.1 ± 0.49b | 52.8 ± 11.3a | 8.7 ± 1.11a | 18.8 ± 4.4a | 12.8 ± 3.03a | 10.8 ± 4.8a | 18.4b | 3.0b |
| DEX | 513 ± 29.0B | 60 ± 6.8A | 7.7 ± 1.87B | 44.5 ± 5.2A | 11.7 ± 3.12B | 13.0 ± 3.1A | 18.8 ± 4.16A | 6.0 ± 4.0A | 1.9b | 5.0b |
| (B) Placentome type in males | ||||||||||
| 50 | ||||||||||
| Control | 69 ± 7.2a | 52 ± 3.8a | 1.3 ± 0.09a | 50.2 ± 3.5a | 2.6 ± 0.12a | 10.5 ± 2.5a | NA | NA | NA | NA |
| DEX | 58 ± 9.0A | 47 ± 3.9A | 1.0 ± 0.16A | 43.6 ± 4.5A | 2.7 ± 0.46A | 8.4 ± 1.9A | NA | NA | NA | NA |
| 100 | ||||||||||
| Control | 501 ± 55.3b | 75 ± 4.9b | 9.9 ± 1.18b, c | 68.6 ± 5.7a | 5.9b | 22.0b | NA | NA | 7.7b | 9.0b |
| DEX | 396 ± 26.1B | 65 ± 6.4B | 5.2 ± 0.42B | 43.5 ± 9.1A | 7.7 ± 1.28B | 15.8 ± 3.0B | 10.6 ± 3.74A | 8.5 ± 3.3A | 9.9 ± 2.10A | 10.5 ± 4.9A |
| 125 | ||||||||||
| Control | 404 ± 33.6b | 76 ± 7.1b | 4.9 ± 0.25a, c | 40.5 ± 11.5a | 5.8 ± 0.68a | 27.0 ± 8.0a | 9.4 ± 3.36a | 10.5 ± 5.7a | 3.2b | 9.0b |
| DEX | 466 ± 42.3B | 80 ± 2.8C | 5.8 ± 0.64B | 42.0 ± 17.7A | 6.1 ± 0.94A, B | 30.0 ± 7.6A, B | 5.5 ± 0.72B | 33.4 ± 6.2B | 8.1 ± 0.71A | 9.2 ± 4.0A |
| 140 | ||||||||||
| Control | 577 ± 46.1b | 71 ± 6.0b | 6.4 ± 0.63b, c | 38.1 ± 10.3a | 6.5.0 ± 1.5a | 25.6 ± 8.8a | 13.3 ± 1.49a | 13.7 ± 3.7a | NA | NA |
| DEX | 571 ± 62.9B | 59 ± 6.9A, B | 9.2 ± 1.77B | 35.8 ± 8.1A | 12.7 ± 2.20C | 14.5 ± 3.8A, B | 12.7 ± 4.42A | 21.5 ± 11.2A, B | NA | NA |
Abbreviations: DEX, dexamethasone; MANOVA, multivariate analysis of variance; NA, no placentomes available; SEM, standard error of the mean.
aData were analyzed with a full factorial model (MANOVA) with dG, gender, placentome subtype, and treatment as factors followed by a pairwise comparison (Holm Sidak) when main effects were P < .05. Data are presented as mean ± SEM per sheep. Placentome weight MANOVA main effects: dG P < .001, type P < .001; interactions: sex × type P = .027, dG × sex × type × treatment P = .009; placentome numbers MANOVA main effects: type P < .001; interaction: dG × type P = .025. Different small letters (a–c) indicate significant results (P < .05) of post hoc analysis (Holm Sidak) across gestation in controls. Different capital letters (A–C) indicate significant results (P < .05) of post hoc analysis (Holm Sidak) across gestation in DEX. Significant differences between control versus DEX are indicated in bold.
bTotal number of placentomes is small, other statistics not reported.
Figure 2.
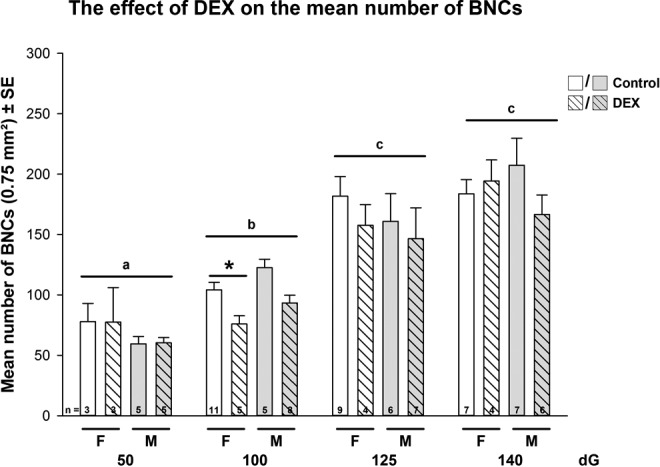
The effect of early dexamethasone (DEX) treatment on the mean number of binucleate cells (BNCs) in sheep placentomes during pregnancy (combined data of placentome subtypes). Data were analyzed by a full factorial model (MANOVA) with treatment, gender, and dG as factors, followed by a pairwise comparison (Holm Sidak) when main effects were P < .05. Data are presented as mean ± standard error of the mean (SEM) per sheep. MANOVA main effects: age P < .001, treatment P < .05; interaction: dG × treatment P < .05. Post hoc P values <.05 are indicated in figure: different letters indicate significant differences in dG, the star indicates significant differences in treatment. n = numbers of animals analyzed.
Table 3.
Summary of the Effects of Early DEX Treatment on Fetal and Placental Development in Sheep and the Role of Placental Apoptosis.a
| 50 dG | 100 dG | 125 dG | 140 dG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | ♂ vs ♀ | Males | Females | ♂ vs ♀ | Males | Females | ♂ vs ♀ | Males | Females | ♂ vs ♀ | Males | |
| Anthropometrics | ||||||||||||
| Weight | ns | Ns | ns | DEX < Con | <(DEX) | ns | ns | ns | ns | ns | ns | ns |
| Crown–rump length | Ns | Ns | ns | DEX < Con | ns | ns | ns | ns | ns | ns | ns | ns |
| Abdominal circum. | Ns | Ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Femur | NA | NA | NA | ns | ns | ns | ns | ns | ns | ns | <(Con) | DEX < CON |
| Ponderal index | Ns | ns | Ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Placenta | ||||||||||||
| Total wt | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Placentome wt | ns | ns | ns | DEX > ConC | ns | ns | ns | ns | ns | DEX > ConC | ns | DEX > ConB |
| Placentome numbers | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| BNC numbers | ns | ns | ns | DEX < ConA | ns | ns | ns | >(DEX)B | ns | ns | ns | ns |
| oPL protein | ||||||||||||
| Mean band | ns | >(Con) | DEX > Con | ns | ns | ns | ns | <(Con) | ns | DEX < Con | ns | ns |
| First band | ns | >(Con)A | DEX > ConA | ns | ns | DEX < ConA | ns | <(Con) | ns | DEX < ConC | ns | DEX < Con |
| Second band | ns | <(Con)B | DEX > ConA | ns | <(Con)A | ns | DEX > ConB | <(Con)A, D | ns | DEX < ConD | >(Con)B, C | ns |
| Ratio ½ band | ns | <(Con) | ns | DEX > Con | <(Con) | DEX < Con | DEX < Con | ns | ns | ns | >(DEX) | ns |
| oPL-plasma levels | ||||||||||||
| Maternal | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Fetal | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Antiapoptotic | ||||||||||||
| PCNA mRNA | ns | ns | ns | DEX < CON | ns | ns | ns | >(DEX) | ns | ns | ns | ns |
| BCL-2 mRNA | ns | <(Con) | ns | ns | ns | ns | ns | <(DEX) | ns | ns | ns | ns |
| Proapoptotic | ||||||||||||
| FAS mRNA | DEX < CON | ns | ns | ns | >(DEX)D | ns | ns | ns | ns | ns | ns | ns |
| Caspase-3 mRNA | ns | ns | ns | DEX > Con | ns | ns | ns | ns | ns | ns | ns | ns |
| Bax mRNA | ns | >(Con) | ns | DEX < ConA | ns | ns | DEX > ConD | ns | ns | ns | ns | ns |
| p53 mRNA | ns | <(Con) | ns | DEX < ConA, B, C | Ns | ns | DEX > ConD | ns | ns | ns | ns | ns |
Abbreviations: ns, not statistically significant; NA, not available; Con, control, DEX, dexamethasone; oPL, ovine placental lactogen; mRNA, messenger RNA; PCNA, proliferating cell nuclear antigen; BCL-2, B-cell lymphoma 2.
aChanges in fetal anthropometric parameters, placenta weight and placental BNC numbers, oPL-protein levels, and anti- and proapoptotic markers as well as maternal and fetal oPL-plasma levels were analyzed with respect to days of gestation (dG), sex, and treatment as well as placentome types (A, B, C, and D) where applicable. In the male and female columns, the greater than sign indicates that the measured parameter in the DEX group is bigger compared to controls; the greater than sign in the gender columns indicates significant differences between males and females in each group; only significant results (P < .05) of post hoc analysis (Holm Sidak) are presented. Capital letter indicates placentome types: A = A types, B = B types, C = C types, and D = D types.
In males, DEX did not affect total placenta weight or total placentome numbers (Table 2). The highest proportion of A subtypes was found at 50 dG, whereas the proportion of B subtypes was highest at 125 dG (Figure 2B). The DEX treatment significantly increased the proportion of C subtypes at 125 dG compared to controls (P < .05). Mean placentome weight in males after DEX treatment was only different from controls in B subtypes at 140 dG (MANOVA main effects: dG P < .001, type P < .001; interactions: gender × type P < .05, dG × gender × type × treatment P < .05; Holm-Sidak post hoc analysis P = .03, Table 2). Analysis of D subtypes was difficult and due to the low numbers, the values are not reported. No significant differences in fetal weight, crown–rump length, abdominal circumference, or ponderal index were observed in males. Only femur length at 140 dG was significantly reduced after DEX compared to controls (control 12.7 ± 0.2 cm vs DEX = 11.8 ± 0.3 cm; MANOVA main effects: dG P < .05, gender P < .05, treatment P < .05; interaction: P > .05; Holm-Sidak post hoc analysis P = .005; Table 3).
Overall, the ratio of placental to fetal weight as a reflection of placental efficiency in controls was not significantly different between males and females, and DEX did not affect the ratio significantly (ANOVA main effects: dG P > .05, treatment P > .05; interaction P > .05).
The Effect of DEX on BNCs
In control groups, the mean number of BNCs increased significantly between 50 and 125 dG, thereafter mean numbers of BNCs did not change toward 140 dG (MANOVA main effects: dG P < .001, type P < .05, treatment P < .05; interaction: dG × treatment P < .05, dG × type P < .05; Figure 3). Dexamethasone decreased significantly in females, the mean number of BNCs compared to controls at 100 dG (Figure 3), predominantly seen in A subtypes (P < .05). The same trend was observed in males, although not significant (P > .05).
Figure 3.
(A-C) The effect of early dexamethasone (DEX) treatment on the mean placental oPL-protein level in sheep. Relative optical density *(ROD) of mean placental oPL-protein level of (A) first band and (B) second band analyzed by full factorial model (MANOVA) with treatment, gender, and dG as factors, followed by a pairwise comparison (Holm Sidak) when main effects were P < .05. oPL first band main effects: dG P < .001, treatment P < .05; interaction: dG × gender P < .05; dG × gender × treatment P < .05; gender × type P < .05; oPL second band main effects: dG P < .001; interactions: dG × gender P < .05; dG × treatment P < .05. Post hoc P values<.05 are indicated in figure: different letters indicate sig. differences in dG, the star indicates significant differences in treatment. + represents post hoc gender differences female versus male in controls, ++ in DEX. n = numbers of animals analyzed.
The Effect of DEX on Placental oPL-Protein Levels
In controls, mean oPL-protein level increased significantly between 50 and 100 dG in both females and males but did not change significantly later in pregnancy (MANOVA main effects: dG P < .001; interaction: dG × gender × treatment P < .05; Holm-Sidak post hoc analysis P < .05). The level of first band oPL-protein increased significantly between 50 and 100 dG, but significantly decreased afterward (MANOVA main effects: dG P < .001, treatment P < .05; interaction: dG × gender P < .05; dG × gender × treatment P < .05; gender × type P < .05; Holm-Sidak post hoc analysis P < .001, Figure 1A). The level of second band oPL-protein increased significantly between 50 and 140 dG (MANOVA main effects: dG P < .001; interactions: dG × gender P < .05; dG × treatment P < .05, gender × type P < .05; Holm-Sidak post hoc analysis P < .05, Figure 1B). The ratio of oPL first to second band in controls was highest at 100 dG and decreased significantly toward 140 dG (MANOVA main effects: dG P < .001; interactions: gender × treatment P < .05; Holm-Sidak post hoc analysis P < .05, Table 3).
Dexamethasone in females significantly increased oPL second band protein levels at 125 dG (mainly in B subtypes) and significantly decreased oPL second band protein levels at 140 dG compared to controls (mean, first mainly in C types and second band mainly in D types; P < .05; Table 3 and Figure 1). Dexamethasone significantly increased the ratio of oPL first to second band at 100 dG but significantly decreased the ratio at 125 dG compared to controls (P < .05, Table 3). In males, DEX increased significantly oPL-protein levels (mean, first and second bands) compared to controls at 50 dG mainly in A subtypes (P < .05; Table 3 and Figure 1). At 100 and 140 dG, oPL-protein levels in A subtypes (100 dG: first band and first/second band ratio; 140 dG: first band) were significantly decreased compared to controls (P < .05; Table 3).
The Effect of DEX on oPL-Plasma Level
In controls, maternal oPL-plasma levels increased significantly across gestation with highest levels at 140 dG and no effect of fetal sex (MANOVA main effects: dG P < .001; interactions P > .05; Holm-Sidak post hoc analysis P < .05, Figure 4A). Fetal oPL-plasma levels in controls increased significantly between 50 and 100 dG but did not change thereafter, regardless of fetal sex (MANOVA main effects: dG P < .05; interactions: P > .05; Holm-Sidak post hoc analysis P < .05, Figure 4B). Dexamethasone did not significantly affect maternal or fetal oPL-plasma levels in either sex.
Figure 4.
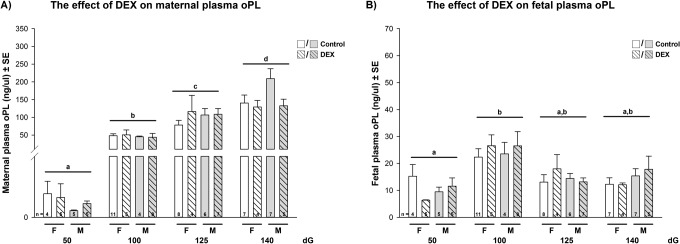
(A and B) The effect of early dexamethasone treatment on the mean maternal (A) and fetal (B) oPL-plasma levels in sheep analyzed by a full factorial model (MANOVA) with treatment, gender, and dG as factors, followed by a pairwise comparison (Holm Sidak) when main effects were P < .05. Maternal oPL main effects: dG P < .001; interactions P > .05; fetal oPL main effects: dG P < .05; interactions: P > .05. Post hoc P values <.05 (Holm-Sidak) are indicated in the figures: different letters indicate significant differences in dG.
The Effect of Early DEX on Markers of Placental Apoptosis
Details on the ontogeny of placental apoptotic markers are shown in Supplement S2. In females, DEX significantly reduced the antiapototic marker PCNA at 100 dG compared to controls (MANOVA main effects: dG P < .05; interactions: dG × treatment P < .05, dG × gender × treatment P < .05; Holm-Sidak post hoc analysis P = .022; Table 3 and Figure S2A). The proapoptotic marker Caspase-3 was significantly increased at 100 dG compared to controls (MANOVA main effects: dG P < .001, treatment P < .05; interaction P > .05; Holm-Sidak post hoc analysis P = .021; Table 3 and Figure S2D). Although DEX induced an decrease in BAX (A type) and p53 (A, B, and C types) mRNA expression levels at 100 dG as compared to controls, at 125 dG, DEX significantly increased those proapoptotic markers in D types (BAX-MANOVA main effects: dG P < .05, treatment P < .05, type P < .05; interactions: dG × treatment P < .05, treatment × type P < .05, dG × treatment × type P < .05; Holm-Sidak post hoc analysis P < .05; p53-MANOVA main effects: dG P < .05, treatment P < .05, type P < .05; interactions: dG × treatment P < .05; Holm-Sidak post hoc analysis P < .05, Table 3). In males, DEX did not significantly affect the measured placental apoptotic markers (Table 3).
The Relationship of BNC Numbers, Placental oPL-Protein, and Plasma Levels With Apoptotic Markers and Fetal and Placental Weight
A detailed description of significant correlations between the parameters analyzed is presented in Table S1. Briefly, in control females, fetal weight was positively correlated with placenta weight, number of BNCs, oPL-protein levels, and maternal oPL-plasma levels and negatively correlated with BCL-2 and FAS mRNA expression levels (Table S1A). After DEX, fetal weight changes were independent of placental oPL-protein expression levels (Table S1A). In males, fetal weight significantly correlated in controls with placenta weight and placentome numbers, BNC numbers, placental oPL-protein levels, and maternal oPL-plasma levels and was negatively correlated with PCNA, BCL-2, and FAS mRNA expression levels (Table S1B). After DEX, fetal weight changes were independent of placentome numbers (Table S1B).
Discussion
This study demonstrated that early DEX treatment is associated with sex-specific alterations in BNC numbers, and, in females, it may be associated with altered placental apoptosis markers, which may contribute to changes in placental and fetal development. The data presented may suggest that the early maternal DEX treatment in sheep, being associated with sex-specific alterations in placental development, BNC numbers, and function, may contribute to the short- and long-term changes in the fetal growth and endocrine axis observed after treatment itself.18–21,55
Fetal growth and development heavily depends on the capacity of the placenta during pregnancy to adapt continuously its function according to fetal demands. Fetal weight correlates strongly with placenta weight,56 as confirmed in the present study. Early exposure to DEX resulted in a sex-dependent decrease in fetal weight and altered placental development, some of which persisted until term.27,57 The distribution and the size of an individual placentome subtype can be influenced by adverse intrauterine conditions58–61 and the presence of an increased number of C and D subtypes has been suggested as a placental adaptation aimed at increasing nutrient delivery to a compromised fetus.62 We have previously shown that GC treatment late in gestation resulted in increased mean number and proportion of A subtypes and decreased numbers of B subtypes at 116 dG in males compared to controls.27 In contrast, in the present study, the proportion of C subtypes (at 125 dG) and mean B subtype weights (at 140 dG) in males was significantly increased after early DEX treatment compared to controls. There is little information about the functional differences between placentome subtypes, but an increased proportion of everted C and D subtypes may have implications for fetal metabolism.58,60,63–65 We have shown that changed proportions of subtypes and differential expression of important placental enzymes (prostaglandin G/H synthase 2) occurred after GC treatment in late pregnancy.27 Early DEX treatment in females in the current study did not change placenta weight or numbers of placentome subtypes significantly compared to controls but increased mean weights of C subtype placentomes compared to controls and may be indicative of placental adaptation to early DEX treatment.
Unique to the ruminant placenta, the trophoectoderm produces BNCs from ∼14 dG and after cell maturation and migration to the fetal–maternal placental interface, BNCs fuse with the maternal epithelium66 to form the maternal–fetal syncytium.67 Binucleate cells account for 10% to 20% of the cells of fetal trophectoderm in sheep,68 and their main function is to deliver fetal hormones (PLs) and effectors (pregnancy associated glycoproteins and prolactin [PRL]-related proteins) to adjust the maternal intrauterine environment to favor the needs of the fetus.67 Ovine PL plays an important role in fetal growth through its actions on maternal metabolism and by regulating fetal substrate availability.36 The oPL containing granules are transferred across the fetal–maternal placental interface and released into both the maternal and fetal circulation.69 Variation in fetal weight has been correlated with placental weight, maternal serum oPL, and cotyledonary oPL mRNA concentrations.39,70 In sheep, GC exposure late in gestation resulted in significantly lower birth weights that were associated with a reduction in the mean number of BNCs, placental oPL-protein, and maternal and fetal oPL-plasma levels.26 In the present study, early DEX resulted in a sex-specific, transient decrease in fetal weight and crown–rump length at 100 dG in female fetuses only.18,19 This decrease in fetal weight in females was associated with significantly lower BNC numbers but was not reflected in changes in placental oPL-protein levels. The underlying mechanism mediating the decrease in BNCs and the oPL output after DEX treatment remain to be elucidated, but an imbalance of pro- and antiapoptotic factors resulting in an increased rate of BNC apoptosis may be involved.26,42 Indeed, placental mRNA expression levels of proapoptotic (Caspase-3 at 100 dG, Bax and p53 at 125 dG) markers were significantly increased and antiapoptotic markers (PCNA at 100 dG) were significantly decreased compared to controls, suggesting an activation of the placental intrinsic apoptosis pathway in females. Glucocorticoid-induced apoptosis may not critically depend on extrinsic pathways.
We recognize that our study is not without limitations. Whole placental homogenates may have masked changes that occurred in protein or mRNA levels—future studies might attempt to separate caruncles and cotyledons. However, this is difficult due to the extensive interdigitation of maternal and fetal tissue, and whole placentome staining may provide a better indication of changes in both fetal and maternal tissue.
Our data on placental oPL-protein levels permit us to relate the effects of DEX on BNC number to their function. For the first time, we were able to analyze the ROD of both oPL-protein bands separately, indicative of a glycosylated and nonglycosylated form.26 Glycosylation is a common posttranslational modification of hormones in the PRL gene family and PL produced during the first half of pregnancy in the mouse, rat, and hamster all appear to be glycosylated.71 Glycosylated forms of PRL have been isolated from the sheep, pig, and human and the receptor binding and biological activities of glycosylated versus nonglycosylated PRLs can be markedly different with decreased binding activities of the glycosylated forms.71 In bovine, it has been suggested that glycosylation of PL may have a small effect on receptor specificity but does not dramatically affect receptor binding or biological activity.71 We are not aware of published information concerning glycosylation of ovine PL. The usual gestation–dependent rise in fetal cortisol was associated with decreased placental first band (=glycosylated) oPL-protein levels between 100 and 140 dG, whereas second band (nongylcosylated) oPL-protein levels increased significantly across gestation, suggesting a decrease in glycosylation across gestation. We suggest that the lack of association between fetal weight and placental oPL-protein levels at 100 dG indicates that DEX may have disrupted the normal relationship in the maternal–fetal–placental units. At 125 dG, fetal weight and crown–rump length in DEX groups in females was restored to normal and was associated with normalized mean number of BNCs and significantly increased placental oPL-protein levels.
Fetal weight in males was significantly correlated in both controls and after early DEX with placenta weight, BNC numbers, placental oPL-protein, and maternal oPL-plasma levels. In males, early DEX treatment did not alter significantly the growth trajectory compared to controls. However at 50 dG, placental oPL-protein levels were significantly increased compared to controls (most prominent in A subtypes), which may indicate an immediate response/stimulation of BNC output. No changes in pro- and antiapoptotic markers were observed, which may be indicative of a protective placental adaptation in males.
Sex-specific adaptations to adverse maternal environments such as antenatal GC treatment have been found in animal and human studies and remain to be explained.72–80 While male fetuses appear to adapt their placental function to maintain continued growth, female fetuses exhibit reduced growth in what is hypothesized to be an attempt to survive any further potential maternal insults.80,81 Consistent with this, we found that male fetal weight was unaffected by early DEX treatment. Females, in contrast, exhibited temporal adaptations to DEX treatment, particularly with respect to placental distribution of subtypes and function, fetal HPA axis activity,18 and postnatal endocrine responsiveness.19,21,55,82 Differences in the distribution of placentome subtypes and/or function may contribute to these sex-specific responses to antenatal GC, and we now show that increased placental apoptosis in females may be a contributing factor. This contributing role of the placenta has also been observed in GC metabolizing enzymes which protect the fetus from high levels of endogenous cortisol83 where in normal term pregnancies, females have higher levels of placental 11βHSD-2 activity compared to males.84 These differences in enzyme activity may suggest that the female fetus could be exposed to lower maternally derived cortisol and thus escapes negative-feedback regulation, facilitating autonomous development of fetal HPA function.85
This study, to our knowledge, is the first study to show that early maternal DEX treatment in sheep was associated with sex-specific alterations in placental development, BNC numbers, and function. These effects may contribute to the short- and long-term changes in the fetal growth and endocrine axis observed after early DEX treatment. In pregnancies with a female fetus, disruption of the fine balance between survival factors and apoptotic markers may influence placental function. The mode of action of GC in the inhibition of placental growth requires further investigation.
Supplementary Material
Acknowledgments
We thank Dr Timothy J.M. Moss and Dr Ilias Nitsos for their assistance in the animal work.
Footnotes
Authors’ Note: Thorsten Braun and Wenbin Meng shared first coauthorship.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by DFG (BR2925/1-1, 3-1, 3-2, PL241/8-2), the Canadian Institutes of Health Research, The Raine Medical Research Foundation of Western Australia, the Australian National Health and Medical Research Council (303261), Women and Infants Research Foundation of Western Australia, and the Child Health Research Foundation of Western Australia Inc.
Supplemental Material: The online supplements are available at http://rs.sagepub.com/supplemental.
References
- 1. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50 (4):515–525. [PubMed] [Google Scholar]
- 2. NIH. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273 (5):413–418. [DOI] [PubMed] [Google Scholar]
- 3. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. [DOI] [PubMed] [Google Scholar]
- 4. Forest MG. Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod Update. 2004;10 (6):469–485. [DOI] [PubMed] [Google Scholar]
- 5. Lajic S, Nordenstrom A, Hirvikoski T. Long-term outcome of prenatal treatment of congenital adrenal hyperplasia. Endocr Dev. 2008;13:82–98. [DOI] [PubMed] [Google Scholar]
- 6. David M, Forest MG. Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr. 1984;105 (5):799–803. [DOI] [PubMed] [Google Scholar]
- 7. Pang SY, Pollack MS, Marshall RN, Immken L. Prenatal treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 1990;322 (2):111–115. [DOI] [PubMed] [Google Scholar]
- 8. Dorr HG, Sippell WG. Prenatal dexamethasone treatment in pregnancies at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency: effect on midgestational amniotic fluid steroid levels. J Clin Endocrinol Metab. 1993;76 (1):117–120. [DOI] [PubMed] [Google Scholar]
- 9. Hirvikoski T, Nordenstrom A, Lindholm T, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92 (2):542–548. [DOI] [PubMed] [Google Scholar]
- 10. Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Lajic S. Long-term follow-up of prenatally treated children at risk for congenital adrenal hyperplasia: does dexamethasone cause behavioural problems? Eur J Endocrinol. 2008;159 (3):309–316. [DOI] [PubMed] [Google Scholar]
- 11. Lajic S, Wedell A, Bui TH, Ritzen EM, Holst M. Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1998;83 (11):3872–3880. [DOI] [PubMed] [Google Scholar]
- 12. Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology. 1995;20 (4):439–449. [DOI] [PubMed] [Google Scholar]
- 13. Dodic M, Hantzis V, Duncan J, et al. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16 (9):1017–1026. [DOI] [PubMed] [Google Scholar]
- 14. Roghair RD, Segar JL, Sharma RV, et al. Newborn lamb coronary artery reactivity is programmed by early gestation dexamethasone before the onset of systemic hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;289 (4):R1169–R1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodic M, McAlinden AT, Jefferies AJ, et al. Differential effects of prenatal exposure to dexamethasone or cortisol on circulatory control mechanisms mediated by angiotensin II in the central nervous system of adult sheep. J Physiol. 2006;571 (pt 3):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab. 2007;293 (1):E75–E82. [DOI] [PubMed] [Google Scholar]
- 17. Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40 (5):729–734. [DOI] [PubMed] [Google Scholar]
- 18. Braun T, Li S, Sloboda DM, et al. Effects of maternal dexamethasone treatment in early pregnancy on pituitary–adrenal axis in fetal sheep. Endocrinology. 2009;150 (12):5466–5477. [DOI] [PubMed] [Google Scholar]
- 19. Li S, Sloboda DM, Moss TJ, et al. Effects of glucocorticoid treatment given in early or late gestation on growth and development in sheep. JDOHaD. 2013;4 (2):146–156. [DOI] [PubMed] [Google Scholar]
- 20. Cox DB. The effect of maternal dexamethasone during early pregnancy on fetal growth, development and the control of glucose homeostasis. J Soc Gynecol Invest. 1999;6 (suppl 1):251. [Google Scholar]
- 21. Li S, Nitsos I, Polglase GR, et al. The effects of dexamethasone treatment in early gestation on hypothalamic-pituitary-adrenal responses and gene expression at 7 months of postnatal age in sheep. Reprod Sci. 2012;19 (3):260–270. [DOI] [PubMed] [Google Scholar]
- 22. Challis JR, Sloboda D, Matthews SG, et al. The fetal placental hypothalamic–pituitary–adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol. 2001;185 (1-2):135–144. [DOI] [PubMed] [Google Scholar]
- 23. Braun T, Challis JR, Newnham JP, Sloboda DM. Early-life glucocorticoid exposure: the hypothalamic–pituitary–adrenal axis, placental function, and long-term disease risk. Endocr Rev. 2013;34 (6):885–916. [DOI] [PubMed] [Google Scholar]
- 24. Ehrhardt RA, Bell AW. Growth and metabolism of the ovine placenta during mid-gestation. Placenta. 1995;16 (8):727–741. [DOI] [PubMed] [Google Scholar]
- 25. Audette MC, Connor KL, Braun T, et al. Maternal glucocorticoid administartion in early pregnancy and the relationship between 11ßHSD2 mRNA in ovine placenta and fetal weight across gestation. Reprod Sci (Suppl). 2008;15(S2):273A. [Google Scholar]
- 26. Braun T, Li S, Moss TJ, et al. Maternal betamethasone administration reduces binucleate cell number and placental lactogen in sheep. J Endocrinol. 2007;194 (2):337–347. [DOI] [PubMed] [Google Scholar]
- 27. Braun T, Li S, Moss TJ, et al. Differential appearance of placentomes and expression of prostaglandin H synthase type 2 in placentome subtypes after betamethasone treatment of sheep late in gestation. Placenta. 2011;32 (4):295–303. [DOI] [PubMed] [Google Scholar]
- 28. Clarke KA, Ward JW, Forhead AJ, Giussani DA, Fowden AL. Regulation of 11 beta-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J Endocrinol. 2002;172 (3):527–534. [DOI] [PubMed] [Google Scholar]
- 29. Clifton VL, Rennie N, Murphy VE. Effect of inhaled glucocorticoid treatment on placental 11beta-hydroxysteroid dehydrogenase type 2 activity and neonatal birthweight in pregnancies complicated by asthma. Aust N Z J Obstet Gynaecol. 2006;46 (2):136–140. [DOI] [PubMed] [Google Scholar]
- 30. Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gatford KL, Owens JA, Li S, et al. Repeated betamethasone treatment of pregnant sheep programs persistent reductions in circulating IGF-I and IGF-binding proteins in progeny. Am J Physiol Endocrinol Metab. 2008;295 (1):E170–E178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hahn T, Barth S, Graf R, et al. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84 (4):1445–1452. [DOI] [PubMed] [Google Scholar]
- 33. Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal `programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3 (6):479–488. [DOI] [PubMed] [Google Scholar]
- 34. Handwerger S, Maurer WF, Crenshaw C, Hurley T, Barrett J, Fellows RE. Development of the sheep as an animal model to study placental lactogen physiology. J Pediatr. 1975;87 (6 pt 2):1139–1143. [DOI] [PubMed] [Google Scholar]
- 35. Vatnick I, Schoknecht PA, Darrigrand R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol. 1991;15 (6):351–356. [PubMed] [Google Scholar]
- 36. Handwerger S, Freemark M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab. 2000;13 (4):343–356. [DOI] [PubMed] [Google Scholar]
- 37. Oliver MH, Harding JE, Breier BH, Evans PC, Gluckman PD. The nutritional regulation of circulating placental lactogen in fetal sheep. Pediatr Res. 1992;31 (5):520–523. [DOI] [PubMed] [Google Scholar]
- 38. Schoknecht PA, McGuire MA, Cohick WS, Currie WB, Bell AW. Effect of chronic infusion of placental lactogen on ovine fetal growth in late gestation. Domest Anim Endocrinol. 1996;13 (6):519–528. [DOI] [PubMed] [Google Scholar]
- 39. Kappes SM, Warren WC, Pratt SL, Liang R, Anthony RV. Quantification and cellular localization of ovine placental lactogen messenger ribonucleic acid expression during mid- and late gestation. Endocrinology. 1992;131 (6):2829–2838. [DOI] [PubMed] [Google Scholar]
- 40. Wooding FB. The role of the binucleate cell in ruminant placental structure. J Reprod Fertil Suppl. 1982;31:31–39. [PubMed] [Google Scholar]
- 41. Wooding FB, Morgan G, Monaghan S, Hamon M, Heap RB. Functional specialization in the ruminant placenta: evidence for two populations of fetal binucleate cells of different selective synthetic capacity. Placenta. 1996;17 (1):75–86. [DOI] [PubMed] [Google Scholar]
- 42. Ward JW, Wooding FB, Fowden AL. The effects of cortisol on the binucleate cell population in the ovine placenta during late gestation. Placenta. 2002;23 (6):451–458. [DOI] [PubMed] [Google Scholar]
- 43. Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. [DOI] [PubMed] [Google Scholar]
- 44. Gaynon PS, Carrel AL. Glucocorticosteroid therapy in childhood acute lymphoblastic leukemia. Adv Exp Med Biol. 1999;457:593–605. [DOI] [PubMed] [Google Scholar]
- 45. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35 (4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol Reprod. 2012;87(1):22, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lyahyai J, Goldammer T, Beattie AE, Zaragoza P, Martin-Burriel I. Positional and functional characterisation of apoptosis related genes belonging to the BCL2 family in sheep. Cytogenet Genome Res. 2005;109 (4):519–526. [DOI] [PubMed] [Google Scholar]
- 48. Cox DB, Fraser M, Challis JRG. Placental development following maternal dexamethasone treatment during early pregnancy. J Soc Gynecol Invest. 1999;6 (suppl 1):120. [Google Scholar]
- 49. Gluckman PD, Kaplan SL, Rudolph AM, Grumbach MM. Hormone ontogeny in the ovine fetus. II. Ovine chorionic somatomammotropin in mid- and late gestation in the fetal and maternal circulations. Endocrinology. 1979;104 (6):1828–1833. [DOI] [PubMed] [Google Scholar]
- 50. Gentili S, Morrison JL, McMillen IC. Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod. 2009;80 (6):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Passmore M, Nataatmadja M, Fraser JF. Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries . BMC Mol Biol. 2009;10 (1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hyatt MA, Gopalakrishnan GS, Bispham J, et al. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J Endocrinol. 2007;192 (1):87–97. [DOI] [PubMed] [Google Scholar]
- 53. Garcia J, Simon MA, Duran M, Canceller J, Aneiros FJ. Differential efficacy of a cognitive-behavioral intervention versus pharmacological treatment in the management of fibromyalgic syndrome. Psychol Health Med. 2006;11 (4):498–506. [DOI] [PubMed] [Google Scholar]
- 54. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li S, Nitsos I, Polglase GR, Newnham JP, Challis JR, Moss TJ. Effects of tail docking and castration on stress responses in lambs and the influence of prenatal glucocorticoid treatment. Reprod Fertil Dev. 2013;25 (7):1020–1025. [DOI] [PubMed] [Google Scholar]
- 56. Bell AW, Hay WW, Jr, Ehrhardt RA. Placental transport of nutrients and its implications for fetal growth. J Reprod Fertil Suppl. 1999;54:401–410. [PubMed] [Google Scholar]
- 57. Braun T, Sloboda DM, Li S, Moss TJ, Newnham JP, Challis JRG. Early prenatal glucocorticoid exposure on placental anthropometry in sheep differs from late gestation exposure. J Dev Origins Health Dis. 2009;1(S1):195. [Google Scholar]
- 58. Penninga L, Longo LD. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 1998;19 (2-3):187–193. [DOI] [PubMed] [Google Scholar]
- 59. Vatnick I, Ignotz G, McBride BW, Bell AW. Effect of heat stress on ovine placental growth in early pregnancy. J Dev Physiol. 1991;16 (3):163–166. [PubMed] [Google Scholar]
- 60. Steyn C, Hawkins P, Saito T, Noakes DE, Kingdom JC, Hanson MA. Undernutrition during the first half of gestation increases the predominance of fetal tissue in late-gestation ovine placentomes. Eur J Obstet Gynecol Reprod Biol. 2001;98 (2):165–170. [DOI] [PubMed] [Google Scholar]
- 61. Gardner DS, Ward JW, Giussani DA, Fowden AL. The effect of a reversible period of adverse intrauterine conditions during late gestation on fetal and placental weight and placentome distribution in sheep. Placenta. 2002;23 (6):459–466. [DOI] [PubMed] [Google Scholar]
- 62. Alexander G. Studies on the placenta of the sheep (Ovis aries L.). Placental size. J Reprod Fertil. 1964;7:289–305. [DOI] [PubMed] [Google Scholar]
- 63. Wintour EM, Alcorn D, McFarlane A, Moritz K, Potocnik SJ, Tangalakis K. Effect of maternal glucocorticoid treatment on fetal fluids in sheep at 0.4 gestation. Am J Physiol. 1994;266 (4 pt 2):R1174–R1181. [DOI] [PubMed] [Google Scholar]
- 64. Ward JW, Forhead AJ, Wooding FB, Fowden AL. Functional significance and cortisol dependence of the gross morphology of ovine placentomes during late gestation. Biol Reprod. 2006;74 (1):137–145. [DOI] [PubMed] [Google Scholar]
- 65. Ward JW, Wooding FB, Fowden AL. Ovine feto-placental metabolism. J Physiol. 2004;554 (pt 2):529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lacroix MC, Bolifraud P, Durieux D, Pauloin A, Vidaud M, Kann G. Placental growth hormone and lactogen production by perifused ovine placental explants: regulation by growth hormone-releasing hormone and glucose. Biol Reprod. 2002;66 (3):555–561. [DOI] [PubMed] [Google Scholar]
- 67. Wooding P, Burton G. Synepitheliochorial placentation: ruminats (ewe and cow). In: Wooding P, Burton G, eds. Comparative Placentation. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 2008:133–167. [Google Scholar]
- 68. Wooding FB, Hobbs T, Morgan G, Heap RB, Flint AP. Cellular dynamics of growth in sheep and goat synepitheliochorial placentomes: an autoradiographic study. J Reprod Fertil. 1993;98 (1):275–283. [DOI] [PubMed] [Google Scholar]
- 69. Wooding FB. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta. 1992;13 (2):101–113. [DOI] [PubMed] [Google Scholar]
- 70. Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572 (pt 1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Byatt JC, Welply JK, Leimgruber RM, Collier RJ. Characterization of glycosylated bovine placental lactogen and the effect of enzymatic deglycosylation on receptor binding and biological activity. Endocrinology. 1990;127 (3):1041–1049. [DOI] [PubMed] [Google Scholar]
- 72. Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144 (7):2775–2784. [DOI] [PubMed] [Google Scholar]
- 73. Reznikov AG, Nosenko ND, Tarasenko LV. Prenatal stress and glucocorticoid effects on the developing gender-related brain. J Steroid Biochem Mol Biol. 1999;69 (1-6):109–115. [DOI] [PubMed] [Google Scholar]
- 74. O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. [DOI] [PubMed] [Google Scholar]
- 75. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2009;22 (3):330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32 (8):570–578. [DOI] [PubMed] [Google Scholar]
- 77. Roberge S, Lacasse Y, Tapp S, et al. Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: systematic review and meta-analysis. J Obstet Gynaecol Can. 2011;33 (3):216–226. [DOI] [PubMed] [Google Scholar]
- 78. Ballard PL, Ballard RA, Granberg JP, et al. Fetal sex and prenatal betamethasone therapy. J Pediatr. 1980;97 (3):451–454. [DOI] [PubMed] [Google Scholar]
- 79. Spinillo A, Capuzzo E, Ometto A, Stronati M, Baltaro F, Iasci A. Value of antenatal corticosteroid therapy in preterm birth. Early Hum Dev. 1995;42 (1):37–47. [DOI] [PubMed] [Google Scholar]
- 80. Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and preterm birth. Placenta. 2013;34 (2):95–99. [DOI] [PubMed] [Google Scholar]
- 81. Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;(31 suppl):S33–S39. [DOI] [PubMed] [Google Scholar]
- 82. Li S, Moss TJ, Nitsos I, et al. The impact of maternal synthetic glucocorticoid administration in late pregnancy on fetal and early neonatal hypothalamic–pituitary–adrenal axis regulatory genes is dependent upon dose and gestational age at exposure. JDOHaD. 2013;4 (1):77–89. [DOI] [PubMed] [Google Scholar]
- 83. Dickinson H, O’Connell B, Walker D, Moritz K. Sex-specific effects of prenatal glucocorticoids on placental development. In: Qian X, ed. Glucocorticoids—New Recognition of Our Familiar Friend. Rijeka, Croatia: InTech; 2012:391–406. [Google Scholar]
- 84. Murphy VE, Gibson PG, Giles WB, et al. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168 (11):1317–1323. [DOI] [PubMed] [Google Scholar]
- 85. Connor KL, Challis JR, van Zijl P, et al. Do alterations in placental 11beta-hydroxysteroid dehydrogenase (11betaHSD) activities explain differences in fetal hypothalamic–pituitary–adrenal (HPA) function following periconceptional undernutrition or twinning in sheep? Reprod Sci. 2009;16 (12):1201–1212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.