Abstract
Allergic rhinitis is a common comorbid condition in pediatric chronic rhinosinusitis (CRS). Testing for aeroallergen sensitization should therefore be considered in the evaluation of children with CRS. At present the aeroallergen sensitivity profile of children with CRS remains uncharacterized. In this study, we retrospectively identify a consecutive series of children with CRS and allergic rhinitis who have undergone joint otolaryngology and allergy evaluation at a single tertiary care center. We describe the aeroallergen sensitivity profiles (based upon formal skin testing) of these children, stratifying them according to co-morbidity status: 1) CRS with cystic fibrosis (CF), 2) CRS with immune deficiency and 3) uncomplicated CRS (without co-morbid CF, immune deficiency or primary ciliary dyskinesia). We identify 208 children (average age 9.3 years, standard deviation 4.8 years) with CRS and allergic rhinitis meeting inclusion criteria, 140 with uncomplicated CRS, 64 with co-morbid immune deficiency and 4 with co-morbid CF. The prevalence of indoor aeroallergen sensitivities (62.9–100.0%) was more common than that of outdoor aeroallergen sensitivities (43.8–50.0%) in all three cohorts of children. In all three cohorts, the most common indoor aeroallergen sensitivity was to dust mites (50.0–75.0%) and the most common outdoor aeroallergen sensitivity was to tree pollens (43.8–50.0%). The aeroallergen sensitivity profile of children with CRS and allergic rhinitis appears to be similar to that of the general pediatric population with allergic rhinitis, and parallels the aeroallergen sensitivities previously described for adults with CRS and allergic rhinitis. Knowledge of the aeroallergen sensitivities in children with CRS and allergic rhinitis will enhance both diagnostic and treatment strategies.
Keywords: Aeroallergen, allergic rhinitis, chronic rhinosinusitis, epidemiology, pediatric, sensitivities, skin testing
Allergic rhinitis (AR) and chronic rhinosinusitis (CRS) are both characterized clinically by sinonasal inflammation and immunologically by shared inflammatory mediators.1 These conditions are commonly comorbid,2,3 and a role for AR is suspected in the pathogenesis and persistence of CRS.4,5 The primary management of pediatric CRS consists of medical treatment aimed, in part, to reduce underlying sinonasal inflammation.6 Because 27% of children with CRS are reported to have concurrent AR,3 knowledge of their aeroallergen sensitivity profile is important to direct therapeutic management strategies. However, the aeroallergen sensitivity profile of children with CRS and AR has not been previously described. We describe here the aeroallergen sensitivity profile of a consecutive series of 208 children, 18 years of age or younger, identified as having both CRS and AR on joint otolaryngology and allergy evaluation. These children comprise the subset of a previously described pediatric CRS cohort with AR,3 who also had available results from formal allergy testing by skin-prick and/or intradermal testing.
Approval for this study was obtained from the Boston Children's Hospital Institutional Review Board. Children included in this study, and their diagnoses of interest, were identified based on their associated International Classification of Diseases, ninth revision codes for CRS (473.*) as well as AR (477.*), asthma (493.*), immune deficiencies (279.*), cystic fibrosis (CF) (277.*), and primary ciliary dyskinesia (PCD) (759.*). The clinical diagnosis of CRS in these children was made by a history of 90 days or more of upper airway and sinonasal symptoms, including but not limited to nasal obstruction, purulent rhinorrhea, and cough.
Children with CRS were considered to have “uncomplicated CRS” if they did not have a concurrent immune deficiency, CF, or PCD.3 Demographic data consisting of age at the time of presentation and gender were recorded. Formal allergy testing by skin-prick testing to commercially available extracts (Greer Laboratories, Lenoir, NC; Hollister-Stier, Spokane, WA) was performed using standard methods and as previously reported.5 Adjunct intradermal testing was used in a limited number of patients. In these patients, the use of intradermal testing was limited only to specific allergens suspected by clinical history that were negative by skin-prick testing. Aeroallergen sensitivities were broadly categorized as mites, molds, grass, trees, weeds, cockroach, mouse, cat, and dog. Mite allergy was based on positive testing to Dermatophagoides pteronyssinus allergen 1, Dermatophagoides farinae allergen 1, or a house dust mix. Mold allergy was based on positive testing to Epicoccum, Alternaria, Hormodendrum, Aspergillus, Penicillium, or Helminthosporium. Grass allergy was tested using a grass mix (Kentucky blue, orchard, red top, timothy, and sweet vernal grass). Tree allergy was based on allergy to pollens from extracts of birch, oak, elm, maple, red cedar, ash, hickory, mulberry, cottonwood, or some mixture of these extracts. Weed allergy was based on allergy to sage brush, lamb's quarter, marsh elder, yellow dock, plantain, or ragweed. Cockroach, mouse, cat, and dog allergies were based on positive testing to standard antigens. Positive histamine and negative saline controls were used in all cases. Wheal diameters were measured 15 minutes after the skin-prick or intradermal test was placed. For both skin-prick and intradermal testing, a wheal diameter at least three millimeters larger than the negative control was considered positive. Medications that might interfere with test results were withheld before skin testing (short-acting antihistamines for 3 days, long-acting antihistamines for 10 days, and cetirizine for 14 days). Patients were excluded if they had a negative histamine control.
Our cohort of children with CRS and AR was 52% male and 48% female with a mean age 9.3 years (standard deviation of 4.8 years). Aeroallergen sensitivity profiles were determined for children with uncomplicated CRS (N = 140) as well as for children with CRS who had comorbid CF (N = 4) or immune deficiencies (N = 64). Only one patient with CRS had PCD, due to the paucity of data, was excluded from further characterization of aeroallergen sensitivity profiles. Multiple aeroallergen sensitizations were documented in all CRS groups (Table 1).
Table 1.
Prevalence of allergen sensitivities in children with CRS
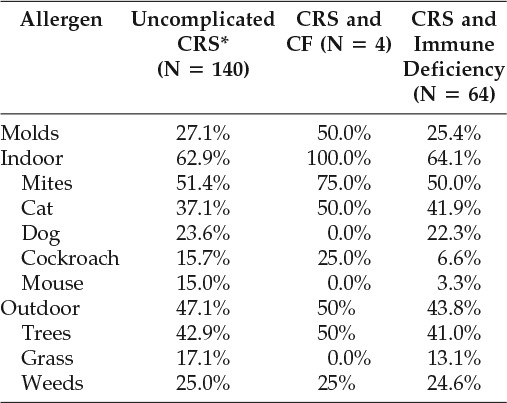
* = Without cystic fibrosis, immune deficiency, and primary ciliary dyskinesia; CRS = chronic rhinosinusitis; CF = cystic fibrosis.
Indoor aeroallergen (mites, cat, dog, cockroach, and mouse) sensitivity was more common than outdoor aeroallergen sensitivity. Indoor aeroallergen sensitivity was found in 63% of children with uncomplicated CRS, 100% of children with CRS and CF, and 64% of children with CRS and an immune deficiency. Sensitivity to dust mites was the most prevalent indoor aeroallergen sensitivity in all groups of children, although sensitivities to molds, the dander of various animals, and cockroach were also common. Overall, 71% of children with uncomplicated CRS and AR were found to be sensitive to perennial environmental aeroallergens, either mold or alternative indoor aeroallergens. Of children with AR and CRS with concurrent immune deficiency, 73% had either mold or indoor aeroallergen sensitivities.
Although less prevalent than indoor aeroallergen sensitivity, outdoor aeroallergen sensitivity nonetheless affected a substantial proportion of children with CRS. Outdoor aeroallergen sensitivities were found in 47% of children with uncomplicated CRS, 50% of children with CRS and concurrent CF, and 44% of children with CRS and concurrent immune deficiency. Of outdoor aeroallergens, sensitivity to tree pollens was most common in all three cohorts.
CRS represents a heterogeneous set of disease processes that phenotypically converge with respect to chronic mucosal inflammation of the paranasal sinuses.1 Previous work has described associations between comorbidities such as CF, immune deficiency and PCD, and the pathogenesis of CRS. AR may also contribute to the development or persistence of CRS via mucosal inflammation of the sinonasal cavity4,5 and is more common than CF, immune deficiency, or PCD in children with CRS.3 However, the aeroallergen sensitivity profile of children with CRS and AR has not been previously described.
The novel characterization of aeroallergen sensitivities in children with CRS and AR presented here reveals a high prevalence of sensitization to all major categories of indoor and outdoor aeroallergens. This study is limited by its retrospective nature and dependence on International Classification of Diseases, ninth revision codes for identifying patients. Our institution's status as a pediatric tertiary care center likely introduces a referral bias as well. These limitations aside, the aeroallergen sensitivity profile of children with CRS was found to be similar to that previously reported for atopic children overall, with indoor aeroallergen sensitivities more common than outdoor aeroallergen sensitivities.7 Moreover, in all cohorts of children, dust mites represent the most common indoor aeroallergen sensitivity, and trees represent the most common outdoor aeroallergen sensitivity. These findings are also consistent with the aeroallergen sensitivity profile previously reported for the adult CRS population.8 The predominance of indoor aeroallergen sensitivities supports a role for persistent AR-driven sinonasal inflammation as an important contributor to the pathophysiology of CRS in atopic children.
In addition to impaired quality of life,9 CRS can also place children at increased risk for other more worrisome sinusitis sequelae such as orbital and intracranial complications.10 Efforts should therefore be made to identify additional sources of sinonasal inflammation in children with CRS. Previous work has demonstrated the prevalence of AR in children with CRS to be greater than the combined prevalence of the more commonly sought comorbid conditions of CF, immune deficiency, and PCD.3 Knowledge of the likely triggers for allergic sinonasal inflammation in children with concurrent AR and CRS is of clinical utility in reducing the disease burden of chronic sinonasal inflammation in such patients.
Footnotes
Financial support: W.P. was supported in part by the National Institutes of Health Grant K24 AI106822
Approval for this study was obtained from the Boston Children's Hospital Institutional Review Board
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Van Crombruggen K, Zhang N, Gevaert P, et al. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol 128:728–732, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Houser SM, Keen KJ. The role of allergy and smoking in chronic rhinosinusitis and polyposis. Laryngoscope 118:1521–1527, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Sedaghat AR, Phipatanakul W, Cunningham MJ. Prevalence of and associations with allergic rhinitis in children with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol 78:343–347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sedaghat AR, Gray ST, Chambers KJ, et al. Sinonasal anatomic variants and asthma are associated with faster development of chronic rhinosinusitis in patients with allergic rhinitis. Int Forum Allergy Rhinol 3:755–761, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Sedaghat AR, Phipatanakul W, Cunningham MJ. Atopy and the development of chronic rhinosinusitis in children with allergic rhinitis. J Allergy Clin Immunol Pract 1:689–691.e1–2, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Wu AW, Shapiro NL, Bhattacharyya N. Chronic rhinosinusitis in children: what are the treatment options? Immunol Allergy Clin North Am 29:705–717, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Sheehan WJ, Rangsithienchai PA, Baxi SN, et al. Age-specific prevalence of outdoor and indoor aeroallergen sensitization in Boston. Clin Pediatr (Phila) 49:579–585, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gutman M, Torres A, Keen KJ, Houser SM. Prevalence of allergy in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg 130:545–552, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham MJ, Chiu EJ, Landgraf JM, Gliklich RE. The health impact of chronic recurrent rhinosinusitis in children. Arch Otolaryngol Head Neck Surg 126:1363–1368, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Sedaghat AR, Wilke CO, Cunningham MJ, Ishman SL. Socioeconomic disparities in the presentation of acute bacterial sinusitis complications in children. Laryngoscope 124:1700–1706, 2014. [DOI] [PubMed] [Google Scholar]


