Abstract
A case report of recalcitrant allergic fungal sinusitis (AFS) refractory to systemic corticosteroids and multiple functional endoscopic sinus surgeries (FESSs) treated with anti-IgE antibody omalizumab is reported. AFS is often classified with chronic rhinosinusitis (CRS). Although similar symptoms are among the two diseases, AFS has a unique pathophysiology. Patients with AFS demonstrate type 1 hypersensitivity to fungal allergens, increased total serum IgE, increased CD8+ T-cell prevalence, and IL-4 and IL-5 response. Omalizumab should be considered in the treatment of AFS.
Keywords: Allergic bronchopulmonary aspergillosis, allergic fungal rhinosinusitis, allergic fungal sinusitis, aspergillosis, Bent, CD8+, IgE, Kuhn, omalizumab, sinobronchial allergic mycosis
Recent publications on allergic fungal sinusitis (AFS) have focused on taxonomy, pathophysiology, and treatment. AFS represents a subclass of chronic rhinosinusitis (CRS). However, AFS patients are disproportionately affected by ophthalmic sequela, i.e., AFS patients account for 50% of all patients with ophthalmic manifestations and sinus disease.1,2 In addition, AFS patients present with greater total serum IgE3,4 and increased prevalence of CD8+ T cells in contrast to CRS patients that present with increased prevalence of CD20+ B lymphocytes.5 IL-4 and IL-5 response is also significantly elevated in AFS patients compared with healthy controls.6 AFS is treated corticosteroids postoperatively,7 and growing evidence suggests that specific immunotherapy8 (SIT) is beneficial. Topical and systemic antifungals, although ineffective in CRS,9 may have utility in AFS.10 This case report describes a patient with AFS refractory to surgery and systemic steroids that was successfully treated with the anti-IgE antibody, omalizumab.
CASE REPORT
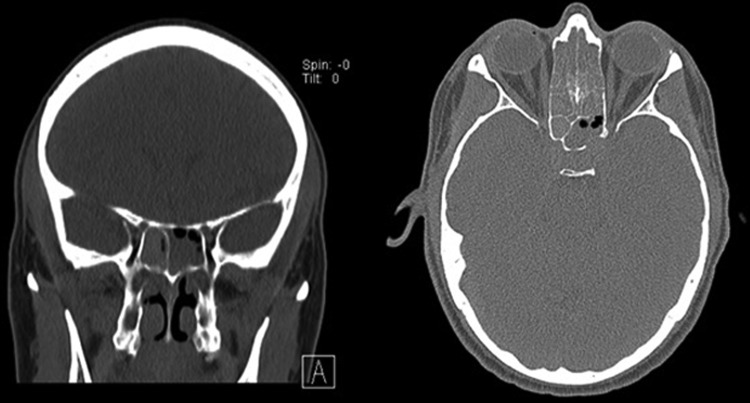
A 41-year-old male with a history of chronic sinusitis, keratoconus, seasonal allergic rhinoconjunctivitis, presumed allergic bronchopulmonary aspergillosis presented with years of headaches, sinus pressure, discolored nasal drainage, anosmia, halitosis, intermittent dyspnea, blurred vision, watery, and itchy eyes. Allergy skin tests showed a 3+ response to Aspergillus fumigatus, Alternaria tenuis, Curvularia, and Bipolaris. Aspergillus fumigatus-specific IgE levels were 6.73 IU/mL, and total serum IgE was 17,258 IU/mL (peak). The eosinophil count was 3200 cells/μL (peak). Computed tomography of the sinuses showed pansinusitis with bone dehiscence in the posterolateral left sphenoid (Fig. 1). Computed tomography of the chest showed moderate central bronchiolar thickening as well as mucoid impaction without consolidation, atelectasis, effusions, or lymphadenopathy. He underwent functional endoscopic sinus surgery (FESS) with polyp removal from the bilateral ethmoid, sphenoid and frontal and maxillary sinuses. Pathology demonstrated allergic mucus with eosinophils, nondematiaceous fungal hyphae, and no evidence of angioinvasion. Sinus, sputum, and blood cultures were growth negative. The disease was refractory to courses of topical and systemic agents. Over the next eight months, courses of oral itraconazole, azithromycin, prednisone, montelukast, cetirizine, and diphenhydramine were prescribed. In addition, sinus irrigation with gentamycin, tobramycin, budesonide, baby shampoo, and vinegar were alternated. The patient had monthly placement of flunisolide gel and sinus debridement under rhinoscopy. Despite the interventions, symptoms remained. The patient continued to complain of persistent headaches, sinus pressure, anosmia, and nasal drainage. Each of the seven rhinoscopies after FESS identified diffuse polypoid mucosa and inspissated allergic mucus consistent with grade II findings (Kupferburg grading system).11 SIT was considered because the patient's sinus symptoms and signs were still present. However, the patient's asthma was not well controlled. After each of three attempts to titrate down to a prednisone dose of 20 mg daily, the first second forced expiratory volume (FEV1) ranged from 42% to 54% of the predicted value. On doses above 40 mg, the FEV1 consistently ranged from 82% to 86% of predicted. A trial of omalizumab for treatment of AFS was felt preferable to SIT given the volatile course of his asthma. Repeat IgE was 5061 IU/mL, and he was started on omalizumab (375 mg biweekly) to control his symptoms. The omalizumab dosing was chosen, because it was the highest dose that was safely studied in clinical trials per package insert. No adverse effect was noted with administration. Rhinoscopy was repeated four weeks later showing resolution of polypoid mucosa without allergic mucus (grade 0 findings). The patient also reported complete resolution of sinus disease, i.e., headaches, sinus pressure, rhinorrhea, and anosmia. Follow-up at eight months after initiating omalizumab revealed that the patient was still without headaches, rhinorrhea, postnasal drip, or anosmia. Physical exam revealed pale nasal mucosa bilaterally without discharge and no visible polyposis. His FEV1 was 81% of predicted despite reduced prednisone dose to 8 mg every other day. SIT was initiated at that time. Twelve months after initiation of omalizumab, off prednisone, his FEV1 was 85% and total serum IgE 1473 IU/mL. At 20 months after initiating omalizumab, his symptoms had not relapsed, and his exam was without change.
Figure 1.
Computerized tomography sinus showing findings of AFS to include: Unilateral sinus expansion, heterogeneous mucous and bone erosion in the left sphenoid sinus.
DISCUSSION
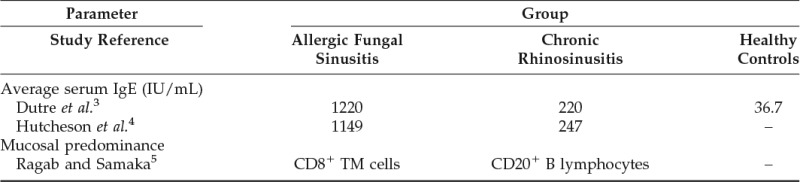
The exact pathophysiology of AFS remains unclear. It is classically thought to be dysregulation of inflammation begetting further inflammation, a variant of CRS. However, there are certain disease manifestations and complications in AFS that are not described with CRS.12 For example, the patient described here presented with sinobronchial allergic mycosis syndrome.13 Although Th2 response to antigens has been implicated in many types of CRS,14 including AFS,6 there remain many differences. Besides CD8+ T-cell predominance, IgE is more elevated in AFS (Table 1). The exact pathophysiologies remain unclear; IgE likely plays a larger role in AFS than it does in CRS. Subsequently, the treatment options and durations differ.
Table 1.
Previous comparisons of immunological features
Patients with AFS tend to require more intensive and prolonged therapy than CRS patients. Apart from initial conservative therapy, postoperative systemic corticosteroids are the standard of care for AFS. SIT has been shown to be safe and may be effective for AFS.
It is notable that omalizumab versus placebo was studied in CRS.15 The investigators did not find statistically significant outcomes comparing the two treatment groups. However, patients with AFS are a small portion of patients with CRS and were underrepresented in the study. The authors state the exclusion criteria prevented patients with serum IgE more than 700 IU/mL from joining. Because the average serum IgE of patients with AFS is much greater, it is likely AFS patients were further underrepresented.
Further studies should be performed to evaluate the efficacy of omalizumab in AFS. Given the likely contribution of IgE to AFS pathophysiology and the efficacy of omalizumab in other forms of rhinosinusitis,16,17 omalizumab should remain a treatment consideration in AFS.
CONCLUSION
AFS is currently treated surgically in combination with steroid administration. This case report demonstrated that omalizumab may be an effective treatment for AFS refractory to FESS and corticosteroids. It suggests that IgE plays an essential role in AFS pathophysiology. This role may be downstream in concordance with recent studies demonstrating the contribution of CD8+ T cells, Th2 cells, IL-4, and IL-5 in AFS pathophysiology.
ACKNOWLEDGMENTS
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the United States Army Medical Department, the United States Army Office of the Surgeon General, the Department of the Army, and Department of Defense, or the United States Government.
Footnotes
Presented at San Antonio Uniformed Services Health Education Consortium Research Day, April 24, 2014. Presented at South Texas American College of Physicians Meeting, May 16, 2014
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Al Anazy FH, Al Dousary SH. Ophthalmic manifestations of paranasal sinus disease: a clinical grading system. Int Forum Allergy Rhinol 2:331–335, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Uri N, Ronen O, Marshak T, et al. Allergic fungal sinusitis and eosinophilic mucin rhinosinusitis: diagnostic criteria. J Laryngol Otol 127:867–871, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Dutre T, Al Dousary S, Zhang N, et al. Allergic fungal rhinosinusitis-more than a fungal disease? J Allergy Clin Immunol 132:487–489.e1, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Hutcheson PS, Schubert MS, Slavin RG. Distinctions between allergic fungal rhinosinusitis and chronic rhinosinusitis. Am J Rhinol Allergy 24:405–408, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Ragab A, Samaka R. Immunohistochemical dissimilarity between allergic fungal and nonfungal chronic rhinosinusitis. Am J Rhinol Allergy 27:168–176, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy 23:281–287, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Rupa V, Jacob M, Mathews MS, Seshadri MS. A prospective, randomised, placebo-controlled trial of postoperative oral steroid in allergic fungal sinusitis. Eur Arch Otorhinolaryngol 267:233–238, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Doellman MS, Dion GR, Weitzel EK, et al. Immunotherapy in allergic fungal sinusitis: the controversy continues. A recent review of literature. Allergy Rhinol (Providence) 4:e32–e35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sacks PL, Harvey RJ, Rimmer J, et al. Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis. Cochrane Database Syst Rev 10:CD008263, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Khalil Y, Tharwat A, Abdou AG, et al. The role of antifungal therapy in the prevention of recurrent allergic fungal rhinosinusitis after functional endoscopic sinus surgery: a randomized, controlled study. Ear Nose Throat J 90:E1–E7, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Kupferberg SB, Bent J, 3rd, Kuhn FA. Prognosis for allergic fungal sinusitis. Otolaryngol Head Neck Surg 117:35–41, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Bozeman S, deShazo R, Stringer S, Wright L. Complications of allergic fungal sinusitis. Am J Med 124:359–368, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Venarske DL, deShazo RD. Sinobronchial allergic mycosis: the SAM syndrome. Chest 121:1670–1676, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Kennedy JL, Borish L. Chronic sinusitis pathophysiology: the role of allergy. Am J Rhinol Allergy 27:367–371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinto JM, Mehta N, DiTineo M, et al. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology 48:318–324, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Tsabouri S, Tseretopoulou X, Priftis K, et al. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract 2:332–340.e1, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 131:110–116, 2013. [DOI] [PubMed] [Google Scholar]