Abstract
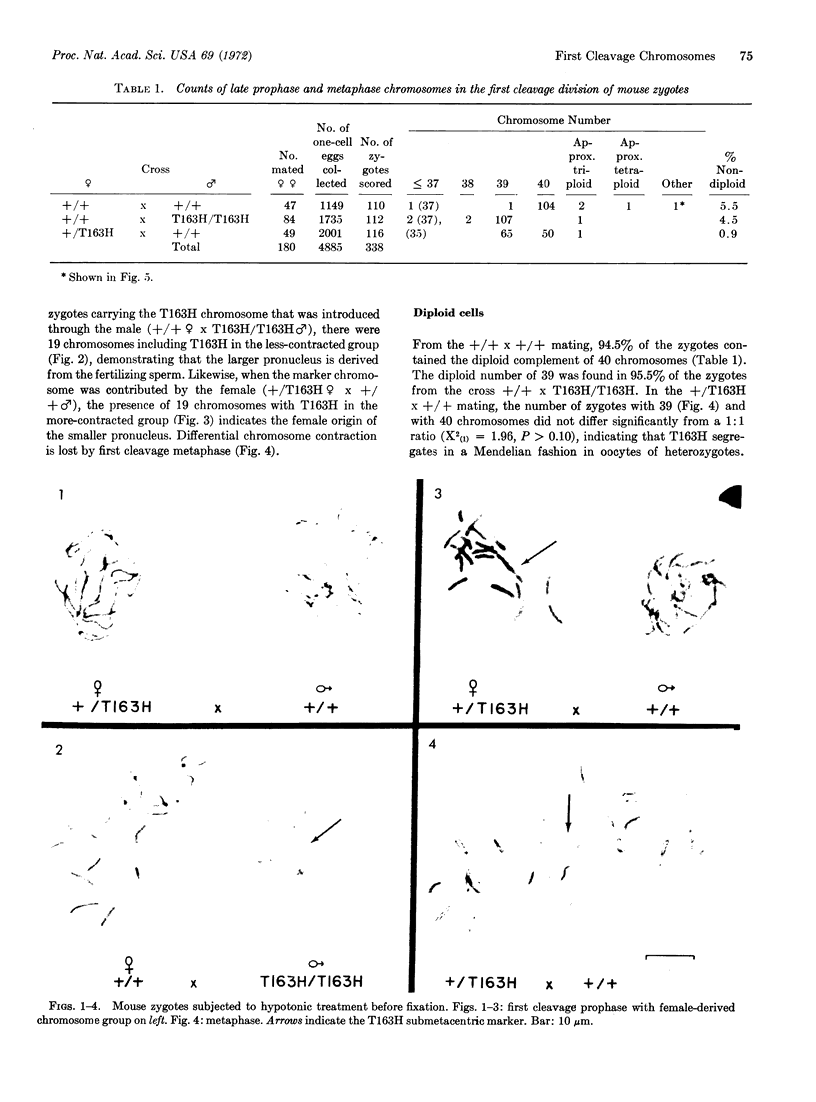
Chromosome counts of 338 mouse zygotes at late prophase and metaphase of the first cleavage division revealed 96.4% diploidy, 1.8% hypodiploidy, 1.2% triploidy, and 0.3% tetraploidy. One additional anomaly might have given rise to an embryo mosaic for maternal but not paternal genes. In zygotes collected without colchicine administration to the mother, one of ten anaphases had a lagging chromosome. Reciprocal crosses with the translation T163H as a marker chromosome at late prophase, when the male- and female-derived chromosome groups have not yet combined, demonstrate that the group derived from the larger pronucleus and having less condensed chromosomes is of paternal origin. T163H undergoes Mendelian segregation in female heterozygotes.
Keywords: fertilization, pronuclei, T163H translocation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R. Anomalies of fertilization leading to triploidy. J Cell Comp Physiol. 1960 Nov;56(Suppl 1):1–15. doi: 10.1002/jcp.1030560404. [DOI] [PubMed] [Google Scholar]
- Carr D. H. Chromosome studies in selected spontaneous abortions. Polyploidy in man. J Med Genet. 1971 Jun;8(2):164–174. doi: 10.1136/jmg.8.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. P., Lyon M. F., Daglish M. A mouse translocation giving a metacentric marker chromosome. Cytogenetics. 1967;6(2):105–119. doi: 10.1159/000129933. [DOI] [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S. Radiation-sensitivity differences within cell-division cycles during mouse cleavage. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;10(2):151–164. doi: 10.1080/09553006614550201. [DOI] [PubMed] [Google Scholar]
- Russell L. B., Woodiel F. N. A spontaneous mouse chimera formed from separate fertilization of two meiotic products of oogenesis. Cytogenetics. 1966;5(1):106–119. doi: 10.1159/000129889. [DOI] [PubMed] [Google Scholar]
- Schindler A. M., Mikamo K. Triploidy in man. Report of a case and a discussion on etiology. Cytogenetics. 1970;9(2):116–130. [PubMed] [Google Scholar]
- Tettenborn U., Gropp A. Meiotic nondisjunction in mice and mouse hybrids. Cytogenetics. 1970;9(4):272–283. doi: 10.1159/000130097. [DOI] [PubMed] [Google Scholar]
- Vickers A. D. Delayed fertilization and chromosomal anomalies in mouse embryos. J Reprod Fertil. 1969 Oct;20(1):69–76. doi: 10.1530/jrf.0.0200069. [DOI] [PubMed] [Google Scholar]