Abstract
There is paucity of information from eastern India with regard to observed dominant micro-organisms causing febrile neutropenia (FN) in patients with haematological malignancies. To identify the prevalence of pathogenic microorganisms associated with FN. A total number of 268 episodes of FN were analysed from September’2010 to October’2013. The blood samples were inoculated into brain heart infusion broth, glucose broth, Hicombi dual performance media (Himedia, LQ-12) at 37° C for 168 h and Bactec method was also performed for these samples. Blood agar, chocolate agar, MacConkey’s agar and cystine lactose electrolyte deficient agar were used for isolation of the microorganisms. A total number of 78 (29.10 %) episodes revealed positive growths. Gram negative bacilli and Gram positive cocci were isolated in 61.53 and 34.61 % cases respectively. The eight commonest isolates were Pseudomonas aeruginosa (14.10 %), methicillin resistant Staphylococcus aureus (MRSA-12.82 %), Acinetobacter sps (11.53 %), coagulase negative Staphylococcus (10.25 %), Klebsiella pneumoniae (8.97 %), Escherichia coli (8.97 %), ESBL E. coli (6.41 %), methicillin sensitive S. aureus (MSSA-6.41 %). Amongst other less common isolates were Citrobacter kosseri (3.84 %), Citrobacter freundii (2.56 %), Ralstonia paucula (2.56 %), Cedecia neteri (1.28 %), methicillin resistant coagulase negative Staphylococcus (2.56 %). Candida spp. including two cases of Candida non-albicans was isolated in 3.84 % of cases. P. aeruginosa was the commonest pathogenic isolates in FN patients associated with haematological malignancies in this study. Gram negative bacteria were the commonest isolates in FN including significant numbers of rare opportunistic micro-organisms.
Keywords: Blood stream infection, Febrile neutropenia, Gram negative organisms
Introduction
Febrile neutropenia (FN) is considered a medical emergency with a mortality rates of up to 70 % if initiation of antibiotics was delayed [1]. Bacteremia is documented in only 10–25 % of FN episodes and clinically documented infections are found in 20–30 % [2]. Signs and symptoms of infection are often subtle in the neutropenic patient [3] so, the empiric therapy is used to cover the most virulent pathogens that may rapidly cause serious or life-threatening infections. There is paucity of data from this part of the country and this study was conducted to identify the prevalence of pathogenic microorganisms causing infection in FN patients.
Aims
To identify the prevalence of pathogenic microorganisms associated with FN.
Materials and Methods
This prospective study was conducted at a tertiary hospital for a period of over 3 years from September’ 2010 to October’2013. Patients admitted in the Hospital with an underlying haematological malignancy and developed FN were included in this study. The medical history including diagnosis, chemotherapy, relapse, duration and severity of neutropenia, granulocyte colony stimulating factor (G-CSF) use and other infection related factors, i.e. antifungal therapy for a probable or proven fungal infection within past 6 months, prophylactic antibiotics or antifungal agents was noted carefully.
Fever was defined as single oral temperature of ≥38.3 °C or oral temperature of ≥38.0 °C that persists for over 1 h. Neutropenia was defined as an absolute neutrophil count (ANC) of ≤500 cells/mm3. Bacteraemia was defined as growth of an organism that was judged not to be a contaminant in a blood culture drawn during a febrile episode. Blood cultures (including urine culture, stool culture etc.) were done as per local protocol.
Total of two hundred and sixty eight blood samples were collected from patients of various ages. Blood was collected aseptically from peripheral vein. 10 ml of blood from an adult and 2–5 ml from a young child was collected and equally dispensed into 2 culture bottles, one containing glucose broth, other brain heart infusion broth and same amount of blood was also taken for BACTEC culture. Culture bottles were clearly labeled with the name, number of the patient and the date and time of collection. Culture bottles were then incubated at 37 °C for up to 7 days or for a maximum of 14 days anaerobically and aerobically. Bottles were macroscopically examined daily for visible evidence of bacterial growth such as turbidity, haemolysis, gas bubbles and clots. For positive blood samples, subcultures were made onto blood agar, chocolate agar and MacConkey agar media. Characteristic colonies thus produced were examined and identified morphologically and biochemically. The organisms were identified through monitoring general biochemical tests such as catalase, oxidase, Triple Sugar Iron agar (TSI), citrate utilization (Simmon’s citrates medium), urease (Christensen’s Urea Agar), indole, motility, H2S production (Sulphide Indole Motility Medium), esculin hydrolysis (Bile esculin agar), and sugar fermentation tests as per standard procedures [4]. All culture Media were provided by Himedia Laboratories Pvt. Ltd., Mumbai; India.
Results
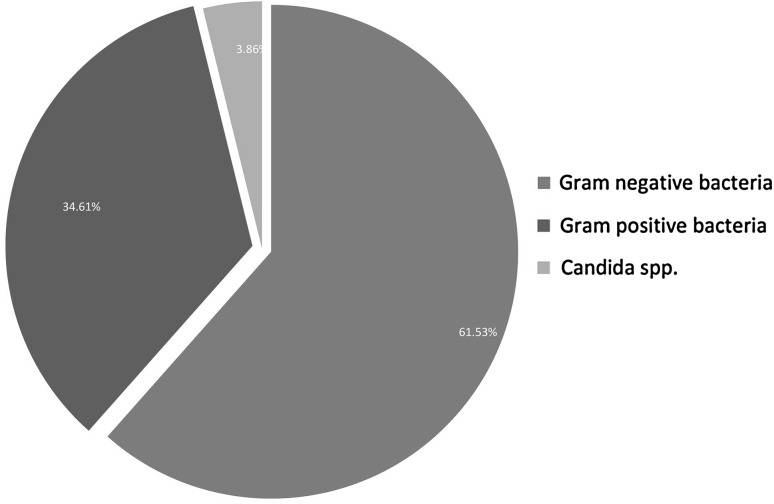
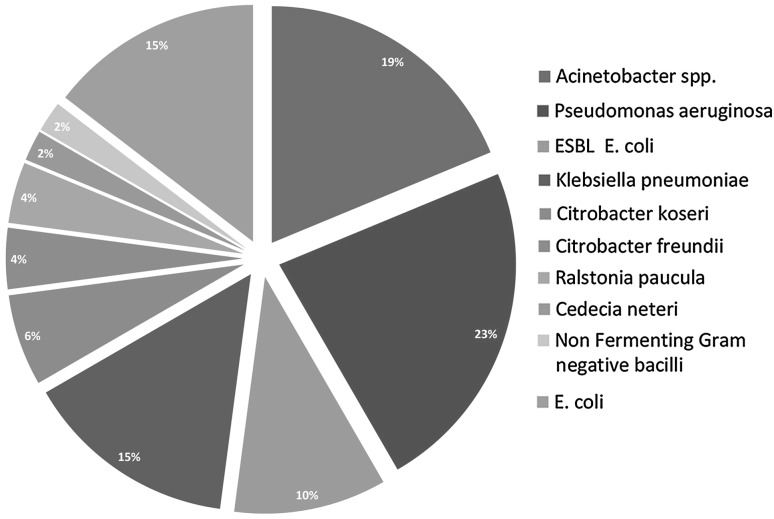
The data analysis of present prospective study revealed that, in a total of 268 blood cultures, 78 yielded microbial growths (Table 1) and in 190 episodes no organism was grown. An incidence of positive blood culture was 29.10 % (78/268) which includes (Fig. 1) Gram negative bacilli 61.53 % (48/78) and Gram positive cocci 34.61 % (27/78). The common Gram negative organisms (Fig. 2) isolated in this group are Pseudomonas aeruginosa (14.10 %), Klebsiella spp. (8.97 %) and Escherichia coli (8.97 %). Acinetobacter spp. was isolated in nine (11.53 %) cases, defined in the group of Enterobacteriaceae. Citrobacter koseri (3.84 %), Citrobacter freundii (2.56 %), Cedecia neteri (1.28 %) are the remote bacteria from the pragmatic patients. Some unusual bacteria like Ralstonia paucula was also found in 2.56 % of gram negative isolates.
Table 1.
Microorganisms isolated
| Sl. No. | Organism | Cases, in respect to total growth positivity (n = 78), No (%) | Cases (%) in respect of total episodes (n = 268) |
|---|---|---|---|
| 1. | Pseudomonas aeruginosa | 11 (14.10) | 4.10 |
| 2. | Methicillin resistance Staphylococcus aureus (MRSA) | 10 (12.82) | 3.73 |
| 3. | Acinetobacter spp. | 9 (11.53) | 3.35 |
| 4. | Coagulase negative Staphylococcus (CONS) | 8 (10.25) | 2.98 |
| 5. | Escherichia coli | 7 (8.97) | 2.61 |
| 6. | Klebsiella pneumoniae | 7 (8.97) | 2.61 |
| 7. | Extended Spectrum Beta Lactamase (ESBL) Escherichia coli | 5 (6.41) | 1.86 |
| 8. | Methicillin sensitive Staphylococcus aureus (MSSA) | 5 (6.41) | 1.86 |
| 9. | Methicillin resistance Coagulase negative Staphylococcus (MR-CONS) | 2 (2.56) | 0.74 |
| 10. | Streptococcus pneumoniae | 1 (1.28) | 0.37 |
| 11. | Candida albicans | 1 (1.28) | 0.37 |
| 12. | Citrobacter koseri | 3 (3.84) | 1.11 |
| 13. | Citrobacter freundii | 2 (2.56) | 0.74 |
| 14. | Ralstonia paucula | 2 (2.56) | 0.74 |
| 15. | Cedecia neteri | 1 (1.28) | 0.37 |
| 16. | Non Fermenting Gram negative bacilli | 1 (1.28) | 0.37 |
| 17. | Streptococcus viridians | 1 (1.28) | 0.37 |
| 18. | Candida nonalbicans | 2 (2.56) | 0.74 |
| Total positive growth | 78 | 29.10 |
Fig. 1.
Different types of organism isolated
Fig. 2.
Different types of Gram negative organisms isolated. ESBL extended spectrum beta lactamase
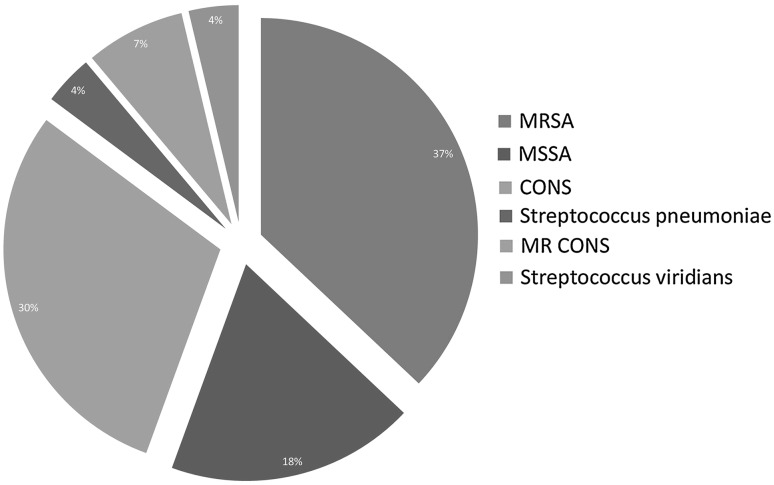
Gram-positive bacteria (Fig. 3) accounted for one third of microbiologically documented infections. Methicillin resistance Staphylococcus aureus (MRSA-12.82 %) and coagulase negative Staphylococcus spp. (CONS-10.25 %) were the main Gram positive pathogens. Of the 27 Gram positive isolates, S. aureus susceptible to methicillin was detected in 6.41 % cases. Candida spp. (both C. albicans and C. nonalbicans) were present in three (3.86 %) cases.
Fig. 3.
Different types of Gram positive organisms isolated. MRSA methicillin resistance Staphylococcus aureus, MSSA methicillin sensitive Staphylococcus aureus, CONS coagulase negative Staphylococcus, MR CONS methicillin resistance coagulase negative Staphylococcus
Discussion
Blood stream infections (BSIs) are a cause of significant morbidity and mortality in neutropenic patients. The causative organisms of BSIs have changed over time. In the 1960s to the 1970s, Gram negative bacteria were more predominant causative agents, but over the last few decades there has been a shift toward Gram positive bacteria due to routine use of anti microbial prophylaxis. Our study however revealed that Gram negative bacteria are predominant isolates from FN patients. Gram-negative bacterial infections represent two third of the microbiologically documented infections in most febrile series of neutropenic patients [5–9]. The high occurrence of non-lactose fermenters especially Pseudomonas spp. and Acinetobacter spp. was a matter of concern. Both of these are associated with a high degree of resistance to antibiotics. It was documented that infections with P. aeruginosa have been associated with increased mortality. The most frequently identified species were P. aeruginosa in this study. P. aeruginosa was isolated in 50 % of neutropenia cases with overall mortality around 10 % in other studies [6, 7, 10–12]. In a study [13] from All India Institute of Medical Sciences, New Delhi, India analyzing for BSIs, pathogen profiles and antimicrobial resistance in FN patients with haematological cancers and bone marrow failure syndromes showed Acinetobacter spp. emerging as common pathogen. Acinetobacter and pseudomonas together were responsible for 42.9 % of all BSIs and both displayed very high resistance to all major classes of antibiotics, including multidrug resistance and ESBL production.
β-lactamases are one of the most important mechanisms of resistance in Gram negative bacteria. Based on this finding, several studies have recommended for first-line empiric antibiotic therapy in gram negative infections [6, 10–12, 14, 15]. Extended Spectrum Beta Lactamase (ESBL) E.coli was encountered in 6.41 % of the total growths in our study. Acinetobacter spp. and Citrobacter spp. have emerged as prominent multi drug resistant (MDR) bacteria and their occurrence in the setting of malignancy could be disastrous. R. paucula and C. neteri were grown in few patients and these opportunistic organisms are very rarely reported in FN cases. Use of gram negative prophylaxis (e.g. ciprofloxacin and levofloxacin) for neutropenic fever has caused the emergence of gram positive organisms as dominant infective pathogens. Among patients with FN, 80 % of gram positive bacteraemia were caused by CONS and viridians Streptococci. CONS are the most common cause of infection in majority of studies [16–19]. Staphylococcal bacteremia results in a low mortality rate, whereas oral streptococci, which are responsible for up to 39 % of infections in neutropenic hosts [20], have been associated with mortality rates of 4–22 % [21, 22]. In a recent study [23] from India retrospectively analyzing the clinical profile and bacterial spectrum and sensitivity patterns of pathogens in febrile neutropenic patients in hematological malignancies revealed gram negative organisms are the predominant organisms with E. coli as major isolate while S. aureus representing the most common gram positive organism.
In addition to gram-negative and gram positive bacteremias, Candida spp. (both C. albicans and non-albicans Candida spp.) were also found in our study. Invasive fungal infection is becoming increasingly important in critically ill patients. Candida has become the fourth most common organism responsible for BSIs in the intensive care unit (ICU) [24, 25].
In conclusion, in approximately one third of cases of FN, causative organism could be identified. Gram negative followed by gram positive organisms and Candida spp. are common isolated pathogens. Isolation of MDR bacteria (Acinetobacter spp. and Citrobacter spp.) and growth of unusual organism (R. paucula and C. neteri) are important findings in this study. Therefore, blood cultures and sensitivity pattern in the locality are essential for surveillance purposes to guide clinicians on proper management of FN patients.
Acknowledgments
Conflict of interest
The authors of this paper have no conflicts of interest.
References
- 1.Schimpff S, Satterlee W, Young VM, Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971;284(19):1061–1065. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- 2.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 3.Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in gran-ulocytopenic patients. Arch Intern Med. 1975;135(5):715–719. doi: 10.1001/archinte.1975.00330050089015. [DOI] [PubMed] [Google Scholar]
- 4.Cheesbrough M. District laboratory practice in tropical countries (part 2) 2. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 5.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationship between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 6.Cometta A, Zinner S, de Bock R, Calandra T, Gaya H, Klastersky J, et al. Piperacillin-tazobactam plus amikacin versus ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. Antimicrob Agents Chemother. 1995;39(2):445–452. doi: 10.1128/AAC.39.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cometta A, Calandra T, Gaya H, Zinner SH, de Bock R, Del Favero A, et al. Monotherapy with meropenem versus combination therapy with ceftazidieme plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto Infection Program. Antimicrob Agents Chemother. 1996;40(5):1108–1115. doi: 10.1128/aac.40.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordonnier C, Pico JL, Gardembas M, Gardembas M, Delmer A, Delain M, et al. Cefepime/amikacin versus cefatzidime amikacin as empirical therapy for febrile episodes in neutropenic patients: a comparative study. Clin Infect Dis. 1997;24(1):41–51. doi: 10.1093/clinids/24.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Glauser M, Boogaerts M, Cordonnier C, Palmblad J, Martino P. Empiric therapy of bacterial infections in severe neutropenia. Clin Microbiol Infect. 1997;3(s1):77–86. doi: 10.1111/j.1469-0691.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 10.Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25(2):247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 11.Hughes WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, Feld R, et al. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Clin Infect Dis. 1997;25(3):551–573. doi: 10.1086/513764. [DOI] [PubMed] [Google Scholar]
- 12.Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 13.Sood P, Seth T, Kapil A, Sharma V, Dayama A, Sharma SK, et al. Emergence of multidrug resistant acinetobacter blood stream infections in febrile neutropenia patients with haematological cancers and bone marrow failure syndromes. J Indian Med Assoc. 2012;110:439–444. [PubMed] [Google Scholar]
- 14.Schimpf S, Satterelee W, Young VM, Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971;284(19):1061–1065. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- 15.Hughes WT, Armstrong DA, Bodey GP, Feld R, Mandell GL, Meyers JD, et al. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. J Infect Dis. 1990;161(3):381–396. doi: 10.1093/infdis/161.3.381. [DOI] [PubMed] [Google Scholar]
- 16.Engelhard D, Elishoov H, Strauss N, Naparstek E, Nagler A, Simhon A, et al. Nosocomial coagulase negative staphylococcal infections in bone marrow transplantation recipients with central vein catheter. A 5-year prospective study. Transplantation. 1996;61:430–434. doi: 10.1097/00007890-199602150-00020. [DOI] [PubMed] [Google Scholar]
- 17.Viscoli C, Castagnola E, Giacchino M, Cesaro S, Properzi E, Tucci F, et al. Bloodstream infections in children with cancer: a multicentre surveillance study of the Italian Association of Paediatric Haematology and Oncology. Supportive Therapy Group-Infectious Diseases Section. Eur J Cancer. 1999;35:770–774. doi: 10.1016/S0959-8049(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 18.Madani TA. Clinical infections and bloodstream isolates associated with fever in patients undergoing chemotherapy for acute myeloid leukemia. Infection. 2000;28(6):367–373. doi: 10.1007/s150100070007. [DOI] [PubMed] [Google Scholar]
- 19.Ninin E, Milpied N, Moreau P, André-Richet B, Morineau N, Mahe B, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001;33:41–47. doi: 10.1086/320871. [DOI] [PubMed] [Google Scholar]
- 20.Reed EC. Infectious complications during autotransplantation. Hematol Oncol Clin North Am. 1993;7(3):717–735. [PubMed] [Google Scholar]
- 21.Bochud PY, Eggiman T, Calandra T, Van Melle G, Saghafi L, Francioli P. Bacteremia due to viridans Streptococcusin neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis. 1994;18(1):25–31. doi: 10.1093/clinids/18.1.25. [DOI] [PubMed] [Google Scholar]
- 22.Kern W, Kurrle E, Schmeiser T. Streptococcal bacteremia in adult patients with leukemia undergoing aggressive chemotherapy: a review of 55 cases. Infection. 1990;18:138–145. doi: 10.1007/BF01642101. [DOI] [PubMed] [Google Scholar]
- 23.Karanwal AB, Parikh BJ, Goswami P, Panchal HP, Parekh BB, Patel KB. Review of clinical profile and bacterial spectrum and sensitivity patterns of pathogens in febrileneutropenic patients in hematological malignancies: a retrospective analysis from a single center. Indian J Med Paediatr Oncol. 2013;34(2):85–88. doi: 10.4103/0971-5851.116184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3(11):685–702. doi: 10.1016/S1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 25.Ostrosky-Zeichner L, Pappas PG. Invasive candidiasis in the intensive care unit. Crit Care Med. 2006;34(3):857–863. doi: 10.1097/01.CCM.0000201897.78123.44. [DOI] [PubMed] [Google Scholar]