Abstract
Whole blood donation is generally a safe procedure, but sometimes adverse reactions of varying severity may occur during or at completion of blood donation process. The aim of the present study was to estimate the frequency and type of adverse events during blood donation. This retrospective study conducted from November 2011 to December 2012 at Department of Blood Transfusion Medicine GMC Jammu. All whole blood donations at our Department was analyzed. All adverse events occurring during or at end of donation were noted using standardized format. Overall 108 adverse events were reported in relation to 29,524 donations, resulting in overall adverse event rate of 0.365 %. Presyncopal reactions in other words vasovagal reactions of mild intensity, were the most commonly observed adverse reactions and accounted for approximately 58/108 (53.70 %) of all adverse reactions noted. Only 0.365 % of blood donations were complicated by adverse events and most of these events were presyncopal symptoms. Our study reinforces that blood donation is a very safe procedure which could be made even more event free by following certain friendly, reassuring and tactful practices.
Keywords: Vasovagal reactions, Adverse donor reactions, Blood donors
Introduction
Blood is the most precious and unique gift that one human being can give to another. The life saving fluid cannot be created artificially. Blood donors are precious resources. Donor retention is directly linked to donor services and donor care. It is important to provide total satisfaction to donor as customers because only then they would become regular donors and remain loyal to system. Most donors tolerate giving blood very well but occasionally adverse reactions may occur [1]. The adverse reactions that occur in donors can be divided into local reactions and systemic reactions.
Local reactions occur predominantly because of problems related to venous access. They are usually hematomas due to extravasation from veins caused by incorrect placement of the needle during venepuncture. Pain, hyperemia and swelling may develop at the site of extravasation. Other local events include pain due to slight trauma to the subcutaneous nerve endings. In most cases these are non fatal complications that do not require any treatment. Local Phlebitis and thrombophlebitis are more serious complications than the foregoing, but are very rare.
The systemic reactions in contrast to the local reactions can be divided into mild or severe. In most cases, they are vasovagal reactions that can be triggered by the pain of venepuncture, by the donor seeing his or her own blood, by the donor seeing another donor unwell,by the anxiety and state of tension of undergoing the donation etc. The systemic reactions are characterized by the appearance of pallor, sweating, dizziness, Abdominal cramps (due to increased gastrointestinal motility due to increased vasovagal effect), nausea, hypotension and bradycardia. Therapeutic intervention must be swift, otherwise this clinical picture typical of vasovagal reaction will progress to an episode of syncope of variable severity, which may or may not be complicated by the onset of Tonic–clonic muscle spasms (convulsive syncope),accompanied by vomiting and loss of sphincter control [2].
The aim of this study was to estimate the frequency and type of adverse events occurring in whole blood donors at our Regional Blood Transfusion Centre from November 2011 to December 2012.
Materials and Methods
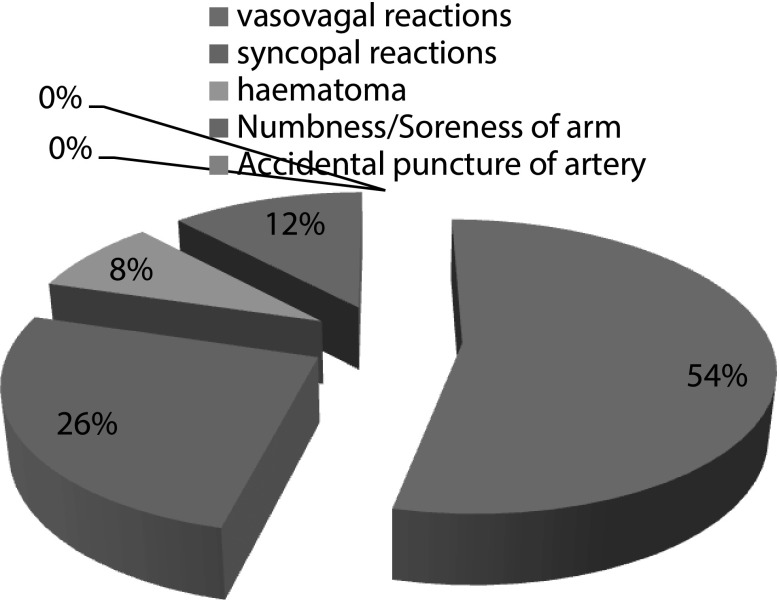
This is a retrospective study, of all adverse reactions related to all the consecutive whole blood donations made between November 2011 to December 2012 at two blood banks of GMC Jammu which is Tertiary Health Care Centre. All donations were collected as per Departmental SOPs. Strict asepsis was maintained by cleaning the site of venipuncture sequentially using betadine and alcohol. The minimum weight required for donation was 45 kg and the lowest acceptable haemoglobin concentration was set at 12.5 g/dl. A warm, friendly and comfortable atmosphere for donors is provided at our department. Those donors who complain of adverse reactions like giddiness, light headedness, pallor are managed by stopping the donation immediately and raising the legs of donor (anti shock position) as pallor, sweating, agitation are harbingers of severe vasovagal reaction which could be prevented by taking corrective measures right at the onset of symptoms. Donors are given refreshment and retained in donor rest room for at least 30 min before being sent away (Fig. 1).
Fig. 1.
Percentage distribution of adverse reactions
The classification scheme employed for recording the adverse events was suggested by the American Red Cross Hemovigilance Program that classifies complications into defined categories with severity ratings (minor/major) for certain types of reaction [3, 4]. Presyncopal symptoms include pallor, sweating or light headedness without loss of consciousness. Syncopal types of complications are classified as minor if there is a transient loss of consciousness lasting less than one minute, while prolonged loss of consciousness for more than a minute or complicated by loss of bowel/bladder control, seizures or convulsions is said to be a major syncopal complication. Local adverse events include haematomas which can be small (<25.8 mm2) or large (>25.8 mm2), bruises, infiltration, allergic reaction and a tingling sensation.
An Adverse Event (AE) was defined as the symptoms or signs of donor discomfort of sufficient severity such that either the donor called for attention of the staff or they were noticed by the staff. Pain at the time of venepuncture was excluded [5].
Results
In our observation, a total of 29,524 donations were recorded amongst which, 26,871 were replacement donors and 2,653 were voluntary donors. Male donors were -29,007 and Female donors were 517. Mean (SD) age of Donors was 32 ± 9 years, with range of 18–60 years. Mean (S.D) weight of donors was 70 ± 9.5 kg, with range of 45–110 kg. Total adverse reactions observed were 108 (0.365 %), amongst which 86 (0.296 %) adverse reactions were seen in males and 22 (4.25 %) adverse reactions were seen in females, p value <0.05, which shows highly significant relationship with respect to sex (Table 1). Mean age of those who experienced adverse donor reactions was 25.94 years. Mean weight of those who experienced adverse donor reactions was 70.25 kg. Repeat blood donors in our study had fewer adverse donor reactions than first time blood donors (1.15 vs 13.429 %), p value <0.05, which shows ADRs have highly significant relationship with respect to first time donation
Table 1.
Adverse donor reactions
| Total | ADR | % ADR | |
|---|---|---|---|
| Male | 29,007 | 86 | 0.296 |
| 1. First time | 7,654 | 65 | 0.849 |
| 2. Repeated | 21,353 | 21 | 0.098 |
| Female | 517 | 22 | 4.25 |
| 1. First time | 143 | 18 | 12.58 |
| 2. Repeated | 374 | 4 | 1.06 |
| Total | 29,524 | 108 |
Presyncopal symptoms, in other words vasovagal reactions of mild intensity were the most commonly observed adverse reactions and accounted for approximately 53.70 % (58/108) of all adverse reactions noted. They affected 0.196 % of donors (58/29,524). Major syncopal reactions accounted for nil. The frequency distribution of the various types of adverse reactions that occurred in donors during the study period is presented in Table 2.
Table 2.
Frequency of various types of adverse donor reactions occurring in donor population
| Type of adverse reaction | Number of donors affected | Percentage |
|---|---|---|
| Systemic complications | ||
| Presyncopal symptoms | 58 | 0.196 |
| Syncopal complications (minor) | 28 | 0.094 |
| Syncopal complications (major) | None | |
| Local complications | ||
| Haematoma | 13 | 0.044 |
| Numbness/tingling/soreness of arm | 9 | 0.030 |
Discussion
From this study, percentage of adverse donor reaction was 0.365 %. This is in accordance with the results of a study conducted all over the world in which rate of adverse donor reaction ranged from 0.3 to 3.8 % [2, 3, 5, 6, 9, 10], Table 3. Male donors reported (86/29007) 0.296 %, adverse donor reactions (ADRs) and female donor reported (22/517) 4.25 % ADRs. Vasovagal reactions of mild intensity were the most commonly observed and accounted for approximately (58/108) 53.70 %. Major syncopal reactions observed was nil in present study, as similar other studies reported very low incidence of severe reactions with no episodes necessitating hospitalization of donor or administration of intravenous fluids [2, 4, 7]. Presyncopal reactions accounted for (58/29,524) 0.196 % and syncopal complications (minor) accounted for (28/29,524) 0.094 %. This is in accordance with the results of a study conducted by Crocco et al. [4] in 2009 who found that vasovagal reactions of mild intensity constituted 71 % of all adverse events.
Table 3.
Comparison of frequency of adverse donor reactions reported in different studies
| S.no | Study | Year | Adverse donor reaction reported |
|---|---|---|---|
| 1 | Crocco et al. | 2007–2009 | 2.2 % |
| 2 | Agnihotri N et al. | 2002–2003 | 2.5 % |
| 3 | Tondon R et al. | 2008 | 1.6 % |
| 4 | Pathak C et al. | 2007–2009 | 0.6 % |
| 5 | Present study | 2008 | 1.6 % |
Among local complications, haematomas was seen in (13/29,524) 0.044 % and numbness/tingling/soreness was seen in (9/29,524) 0.030 % of cases. Local reactions are mainly caused by blood donation related neurological needle injuries which are commonly experienced by the donors after the donation in the form of hematomas, numbness/tingling, excessive or radiating pain, loss of arm/hand strength. The time to recover from these complications can range from less than 3 days to more than 6 months [8].
In our study men were half as likely to have adverse events (ADR-males 0.296 % vs ADR-females 4.25 %). p value <0.05, which shows ADRs have highly significant relationship with respect to sex. Repeat blood donors had fewer ADRs than first time blood donors (1.15 vs 13.429 %). p value <0.05, which shows ADRs have highly significant relationship with respect to first time donation. This is in accordance to study of D.O Kasprisin in which first time donors have higher frequency of reactions 1.7 % than do repeat donors 0.19 % [10, 11].
As only 0.365 % of whole blood donations were complicated by adverse events and most of these events were pre syncopal symptoms, thus our study confirms that blood donation is a very safe procedure which could be made more event free by following, certain friendly, reassuring and tactful practices. In conclusion, blood donations have an obligation to constantly monitor risks of blood donation and to make a concerted and committed effort to achieve the lowest possible rate of complications.
Acknowledgments
We acknowledge Dr. Dinesh Kumar MD, Department of Community Medicine, Government Medical College, Jammu for statistical analysis.
References
- 1.Lung JA, Wilson SD. Development of arteriovenous fistula following blood donation. Transfusion. 1971;11:145. doi: 10.1111/j.1537-2995.1971.tb04392.x. [DOI] [PubMed] [Google Scholar]
- 2.Crocco A, D’Elia D. Adverse reactions during voluntary donation of blood and/or blood components. A statistical–epidemiological study. Blood Transfus. 2007;5:143–152. doi: 10.2450/2007.0005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathak C, Pujani M, Pahuja S, Jain M. Adverse reactions in whole blood donors: an Indian scenario. Blood Transfus. 2011;9:46–49. doi: 10.2450/2010.0002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crocco I, Franchini M, Garozzo G, et al. Adverse reactions in blood and apheresis donors: experience of two Italian transfusion centres. Blood Transfus. 2009;7:35–38. doi: 10.2450/2008.0018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnihotri N, Marwaha N, Sharma RR. Analysis of adverse events and predisposing factors in voluntary and replacement whole blood donors : a study from north India. Asian J Transfus Sci. 2012;6:155–160. doi: 10.4103/0973-6247.98922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eder AF, Hillyer CD, Dy BA, et al. Adverse reactions to allogeneic whole blood donation by 16–17-year-olds. JAMA. 2008;299:2279–2286. doi: 10.1001/jama.299.19.2279. [DOI] [PubMed] [Google Scholar]
- 7.Lin JT, Ziegler DK, Lai CW, Bayer W. Convulsive syncope in blood donors. Ann Neurol. 1982;11:525–528. doi: 10.1002/ana.410110513. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen BS, Johnsen SP, Jorgensen J. Complications related to blood donation: a population based study. Vox Sang. 2008;94:132–137. doi: 10.1111/j.1423-0410.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 9.Newman BH, Waxman DA. Blood donation related neurologica needle injury: evaluation of 2 years worth of data from a large blood center. Transfusion. 1996;3:213–215. doi: 10.1046/j.1537-2995.1996.36396182137.x. [DOI] [PubMed] [Google Scholar]
- 10.Newman BH, Pichette S, Pichette D, Dzaka E. Adverse effects in blood donors after whole-blood donation: a study of 1,000 blood donors interviewed 3 weeks after whole-blood donation. Transfusion. 2003;43:598–603. doi: 10.1046/j.1537-2995.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 11.Kasprisin DO, Glynn SH, Taylor F, Miller KA. Moderate and severe reactions in blood donors. Transfusion. 1992;32:23–26. doi: 10.1046/j.1537-2995.1992.32192116426.x. [DOI] [PubMed] [Google Scholar]